Abstract
Human radial glia (RG) share many of the features described in rodents, but also have a number of characteristics unique to the human brain. Results obtained from different mammalian species including human and non-human primates reveal differences in the involvement of RG in neurogenesis and oligodendrogenesis and in the timing of the initial expression of typical RG immunomarkers. A common problem in studying the human brain is that experimental procedures using modern molecular and genetic methods, such as in vivo transduction with retroviruses or creation of knockout or transgenic mutants, are not possible. Nevertheless, abundant and valuable information about the development of the human brain has been revealed using postmortem human material. Additionally, a combination and spectrum of in vitro techniques are used to gain knowledge about normal developmental processes in the human brain, including better understanding of RG as progenitor cells. Molecular and functional characterization of multipotent progenitors, such as RG, is important for future cell replacement therapies in neurological and psychiatric disorders, which are often resistant to conventional treatments. The protracted time of development and larger size of the human brain could provide insight into processes that may go unnoticed in the much smaller rodent cortex, which develops over a much shorter period. With that in mind, we summarize results on the role of RG in the human fetal brain.
Keywords: Fetal human brain, Neurogenesis, Pax6, Electrical properties
Classical papers, dating from more than 100 years ago (Magini 1888), describe radial glia (RG) as scaffolding for young neurons during their migration into the developing neocortex (reviewed in Bentivoglio and Mozzarello 1999). This view was based on their characteristic orientation in the fetal telencephalon as seen in Golgi impregnated human fetal brains. Later, studies using various experimental methods demonstrated specific cellular and molecular mechanisms of this neuronal-glial interaction (Rakic 1972, 1988, 2003; Hatten 1993; Anton and others 1996). After neurogenesis and neuronal migration are finished, RG cells transform into astrocytes (Rakic 1972; Sidman and Rakic 1973; Hatten 1993).
With the aid of genetic fate mapping, researchers in multiple laboratories almost simultaneously discovered that RG cells produce postmitotic, pyramidal neurons in rodents (Malatesta and others 2000; Miyata and others 2001; Noctor and others 2001). Thus, new evidence clearly indicates that the role of RG is much more complex than being mere guides for migrating cortical neurons. However, studies that corroborate human RG as neuronal progenitors have lagged behind animal research due to difficulties in applying cell fate mapping experiments in humans. Although subhuman mammals provide excellent models of human physiology, extrapolating directly from rodents to humans is not without risk. The human neocortex is structurally and functionally far more complex than that of rodents. The birth of new neurons in the human brain (neurogenesis) occurs over the course of months, not days as in the rodent brain. More importantly, the proteomics of neurogenesis differs between humans and rodents, further indicating that the cellular regulatory processes governing neural progenitors are not identical across species. Although immunohistochemical studies of the human embryonic and fetal neurogerminal layers, the ventricular zone/subventricular zone (VZ/SVZ), suggest that RG give rise to both astrocytes and neurons (Zecevic 2004; Howard and others 2006), direct evidence of this lineage relationship has been lacking. Recently, however, we demonstrated that RG derived from human midgestational brain tissue are the direct parental cells of a subpopulation of forebrain neurons (Mo and others 2007).
This discovery not only confirmed the results of animal studies, but, more importantly, has brought to the forefront a series of interesting questions regarding the role of progenitor cells in the development of the human brain in health and disease. In this review, we will focus on the role of RG as neural progenitor cells in human cortical development while contrasting this idea with studies in various other species.
RG in the Human Fetal Brain
In both primates and subprimate vertebrates, RG cells are derived from the earliest cells found in the wall of the neural tube, called neuroepithelial cells. Transformation of neuroepithelial cells into RG starts at the beginning of neurogenesis (Götz and Huttner 2005). During early stages of human development, 5 to 7 gestation weeks (gw), when the width of the telencephalic wall is narrow, RG span from the ventricular to pial surface, making them morphologically indistinguishable from bipolar neuroepithelial cells (Fig. 1A, B). Later, as the width of the human cerebral wall increases and incipient convolutions begin to develop (around 15 gw), RG elongate and obtain a bipolar shape with their cell bodies situated in the VZ (Fig. 1). The shorter apical process attaches to the ventricle, whereas the radially oriented, longer process becomes more complex and curved with terminal branching into multiple endfeet at the pial surface, which is characteristic of RG in primates (e.g., Marin-Padilla 1988, 1995; Rakic 1995). Ultrastructural and immunohistochemical characteristics, including electrolucent cytoplasm, lamellate expansions, the presence of intermediate filaments, glycogen granules in the endfeet, and typical immunomarkers confirm the glial nature of these cells (Choi 1986; Rakic 1972, 1995; Götz 2003; Zecevic 2004).
Fig. 1.
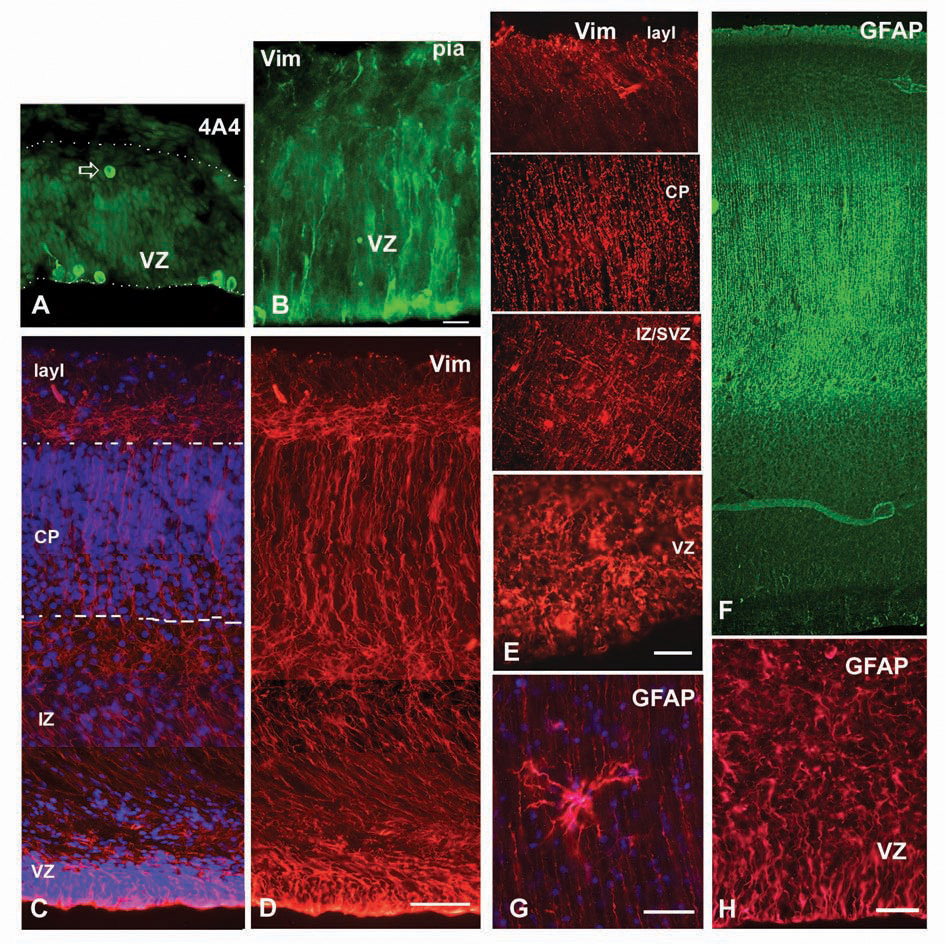
Astroglial markers in the embryonic and fetal human forebrain. (A) 4A4+ cells in the ventricular zone (VZ) of the 4.5-gestation week (gw) embryo. Note a cell close to pia (arrow). Dotted lines mark pia and ventricular surface. (B) At 5 gw, radially oriented vimentin+ fibers and dividing cells by the VZ surface. (C, D) At 15 gw, long vimentin+ fibers span the entire thickness of the medial telencephalic wall. Dashed lines mark cortical plate (CP). (E) Four regions along the telencephalic wall at midgestation (22 gw) show differential orientation of vimentin+ fibers. Note a “meshlike” distribution in the intermediate zone (IZ). (F) Glial fibrillary acidic protein (GFAP) labels radial glia at midgestation and (G) an occasional mature looking astrocyte in the lower portion of the cortical plate. (H) Higher magnification of GFAP-labeled ventricular zone/subventricular zone (VZ/SVZ) at midgestation. Blue-bisbenzamide labeling. Scale bars: A, B, C, D, E, H, 50 µm; G, 20 µm.
Immunohistochemical Characteristics of RG in Different Species
Variation in the expression of antigenic proteins across the spectrum of species accounts for some of the earliest evidence that the neural progenitor population differs taxonomically. For example, in primates, the intermediate filament proteins, glial fibrillary acidic protein (GFAP) and vimentin, are expressed concomitantly in RG from the initiation of neurogenesis at 5 to 6 gw in humans (Levitt and others 1981; Kadhim and others 1988; Cameron and Rakic 1991; Zecevic and others 1999; Zecevic 2004; Howard and others 2006). In contrast, in rodent RG, GFAP is expressed in cells of astrocytic lineage only after neurogenesis is complete and vimentin is no longer detectable (Voigt 1989; Cameron and Rakic 1991).
Markers that distinguish RG from neuroepithelial cells in rodents, such as vimentin (e.g., Dahl and others 1981), astrocyte-specific glutamate transporter (GLAST) (Shibata and others 1997), and brain lipid-binding protein (BLBP) (Feng and others 1994), also label human RG cells often in a nonoverlapping fashion (Howard and others 2006). Similarly, various subpopulations of RG cells are distinguished in rodents by the expression of transcription factors (Götz and others 1998), molecular markers (Hartfuss and others 2001; Liour and Yu 2003; Fishell and Kriegstein 2003; Gongidi and others 2004), and morphologies (Alves and others 2002). Notably, RG markers change during the course of normal development as well as with the region of the brain, which is of particular interest because these parameters may be predictive of neuronal, glial, or multipotent potential. For example, cells expressing BLBP may predict a neurogenic fate, and those expressing GLAST, a gliogenic fate (Hartfuss and others 2001). Yet, other studies argue that BLBP expression does not define a separate, gliogenic subclass, but rather signifies a developmental shift in all RG cells toward production of neurons (Anthony and others 2004). Experiments with acutely cultured midgestational VZ/SVZ cells have shown that the human RG population is heterogeneous with respect to BLBP and GLAST expression (Fig. 4 in Howard and others 2006). Furthermore, BLBP+ cells from human fetal VZ/SVZ are heterogeneous and generate both cortical neurons and astrocytes in vitro (see below; Mo and others 2007).
Fig. 4.
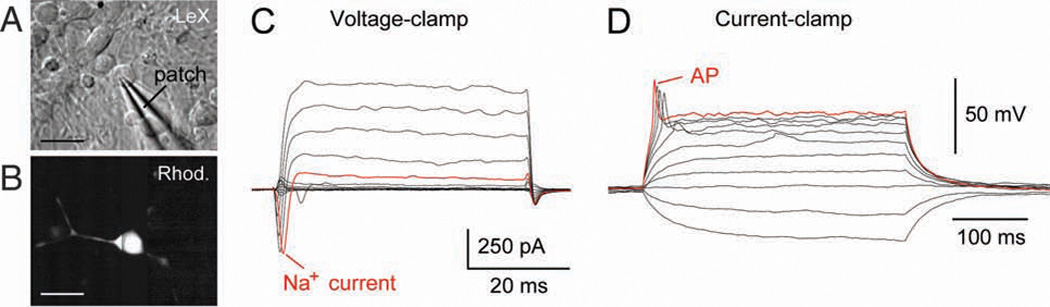
Electrophysiological characterization of human cells in vitro. (A) Infrared image of a human fetal LeX+ cell in the culture dish. Electrical recording is carried out using a glass pipette (patch) in whole-cell configuration. (B) Same cells as in A after the injection of fluorescent dye rhodamine. (C) Transient inward current (Na+ current) obtained from a human LeX+ cell in culture. (D) The same cell as in C except the recoding configuration is switched from voltage to current clamp. A series of current pulses is injected into the cell to study the membrane excitability. Triggering of an action potential (AP) suggests neuronal lineage. Scale bar: A, B, 10 µm
Actively dividing RG cells are visualized using 4A4 antibody, a property conferred by vimentin phosphorylation during M-phase (Kamei and others 1998). Using 4A4 antibody, RG are revealed in the human embryo between 5 and 6 gw, in a distinct caudorostral gradient, from the nascent spinal cord and rhombencephalon, to the telencephalon. Anti-vimentin antibody identifies quiescent and dividing RG, whereas 4A4+ cells lay along the VZ surface (Howard and others 2006) and occasionally under the pia (Fig. 1A, B). Subpial 4A4+ cells possibly correspond to basal progenitors described in mice (Haubensak and others 2004). By 4.5 gw, SMI-31, an antibody specific for neuronal lineage, labels cells below the pia (Fig. 2, inset), probably representing so-called predecessor neurons demonstrated at Carnegie stage 12 (31 days) in the rostral end of the human neural tube. Interestingly, predecessor neurons are believed to have noncortical origin (Bystron and others 2006).
Fig. 2.
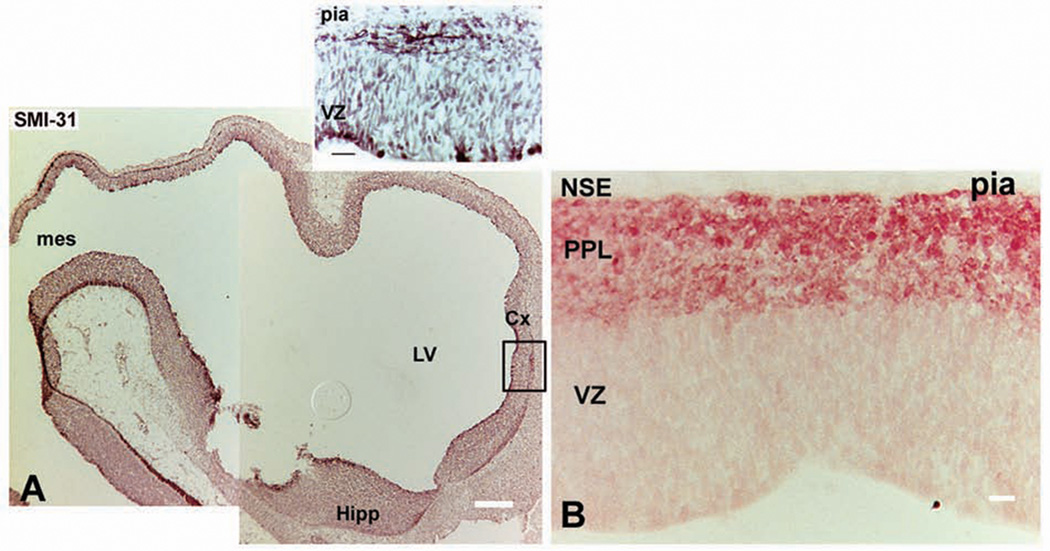
(A) Immunolabeling with neuronal marker SMI-31 at the rostral end of neural tube at 4.5 gestation weeks (gw). (Inset, boxed area in A) SMI-31+ cells dividing in the ventricular zone (VZ) and processes in the emerging primordial plexiform layer (PPL), below the pia. (B) Neuron-specific enolase (NSE) labels young neurons in the PPL at 7 to 8 gw. Scale bars: A, 200 µm; inset, 20 µm; B, 20 µm.
At 5 to 6 gw, with initiation of cortical neurogenesis, young neurons are distributed in the primordial plexiform layer (PPL; Rakic and Zecevic 2003; Zecevic 2004; Howard and others 2006). Two weeks later, at the emergence of the cortical plate, cells stained with NeuN and neuron-specific enolase (NSE), markers of mature neurons, reside in the PPL (Fig. 2B). It is tempting to speculate that these neurons, besides mature molecular composition, are endowed with additional features of mature neurons, such as the ability to generate sodium and calcium spikes, which may guide the migration of cortical neurons. Namely, various patterns of endogenously generated neural activity may sculpt the precise circuits of the developing brain (Purves and Lichtman 1985).
By midgestation, at 20 gw, the bulk of neurogenesis in the human cerebral cortex has occurred and RG start transforming into GFAP+ astrocytes in the intermediate zone (IZ) and in the cortical plate (Fig. 1G; de Azevedo and others 2003; Zecevic 2004). Although this indicates that RG have begun the shift from generating neurons to astroglia, RG with bipolar morphology and processes labeled with GFAP and vimentin still span the telencephalic wall (Fig. 1E, F), suggesting their retained neurogenic potential at this period. This is consistent with the fact that the human SVZ remains a major proliferative region in primate brains at later stages of corticogenesis (Smart and others 2002; Zecevic and others 2005). Direct evidence for the neurogenic potential of RG at 20 gw will be elucidated below. At this age, radial and tangentially running processes of vimentin+ RG form a gridlike structure at the border of the IZ and SVZ (Fig. 1E; Ulfig and others 1999; Zecevic 2004). This organization of RG fibers suggests that they may assist tangential migration of interneurons through the IZ/SVZ (Zecevic 2004).
Where Do Human Cortical Neurons Come From?
Neural stem cells (NSCs) generate mature neurons, astrocytes, and oligodendrocytes through a stepwise series of progenitor cell divisions that progressively yield more restricted daughter cells (Levitt and others 1981; Luskin and McDermott 1994; McConnell 1995; Tan and others 1998). The debate lies in determining which cell type retains NSC properties of pluripotency and immortality and which type is the first to become restricted. Whether RG are truly stem cells is a topic that is currently being deliberated. RG, in their familiar, developmental form, terminally differentiate either into astrocytes or intermediate progenitors (IPs) that generate two neurons in the SVZ (Noctor and others 2004; Miyata and others 2004; Martinez-Cerdeno and others 2006). In addition, in the adult SVZ, direct descendants of RG, termed type B astrocytes, self-renew limitlessly and retain neuropoietic capacity, indicating that indeed at least a subpopulation of RG fulfill neural stem cell criteria (Alvarez-Buylla and others 2001; Laywell and others 2000; Alves and others 2002; Merkle and others 2004; Sanai and others 2004).
Numerous reports using rodent models identify RG cells as the only cell with the capability to give rise to cortical neurons (Malatesta and others 2000; Miyata and others 2001; Noctor and others 2001, 2004; Weissman and others 2003; reviewed by Kessaris and others 2006). Comparatively in humans, in addition to RG, which have the capability to give rise to all three neural lineages, restricted neuronal progenitors appear to be present from the earliest stages of corticogenesis (Zecevic 2004; Howard and others 2006; Mo and others 2007).
RG and Cortical Neurons in the Developing Human Brain
It is worth noting that, until this past year, RG were not directly shown to generate neurons in humans, and the belief that RG are neurogenic in man was extrapolated from studies in rodents (Malatesta and others 2000; Miyata and others 2001; Noctor and others 2001, 2004; Weissman and others 2003) and immunohistochemical experiments using fixed human embryonic and fetal tissue (Weissman and others 2003; Zecevic, 2004; Howard and others 2006). In vitro experiments with human fetal RG isolated from midgestational VZ/SVZ by immunopanning showed neurons to be the immediate progeny of RG in humans (Mo and others 2007). Immunopanning was done with an antibody against LeX (fucose N-acetyl lactosamine, otherwise called SSEA1 or CD15), a cell surface molecule expressed by mouse embryonic stem cells (e.g., Kim and Morshead 2003; Capela and Temple 2006). This antibody also identified human fetal VZ/SVZ cells that self-renewed and had high levels of nestin mRNA expression, typical of stem-like cells (Mo and others 2007). In our in vitro experiments, the purity of immunopanned cells was 95%, as determined by subsequent LeX immunolabeling (Fig. 3A–C). LeX+ cells were highly prolific, which was consistent with their high frequency (40%) of neurosphere formation in uncoated dishes (Fig. 3D). Neurospheres derived from LeX+ cells, when allowed to differentiate, generated mainly astrocytes (84%) and a small number of β-III-tubulin+ neurons (13%; Fig. 3E).
Fig. 3.
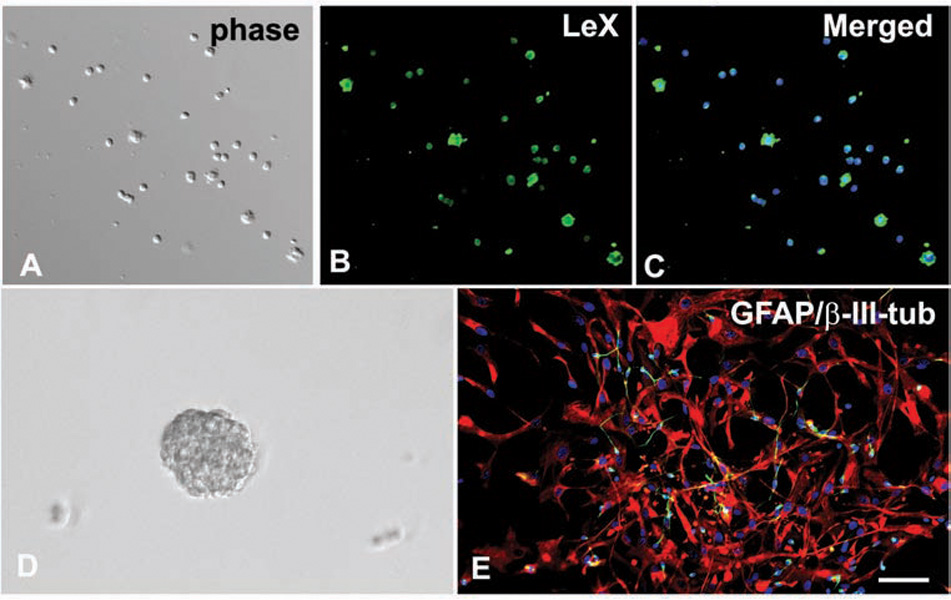
LeX+ cells isolated from the human forebrain at 20 gestation weeks (gw). (A) Acute cell culture under phase contrast. (B, C) The cells are immunostained with LeX antibody (green). (D) Floating neurospheres derived from LeX+ cells form in expansion medium after 7 days in vitro. (E) In differentiation medium, neurospheres attach to the surface and differentiate mainly into GFAP+ astrocytes (red) and occasionally into neurons labeled with β-III-tubulin (green). Blue-cell nuclei are stained with bisbenzimide. Scale bar: 50 µm.
Whole-cell recordings are emerging as a powerful experimental tool for studying the developing brain (Kerkovich and others 1999; Noctor and others 2001; Picken Bahrey and Moody 2003; Noctor and others 2004; Zhang 2004). Biophysical characteristics of the cell membrane, such as the amplitude and dynamics of transmembrane currents, can be used to complement cell type identification in mixed cultured systems and other in vitro preparations (Chiu and others 1994; Kerkovich and others 1999; Piper and others 2000; Calhoun and others 2003; Balasubramaniyan and others 2004; Pagani and others 2006; Johnson and others 2007). In the example shown in Figure 4, a cultured human LeX+ cell was patched in whole-cell configuration (Fig. 4A). The fluorescent dye rhodamine was injected into the cytoplasm to trace the processes and reveal cellular morphology (Fig. 4B). While the cell was slowly injected with the dye, electrical recordings were performed to assess membrane properties. Voltage-clamp recordings detected a substantial tetrodotoxin (TTX)-sensitive Na+ current in 7 of 19 human LeX+ cells (Fig. 4C). These cells were capable of firing an action potential (AP) upon direct current depolarization (Fig. 4D). Since neurons are the only cellular elements in the CNS capable of firing a sodium action potential, the results shown in Figure 4 unequivocally demonstrate that a substantial fraction of LeX+ cells were dedicated neuron progenitors.
In addition, a series of immunocytochemical and electro-physiological experiments confirmed that β-III-tubulin was specific for neuronal cells and not transiently expressed in RG destined to become astrocytes. When LeX+ cells with neuronal morphology were patched with rhodamine-filled pipettes, they produced transient sodium current in voltage-clamp mode, and a regenerative spikelet in response to direct current injection. Antibody to β-III-tubulin subsequently labeled the rhodamine-filled cells, indicating that LeX+/β-III-tubulin+ cells were neuronal progenitors (Mo and others 2007).
To confirm that these LeX+ progenitors that generated neurons were, in fact, RG, we transfected them with RG-specific plasmids, enhanced green fluorescent protein (EGFP) driven by the BLBP (Schmid and others 2006) or the human GFAP (hGFAP) promoter (de Leeuw and others 2006). When transfected (green) cells differentiated, the vast majority became GFAP+ astroglia (Fig. 5A–C), but occasional cells (0.5%) were colabeled with β-III-tubulin and had morphology of young neurons (Fig. 5D–F). Finally, since BLBP-EGFP and hGFAP-EGFP were presumably downregulated in transfected cells that differentiated into neurons, LeX+ cells were cotransfected with BLBP-Cre and CAGGS-loxP-LacZ-loxP-YFP (β-actin-based promoter). This allowed differentiated RG progeny to be followed prospectively. Initially, YFP was present only in GFAP+ cells, and not in GFAP− cells. Transfected RG cells, however, gradually differentiated into young, β-III-tubulin+ neurons, and finally mature neurons labeled with NeuN (Mo and others 2007). Taken together, these data directly demonstrated that human RG differentiate into mature, cortical neurons, as also reported in mice. Additional studies, however, indicate that the cortical progenitor pool is more complex in human and that multiple progenitors likely produce cortical neurons.
Fig. 5.
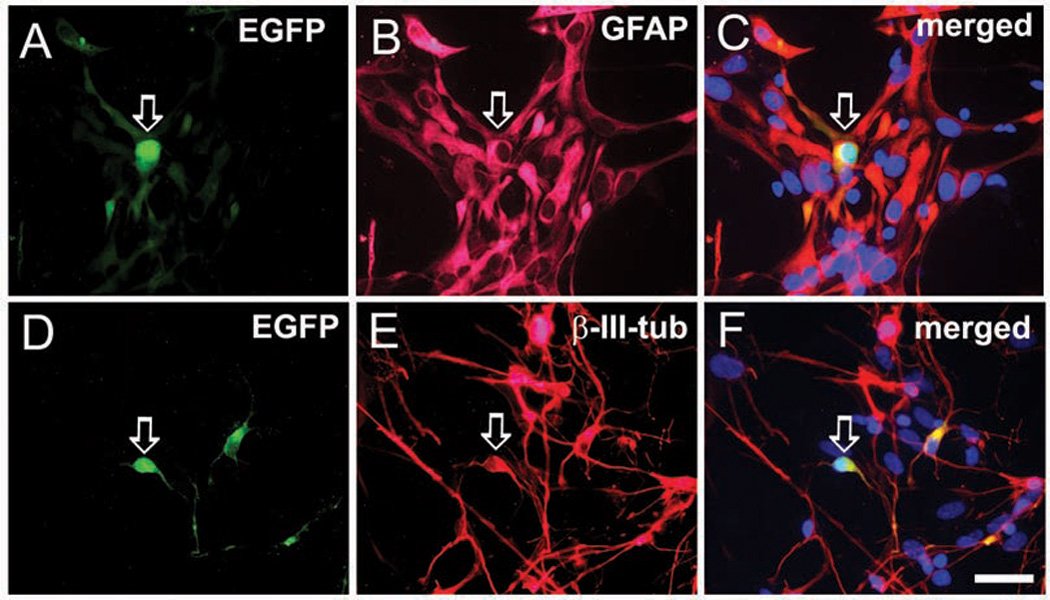
Progeny of radial glia (RG) cells transfected with brain lipid-binding protein/enhanced green fluorescent protein (BLBP-EGFP) plasmid. After 7 days in vitro in differentiation medium, the green cells are either (A–C) colabeled with glial marker GFAP (arrow), or occasionally (D–F) neuronal marker β-III-tubulin (arrow). Scale bar: 25 µm.
Neuron Restricted Progenitors
Studies using cell-type-specific markers suggest that neuron-restricted progenitors are present in the human cortical VZ/SVZ from early embryonic stages of development (Zecevic 2004; Howard and others 2006). In human embryos, three dividing cell types are identified as early as 4.5 gw, immediately prior to neurogenesis (Howard and others 2006). The first cell type colabels with 4A4 and anti-SMI-31. This phenotypic profile is consistent with the ability of RG to produce both glia and neurons (Mo and others 2007). The second progenitor cell type is labeled by neuron-specific antibodies such as SMI-31 and MAP2, but not labeled by RG or astrocytic markers (e.g., 4A4, vimentin, or GFAP). The final cell type that divides in the wall of the neural tube is marked by bisbenzimide, which labels all cell nuclei, but is not identified with a panel of neuronal or glial markers including SMI-31, MAP2, and 4A4. Whether these cells represent neuroepithelial cells, an intermediate progenitor cell type between neuroepithelium and RG, or a derivative that never progresses through an RG lineage, is difficult to determine in the fixed human embryonic CNS.
In addition to their antigenic properties, the spatial distribution of the three aforementioned progenitor types also suggests that neuron-restricted progenitor cells coexist with RG in the human neural tube (Howard and others 2006). When followed chronologically, nascent RG (4A4+/SMI-31+ cells) extend from caudal and dorsal positions at 4.5 gw to rostral and ventral locations at 6 gw, by which point RG are present in the wall of the entire CNS. At 4.5 gw, however, putative neuron-restricted (SMI-31+/4A4−) dividing cells are present throughout the entire neural tube, including the most rostral part. It is unlikely that SMI-31+ dividing cells in the prosencephalon are derived from RG, given that RG are not yet present in the rostral forebrain of the 4.5-gw embryo and other known developmental gradients in the CNS (including that for RG) proceed caudally to rostrally. Equally unlikely is the notion that SMI-31+/4A4− dividing cells “de-differentiate” by up-regulating 4A4 to become multipotent. Indeed, other authors have described restricted progenitor subtypes that exist from the beginning of neurogenesis in primates (Levitt and Rakic 1980; McCarthy and others 2001; Zecevic 2004; Bystron and others 2006) and rodents (Mayer-Proschel and others 1997; McCarthy and others 2001; Haubensak and others 2004; Gal and others 2006).
Moreover, at all examined ages of the human fetal development (5–22 gw), we and others have observed a small number of cells that double-label with RG and neuronal markers (Skogh and others 2001; Zecevic 2004; Howard and others 2006; Mo and others 2007). These cells may represent multipotent progenitors, neurogenic RG cells, or, at later stages of development, intermediate progenitors (IPs) on their way to differentiate into neurons (Noctor and others 2004; Miyata and others 2004; Martinez-Cerdeno and others 2006). Indeed IPs contribute to the total cell population in our VZ/SVZ cultures at midgestation, as demonstrated by immunostaining for the IP marker Tbr-2 (see below; Hevner and others 2001).
These data indicate several possibilities: 1) pluripotent neuroepithelial cells give birth to both RG and a separate neuron-restricted progenitor line prior to 4.5 gw; 2) neuroepithelial cells transform exclusively into RG that then give off a separate neuron-restricted progenitor line, which in turn contributes new, postmitotic, migratory neurons to the developing cerebral cortex in parallel with RG; and 3) there is a single, or series of, common progenitors with pluripotent potential between the neuroepithelial and radial glial stages that have already broken off into at least two progenitor lines (RG and neuron restricted) by the onset of corticogenesis. Although difficult to discern exactly, it seems that in the human embryonic brain RG and neuron-restricted progenitors derive from a common pluripotent ancestor, but are, in fact, separate cell lines.
Experiments comparing LeX+ (RG) and LeX− (cells that remained after immunopanning with LeX antibody) cell populations further indicated that both RG and neuron-restricted progenitors within the VZ/SVZ supply the incipient human cortex with neurons (Mo and others 2007). LeX− cell cultures differed from LeX+ cell cultures in several important aspects: 1) they contained four times as many dividing β-III-tubulin+ cells, demonstrated by double-labeling with Ki67 (Fig. 6A, B); 2) doublecortin (DCX), another neuronal marker, labeled many more cells in the LeX− culture, none of which was costained with the glial marker 4A4; 3) as many as one-third (28%) of these DCX+ cells were proliferating in LeX− cultures, in contrast to 3% in the Lex+ cultures, as seen with BrdU colabeling (Fig. 6C, D). That 97% of DCX+ cells in the LeX+ culture were quiescent indicates that these putative neurons were likely derived from RG and correlate to postmitotic migratory neurons in vivo; whereas DCX+ dividing cells in the LeX− culture were likely neuron-restricted progenitors; and 4) Lex− cultures contained only a few proliferating RG (4A4+ cells) and had reduced capability to form neurospheres, indicating limited numbers of stemlike progenitors (Capela and Temple 2006). Concurrently, the majority of cells in LeX− cultures were lineage-restricted progenitors that rarely formed neurospheres. Adding further complexity, clonal analysis confirmed that human fetal LeX+ cells were a heterogenous population that consisted of cell-type–restricted progenitors that differentiated into either neurons or glia, and multipotent progenitors that produced both neurons and glia (Fig. 7; Mo and others 2007).
Fig. 6.
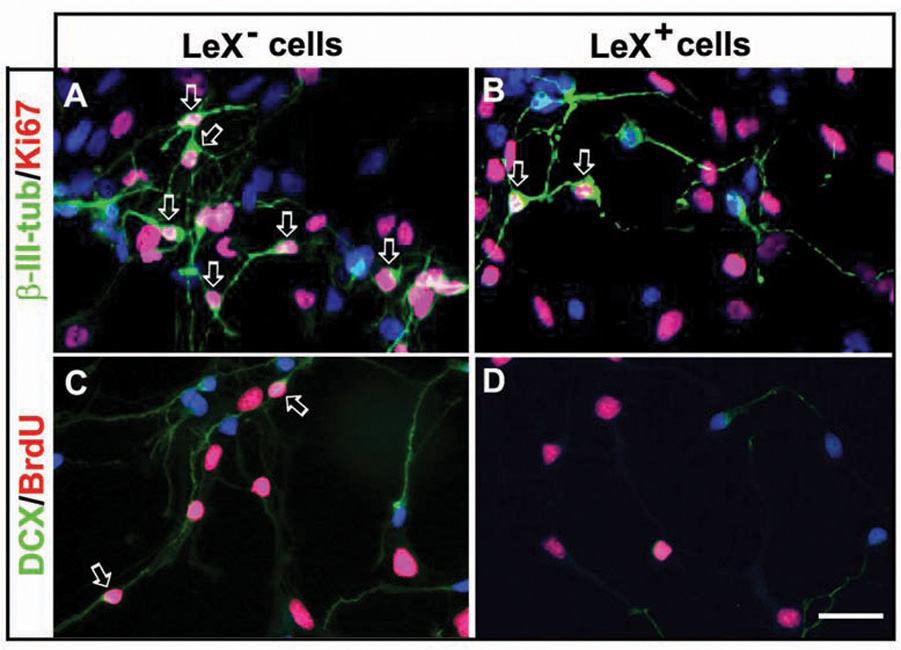
Proliferative neuronal restricted progenitor cells. (A, B) Cells labeled with β-III-tubulin/Ki67. (A) LeX− cells cultured in expansion medium, numerous β-III-tubulin+ cells (green) are proliferating as indicated by their colabeling with Ki67 (red, arrows). (B) Under the same in vitro conditions, in LeX+ cultures, dividing β-III-tubulin+ cells are rare (arrows). (C) In LeX− cultures, numerous DCX+ cells (green) are observed, and some of them incorporate BrdU (red, arrows). (D) In LeX+ cell cultures, however, the number of DCX+ cells is small and BrdU rarely incorporates into these cells (red). Scale bar: 25 µm.
Fig. 7.
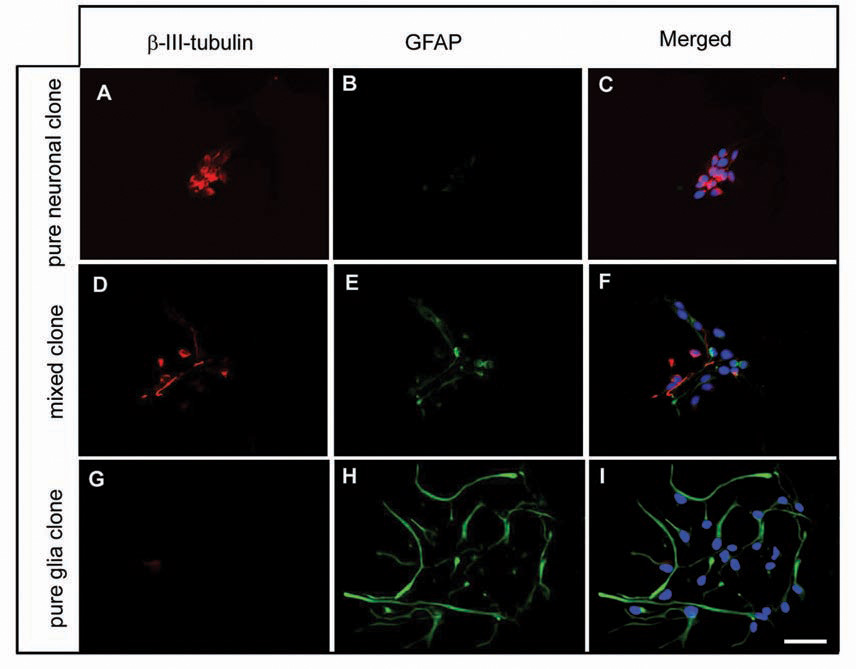
Clonal analysis of LeX+ cells. Clonal analysis reveals that a single LeX+ cell generates clones that consist of (A–C) only β-III-tubulin+ cells, (D–F) mixed clones, (G–I) only GFAP+ cells. In all pictures cell nuclei are stained with bisben-zimide (blue). Scale bar: 25 µm.
In fact, evidence across experimental models with various species is beginning to confirm that NSCs give rise to multiple progenitors, including multipotent RG and neuron-restricted progenitors early in development, prior to the onset of neurogenesis. In culture, human embryonic stem cells (hES) yield both neuron-specific and multipotent progenitors (Carpenter and others 2001). In mice, experiments in green fluorescent protein (GFP) knock-in embryos suggest that cortical neurons are derived from a neuron-specific progenitor population that divide symmetrically to produce two postmitotic neurons located at the basal border of the VZ; whereas a population of mitotic cells in the SVZ divide symmetrically to produce two new progenitors or asymmetrically to yield a postmitotic neuron and a progenitor, much like RG asymmetric divisions (Haubensak and others 2004). Similarly, two progenitor populations, dividing RG and precursors lacking long radial processes that reach pia, termed by the authors “short neural precursors” that are morphologically, ultrastructurally, and molecularly distinct from dividing RG, were demonstrated in the mouse VZ (Gal and others 2006).
Oligodendrocyte Progenitors Derive from RG
Thorough understanding of oligodendrocyte (OL) development, and consequently the process of primary myelination in humans, is a prerequisite step in exploring new therapeutic approaches for myelin recovery in developmental disorders as well as autoimmune diseases and trauma.
In rodents, most OLs are generated during late embryo-genesis and early postnatal life (Pringle and Richardson 1993; Timsit and others 1995; Olivier and others 2001; Rowitch 2004; Vallstedt and others 2005; Kessaris and others 2006). Several well-established markers are used to demonstrate gradual differentiation in the OL lineage: from early oligodendrocyte progenitor cells (OPCs), immunolabeled by antibodies to chondroitin sulfate proteoglycan (NG2; Stallcup and Beasley 1987) and platelet-derived growth factor receptor-α (PDGFRα Lu and others 2000), to late OPCs labeled with O4 antibody (Sommer and Schashner 1981), and finally to premyelinating and myelinating OLs, identified by expression of myelin proteins, myelin basic protein (MBP) and myelin oligoden-drocyte glycoprotein (MOG; Hardy and Reynolds 1991; Pfeiffer and others 1993).
Initial OL progenitors in rodents include a common OL and astrocyte progenitor (2A-O cells) isolated from the optic nerve (Raff and others 1983), glial-restricted precursors (GRPs) identified in spinal cord cell cultures (Rao and others 1998; Gregori and others 2002), a common neuron-oligodendrocyte progenitor in the ventral spinal cord (Richardson and others 1997, 2001; Rowitch and others 2002), and RG cells (Malatesta and others 2003; Fogarty and others 2005; Casper and McCarthy 2006; Ganat and others 2006).
Our previous immunolabeling studies demonstrate the distribution of OPCs in the human fetal forebrain (Jakovcevski and Zecevic 2005a), and that a subset of RG are labeled with OPC markers, which indicates that RG have the potential to differentiate into early OPCs (Jakovcevski and Zecevic 2005b). Direct proof of this lineage relationship, however, was missing until recently.
Using in vitro methods similar to those for obtaining cortical neurons (Mo and others 2007), we established that RG, enriched from the human fetal VZ/SVZ and kept in a medium permissive for OL differentiation, generate cells along the OL lineage (Mo Z and Zecevic N, unpublished data).
Fate Determination of RG Progeny
Combined results from a number of laboratories show that both intrinsic and extrinsic factors influence fate determination of RG progeny (Fishell 1995; Hartfuss and others 2001; Götz and others 2002; Malatesta and others 2003; Anthony and others 2004).
Regional Determination of Cell Fate
Regional determination of progenitor identity is stage dependent. Experiments with mouse cortical progenitors in which diverse cell types are produced according to a precise time schedule illustrate this point nicely. The generation of neurons always precedes that of glia, although glial progenitors are present early but differentiate late in development (Qian and others 1998, 2000; Abramova and others 2005). This trend is in agreement with results from our clonal study of two fetal ages, 14 and 20 gw. As was expected at this developmental stage, individual LeX+ cells generated predominately gliogenic clones as cortical neurogenesis was tapering off (e.g., Sidman and Rakic 1973). However, when the two studied ages were compared, the number of neuronal clones was higher at 14 gw than at 20 gw, which followed the predicted time table whereby neurons are generated prior to glia. Interestingly, ~10% of LeX+ cells formed mixed clones, similar to results reported in animal studies (e.g., Parnavelas and others 1991; Luskin and others 1993; McCarthy and others 2001). Thus, multipotent progenitors were still present at a relatively late stage of human corticogenesis, which may have relevance for childhood tumors, such as glioma and meduloblastoma (Ignatova and others 2002; Lim and others 2007). In neurological tumors of adulthood, primarily astrocytomas, a link between brain tumor stem cells and “normal” neural stem cells that have taken on oncogenic properties is at the vanguard of oncogenesis research (Sanai and others 2005; Lee and others 2006; Pfenninger and others 2007; reviewed in Vescovi and others 2006).
Coculture experiments that we performed suggest that both the stage of development and the local environment play a role in determining RG progeny in the human brain. When GFP-infected Lex+ cells from an earlier age (14 gw) were cocultured with the ganglionic eminence (GE) more neurons were generated, but Lex+ cells derived from fetuses that were 1.5 months older (20 gw) produced more neurons in coculture with cortical VZ/SVZ (Mo and others 2007). This pattern is in accord with results from animal experiments (Anthony and others 2004).
Regional cues influence not only the total number, but also the type of neurons generated from neurogenic RG (Skogh and others 2001; Hall and others 2003). In primates, cortical interneurons are generated both from GE and cortical VZ/SVZ progenitors (Letinic and others 2002; Rakic and Zecevic 2003). This was confirmed in our in vitro experiments, where calretinin+ interneurons were generated when human LeX+ cells obtained from cortical VZ/SVZ were cocultured with either the GE or cortical VZ/SVZ. The number of interneurons produced, however, was higher in GE cocultures (50%) than in cortical VZ/SVZ cocultures (20%; Mo and others 2007). This may be explained by variation in growth factors in these brain regions. In animal studies, growth factors are reported to influence progenitor and stemlike cells, and subsequently neurogenesis (e.g., Qian and others 1998; Vescovi and others 1993; Vaccarino and others 1999; Tropepe and others 1999; Sun and others 2005; Marie and others 2007).
Indeed, the level of mRNA for two growth factors, epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) in our cultured cells were differentially distributed related to brain region and stage of development. At 14 gw, messenger RNAs (mRNAs) for both EGF and FGF2 were higher in cells obtained from the GE than cortical VZ/SVZ cells. In contrast, at a later developmental age (20 gw), the level of EGF mRNA was higher in the cortical VZ/SVZ, whereas the level of FGF2 mRNA was the same in the two regions (Mo and others 2007). Hence, elevated levels of both mRNA EGF and FGF2 in cultures relate to the forebrain region and developmental stage and coincide with periods of greater neurogenesis from RG.
Surrounding cells also influence fate determination of RG cells, as demonstrated in a set of in vitro experiments. A population of human fetal neurons enriched at midgestation consisted of 68 ± 4.5% β-III tubulin+ neurons and 29.5 ± 2.1% GFAP+ RG-like cells (Fig. 8A–C). Treatment of these cultures with cytosine arabinoside (Ara-C) eliminated highly proliferative GFAP+ cells by 70%. Neuronal number, however, also decreased in these cultures by 30%, suggesting that either the number of neurogenic RG was reduced and/or astrocytes secrete factors needed for neuronal survival, such as FGF2 (Vaccarino and others 1999; Mo and others 2007). Indeed, in primary cell cultures grown without FGF2, neuronal progenitors (β-III-tubulin+) did not divide (Fig. 8G), whereas GFAP+ astroglia proliferated. In contrast, in primary cell cultures, in the presence of FGF2, numerous β-tubulin+ cells also divided (Fig. 6), suggesting the importance of FGF2 signaling in proliferation of neuronal progenitors.
Fig. 8.
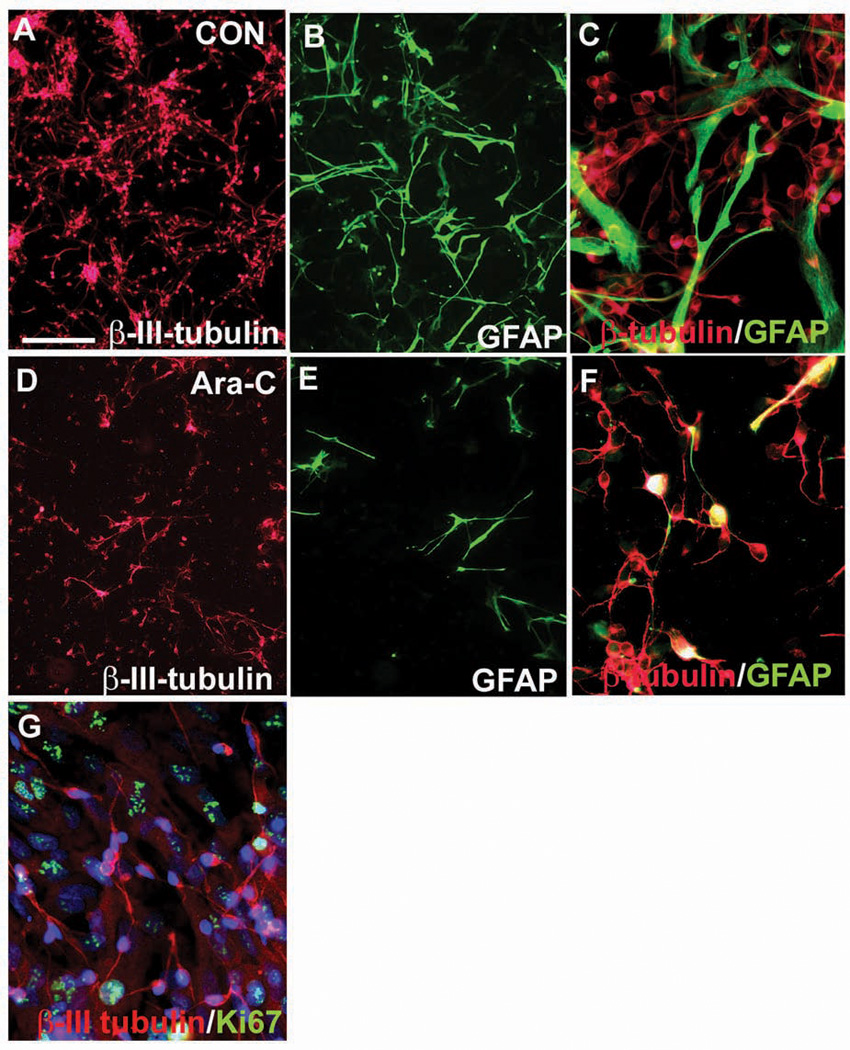
(A–C) Enriched human, fetal, neuronal cultures in control conditions. (A) Neurons stained with β-III tubulin comprise two-thirds, whereas (B) glial fibrillary acidic protein+ (GFAP+) astroglia account for one-third of cells in these cultures. (C) Higher magnification of cells double-stained with β-III tubulin and GFAR. (D–F) Cytosine arabinoside (Ara-C)-treated cultures. (D) Neuronal number declines less than (E) the number of highly proliferative astrocytes. (F) Higher magnification. (G) β-III tubulin+ neurons (in red) are not proliferating, as seen with Ki67 colabeling (in green). Scale bar in A: 50 µm
Transcription Factors Influence RG Neurogenesis
The expression of homeobox transcription factors is important to establish ventrodorsal and mediolateral boundaries in the brain during development (Lindsay and others 2005). One of these transcription factors, paired box 6 (Pax6), is expressed in dorsal regions (Puelles and others 2000; Stoykova and others 2000; Yun and others 2001), whereas oligodendrocyte lineage genes 1 and 2 (Oligl and 2) are found in ventral regions (e.g., Ross and others 2003). The role of Pax6 in CNS development has been studied mainly in rodents (e.g., Puelles and Rubenstein 2003; Götz and others 1998), with studies in humans limited to the developing eye (Glaser and others 1994; reviewed in Chi and Epstein 2002) and adult SVZ (Baer and others 2007).
Notably, Pax6 expression by RG cells in the mouse dorsal telencephalon is a determinant of neurogenetic fate (Götz and others 1998). This is illustrated in Sey mutant mice with nonfunctional Pax6, in which both RG cells and cortical neurons are reduced by 50% (Heins and others 2002). In vitro, Pax6 instructs neuronal fate of all progenitors in embryonic and adult neurosphere preparation (Hack and others 2004). In rodents, Pax6 is down-regulated as RG cells differentiate into Tbr2+ IPs and Tbr-1+ cortical neurons (Hevner and others 2001; Englund and others 2005). This, however, is not the case in human fetal cells, where not only RG but also a subpopulation of neuronal progenitor cells and young neurons also express Pax6 at midgestation (Fig. 9).
Fig. 9.
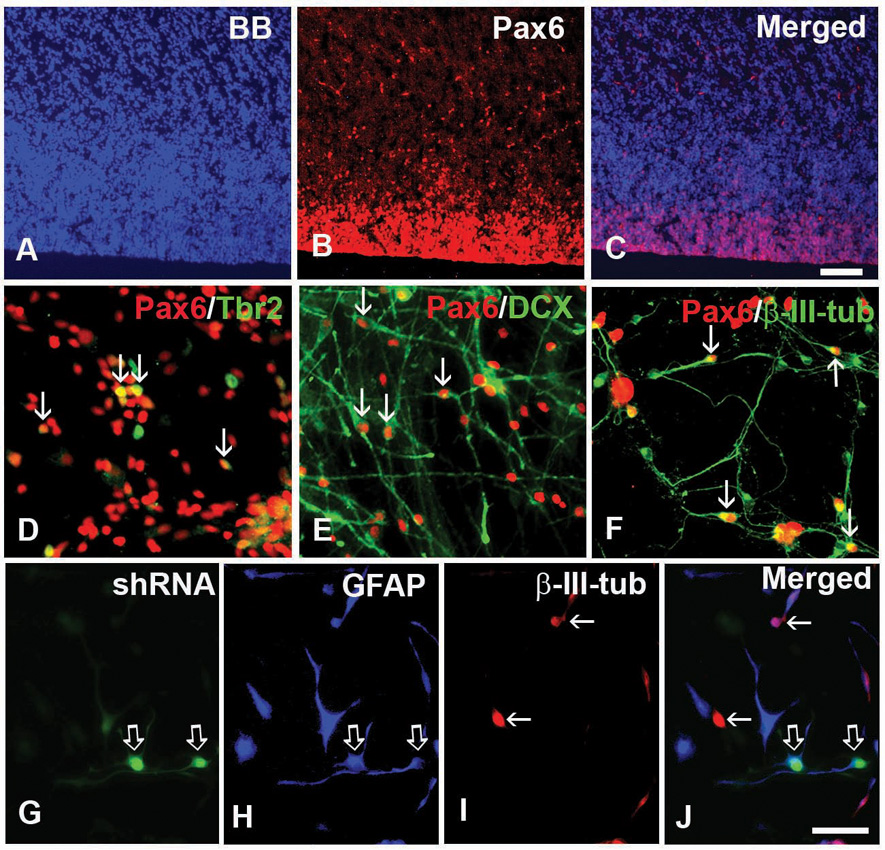
Paired box 6 (Pax6) is critical for neurogenesis. (A–C) Pax6 (red) is expressed in the ventricular zone (VZ) and subventricular zone (SVZ) on cryosections frontally cut from the human fetal forebrain at 15 gestation weeks. Blue-bisbenzimide (BB)-labeled cell nuclei. (D–F) Coexpression of Pax6 and (D) intermediate progenitor marker Tbr2, or (E, F) neuronal markers. (E) Doublecortin (DCX) and (F) β-III-tubulin (arrows) after 3 days in vitro (div). (G–J) LeX+ cells cultured 3 div in expansion medium after transfection with Pax6 shRNA followed by 5 div in differentiation medium. (G) Pax6 knockdown cells (green, open arrows) are labeled with (H) glial fibrillary acidic protein (GFAP, open arrows; blue), but not with I) β-III-tubulin (red, arrows). (J) Merged image. Scale bars: A–C, 100 µm; D–J, 25 µm.
In the human fetal forebrain at midgestation (17–22 gw), Pax6 is widely expressed, both dorsally in the cortical VZ/SVZ and ventrally in the GE (Mo and Zecevic 2007). Thus, the distinction of typical dorsal versus ventral factors reported in rodents is blurred at this developmental age in the human fetal brain.
Moreover, in contrast to rodents, numerous cells in the cortical VZ/SVZ and GE coexpress both Pax6 and O1ig2, which suggests, again, a lineage relationship between RG and oligodendrocytes. To study whether Pax6 plays an equally important role in human neurogenesis as it does in rodents, the expression of Pax6 was knocked down in RG cells isolated from VZ/SVZ at midgestation by the interference RNA (Pax6 shRNA). Cells transfected with shRNA lost their ability to proliferate and generate either intermediate progenitor cells or neurons (Fig. 9G–J). Notably, loss of Pax6 reduced proliferation of RG cells, similar to reports in the developing retina (Marquardt and others 2001), but in contrast to findings in the rodent cerebral cortex (Götz and others 1998; Heins and others 2002). In addition, loss of Pax6 reduced the number of both projection neurons and interneurons regardless of the brain region (Mo and Zecevic 2007). This suggests that the role of the transcription factor Pax6 in RG-driven neurogenesis is well maintained from rodents to humans. In humans, however, the role of Pax6 is more complex, regulating the neurogenic potential of RG both in the dorsal and ventral telencephalon, as well as mediating generation of interneurons in addition to projection neurons. Interestingly, Pax6 has also been shown to favor neurogenic potential of type B cells, the adult neural stem cells (Hack and others 2005).
Concluding Remarks
Nearly a decade has passed since RG cells were first described to be the direct predecessors of pyramidal neurons in the rodent telencephalic wall. Yet, not until this past year were RG shown to be definitively neurogenic in human fetal development (Mo and others 2007). Despite the obvious difficulties in studying the human fetal brain from a technical perspective, all that can be gleaned directly about human nervous system development is of incalculable value as related to the prevention and treatment of neurological and psychiatric disorders.
The assumption can be made that the complex organization of the human cortex has its foundation in the early complexity of cortical progenitors. Studying development of the human brain in vivo is not yet possible with the techniques currently available. Studies based on double-labeling immunohistochemistry, however, indicate that various subtypes of dividing cells are present in the fetal VZ/SVZ region. In vitro studies, using antigenic and electrical properties, more convincingly reveal that RG are multipotent progenitor cells that can generate all three neural cell types: neurons, astrocytes, and oligodendrocytes. Many questions, however, still remain. When is the decision made to restrict cell potential and what are the determinants of a particular cell fate? How do progenitors vary during development and across forebrain regions? Transcription factors, such as Pax6 and Oligl and 2, are a few examples of multiple factors that determine neuronal and oligodendrocyte fate. The role of surrounding cell types, growth factors, or spontaneous activity in these processes must still be better explored. Studies on brain slice cultures, which contain human cortical progenitors in their normal microenvironment, must be more extensive. Furthermore, noninvasive imaging techniques, refined to the point of resolution adequate to observe individual cell groups in vivo, will greatly facilitate our understanding of the structural and functional characterization of human cortical progenitors (Manganas and others 2007).
The brain, once believed to be completely differentiated, is now known to contain adult NSCs that directly descend from RG. These cells have been implicated as the initiating cells in malignant gliomas (reviewed in Sanai and others 2005), and research in this arena has recently focused heavily on the connection between neurogenesis and tumorigenesis.
As we review the achievements of the past decade, researchers in the field of human neurodevelopment are undoubtedly working to significantly advance our understanding of RG/NSCs. Comprehending the biology of these unique cells will be paramount to preventing diseases caused by stem cells that have gone awry (tumors) and devising treatments for diseases with neuronal loss, restoring the normal cytoarchitecture.
References
- Abramova N, Charniga C, Goderie SK, Temple S. Stage-specific changes in gene expression in acutely isolated mouse CNS progenitor cells. Dev Biol. 2005;283:269–281. doi: 10.1016/j.ydbio.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alves JA, Barone P, Engelender S, Froes MM, Menezes JR. Initial stages of radial glia transformation in early postnatal anterior subventricular zone. J Neurobiol. 2002;52:251–265. doi: 10.1002/neu.10087. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Anton SA, Cameron RS, Rakic P. Role of neuron-glia junctional proteins in the maintenance and termination of neuronal migration across the embryonic cerebral wall. J Neurosci. 1996;16:2283–2293. doi: 10.1523/JNEUROSCI.16-07-02283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Eriksson PS, Faull RL, Rees MI, Curtis MA. Sox-2 is expressed by glial and progenitor cells and Pax-6 is expressed by neuroblasts in the human subventricular zone. Exp Neurol. 2007;204:828–831. doi: 10.1016/j.expneurol.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Balasubramaniyan V, de Haas AH, Bakels R, Koper A, Boddeke HW, Copray JC. Functionally deficient neuronal differentiation of mouse embryonic neural stem cells in vitro. Neurosci Res. 2004;49:261–265. doi: 10.1016/j.neures.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Mozzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnar Z, Blakemore C. The first neurons of the human cerebral cortex. Nat Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Calhoun JD, Lambert NA, Mtalipova MM, Noggle SA, Lyons I, Condie BG, et al. Differentiation of rhesus embryonic stem cells to neural progenitors and neurons. Biochem Biophys Res Commun. 2003;306:191–197. doi: 10.1016/s0006-291x(03)00937-9. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex— a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev Biol. 2006;291:300–313. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Ikonuma MS, Denham J, Mujtaba T, Chiu C-P, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002;18:41–47. doi: 10.1016/s0168-9525(01)02594-x. [DOI] [PubMed] [Google Scholar]
- Chiu FC, Rozental R, Bassallo C, Lyman WD, Spray DC. Human fetal neurons in culture: intercellular communication and voltage- and ligand-gated responses. J Neurosci Res. 1994;38:687–697. doi: 10.1002/jnr.490380611. [DOI] [PubMed] [Google Scholar]
- Choi BH. Glial fibrillary acid protein in radial glia of early human fetal cerebrum: a light and electron immunocytochemical study. J Neuropathol Exp Neurol. 1986;45:408–418. doi: 10.1097/00005072-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Dahl D, Rueger DC, Bignami A, Weber K, Osborn M. Vimentin, the 57.000 molecular weight protein of fibroblast filaments, is the major cytoskeleton component in immature glia. Eur J Cell Biol. 1981;24:191–196. [PubMed] [Google Scholar]
- de Azevedo LC, Fallet C, Maura-Neto V, Daumas-Duport C, Hedin-Pareira C, Lent R. Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol. 2003;55:288–298. doi: 10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- de Leeuw B, Su Mu, ter Horst M, Iwata S, Rodijk M, Hoeben RC, et al. Increased glia-specific transgene expression with glial fibrillary acidic protein promoters containing multiple enhancer elements. J Neurosci Res. 2006;83:744–753. doi: 10.1002/jnr.20776. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, et al. Pax6, Tbr2, and Tbrl are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain-lipid binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Fishell G. Striatal precursors adopt cortical identities in response to local cues. Development. 1995;121:803–812. doi: 10.1242/dev.121.3.803. [DOI] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132(8):1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Ton CC, Mueller R, Petzl-Erler ML, Oliver C, Nevin NC, et al. Absence of PAX6 gene mutations in Gillespie syndrome (partial aniridia, cerebellar ataxia, and mental retardation) Genomics. 1994;19:145–148. doi: 10.1006/geno.1994.1024. [DOI] [PubMed] [Google Scholar]
- Gongidi V, Ring C, Moody M, Brekken R, Sage EH, Rakic P, et al. SPARC-like 1 regulates the terminal phase of radial glia-guided migration in the cerebral cortex. Neuron. 2004;41:57–69. doi: 10.1016/s0896-6273(03)00818-3. [DOI] [PubMed] [Google Scholar]
- Götz M. Glial cells generate neurons-master control within CNS regions: developmental perspectives on neural stem cells. Neuroscientist. 2003;9:379–397. doi: 10.1177/1073858403257138. [DOI] [PubMed] [Google Scholar]
- Götz M, Hartfuss E, Malatesta P. Radial glia cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res Bull. 2002;57:777–788. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Gregori N, Proschel C, Noble M, Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, Nakafuku M, Götz M. Regionalization and fate specification in neurospheres: the role of O1ig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Hall AC, Mira H, Wagner J, Arena E. Region-specific effects of glia on neuronal induction and differentiation with a focus on dopaminergic neurons. Glia. 2003;43:47–51. doi: 10.1002/glia.10229. [DOI] [PubMed] [Google Scholar]
- Hardy R, Reynolds R. Proliferation and differentiation potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo. Development. 1991;111:1061–1080. doi: 10.1242/dev.111.4.1061. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Götz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hatten ME. The role of migration in central nervous system neuronal development. Curr Opin Neurobiol. 1993;3:38–44. doi: 10.1016/0959-4388(93)90033-u. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, et al. Tbrl regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005a;49:480–491. doi: 10.1002/glia.20134. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005b;25:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim HJ, Gadisseux J-F, Edvrard P. Topographical and cytological evolution of the glial phase during prenatal development of the human brain: histochemical and electron microscopic study. J Neuropathol Exp Neurol. 1988;47:166–188. doi: 10.1097/00005072-198803000-00009. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Inagaki N, Nishizawa M, Tsutsumi O, Taketani Y, Inagaki M. Visualization of mitotic radial glial lineage cells in the developing rat brain by Cdc2 kinase-phosphorylated vimentin. Glia. 1998;23(3):191–199. doi: 10.1002/(sici)1098-1136(199807)23:3<191::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kerkovich DM, Sapp D, Weidenheim K, Brosnan CF, Pfeiffer SE, Yeh HH, et al. Fetal human cortical neurons grown in culture: morphological differentiation, biochemical correlates and development of electrical activity. Int J Dev Neurosci. 1999;17:347–356. doi: 10.1016/s0736-5748(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci. 2003;23:10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler D. A Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acid protein in radial glial cells and astrocytes of the developing rhesus monkey brain. Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neural and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncology. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S, Sarma S, Martinez-de-la-Torre M, Kerwin J, Scott M, Luis Ferran J, et al. Anatomical and gene expression mapping of the ventral pallium in a three-dimensional model of developing human brain. Neuroscience. 2005;136:625–632. doi: 10.1016/j.neuroscience.2005.06.093. [DOI] [PubMed] [Google Scholar]
- Liour SS, Yu RK. Differentiation of radial glia-like cells from embryonic stem cells. Glia. 2003;42:109–117. doi: 10.1002/glia.10202. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D-I, Alberta JA, Zhu Z, Pawlitzky I, Chan J, et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. and others. [DOI] [PubMed] [Google Scholar]
- Luskin MB, McDermott K. Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia. 1994;11:211–226. doi: 10.1002/glia.440110302. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magini G. Sur la nevroglie et les cellules nerveuses cerebrales chez les foetus. Arch Ital Biol. 1888;9:59–60. [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchoff F, et al. Neuronal and glia progeny: regional didfferences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 9:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;9:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie D, Fiorio Pla A, Chang YH, Barker JL. Self-renewing and differentiation properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J Neurosci. 2007;27:1836–1852. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Early ontogenesis of human cerebral cortex. In: Peters A, Jones EG, editors. Cerebral cortex: development and maturation of cerebral cortex. New York: Plenum Press; 1988. pp. 479–509. [Google Scholar]
- Marin-Padilla M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a golgi study. J Comp Neurol. 1995;357:554–572. doi: 10.1002/cne.903570407. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemont F, Guss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16:1152–1161. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19(4):773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Turnbull DH, Walsh CA, Fishell G. Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J Neurosci. 2001;21:6772–6781. doi: 10.1523/JNEUROSCI.21-17-06772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. Strategies for the generation of neuronal diversity in the developing central nervous system. J Neurosci. 1995;15:6987–6998. doi: 10.1523/JNEUROSCI.15-11-06987.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawagushi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawagushi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface dividing cortical progenitor cells. Development. 2004;130:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, et al. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27:4132–4145. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Is Pax6 critical for neurogenesis in the human fetal brain? Cereb Cortex. 2007 doi: 10.1093/cercor/bhm181. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glia cells establish radial units in the neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise on symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Olivier C, Cobos I, Perez Villegas EM, Spassky N, Zalc B, Martinez S, et al. Monofocal origin of telencephalic oligodendrocytes in the anterior entopeduncular area of the chick embryo. Development. 2001;128:1757–1769. doi: 10.1242/dev.128.10.1757. [DOI] [PubMed] [Google Scholar]
- Pagani F, Lauro C, Fucile S, Catalano M, Limatola C, Eusebi F, et al. Functional properties of neurons derived from fetal mouse neurospheres are compatible with those of neuronal precursors in vivo. J Neurosci Res. 2006;83:1494–1501. doi: 10.1002/jnr.20835. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cereb Cortex. 1991;1:463–491. doi: 10.1093/cercor/1.6.463. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pfenninger CV, Roschupkina T, Hertwig F, Kottwitz D, Englund E, Bengzon J, et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- Picken Bahrey HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol. 2003;89:1761–1773. doi: 10.1152/jn.00972.2002. [DOI] [PubMed] [Google Scholar]
- Piper DR, Mujtaba T, Rao MS, Lucero MT. Immunocytochemical and physiological characterization of a population of cultured human neural precursors. J Neurophysiol. 2000;84:534–548. doi: 10.1152/jn.2000.84.1.534. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Qian X, Goderie SK, Shen Q, Stern JH, Temple S. Intrinsic programs of patterned cell lineages in isolated vertebrate CNS ventricular zone cells. Development. 1998;125:3143–3152. doi: 10.1242/dev.125.16.3143. [DOI] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey cortex. J Comp Neurol. 1972;145:61–84. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial glial cells: scaffolding for brain construction. In: Ketterman H, Ransom BR, editors. Neuroglial cells. New York: Oxford University Press; 1995. pp. 746–762. [Google Scholar]
- Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of cortical layer I in humans. Cereb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci USA. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Pringle NP, Yu WP, Hall AC. Origins of spinal cord oligodendrocytes: possible developmental and evolutionary relationships with motor neurons. Dev Neurosci. 1997;19:58–68. doi: 10.1159/000111186. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Smith HK, Sun T, Pringle NP, Hall A, Woodruff R. Oligodendrocyte lineage and the motor neuron connection. Glia. 2001;29:136–142. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Lu QR, Kessaris N, Richardson WD. An “oligarchy” rules neural development. Trends Neurosci. 2002;25:417–422. doi: 10.1016/s0166-2236(02)02201-4. [DOI] [PubMed] [Google Scholar]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Qulnones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Yokota Y, Anton ES. Generation and characterization of brain lipid-binding protein promoter-based transgenic mouse models for the study of radial glia. Glia. 2006;53:345–351. doi: 10.1002/glia.20274. [DOI] [PubMed] [Google Scholar]
- Shibata T, Yamada K, Watanabe K, Ikenaka M, Wada K, Tanaka K, et al. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Skogh C, Eriksson C, Kokaia M, Meijer LU, Wictorin K, Campbell K. Generation of regionally specified neurons in expaned glial cultures derived from the mouse and human lateral ganglionic eminence. Mol Cell Neurosci. 2001;17:811–820. doi: 10.1006/mcne.2001.0973. [DOI] [PubMed] [Google Scholar]
- Smart IHM, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (Ol to 04) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;9:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Tan S-S, Kaloniatis M, Sturm K, Tarn PPL, Reese BE, Faulkner-Jones BE. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- Timsit S, Martinez S, Allinquant B, Peyron F, Puelles L, Zalc B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J Neurosci. 1995;15:1012–1024. doi: 10.1523/JNEUROSCI.15-02-01012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Neudorfer F, Bohl J. Distribution patterns of vimentin-immunoreactive structures in the human prosencephalon during the second half of gestation. J Anat. 1999;195:87–100. doi: 10.1046/j.1469-7580.1999.19510087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Rababllo R, Nilsen J, Rhee J, Zhou M, et al. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraier DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical sub-ventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Mlosevic A, Rakic S, Marin-Padilla M. The human neocortex: early development and composition of the primordial plexiform layer. An immunohistochemical study. J Comp Neurol. 1999;412:241–254. [PubMed] [Google Scholar]
- Zhang ZW. Maturation of layer V pyramidal neurons in the rat prefrontal cortex: intrinsic properties and synaptic function. J Neurophysiol. 2004;91:1171–1182. doi: 10.1152/jn.00855.2003. [DOI] [PubMed] [Google Scholar]


