Abstract
Background
Collaterals may sustain penumbra prior to recanalisation yet the influence of baseline collateral flow on infarct growth following endovascular therapy remains unknown.
Methods
Consecutive patients underwent serial diffusion and perfusion MRI before and after endovascular therapy for acute cerebral ischaemia. We assessed the relationship between MRI diffusion and perfusion lesion indices, angiographic collateral grade and infarct growth. Tmax perfusion lesion maps were generated and diffusion–perfusion mismatch regions were divided into Tmax ≥4 s (severe delay) and Tmax ≥2 but <4 s (mild delay).
Results
Among 44 patients, collateral grade was poor in 7 (15.9%), intermediate in 20 (45.5%) and good in 17 (38.6%) patients. Although diffusion–perfusion mismatch volume was not different depending on the collateral grade, patients with good collaterals had larger areas of milder perfusion delay than those with poor collaterals (p = 0.005). Among 32 patients who underwent day 3–5 post-treatment MRIs, the degree of pretreatment collateral circulation (r = −0.476, p = 0.006) and volume of diffusion–perfusion mismatch (r = 0.371, p = 0.037) were correlated with infarct growth. Greatest infarct growth occurred in patients with both non-recanalisation and poor collaterals. Multiple regression analysis revealed that pretreatment collateral grade was independently associated with infarct growth.
Conclusion
Our data suggest that angiographic collateral grade and penumbral volume interactively shape tissue fate in patients undergoing endovascular recanalisation therapy. These angiographic and MRI parameters provide complementary information about residual blood flow that may help guide treatment decision making in acute cerebral ischaemia.
In the setting of acute ischaemic stroke, arterial recanalisation to restore antegrade perfusion to the ischaemic territory remains the principal therapeutic approach. Prior to attempted recanalisation, the prediction of final infarct volume should recanalisation not occur may facilitate selection of candidates. Multiparametric MRI, including diffusion weighted (DWI) and perfusion weighted (PWI) imaging, has increasingly been used for selection of patients for recanalisation therapy in clinical practice.1 2 DWI can demonstrate ischaemic changes within minutes of onset whereas PWI defines area of hypoperfusion. Thus the mismatch areas between PWI and DWI have been suggested as the penumbra zone, although many uncertainties exist.
Although the pathophysiological recruitment of the cerebral collateral circulation in the setting of chronic haemodynamic insufficiency has been explored,3 relatively little attention has been devoted to the role of pretreatment collateral circulation in patients with acute ischaemic stroke who are candidates for recanalisation therapy.4 5 Collaterals may sustain the penumbra prior to recanalisation, yet the influence of baseline collateral flow on infarct growth following endovascular therapy remains unknown. Recently, a retrospective CT based volumetric analysis reported that infarct volume and clinical severity at discharge were lower for patients with better pretreatment pial collateral formation.6 However, pretreatment neuroimaging findings, such as pretreatment DWI lesion volumes, were not considered.6 Furthermore, the relationship between diffusion–perfusion mismatch and collateral circulation has not yet been reported.
We evaluated the relationship between pretreatment parameters of collateral perfusion, evidenced by diffusion–perfusion mismatch and angiographic collaterals, and assessed how these pretreatment variables influence tissue fate after ischaemic injury controlling for the degree of recanalisation.
PATIENTS AND METHODS
Patient selection
The present analysis was performed on data collected in a large prospective registry of patients evaluated with diffusion–perfusion MRI and receiving recanalisation therapy (intravenous or intra-arterial thrombolytic therapy, or mechanical clot retrieval therapy) for acute cerebral ischaemia. For mechanical clot retrieval therapy, a mechanical thrombectomy device (MERCI Retrieval System, Concentric Medical, Inc., Mountain View, California, USA) was used. This study analysed consecutive patients encountered at a university medical centre from October 2002 to June 2006. Patients were included in this study if: (1) they presented with symptoms of acute cerebral ischaemia within the middle cerebral artery territory; (2) they underwent conventional angiography; and (3) pretreatment DWI and PWI were performed. The local institutional review board approved the study.
MRI methods and image analysis
All patients underwent MRI (1.5 T; Siemens Medical System, Erlangen, Germany) before recanalisation therapy. The MRI protocol included DWI, gradient recalled echo, fluid attenuated inversion recovery and PWI sequences, with previously described MRI methodology. 7 We selected a definition based on the perfusion parameter Tmax, which is the time to peak MR signal intensity change after deconvolution; Tmax perfusion lesion maps were generated by deconvolution of an arterial input function and tissue concentration curves based on previous methods.8
Data analysis was carried out with software developed inhouse using the Interactive Data Language produced by ITT Visual Systems (Boulder, Colorado, USA). MRI volume measurements were performed by one of the authors (YSR) blinded to the clinical information. One study noted that Tmax ≥2 s may tend to overestimate and Tmax ≥4 s to underestimate penumbral regions.9 Tmax ≥4 s was used as the threshold distinguishing the penumbra from “benign oligaemia”. “Benign oligaemia” was considered present if Tmax was ≥2 but <4 s. Penumbral regions were approximated as regions showing perfusion–diffusion mismatch, with voxels showing Tmax ≥4 s but no diffusion abnormality. For each patient, DWI and PWI lesion volumes were outlined automatically with subsequent manual correction, and volumes were calculated with a computer assisted volumetric analysis program (Medical Image Processing, Analysis and Visualisation, V.2.1, CIT, NIH).
Angiographic study
All patients underwent comprehensive diagnostic cerebral angiography, including injection of both internal carotid arteries and the dominant vertebral artery, with assessment through the late venous phase to assess collateral flow from all possible sources. Angiographic collateral grade was evaluated with the ASITN/SIR Collateral Flow Grading System on pretreatment angiography.10 This angiographic scale assigns patients to grade 0 (no collaterals visible to the ischaemic site), 1 (slow collaterals to the periphery of the ischaemic site with persistence of some of the defect), 2 (rapid collaterals to the periphery of ischaemic site with persistence of some of the defect and to only a portion of the ischaemic territory), 3 (collaterals with slow but complete angiographic blood flow of the ischaemic bed by the late venous phase) and 4 (complete and rapid collateral blood flow to the vascular bed in the entire ischaemic territory by retrograde perfusion). In this study, a score of 0–1 was designated as poor, 2–3 as intermediate and 4 as good collateral flow. Vascular reperfusion was based on the Thrombolysis in Myocardial Infarction (TIMI) scale, with assignments of 3 (complete recanalisation), 2 (partial recanalisation), 1 (minimal recanalisation) and 0 (no recanalisation).
Statistical analysis
The relationships of mismatch volume and angiographic parameters to infarct growth during the follow-up period were evaluated using the Kruskall–Wallis test for continuous variables and Pearson’s χ2 or Fisher’s exact test for categorical variables. In addition, multiple linear regression models (step-wise method, SPSS V.13.0) were developed to predict the independent contribution of factors that influenced infarct growth; age, pretreatment mismatch volume, pretreatment collateral grade, TIMI scale and time from symptom onset to recanalisation therapy served as predictor variables. Probability values less than 0.05 were accepted as significant.
RESULTS
Among 65 patients who received endovascular recanalisation therapy for acute cerebral ischaemia within the middle cerebral artery (MCA) territory during the study period, 44 patients were included in this study: pretreatment MRI was not performed in 21 patients. Pretreatment MRI was performed at 3.9 (2.0) h (range 1.1–8.3) and recanalisation therapy was performed at 5.6 (2.1) h (range 2.0–11.5) after the last known well time. Nineteen men and 25 women with an average age of 65.0 (20.6) years (range 15–95) were included. Ten patients received thrombolysis (two intravenous, seven intra-arterial and one both intravenous and intra-arterial tissue plasminogen activator) only, 27 endovascular thrombectomy therapy only and seven combined thrombolysis and endovascular thrombectomy. Outcome MRI was performed in 32 patients at 4.9 (2.1) days (range 1.9–9.2) after recanalisation therapy.
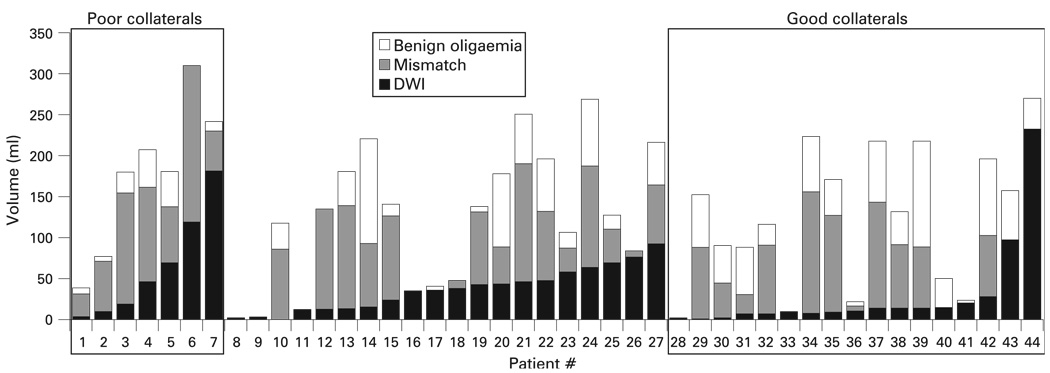
Patient characteristics and treatment response depending on the degree of angiographic collateral circulation are illustrated in table 1. DWI or mismatch lesion volumes and collateral grade were not correlated with imaging time after symptom onset (p>0.05 in all cases). Although DWI lesion and mismatch lesions volumes were numerically higher in patients with poorer collaterals, these differences did not reach statistical significance. No significant difference was found between diffusion–perfusion mismatch and pretreatment collaterals; there was no significant relation of collateral circulation with either the volumes (in ml) of diffusion–perfusion mismatch (p = 0.285) or the degree of mismatch, defined as pretreatment PWI lesion volume exceeding pretreatment DWI lesion volume [(PWI volume–DWI volume)/PWI volume × 100] (p = 0.466). However, when mismatch presented on MRI, the severity of perfusion delay differed depending on pretreatment collaterals; patients with poor collaterals had larger extents of penumbral mismatch area versus “benign oligaemic” areas, whereas those with good collaterals had larger regions of “benign oligaemic” area versus penumbral mismatch area (p = 0.005) (table 1, fig 1).
Table 1.
Patient characteristics
| Collateral grading | ||||
|---|---|---|---|---|
| Poor (grade 0–1) (n = 7) |
Intermediate (grade 2–3) (n = 20) |
Good (grade 4) (n = 17) |
p Value | |
| Male sex (n (%)) | 5 (71.4) | 7 (35.0) | 7 (41.2) | 0.288 |
| Age (y) | 60.3 (16.0) | 72.2 (13.8) | 58.5 (24.5) | 0.106 |
| NIHSS score | 15.4 (6.5) | 18.5 (6.6) | 14.5 (5.6) | 0.148 |
| Site of occlusion | ||||
| Carotid bifurcation | 1 | 1 | 2 | |
| Carotid intracranial | 2 | 6 | 2 | |
| Proximal M1 | 2 | 4 | 9 | |
| Distal M1 | 1 | 4 | 4 | |
| Distal MCA | 1 | 5 | – | |
| Mechanisms of stroke | 0.166 | |||
| Cardioembolic | 4 | 16 | 9 | |
| Large artery atherosclerotic | 3 | 4 | 5 | |
| Other | – | – | 3 | |
| Mode of treatment | ||||
| Fibrinolysis | 1 | 6 | 3 | |
| Endovascular thrombectomy | 5 | 12 | 10 | |
| Combined | 1 | 2 | 4 | |
| TIMI grade | ||||
| TIMI 0–1 | 3 | 5 | 1 | |
| TIMI 2 | 4 | 10 | 9 | |
| TIMI 3 | 0 | 5 | 7 | |
| Pretreatment ischaemic zone (ml) | ||||
| Initial DWI lesion volume | 62.9 (65.2) | 35.8 (26.4) | 27.9 (56.6) | 0.052 |
| Initial penumbra volume | 92.8 (57.0) | 57.9 (50.4) | 51.0 (51.5) | 0.285 |
| Initial “benign oligaemia” volume | 19.7 (18.3) | 30.3 (36.8) | 45.8 (34.7) | 0.170 |
| Degree of mismatch (%)* | 67.6 (25.6) | 48.8 (33.4) | 55.3 (43.8) | 0.466 |
| Presence of mismatch (n (%))† | 7 (100) | 14 (70) | 11 (64.7) | 0.258 |
| Severity of perfusion defect‡ | 4.8 (3.6) | 3.8 (4.4) | 1.0 (1.2) | 0.005 |
Values are mean (SD) unless otherwise stated.
Pretreatment PWI lesion volume exceeding pretreatment DWI lesion volume: [(PWI volume–DWI volume)/PWI volume×100].
When the area of PWI lesion is greater than DWI lesion by 20% or more.
Ratio of initial penumbra volume versus initial “benign oligaemia” volume: [PWI volume of Tmax ≥4 s/PWI volume of Tmax ≥2 but <4 s). A high score means that the area of hypoperfusion consisted of severely hypoperfused regions in terms of severity. DWI, diffusion weighted imaging; M1, proximal portion of middle cerebral artery; MCA, middle cerebral artery; NIHSS, National Institute of Health Stroke Scale; PWI, perfusion weighted imaging; TIMI scale, Thrombolysis in Myocardial Infarction scale.
Figure 1.
Volumes of diffusion weighted imaging (DWI), penumbra and “benign oligaemia” of 44 patients with acute middle cerebral artery infarctions.
Among 32 patients who underwent follow-up MRIs, collateral grade was poor in 6, intermediate in 13 and good in 13 patients. Degree of collateral circulation on pretreatment angiography correlated with infarct growth, and patients with good collateral circulation showed lesser degrees of infarct growth than those with poor collaterals (21.8 (28.6) vs 118.9 (114.7) ml; p = 0.003). Infarct growth in patients with intermediate collaterals was 27.9 (35.2) ml. Simple regression analysis showed that the degree of pretreatment collateral circulation (grade 0–1 vs 2–3 vs 4) (r = 20.476, r2 = 23%, p = 0.006) and diffusion–perfusion mismatch volume (r = 0.371, r2 = 0.14%, p = 0.037), correlated with infarct growth.
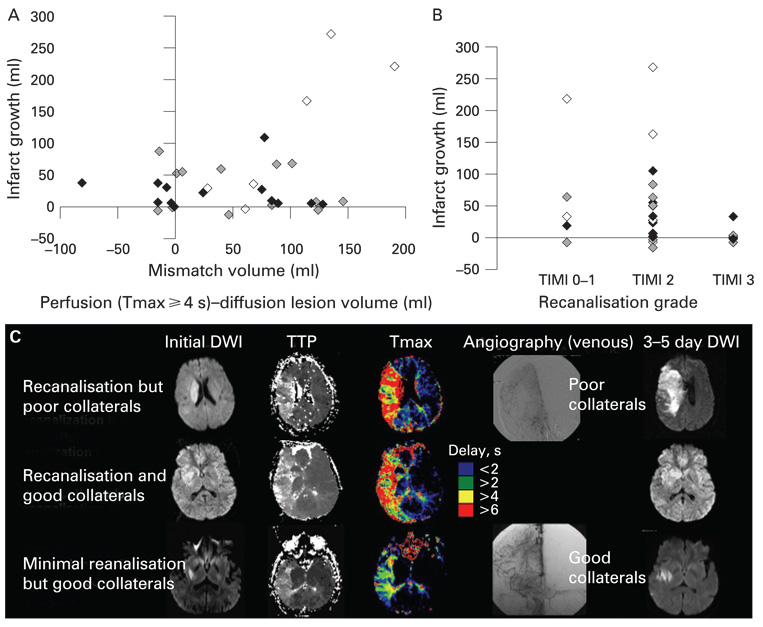
The impact of pretreatment collateral flow on infarct growth interacted with both volume of diffusion–perfusion mismatch and degree of recanalisation after recanalisation therapy (fig 2). No or poor reperfusion (TIMI 0–1) was observed in five patients whereas partial (TIMI 2) or complete (TIMI 3) reperfusion was observed in 20 and seven patients, respectively. Marked enlargement in DWI lesion volume was observed only in patients with poor collaterals and large mismatch area on pretreatment MRI (fig 2A). A patient with good pretreatment collateral flow did not show infarct growth despite non-recanalisation after treatment (fig 2B, 2C). In contrast, some patients with poor collaterals showed infarct growth despite achievement of substantial recanalisation, likely due to lesion progression in the interval between the baseline MRI study and the therapeutic intervention (fig 2B, 2C).
Figure 2.
Impact of angiographic collateral grade on infarct growth. Displayed are scattered plots of (A) pretreatment diffusion–perfusion mismatch volume versus infarct growth and (B) the degree of recanalisation after treatment versus infarct growth. Filled diamonds indicate patients with good collaterals (grade 4), grey diamonds those with intermediate collaterals (grade 2–3) and open diamonds those with poor collaterals (grade 0–1). (C) Pretreatment diffusion and perfusion MRI findings and final diffusion weighted imaging (DWI) findings of patients with (i) recanalisation (Thrombolysis in Myocardial Infarction scale (TIMI) 2) but poor collaterals (grade 1), (ii) recanalisation (TIMI 3) but good collaterals (grade 4) and (iii) minimal recanalisation (TIMI 1) but good collaterals (grade 4). The initial DWI lesion volume, time to peak (TTP) delayed perfusion volume and mismatch area was similar among the patients. However, the patient with good collaterals showed minimal/no marked infarct growth, regardless of the occurrence of recanalisation, whereas infarct growth with clinical deterioration was observed in the patient with poor collaterals.
There was a trend that patients with a greater extent of pretreatment collaterals more frequently achieved therapeutic recanalisation (p = 0.130). Complete reperfusion was achieved in 0% of poor collateral patients, 25% of intermediate collateral patients and 41% of good collateral patients (table 1).
Multiple regression analysis was performed to further evaluate the independent predictor for infarct growth (table 2). Although pretreatment diffusion–perfusion mismatch and posttreatment TIMI scale were associated with infarct growth (model 1), no such relationships were observed when pretreatment collateral grade was entered into the same model (model 2) (table 2). Multiple regression analysis revealed that pretreatment collateral circulation (grade 0–1 vs 2 vs 3) independently correlated with infarct growth (β = 241.57; p = 0.009), adjusting for other variables. Other factors, including pretreatment mismatch volume (p = 0.108) and TIMI scale after recanalisation therapy (p = 0.354), did not significantly add value to pretreatment collateral grade. There was no significant correlation between the individual factors.
Table 2.
Multiple linear regression analysis for infarct growth
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variable | β coefficient | p Value | β coefficient | p Value |
| Constant | 84.72 | 0.033 | 134.78 | 0.001 |
| Collateral grade | −41.57 | 0.009 | ||
| Mismatch volume (ml) | 0.552 | 0.009 | 0.290 | 0.108 |
| TIMI scale | −36.70 | 0.041 | −0.167 | 0.354 |
| Time from onset to treatment (h) |
0.210 | 0.206 | 0.201 | 0.245 |
| Age (y) | −0.153 | 0.355 | −0.185 | 0.282 |
| R square (%) | 18 | 22 | ||
TIMI Scale, Thrombolysis in Myocardial Infarction scale.
DISCUSSION
The haemodynamic effects of the collateral circulation are important in maintaining perfusion to penumbral regions.4 5 In this study, there was a complex interplay between the degree of angiographically defined collaterals, the presence of penumbra tissue, the achievement of recanalisation and the occurrence of infarct growth. Our data illustrate that collaterals and mismatch represent related, yet distinct, aspects of ischaemic pathophysiology. Angiographic collaterals principally reflect arterial inflow (one component of collateral perfusion) and mismatch measures may be selectively defined to investigate other aspects of collateral perfusion (eg, Tmax, cerebral blood volume, etc) before recanalisation. Furthermore, collaterals may influence “the severity of ischaemic injury” over the hypoperfused region, whereas mismatch areas demonstrated by DWI–Tmax may represent penumbral extent. Our data suggest that collateral blood flow minimises the degree of residual tissue hypoperfusion after arterial occlusion,5 possibly due to ischaemic preconditioning.11 Patients with good collaterals had relatively larger areas of only mildly hypoperfused tissue than those with poor collaterals, and infarct growth within the penumbral zone was smaller when collaterals were better.
Our results indicate that poor collateral flow is an important determinant of tissue fate, particularly in the setting of poor/partial recanalisation after treatment and extensive diffusion–perfusion mismatch on pretreatment MRI. In the present study, the correlation between pretreatment diffusion–perfusion mismatch and infarct growth was modest; the correlation coefficient was 0.37 (r2 = 14%), indicating that only 14% of the variability in the infarct growth can be explained by the pretreatment diffusion–perfusion mismatch. The small r2 value could be caused by technical related reasons; the diffusion–perfusion criteria used to define the penumbra may not be appropriate. Our results suggested that this relatively poor correlation between them may also be attributable to several factors, including the pretreatment collaterals as well as recanalisation after treatment; in our data the r2 value increased to 18% and 22% by considering recanalisation and pretreatment collaterals in the model. The larger enlargement of DWI lesion volumes were observed with an increase in diffusion–perfusion mismatch volume in patients with poor collaterals, compared with patients with good collaterals. Moreover, collaterals have previously been shown to be potentially more important than recanalisation in some cases. Our results are in good agreement with a previous study showing that poor collaterals are an important determinant, irrespective of the degree of recanalisation. 6 In the present study, when complete recanalisation was not achieved, infarct growth was usually not observed if good collaterals were noted.
Despite these influential aspects of collateral flow, collaterals at angiography are often only considered an interesting curiosity and are not typically used for decision making in endovascular management.5 Angiographic collaterals and the presence of mismatch should be considered individually in such patients. Absence or relative paucity of collaterals may be used to predict unlikely recanalisation despite aggressive therapeutic endovascular strategies. Alternatively, the presence of robust collateral flow at angiography may persuade clinicians to attempt further recanalisation efforts, as collateral flow may be a marker for eventual success of such approaches. In cases where collateral flow is marginal, evolving haemodynamic strategies to improve ischaemia through augmentation of collateral perfusion may be warranted.12 Therapeutic strategies enhancing collaterals may ultimately be as important as recanalisation. Infarct growth may be minimised with the use of collateral therapeutic strategies, particularly in the setting of persistent arterial occlusion despite attempted recanalisation. Collateral therapeutics may entail the use of readily available haemodynamic manipulations such as head positioning, hypervolaemia or hypertensive therapy in selected cases.12 Endovascular device strategies, such as partial aortic obstruction, may also be used for this purpose. Considering that recanalisation is often achieved in only a fraction of cases undergoing endovascular therapy, further research on collateral circulation and related therapeutic approaches is warranted.
The results of this study should be interpreted with caution because of the limited sample size with different treatment modalities, the extensive age range and data from a single centre. In addition, day 3–5 post-treatment MRIs could not be performed in several patients (12 patients, 27%). However, it should be noted that none of the clinical (age, sex and NIHSS score), MRI (volumetric data including diffusion–perfusion mismatch) or angiographic parameters (collateral grade and TIMI scales) were different between patients who underwent post-treatment MRI and those who did not (see supplementary table online).
Our results indicate that aside from the current diffusion–perfusion mismatch concept, the status of collateral flow at angiography may be an important factor in tissue fate, regardless of the occurrence of recanalisation after treatment. Collateral enhancement paradigms may ultimately be important, yet such approaches will depend on ongoing research to better characterise the nature of collateral perfusion, including the basis of “benign oligaemia”, penumbral flow heterogeneity and low perfusion hyperaemia.
Acknowledgements
NIH/NINDS K23NS054084 (DSL), P50 NS044378.
UCLA Collateral Investigators: Oh Young Bang, MD, PhD; Jeffrey L Saver, MD; Brian H Buck, MD; Jeffry R Alger, PhD; Sidney Starkman, MD; Bruce Ovbiagele, MD; Doojin Kim, MD; Latisha K Ali, MD; Nerses Sanossian, MD; Paul M Vespa, MD; Reza Jahan, MD; Noriko Salamon, MD; Gary R Duckwiler, MD; J Pablo Villablanca; Sa Rah Yoon, MD; Fernando Viñuela, MD; and David S Liebeskind, MD.
Footnotes
To order reprints of this article go to: http://journals.bmj.com/cgi/reprintform
The supplementary table is published online only at http://jnnp.bmj.com/content/vol79/issue6
Competing interests: None.
Ethics approval: The local institutional review board approved the study.
REFERENCES
- 1.Hjort N, Butcher K, Davis SM, et al. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- 2.Kohrmann M, Juttler E, Fiebach JB, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: A cohort study. Lancet Neurol. 2006;5:661–667. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 5.Liebeskind DS. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. x. [DOI] [PubMed] [Google Scholar]
- 6.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 7.Liebeskind DS, Kidwell CS. Advanced MR imaging of acute stroke: The university of California at Los Angeles endovascular therapy experience. Neuroimaging Clin N Am. 2005;15:455–466. doi: 10.1016/j.nic.2005.06.002. xiii. [DOI] [PubMed] [Google Scholar]
- 8.Ostergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 9.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion–diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 10.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa K, Yagita Y, Sasaki T, et al. Chronic mild reduction of cerebral perfusion pressure induces ischemic tolerance in focal cerebral ischemia. Stroke. 2005;36:2270–2274. doi: 10.1161/01.STR.0000181075.77897.0e. [DOI] [PubMed] [Google Scholar]
- 12.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–265. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]