Abstract
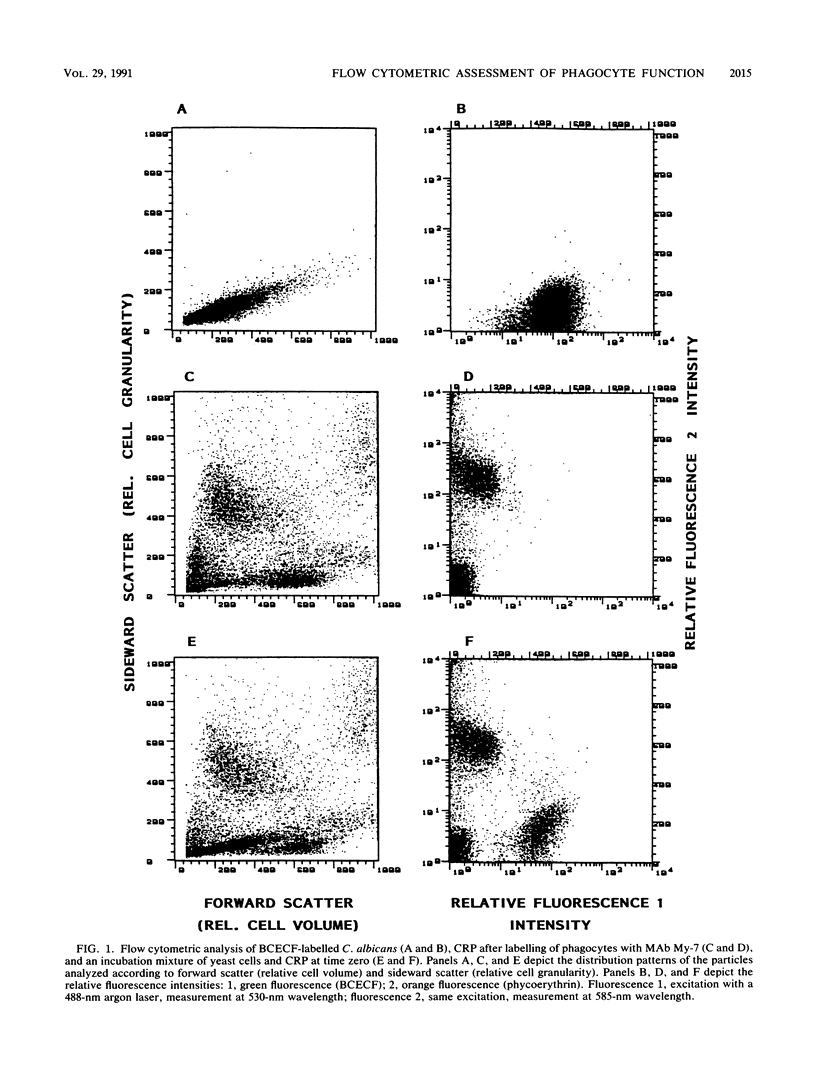
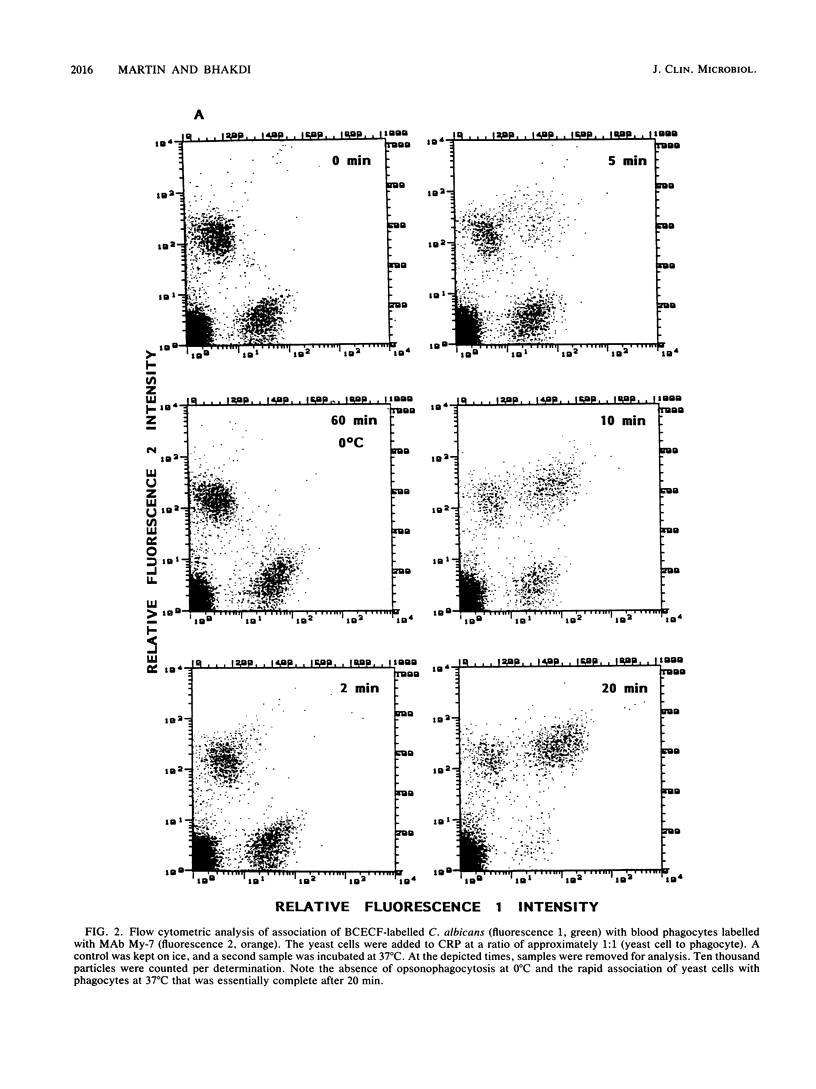
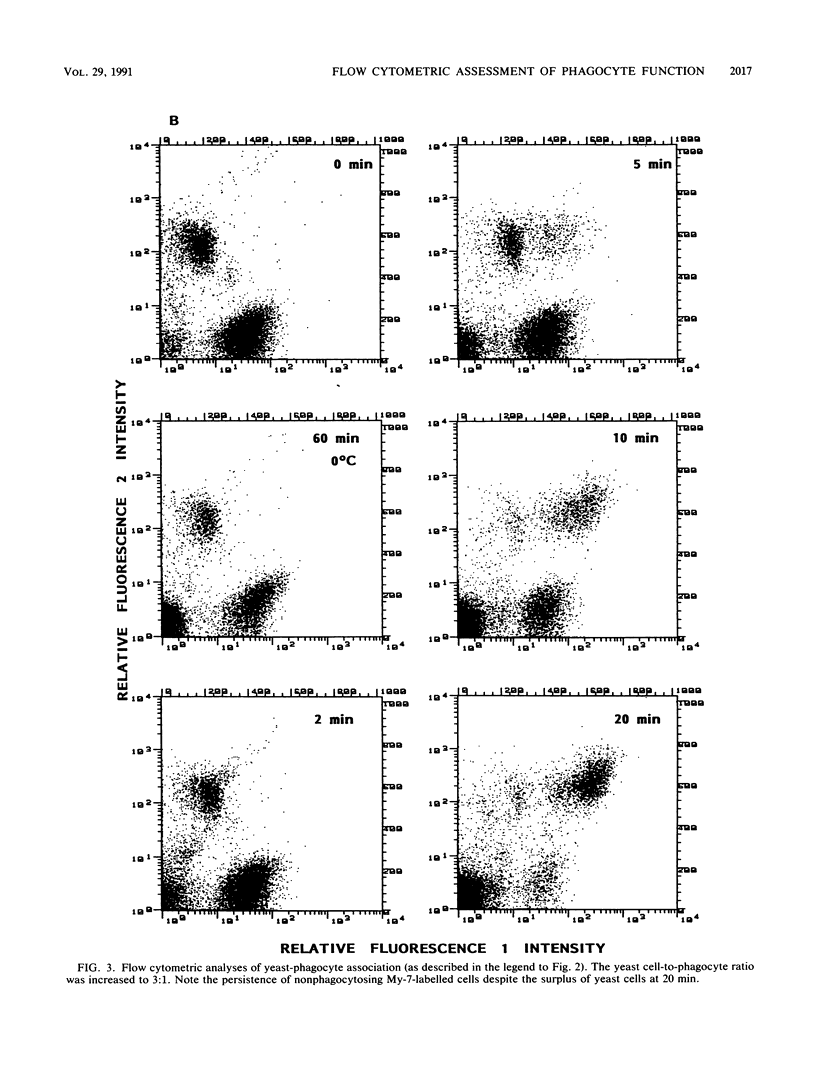
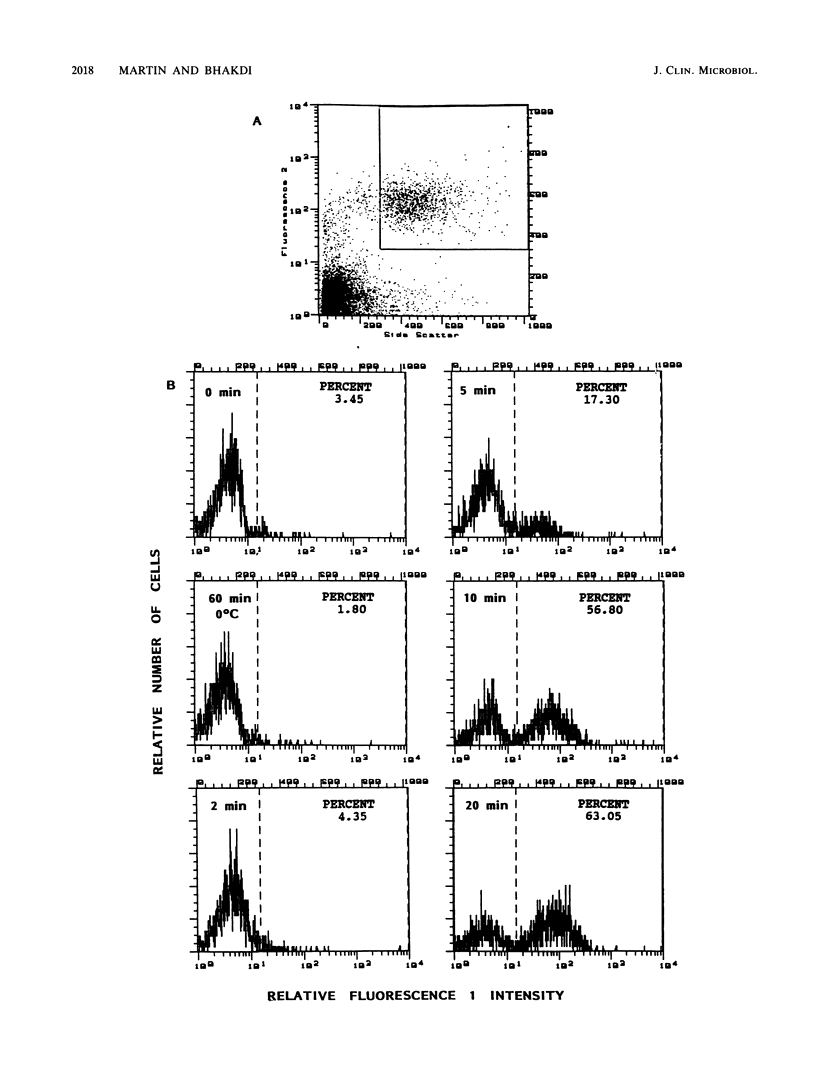
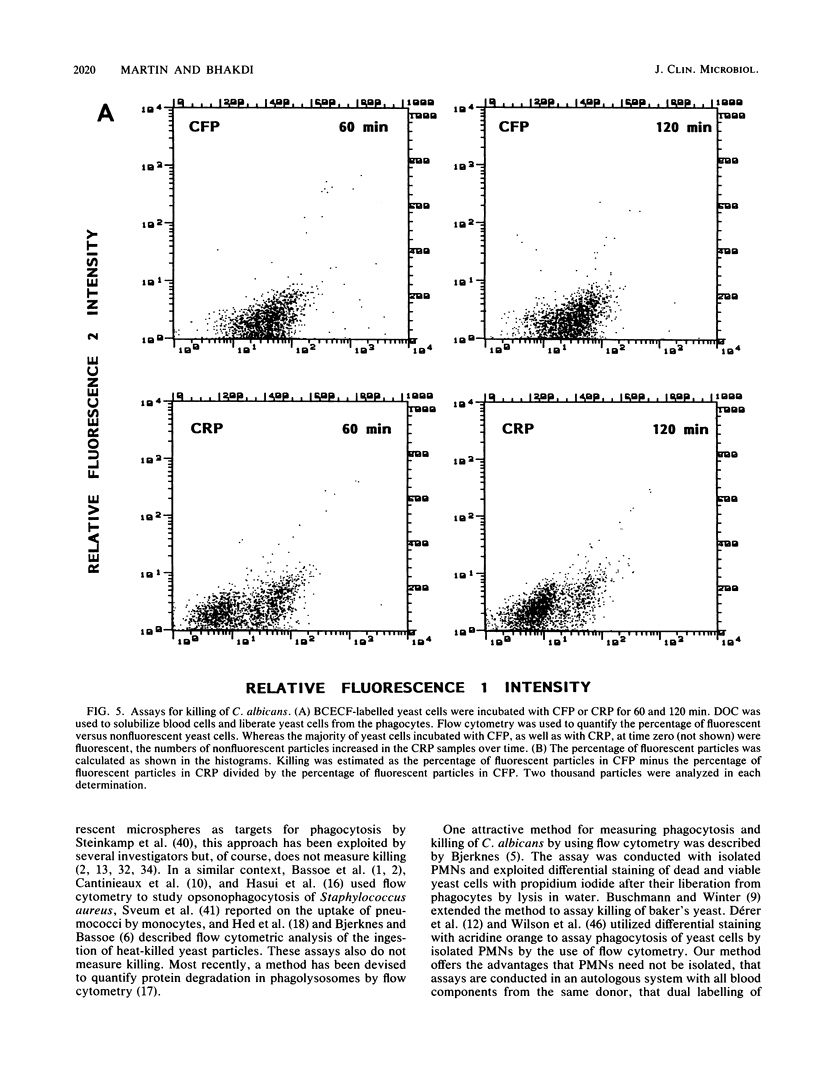
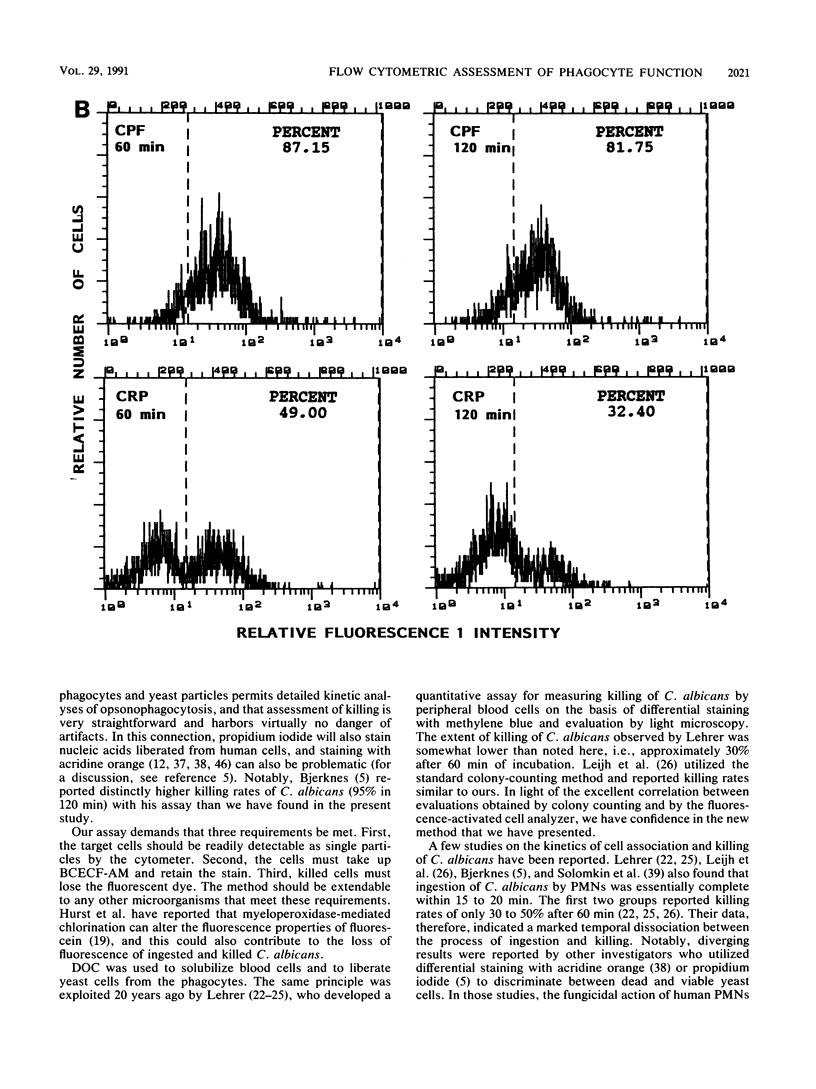
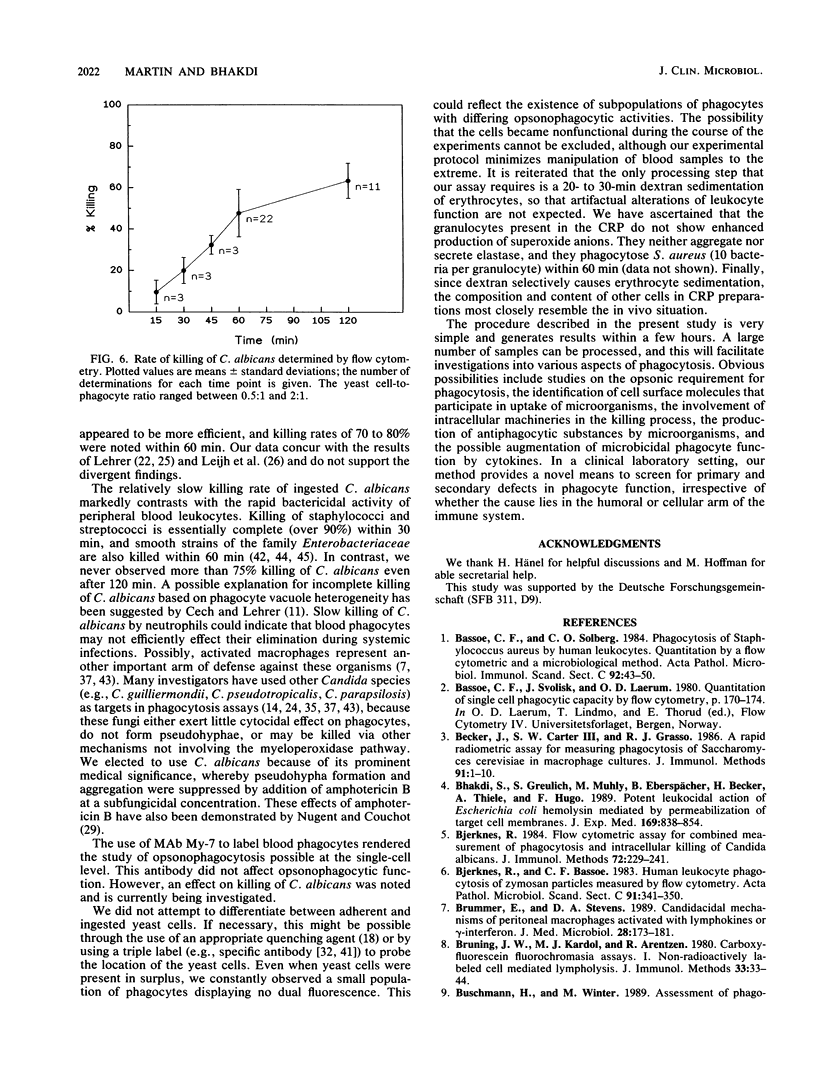
We describe a simple, rapid, automated procedure for measuring opsonophagocytosis and killing of Candida albicans by human peripheral blood leukocytes. Yeast cells are labelled by allowing uptake and cleavage of membrane-permeable bis-carboxyethyl-carboxyfluorescein pentaacetoxymethylester to its membrane-impermeable fluorescent derivative bis-carboxyethyl-carboxyfluorescein. The yeast cells are added to cell-rich plasma obtained after dextran sedimentation of erythrocytes. Opsonophagocytosis and killing are quantified by using automated fluorescent cell analysis, and the following parameters can be obtained: (i) relative percentage of phagocytes that participate in opsonophagocytosis, (ii) relative percentage of yeast cells that become associated with phagocytes, and (iii) percentage of killing of C. albicans. The first two parameters are obtained through the additional use of a phycoerythrin-conjugated monoclonal antibody that selectively labels monocytes and polymorphonuclear granulocytes in peripheral blood. Killing is assessed by solubilizing blood cells with deoxycholate to liberate yeast cells from the phagocytes. Viable yeast cells retain carboxyfluorescein, but nonviable cells lose the fluorescent marker; thus, the reduction in number of fluorescent particles directly reflects phagocytic killing. Results obtained by the present method correlated excellently with parallel enumerations by colony counting. Test results with seven healthy individuals revealed a marked dissociation between the process of opsonophagocytosis, which was essentially complete after 20 min at 37 degrees C, and killing rates, which were 48% +/- 11% and 63% +/- 9% (standard deviation) after 1 and 2 h, respectively, when yeast cell-to-phagocyte ratios were in the range of 0.5:1 to 2:1. The described assay is unrivaled in simplicity, rapidity, and reproducibility and generates results for a large number of samples within hours.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassøe C. F., Solberg C. O. Phagocytosis of Staphylococcus aureus by human leukocytes: quantitation by a flow cytometric and a microbiological method. Acta Pathol Microbiol Immunol Scand C. 1984 Feb;92(1):43–50. doi: 10.1111/j.1699-0463.1984.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Becker J., Carter S. W., 3rd, Grasso R. J. A rapid radiometric assay for measuring phagocytosis of Saccharomyces cerevisiae in macrophage cultures. J Immunol Methods. 1986 Jul 11;91(1):1–10. doi: 10.1016/0022-1759(86)90095-5. [DOI] [PubMed] [Google Scholar]
- Bjerknes R., Bassøe C. F. Human leukocyte phagocytosis of zymosan particles measured by flow cytometry. Acta Pathol Microbiol Immunol Scand C. 1983 Oct;91(5):341–348. [PubMed] [Google Scholar]
- Bjerknes R. Flow cytometric assay for combined measurement of phagocytosis and intracellular killing of Candida albicans. J Immunol Methods. 1984 Aug 3;72(1):229–241. doi: 10.1016/0022-1759(84)90451-4. [DOI] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Candidacidal mechanisms of peritoneal macrophages activated with lymphokines or gamma-interferon. J Med Microbiol. 1989 Mar;28(3):173–181. doi: 10.1099/00222615-28-3-173. [DOI] [PubMed] [Google Scholar]
- Bruning J. W., Kardol M. J., Arentzen R. Carboxyfluorescein fluorochromasia assays. I. Non-radioactively labeled cell mediated lympholysis. J Immunol Methods. 1980;33(1):33–44. doi: 10.1016/0022-1759(80)90080-0. [DOI] [PubMed] [Google Scholar]
- Cantinieaux B., Hariga C., Courtoy P., Hupin J., Fondu P. Staphylococcus aureus phagocytosis. A new cytofluorometric method using FITC and paraformaldehyde. J Immunol Methods. 1989 Jul 26;121(2):203–208. doi: 10.1016/0022-1759(89)90161-0. [DOI] [PubMed] [Google Scholar]
- Cech P., Lehrer R. I. Heterogeneity of human neutrophil phagolysosomes: functional consequences for candidacidal activity. Blood. 1984 Jul;64(1):147–151. [PubMed] [Google Scholar]
- Dunn P. A., Tyrer H. W. Quantitation of neutrophil phagocytosis, using fluorescent latex beads. Correlation of microscopy and flow cytometry. J Lab Clin Med. 1981 Sep;98(3):374–381. [PubMed] [Google Scholar]
- Dérer M., Walker C., Kristensen F., Reinhardt M. C. A simple and rapid flow cytometric method for routine assessment of baker's yeast uptake by human polymorphonuclear leukocytes. J Immunol Methods. 1983 Jul 29;61(3):359–365. doi: 10.1016/0022-1759(83)90232-6. [DOI] [PubMed] [Google Scholar]
- El-Maalem H., Fletcher J. Defective neutrophil function in chronic granulocytic leukaemia. Br J Haematol. 1976 Sep;34(1):95–103. doi: 10.1111/j.1365-2141.1976.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Harvey D. M., Sheppard K. J., Fletcher J. A method for measuring rate of neutrophil phagocytosis of Staphylococcus epidermidis or Candida guilliermondii using uptake of tritiated uridine. J Immunol Methods. 1986 Nov 6;93(2):259–264. doi: 10.1016/0022-1759(86)90198-5. [DOI] [PubMed] [Google Scholar]
- Hasui M., Hirabayashi Y., Kobayashi Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. J Immunol Methods. 1989 Feb 8;117(1):53–58. doi: 10.1016/0022-1759(89)90118-x. [DOI] [PubMed] [Google Scholar]
- Haynes A. P., Fletcher J., Garnett M., Robins A. A novel flow cytometric method for measuring protein digestion within the phagocytic vacuole of polymorphonuclear neutrophils. J Immunol Methods. 1990 Dec 31;135(1-2):155–161. doi: 10.1016/0022-1759(90)90268-z. [DOI] [PubMed] [Google Scholar]
- Hed J., Hallden G., Johansson S. G., Larsson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods. 1987 Jul 16;101(1):119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- Hurst J. K., Albrich J. M., Green T. R., Rosen H., Klebanoff S. Myeloperoxidase-dependent fluorescein chlorination by stimulated neutrophils. J Biol Chem. 1984 Apr 25;259(8):4812–4821. [PubMed] [Google Scholar]
- Husseini R. H., Hoadley M. E., Hutchinson J. J., Penn C. W., Smith H. Intracellular killing of Candida albicans by human polymorphonuclear leucocytes: comparison of three methods of assessment. J Immunol Methods. 1985 Aug 2;81(2):215–221. doi: 10.1016/0022-1759(85)90206-6. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L. Paraformaldehyde fixation of hematopoietic cells for quantitative flow cytometry (FACS) analysis. J Immunol Methods. 1981;47(1):25–30. doi: 10.1016/0022-1759(81)90253-2. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of Candidacidal Activity of Specific Leukocyte Types in Mixed Cell Populations II. Normal and Chronic Granulomatous Disease Eosinophils. Infect Immun. 1971 Jun;3(6):800–802. doi: 10.1128/iai.3.6.800-802.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J., Diamond R. D. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J Immunol Methods. 1987 Jul 16;101(1):37–42. doi: 10.1016/0022-1759(87)90213-4. [DOI] [PubMed] [Google Scholar]
- Louria D. B., Smith J. K., Brayton R. G., Buse M. Anti-Candida factors in serum and their inhibitors. I. Clinical and laboratory observations. J Infect Dis. 1972 Feb;125(2):102–114. doi: 10.1093/infdis/125.2.102. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Couchot K. R. Effects of sublethal concentrations of amphotericin B on Candida albicans. J Infect Dis. 1986 Oct;154(4):665–669. doi: 10.1093/infdis/154.4.665. [DOI] [PubMed] [Google Scholar]
- Oben J. A., Foreman J. C. A simple quantitative fluorimetric assay of in vitro phagocytosis in human neutrophils. J Immunol Methods. 1988 Aug 9;112(1):99–103. doi: 10.1016/0022-1759(88)90039-7. [DOI] [PubMed] [Google Scholar]
- Oda T., Maeda H. A new simple fluorometric assay for phagocytosis. J Immunol Methods. 1986 Apr 17;88(2):175–183. doi: 10.1016/0022-1759(86)90004-9. [DOI] [PubMed] [Google Scholar]
- Ogle J. D., Noel J. G., Sramkoski R. M., Ogle C. K., Alexander J. W. Phagocytosis of opsonized fluorescent microspheres by human neutrophils. A two-color flow cytometric method for the determination of attachment and ingestion. J Immunol Methods. 1988 Nov 25;115(1):17–29. doi: 10.1016/0022-1759(88)90305-5. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Rolland A., Merdrignac G., Gouranton J., Bourel D., Le Verge R., Genetet B. Flow cytometric quantitative evaluation of phagocytosis by human mononuclear and polymorphonuclear cells using fluorescent nanoparticles. J Immunol Methods. 1987 Feb 11;96(2):185–193. doi: 10.1016/0022-1759(87)90313-9. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid L., Brune K. Assessment of phagocytic and antimicrobial activity of human granulocytes. Infect Immun. 1974 Nov;10(5):1120–1126. doi: 10.1128/iai.10.5.1120-1126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit K. E. Phagocytosis and intracellular killing of pathogenic yeasts by human monocytes and neutrophils. Infect Immun. 1979 Jun;24(3):932–938. doi: 10.1128/iai.24.3.932-938.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Rommel F. A rapid micro method for the simultaneous determination of phagocytic-microbiocidal activity of human peripheral blood leukocytes in vitro. J Immunol Methods. 1977;17(3-4):241–247. doi: 10.1016/0022-1759(77)90106-5. [DOI] [PubMed] [Google Scholar]
- Solomkin J. S., Mills E. L., Giebink G. S., Nelson R. D., Simmons R. L., Quie P. G. Phagocytosis of Candida albicans by human leukocytes: opsonic requirements. J Infect Dis. 1978 Jan;137(1):30–37. doi: 10.1093/infdis/137.1.30. [DOI] [PubMed] [Google Scholar]
- Steinkamp J. A., Wilson J. S., Saunders G. C., Stewart C. C. Phagocytosis: flow cytometric quantitation with fluorescent microspheres. Science. 1982 Jan 1;215(4528):64–66. doi: 10.1126/science.7053559. [DOI] [PubMed] [Google Scholar]
- Sveum R. J., Chused T. M., Frank M. M., Brown E. J. A quantitative fluorescent method for measurement of bacterial adherence and phagocytosis. J Immunol Methods. 1986 Jun 24;90(2):257–264. doi: 10.1016/0022-1759(86)90083-9. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Bistoni F., Cenci E., Perito S., Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985 Oct;23(5):377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- Vel W. A., Namavar F., Verweij A. M., Pubben A. N., MacLaren D. M. Killing capacity of human polymorphonuclear leukocytes in aerobic and anaerobic conditions. J Med Microbiol. 1984 Oct;18(2):173–180. doi: 10.1099/00222615-18-2-173. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. M., Galvin A. M., Robins R. A., Reeves W. G. A flow cytometric method for the measurement of phagocytosis by polymorphonuclear leucocytes. J Immunol Methods. 1985 Feb 11;76(2):247–253. doi: 10.1016/0022-1759(85)90301-1. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Boler J., Valdimarsson H. Phagocytosis measured as inhibition of uridine uptake by Candida albicans. J Immunol Methods. 1977;14(1):19–24. doi: 10.1016/s0022-1759(97)90016-8. [DOI] [PubMed] [Google Scholar]


