Abstract
Context
Despite concerns about drug safety, current information on older adults’ use of prescription and over-the-counter medications and dietary supplements is limited.
Objective
To estimate the prevalence and patterns of medication use among older adults (including concurrent use), and potential major drug-drug interactions.
Design, Setting, and Participants
Three thousand five community-residing individuals, aged 57 through 85 years, were drawn from a cross-sectional, nationally representative probability sample of the United States. In-home interviews, including medication logs, were administered between June 2005 and March 2006. Medication use was defined as prescription, over-the-counter, and dietary supplements used “on a regular schedule, like every day or every week.” Concurrent use was defined as the regular use of at least 2 medications.
Main Outcome Measure
Population estimates of the prevalence of medication use, concurrent use, and potential major drug-drug interactions, stratified by age group and gender.
Results
The unweighted survey response rate was 74.8% (weighted response rate, 75.5%). Eighty-one percent (95% confidence interval [CI], 79.4%–83.5%) used at least 1 prescription medication, 42% (95% CI, 39.7%–44.8%) used at least 1 over-the-counter medication, and 49% (95% CI, 46.2%–52.7%) used a dietary supplement. Twenty-nine percent (95% CI, 26.6%–30.6%) used at least 5 prescription medications concurrently; this was highest among men (37.1%; 95% CI, 31.7%–42.4%) and women (36.0%; 95% CI, 30.2%–41.9%) aged 75 to 85 years. Among prescription medication users, concurrent use of over-the-counter medications was 46% (95% CI, 43.4%–49.1%) and concurrent use of dietary supplements was 52% (95% CI, 48.8%–55.5%). Overall, 4% of individuals were potentially at risk of having a major drug-drug interaction; half of these involved the use of nonprescription medications. These regimens were most prevalent among men in the oldest age group (10%; 95% CI, 6.4%–13.7%) and nearly half involved anticoagulants. No contraindicated concurrent drug use was identified.
Conclusions
In this sample of community-dwelling older adults, prescription and nonprescription medications were commonly used together, with nearly 1 in 25 individuals potentially at risk for a major drug-drug interaction.
Rates of per-capita prescription medication use have increased considerably over the last several decades,1 as have the rates of use of over-the-counter medications2 and dietary supplements.3 Older adults are the largest per capita consumers of prescription medications1 and the most at risk for medication-related adverse events.4 Implementation of the Medicare Part D Prescription Drug Benefit and efforts by Congress and the US Food and Drug Administration to enhance postmarketing surveillance to better safeguard public health5,6 have also focused attention on use of prescription medications among older adults. Despite concerns about drug safety and new federal policies to improve older adults’ access to medications, current information on their concurrent use of prescription medications, over-the-counter medications, and dietary supplements is limited.
Most epidemiologic studies examining medication use among older adults are nearly a decade old and aggregate persons 65 years and older.7,8 More than 200 new drugs have come to market in the United States since most of these studies were conducted.9 In addition, many studies use pharmacy claims or aggregate sales data to approximate use of prescription medications, thus measuring prescription acquisition rather than use.10 These data overestimate prescription medication use due to non-adherence,11,12 are limited to patients who purchase at least 1 prescription medication, and almost always exclude nonprescription therapies. This is important because medication use among older adults commonly involves multiple medications and the concurrent use of prescription medications with nonprescription therapies.13,14 The Slone survey,8,15 an annual telephone-based survey of the US population 18 years and older, does provide information regarding the use of prescription and nonprescription medications. However, the most recent Slone survey, conducted in 2006, reports little change in overall medication use since 1998 but does report that the rate of polypharmacy (ie, the use of ≥5 medicines) has increased.15
In the current study, we build on prior reports with a particular focus on older adults. In addition, we consider the potential impact of medication use on clinical outcomes that may result from drug-drug interactions, including interactions between prescription and nonprescription therapies. To do so, we used data from the National Social life, Health and Aging Project (NSHAP), a recent population-based survey of community-dwelling older adults in the United States.
METHODS
Participants
The NSHAP study protocol is a nationally representative probability sample of community-dwelling persons aged 57 through 84 years (at the time of screening in 2004) from households across the United States, as previously described.16 Blacks, Hispanics, men, and individuals aged 75 through 84 years at the time of screening were oversampled. Of 4400 individuals identified, 383 were not in the target population, and 1012 were not interviewed (of whom 834 refused and 147 were in poor health and could not participate). Therefore, 4017 persons were eligible, of whom 3005 were successfully interviewed, yielding an unweighted response rate of 74.8% and a weighted response rate of 75.5%.
Professional interviewers conducted in-home interviews and compiled medication logs in English and Spanish between July 2005 and March 2006. Participants who in the judgment of the interviewer were unable to adequately understand and complete the informed consent process were not eligible for participation in the study (categorized above as in “poor health”). The protocol was approved by the University of Chicago and NORC institutional review boards, and all respondents provided written informed consent.
Data
Data on medication use were collected during the household interview by direct observation of medication bottles using a computer-based log. Participants were asked to provide the interviewer with the containers for all medications used “on a regular schedule, like every day or every week” and were instructed to include “prescription and non-prescription medications, over-the-counter medicines, vitamins, and herbal and alternative medicines.” In the fewer than 5% of cases in which neither packaging nor the actual medication was available for direct observation, the medication name was obtained by respondent self-report.
The interviewer directly recorded up to 20 medication names into a laptop computer; fewer than 1% of respondents reported the use of 20 medications. All identifiable drug names for prescription and over-the-counter medications as well as dietary supplements were coded. A clinical pharmacist (D.M.Q.) reviewed all unique drug entries and recoded proprietary medication names (eg, Lopressor) to ingredient drug names (eg, metoprolol) when possible to facilitate matching to a drug database used to assign drug names.17 A total of 15 389 individual medications were reported by 2976 respondents. Of these, 15 224 (99%) were coded and linked to the drug database; of these, 702 did not originally appear in the database and therefore were classified (D.M.Q.) using the existing database categories. Most of these drug names were dietary supplements, since the coverage of this category of medications in the drug database is not complete. Additional details on the method of drug coding have been previously described.11
Medications were coded by type (prescription, over-the-counter, or dietary supplement) and by the formulation of each compound (eg, single vs multicomponent products). Medication use was defined as the regular use (at least daily or weekly) of at least 1 prescription or over-the-counter medication or dietary supplement. Use of nonprescription medications included over-the-counter medications and dietary supplements. Concurrent use was defined as the regular use of 2 or more medications at least daily or weekly.
We used Thomson Micromedex18 to determine potential major medication interactions among the 20 most common prescription and over-the-counter medications and the 20 most common dietary supplements. Micromedex was used to identify potential interactions among the list of common medications and provided a measure of the severity of the interaction (contraindicated [the drugs are contraindicated for concurrent use]; major [the interaction may be life-threatening, require medical intervention to minimize or prevent serious adverse events, or both]; moderate [the interaction may result in the exacerbation of the patient’s condition, require an alternation in therapy, or both]; and minor [the interaction would have limited clinical effects]). In our analysis, we focus on those drug-drug interactions considered to be potentially of major severity.
We selected age intervals (57–64 years, 65–74 years, and 75–85 years; some individuals aged 84 years at enrollment had turned 85 during the study) for consistency with previous studies and the age structure of the study design. We determined race or ethnic group on the basis of the questions: “Do you consider yourself primarily white or Caucasian, black or African American, American Indian, Asian, or something else?” and “Do you consider yourself Hispanic or Latino?” Race/ethnicity was considered important for this study because previous studies have documented racial and ethnic differences in the prevalence of medication use.19,20
We asked respondents to rate their physical health using the standard 5-point scale with the responses excellent, very good, good, fair, or poor. We also asked respondents whether a physician had ever told them they had any of several common medical conditions, including high blood pressure, stroke, myocardial infarction, heart failure, diabetes, thyroid problems, Alzheimer disease or dementia, cancer, and any type of arthritis. We used the responses to these questions to compute a previously validated version of the Charlson Comorbidity Index.21
Information about insurance status was ascertained by asking, “Are you currently covered by any of the following health insurance programs (Medicare, Medicaid, private insurance, Veterans Administration, or other)?” We computed household income as percentage of federal poverty level (FPL), computed based on reported household income and household size. In 2006, the FPL was $9669 for a single person 65 years or older and $12 201 for a household of 2 adults.22 We considered respondents who reporting being currently married as a household of 2 adults; all other respondents were considered single persons. We defined poor respondents as those having a household income at or below 100% of the FPL, near-poor respondents as those having a household income between 101% through 200% of the FPL, and nonpoor as those having a household income above 200% of the FPL.
Analysis
For each analysis, we used weights included in the NSHAP data set to adjust for differential probability of selection and differential nonresponse.23 Reported confidence intervals (CIs) were constructed by inverting the corresponding Wald test and do not include any adjustment for multiple testing. Descriptive statistics were used to estimate the prevalence of use of prescription medications, over-the-counter medications, or dietary supplements; concurrent medication use; and medication interactions among the entire sample and stratified by age and gender. Logistic regression was used to compare prevalence between subgroups. All P values reported are 2-sided. We also used logistic regression to assess which variables were significantly (P <.05) associated with no regular medication use. We selected all variables listed in Table 1 to be tested in our adjusted model, since these variables have previously been shown to be related to the probability of medication use. The variables were entered into the model simultaneously, and all analyses were performed using Stata version 10.1.24 All CIs and tests use design-based estimates of variance.
Table 1.
Weighted Prevalence Estimates of Medication Use by Sociodemographic and Health-Related Characteristicsa
| Characteristic | Estimated Prevalence, % (95% CI) | ||||
|---|---|---|---|---|---|
| Overall Sample (N = 2976) |
No Medication (n = 259) |
Prescription Medication (n = 2455) |
Over-the-counter Medication (n = 1253) |
Dietary Supplementb (n = 1425) |
|
| Overall | 100 | 9 (7.5–10.0) | 81 (79.4–83.5) | 42 (39.7–44.8) | 49 (46.2–52.7) |
| Weighted No. | 2979 | 261 | 2426 | 1258 | 1473 |
| Age, y | |||||
| 57–64 | 42 (39.0–44.1) | 13.3 (10.7–15.9) | 74.3 (69.9–78.7) | 36.1 (32.4–39.9) | 43.6 (40.0–47.2) |
| Weighted No. | 1237 | 165 | 920 | 447 | 539 |
| 65–74 | 35 (32.6–37.1) | 6.0 (4.6–7.3) | 84.2 (81.9–86.5) | 46.0 (42.6–49.5) | 53.2 (49.0–57.4) |
| Weighted No. | 1038 | 62 | 875 | 478 | 553 |
| 75–85 | 24 (21.7–25.5) | 4.9 (3.1–6.6) | 89.7 (87.4–92.1) | 47.3 (43.1–51.5) | 54.2 (49.2–59.2) |
| Weighted No. | 703 | 34 | 631 | 333 | 381 |
| Gender | |||||
| Men | 49 (46.2–51.0) | 11.0 (9.1–13.0) | 76.6 (73.1–80.1) | 42.6 (39.5–45.7) | 43.1 (39.5–46.8) |
| Weighted No. | 1448 | 160 | 1109 | 617 | 624 |
| Women | 51 (49.0–53.8) | 6.6 (5.0–8.2) | 86.0 (83.8–88.2) | 41.9 (38.7–45.1) | 55.4 (51.7–59.2) |
| Weighted No. | 1531 | 101 | 1317 | 641 | 849 |
| Education | |||||
| <High school | 18 (15.2–21.6) | 10.8 (7.4–14.2) | 82.1 (78.3–86.0) | 45.8 (41.0–50.5) | 44.5 (39.3–49.7) |
| Weighted No. | 548 | 59 | 450 | 251 | 244 |
| High school or equivalent | 27 (24.4–29.5) | 8.8 (6.3–11.3) | 81.3 (77.7–85.0) | 42.7 (38.8–46.5) | 48.1 (43.8–52.3) |
| Weighted No. | 803 | 71 | 653 | 343 | 386 |
| Some college | 30 (27.3–32.8) | 8.0 (5.3–10.6) | 83.0 (79.7–86.3) | 41.6 (36.4–46.8) | 52.3 (46.0–58.7) |
| Weighted No. | 895 | 71 | 743 | 372 | 469 |
| ≥Bachelor’s degree | 25 (21.3–27.9) | 8.1 (5.5–10.7) | 79.1 (74.9–83.3) | 39.8 (35.8–43.9) | 51.1 (45.4–56.7) |
| Weighted No. | 732 | 60 | 579 | 292 | 374 |
| Race/ethnicityc | |||||
| White, non-Hispanic | 81 (77.0–84.4) | 8.0 (6.7–9.3) | 82.0 (79.8–84.2) | 44.1 (40.8–47.4) | 51.7 (48.1–55.4) |
| Weighted No. | 2397 | 191 | 1966 | 1056 | 1240 |
| Black, non-Hispanic | 10 (7.6–12.1) | 8.5 (5.2–11.7) | 84.0 (79.6–88.5) | 36.1 (28.3–43.9) | 37.3 (30.3–44.3) |
| Weighted No. | 294 | 25 | 247 | 106 | 110 |
| Hispanic, any race | 7 (3.5–10.2) | 18.1 (12.3–23.8) | 71.4 (65.2–77.6) | 30.6 (23.9–37.3) | 41.9 (34.5–49.3) |
| Weighted No. | 204 | 37 | 146 | 62 | 85 |
| Other | 3 (1.6–3.4) | 11.1 (3.6–18.6) | 76.9 (65.2–88.7) | 37.1 (26.1–48.0) | 43.7 (31.8–55.5) |
| Weighted No. | 74 | 8 | 57 | 28 | 32 |
| Household incomed | |||||
| Poor (≤100% FPL) | 8.4 (5.5–11.2) | 13.4 (7.8–18.9) | 78.6 (72.7–84.6) | 40.9 (34.8–46.9) | 49.4 (42.4–56.4) |
| Weighted No. | 177 | 24 | 139 | 72 | 87 |
| Near poor (101%–200% FPL) | 16.7 (14.7–18.8) | 8.7 (5.1–12.2) | 83.5 (79.4–87.6) | 47.2 (39.9–54.5) | 47.1 (40.1–54.1) |
| Weighted No. | 354 | 31 | 295 | 167 | 167 |
| Nonpoor (>200% FPL) | 74.9 (71.1–78.7) | 6.9 (5.3–8.4) | 81.9 (78.9–84.8) | 44.2 (40.2–48.1) | 52.4 (48.6–56.3) |
| Weighted No. | 1582 | 109 | 1295 | 699 | 829 |
| Health insurancee | |||||
| None | 5.1 (3.9–6.3) | 14.8 (7.2–22.5) | 73.5 (64.9–82.2) | 40.5 (30.7–50.4) | 39.0 (31.3–46.6) |
| Weighted No. | 131 | 19 | 97 | 53 | 51 |
| Medicare | 57.7 (54.8–60.7) | 5.0 (3.7–6.4) | 87.6 (85.8–89.4) | 47.9 (44.1–51.7) | 54.2 (49.5–58.9) |
| Weighted No. | 1478 | 74 | 1295 | 708 | 802 |
| Private insurance | 30.3 (27.2–33.5) | 12.8 (8.7–16.8) | 74.0 (68.4–79.7) | 36.5 (31.7–41.4) | 45.4 (40.8–50.0) |
| Weighted No. | 777 | 99 | 575 | 284 | 353 |
| Medicaid, VA, other | 6.8 (5.6–8.0) | 14.1 (7.4–20.8) | 74.8 (66.2–83.5) | 39.0 (28.4–49.5) | 52.9 (43.0–62.8) |
| Weighted No. | 174 | 24 | 130 | 68 | 92 |
| Self-reported health | |||||
| Poor | 6.7 (5.4–8.1) | 6.2 (1.5–10.8) | 89.9 (83.9–95.9) | 51.0 (42.4–59.5) | 49.1 (41.8–56.4) |
| Weighted No. | 200 | 12 | 180 | 102 | 98 |
| Fair | 17.9 (15.9–19.9) | 3.8 (1.9–5.7) | 89.6 (84.9–94.3) | 50.5 (45.4–55.6) | 50.1 (43.7–56.6) |
| Weighted No. | 533 | 20 | 478 | 269 | 267 |
| Good | 29.6 (27.5–31.7) | 5.7 (3.8–7.8) | 87.2 (84.2–90.2) | 45.2 (41.0–49.4) | 48.0 (43.0–53.0) |
| Weighted No. | 879 | 51 | 767 | 397 | 422 |
| Very good | 32.6 (30.5–34.7) | 10.6 (8.3–12.9) | 77.3 (74.1–80.4) | 38.2 (34.6–41.7) | 50.7 (46.8–54.7) |
| Weighted No. | 969 | 103 | 749 | 370 | 492 |
| Excellent | 13.1 (11.3–14.9) | 19.2 (14.0–24.5) | 62.8 (56.4–69.1) | 29.2 (22.3–36.1) | 48.1 (40.7–55.5) |
| Weighted No. | 389 | 75 | 244 | 114 | 187 |
| Comorbidity indexf | |||||
| 0 | 24.7 (22.9–26.4) | 19.5 (15.7–23.3) | 63.4 (58.7–68.1) | 29.1 (22.8–35.4) | 45.1 (40.8–49.4) |
| Weighted No. | 734 | 143 | 466 | 214 | 331 |
| 1–4 | 68.8 (66.8–70.8) | 5.7 (4.5–6.9) | 86.3 (84.1–88.5) | 45.2 (42.6–47.8) | 50.0 (46.3–53.7) |
| Weighted No. | 2049 | 117 | 1768 | 926 | 1025 |
| ≥5 | 6.6 (5.5–7.7) | 0.6 (0 to 1.4) | 98.2 (96.5–99.9) | 60.2 (52.9–67.5) | 59.8 (52.7–66.9) |
| Weighted No. | 196 | 1 | 192 | 118 | 117 |
Abbreviations: CI, confidence interval; FPL, federal poverty level; VA, Veterans Administration.
Percentages and numbers of respondents are weighted estimates to account for differential probabilities of selection and differential nonresponse; CIs based on inversion of Wald tests constructed with the use of design-based SEs.
Includes the use of nutritional products and alternative therapies.
Excludes 12 respondents who refused to answer or answered “don’t know.”
Excludes 860 respondents for whom information on household income was not collected.
Excludes 473 respondents for whom information on health insurance was not collected. “Private insurance” and “Medicaid, VA, other” exclude respondents with Medicare. Insurance categories are mutually exclusive.
Range of possible scores, 0–10.
RESULTS
The weighted distribution of demographic and health characteristics in the NSHAP sample correspond closely to those of the population and other national samples.16 Of the 3005 respondents, 2976 (99%) completed the medication log.
Table 1 reports the prevalence of medication use by type, both overall and within sociodemographic and health subgroups. During 2005 to 2006, 91% (95% CI, 90.0%–92.5%) of older adults, corresponding to 50.5 million adults aged 57 to 85 years, regularly used at least 1 medication. Among all medication types, prescription medication use was the most prevalent, used by 81% (95% CI, 79.4%–83.5%), or 44.9 million older adults. Nearly one-half of older adults regularly used at least 1 over-the-counter medication or dietary supplement. The prevalence of prescription medication use was highest among the oldest age group; 89.7% (95% CI, 87.4%–92.1%) of persons aged 75 to 85 years used at least 1 prescription medication, compared with 74.3% (95% CI, 69.9%–78.7%) of those aged 57 through 64 years. Women were more likely to use prescription medications (86.0%; 95% CI, 83.8%–88.2%) than men (76.6%; 95% CI, 73.1%–80.1%). While the prevalence of dietary supplement use was greater in women (55.4%; 95% CI, 51.7%–59.2%) compared with men (43.1%; 95% CI, 39.5%–46.8%), the prevalence of over-the counter medication use was similar among women (41.9%; 95% CI, 38.7%–45.1%) and men (42.6%; 95% CI, 39.5%–45.7%).
Table 2 depicts the likelihood of no regular medication use by individuals’ sociodemographic and health characteristics. As expected, medication use was more likely among those with poorer self-reported health and more comorbid conditions. In addition, medication use was more likely among nonpoor respondents and those with greater formal education.
Table 2.
Sociodemographic and Health-Related Factors Associated With No Regular Medication Use Among Older Adults in the United States
| Characteristic | OR (95% CI) | |
|---|---|---|
| Unadjusted | Adjusteda | |
| Age, y | ||
| 57–64 | 1 [Reference] | 1 [Reference] |
| 65–74 | 0.41 (0.30–0.57)b | 0.23 (0.11–0.45)b |
| 75–85 | 0.33 (0.21–0.53)b | 0.34 (0.13–0.85)b |
| Gender | ||
| Men | 1 [Reference] | 1 [Reference] |
| Women | 0.57 (0.41–0.79)b | 0.40 (0.25–0.64)b |
| Education | ||
| <High school | 1 [Reference] | 1 [Reference] |
| High school or equivalent | 0.80 (0.49–1.3) | 0.62 (0.34–1.1) |
| Some college | 0.71 (0.43–1.2) | 0.66 (0.33–1.3) |
| Bachelor’s degree or more | 0.73 (0.45–1.2) | 0.44 (0.24–0.8)b |
| Race/ethnicity | ||
| White, non-Hispanic | 1 [Reference] | 1 [Reference] |
| Black, non-Hispanic | 1.1 (0.68–1.69) | 0.63 (0.28–1.4) |
| Hispanic, any race | 2.6 (1.67–3.9)b | 1.3 (0.76–2.3) |
| Other | 1.4 (0.67–3.1) | 1.6 (0.59–4.3) |
| Household income | ||
| Poor (≤ 100% FPL) | 1 [Reference] | 1 [Reference] |
| Near poor (101%–200% FPL) | 0.61 (0.32–1.2) | 0.87 (0.43–1.8) |
| Nonpoor (>200% FPL) | 0.48 (0.3–0.77)b | 0.37 (0.17–0.79)b |
| Health insurance | ||
| None | 1 [Reference] | 1 [Reference] |
| Medicare | 0.30 (0.17–0.55)b | 0.65 (0.36–1.2) |
| Private insurance | 0.84 (0.40–1.77) | 0.46 (0.18–1.2) |
| Medicaid, VA, other | 0.94 (0.42–2.1) | 0.58 (0.24–1.4) |
| Self-reported physical health | ||
| Poor | 1 [Reference] | 1 [Reference] |
| Fair | 0.60 (0.25–1.43) | 0.73 (0.18–3.0) |
| Good | 0.93 (0.36–2.43) | 1.7 (0.5–5.5) |
| Very good | 1.8 (0.87–3.74) | 2.9 (1.0–8.6)b |
| Excellent | 3.6 (1.53–8.51)b | 6.2 (1.5–25.1)b |
| Comorbidity indexc | ||
| 0 | 1 [Reference] | 1 [Reference] |
| 1–4 | 0.25 (0.18–0.34)b | 0.33 (0.20–0.53)b |
| ≥5 | 0.024 (0.01–0.11)b | 0.04 (0.01–0.38)b |
Abbreviations: CI, confidence interval; FPL, federal poverty level; OR, odds ratio; VA, Veterans Administration.
Model included the variables age group, gender, education, racial/ethnic group, household income, health insurance (“private insurance” and “Medicaid, VA, other” exclude respondents with Medicare), self-reported health, and comorbidity index.
Statistically significant (P<.05).
Range of possible scores, 0–10.
Commonly Used Prescription and Nonprescription Medications
The most commonly used prescription or over-the-counter medications, either as single or multi component products, were cardiovascular agents, including antihyperlipidemics and anticoagulants such as aspirin, hydrochlorothiazide, atorvastatin, lisinopril, metoprolol, simvastatin, atenolol, amlodipine, furosemide, ezetimibe, valsartan, warfarin, and clopidogrel (Table 3). Across all age groups, the prevalence of use of the statins atorvastatin and simvastatin was higher among men compared with women.
Table 3.
Weighted Prevalence Estimates of the Most Commonly Used Prescription and Over-the-counter (OTC) Medications by Age and Gendera
| Medication | Estimated Prevalence, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Aged 57–64 y (n = 1016) |
Aged 65–74 y (n = 1082) |
Aged 75–85 y (n = 878) |
Total (N = 2976) |
||||
| Men (n = 525) |
Women (n = 491) |
Men (n = 543) |
Women (n = 539) |
Men (n = 377) |
Women (n = 501) |
||
| Aspirin (OTC available) | 23.8 (19.4–28.3) | 20.5 (15.5–25.5) | 34.5 (29.9–39.1) | 26.6 (23.0–30.1) | 39.1 (32.1–46.1) | 31.2 (26.0–36.5) | 28.0 (25.3–30.7) |
| Weighted No. | 151 | 124 | 175 | 142 | 121 | 123 | 835 |
| Hydrochlorothiazide | 9.1 (6.1–12.2) | 16.6 (11.8–21.4) | 15.2 (10.9–19.5) | 16.9 (13.0–20.7) | 19.4 (15.1–23.7) | 20.0 (15.2–24.8) | 15.6 (13.4–17.7) |
| Weighted No. | 58 | 100 | 77 | 90 | 60 | 79 | 464 |
| Atorvastatin | 15.8 (11.7–19.8) | 9.6 (6.8–12.3) | 15.5 (11.9–19.0) | 13.4 (10.1–16.7) | 15.6 (11.8–19.4) | 11.5 (7.6–15.3) | 13.4 (11.9–15.0) |
| Weighted No. | 100 | 58 | 78 | 71 | 48 | 45 | 401 |
| Levothyroxine | 3.1 (1.8–4.5) | 16.4 (11.7–21.1) | 7.6 (5.2–10.0) | 18.8 (15.7–21.9) | 9.6 (5.8–13.3) | 20.9 (17.2–24.6) | 12.4 (10.8–14.0) |
| Weighted No. | 20 | 99 | 38 | 100 | 30 | 82 | 369 |
| Lisinopril | 9.9 (6.8–13.0) | 9.6 (5.4–13.8) | 14.8 (11.5–18.2) | 10.0 (7.2–12.8) | 21.1 (17.5–24.7) | 12.8 (9.5–16.1) | 12.2 (10.5–14.0) |
| Weighted No. | 63 | 58 | 75 | 53 | 65 | 51 | 365 |
| Metoprolol | 9.6 (7.6–11.7) | 8.0 (5.0–11.1) | 12.1 (8.5–15.6) | 9.4 (7.0–11.7) | 15.2 (10.9–19.6) | 14.4 (11.7–17.2) | 10.9 (9.6–12.2) |
| Weighted No. | 61 | 49 | 61 | 50 | 47 | 57 | 324 |
| Simvastatin | 9.3 (7.0–11.7) | 5.3 (3.0–7.7) | 11.6 (8.7–14.5) | 8.7 (6.0–11.4) | 18.0 (13.2–22.8) | 8.6 (5.3–11.8) | 9.6 (8.5–10.7) |
| Weighted No. | 59 | 32 | 59 | 46 | 56 | 34 | 286 |
| Atenolol | 6.2 (3.7–8.8) | 6.9 (4.5–9.3) | 9.5 (7.1–11.8) | 8.4 (5.3–11.5) | 13.3 (8.8–17.8) | 11.0 (8.3–13.8) | 8.7 (7.5–9.9) |
| Weighted No. | 39 | 42 | 48 | 44 | 41 | 44 | 258 |
| Amlodipine | 6.8 (3.6–9.9) | 7.9 (5.2–10.7) | 11.1 (8.2–14.0) | 7.5 (5.1–9.8) | 8.1 (4.7–11.4) | 9.3 (6.5–12.1) | 8.3 (7.5–9.2) |
| Weighted No. | 43 | 48 | 56 | 40 | 25 | 37 | 249 |
| Metformin | 9.7 (6.3–13.1) | 8.4 (5.6–11.2) | 8.7 (6.2–11.3) | 8.5 (6.4–10.6) | 6.0 (3.3–8.6) | 6.0 (3.9–8.2) | 8.2 (7.0–9.4) |
| Weighted No. | 61 | 51 | 44 | 45 | 18 | 24 | 244 |
| Acetaminophen (OTC available) | 3.5 (1.6–5.5) | 9.4 (5.5–13.3) | 6.7 (4.2–9.2) | 9.5 (6.7–12.2) | 5.8 (3.2–8.4) | 10.3 (7.8–12.8) | 7.5 (6.0–9.0) |
| Weighted No. | 22 | 57 | 34 | 50 | 18 | 41 | 222 |
| Furosemide | 2.3 (1.1–3.5) | 5.6 (2.7–8.5) | 8.7 (6.1–11.3) | 5.6 (3.7–7.5) | 8.4 (5.3–11.5) | 10.5 (7.7–13.2) | 6.4 (5.3–7.5) |
| Weighted No. | 15 | 34 | 44 | 30 | 26 | 41 | 190 |
| Ezetimibe | 4.8 (2.7–7.0) | 3.6 (1.8–5.4) | 6.9 (4.6–9.2) | 5.4 (3.1–7.7) | 5.1 (1.8–8.3) | 5.9 (2.8–9.1) | 5.2 (4.2–6.2) |
| Weighted No. | 31 | 22 | 35 | 29 | 16 | 23 | 155 |
| Valsartan | 2.7 (1.0–4.4) | 5.3 (2.8–7.8) | 3.7 (2.0–5.5) | 7.0 (4.7–9.2) | 3.4 (0.9–6.0) | 5.4 (3.6–7.2) | 4.6 (3.8–5.4) |
| Weighted No. | 17 | 32 | 19 | 37 | 11 | 21 | 137 |
| Alendronate | 0.3 (0–0.8) | 5.4 (3.1–7.8) | 1.2 (0.5–1.8) | 9.3 (6.0–12.5) | 1.8 (0.1–3.5) | 9.4 (6.7–12.1) | 4.5 (3.6–5.4) |
| Weighted No. | 2 | 33 | 6 | 49 | 6 | 37 | 133 |
| Warfarin | 2.5 (1.2–3.9) | 1.7 (0.7–2.7) | 4.4 (2.8–6.1) | 2.7 (1.4–3.9) | 12.7 (8.5–16.9) | 7.3 (4.8–9.9) | 4.4 (3.7–5.1) |
| Weighted No. | 16 | 10 | 22 | 14 | 39 | 29 | 131 |
| Omeprazole (OTC available) | 2.9 (1.4–4.5) | 4.2 (1.5–6.9) | 5.4 (3.1–7.6) | 3.8 (1.9–5.7) | 7.1 (3.8–10.3) | 4.4 (2.0–6.8) | 4.4 (3.4–5.4) |
| Weighted No. | 19 | 25 | 27 | 20 | 22 | 17 | 130 |
| Clopidogrel | 3.4 (1.7–5.1) | 3.9 (0.8–6.9) | 6.1 (3.8–8.5) | 3.3 (1.5–5.1) | 5.3 (3.1–7.5) | 4.8 (2.6–6.9) | 4.3 (3.3–5.3) |
| Weighted No. | 22 | 23 | 31 | 18 | 16 | 19 | 129 |
| Albuterol | 2.3 (0.6–4.0) | 5.9 (3.3–8.4) | 6.1 (3.7–8.6) | 4.3 (1.9–6.7) | 3.9 (1.5–6.3) | 3.3 (2.0–4.7) | 4.3 (3.3–5.3) |
| Weighted No. | 14 | 35 | 31 | 23 | 12 | 13 | 129 |
| Conjugated estrogens | NA | 9.1 (6.1–12.2) | NA | 9.2 (5.6–12.8) | NA | 5.1 (2.9–7.4) | 4.2 (3.1–5.2) |
| Weighted No. | NA | 55 | NA | 49 | NA | 20 | 124 |
Abbreviations: CI, confidence interval; NA, not applicable.
Estimates (numbers and percentages) weighted to account for differential probabilities of selection and differential nonresponse; CIs based on inversion of Wald tests constructed with the use of design-based SEs. Medications used by respondent as a single or multicomponent product. Prescription-only and OTC medications were combined, because prescription status varies for several medications depending on formulation and strength and because medication data collection was limited to drug names.
The most commonly used dietary supplements were nutritional products, including multivitamins and individual vitamins or minerals (table 4). Alternative therapies intended for use in improving cardiovascular health (eg, omega-3 fatty acids, garlic, coenzyme Q) and other age-related chronic conditions (eg, “eye vitamins” for macular degeneration, glucosamine-chondroitin, saw palmetto) were also widely used. The prevalence of dietary supplement use tended to be lowest in the youngest age group. Across all age groups, calcium, vitamin D, and glucosamine-chondroitin supplements were more commonly used among women than men, while niacin was more commonly used among men.
Table 4.
Weighted Prevalence Estimates of the Most Commonly Used Dietary Supplements by Age and Gendera
| Supplement | Estimated Prevalence, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Aged 57–64 y (n = 1016) |
Aged 65–74 y (n = 1082) |
Aged 75–85 y (n = 878) |
Total (N = 2976) |
||||
| Men (n = 525) |
Women (n = 491) |
Men (n = 543) |
Women (n = 539) |
Men (n = 377) |
Women (n = 501) |
||
| Multivitamin/minerals | 20.0 (15.7–24.3) | 32.3 (27.2–37.4) | 29.6 (23.9–35.3) | 33.5 (28.1–38.8) | 23.7 (16.8–30.5) | 28.3 (23.3–33.2) | 28.0 (24.7–31.3) |
| Weighted No. | 127 | 196 | 150 | 178 | 73 | 111 | 835 |
| Calcium | 6.3 (3.1–9.5) | 25.7 (20.8–30.5) | 8.7 (6.0–11.3) | 30.1 (26.1–34.1) | 6.2 (3.4–9.0) | 25.6 (21.3–29.9) | 17.4 (15.8–19.0) |
| Weighted No. | 40 | 155 | 44 | 160 | 19 | 101 | 519 |
| Vitamin C | 7.5 (4.8–10.2) | 9.6 (5.9–13.2) | 9.4 (6.6–12.1) | 12.3 (8.8–15.7) | 5.7 (3.0–8.4) | 8.2 (5.3–11.1) | 9.0 (7.5–10.5) |
| Weighted No. | 47 | 58 | 47 | 65 | 18 | 32 | 268 |
| Vitamin E | 2.8 (1.3–4.2) | 9.7 (5.5–13.9) | 10.6 (5.8–15.4) | 11.6 (8.0–15.1) | 7.6 (4.2–10.9) | 8.8 (5.8–11.8) | 8.4 (6.7–10.1) |
| Weighted No. | 18 | 58 | 54 | 62 | 23 | 35 | 250 |
| Any vitamin Bb | 4.3 (2.5–6.1) | 9.1 (5.5–12.7) | 8.5 (5.8–11.2) | 8.3 (5.5–11.2) | 9.3 (5.3–13.4) | 8.0 (5.2–10.8) | 7.7 (6.4–9.0) |
| Weighted No. | 27 | 55 | 43 | 44 | 29 | 32 | 230 |
| Chondroitin–glucosamine | 5.8 (2.6–8.9) | 8.8 (5.6–11.9) | 5.1 (2.8–7.5) | 10.0 (7.1–12.8) | 5.9 (2.5–9.4) | 8.4 (5.3–11.5) | 7.4 (6.3–8.5) |
| Weighted No. | 37 | 53 | 26 | 53 | 18 | 33 | 220 |
| Potassium supplementsc | 2.8 (1.0–4.6) | 8.2 (4.6–11.9) | 5.5 (3.3–7.8) | 6.6 (4.4–8.7) | 8.5 (5.3–11.7) | 11.3 (8.2–14.5) | 6.8 (5.9–7.6) |
| Weighted No. | 17 | 50 | 28 | 35 | 26 | 45 | 201 |
| Folic acid | 2.6 (1.2–4.0) | 4.9 (2.3–7.5) | 6.0 (4.0–7.9) | 5.9 (3.6–8.2) | 7.5 (3.5–11.5) | 6.2 (4.3–8.2) | 5.2 (4.2–6.2) |
| Weighted No. | 17 | 30 | 30 | 31 | 23 | 25 | 156 |
| Omega-3 fatty acids | 2.0 (0.6–3.4) | 6.8 (4.5–9.1) | 5.7 (3.1–8.3) | 4.7 (2.1–7.3) | 3.8 (1.3–6.3) | 3.5 (1.7–5.3) | 4.5 (3.1–5.8) |
| Weighted No. | 13 | 41 | 29 | 25 | 12 | 14 | 133 |
| Vitamin D | 2.9 (0–6.1) | 4.4 (2.2–6.6) | 2.3 (0.7–4.0) | 7.0 (4.4–9.6) | 2.3 (0.6–4.1) | 8.3 (4.8–11.7) | 4.5 (3.3–5.7) |
| Weighted No. | 18 | 27 | 12 | 37 | 7 | 33 | 134 |
| Magnesium | 2.6 (0–5.9) | 3.5 (1.6–5.4) | 1.5 (0.4–2.6) | 6.1 (3.3–9.0) | 1.3 (0.2–2.5) | 1.8 (0.6–3.0) | 3.0 (1.9–4.1) |
| Weighted No. | 17 | 21 | 8 | 32 | 4 | 7 | 90 |
| Eye vitaminsd | 0.7 (0.3–1.1) | 2.4 (0.8–4.0) | 2.6 (0.5–4.6) | 2.7 (1.2–4.2) | 3.3 (1.5–5.1) | 4.9 (2.5–7.3) | 2.6 (1.9–3.2) |
| Weighted No. | 4 | 15 | 13 | 15 | 10 | 19 | 76 |
| Zinc | 3.5 (0.2–6.7) | 1.1 (0.1–2.1) | 3.3 (1.1–5.6) | 3.7 (1.4–6.1) | 1.8 (0.4–3.1) | 1.7 (0.3–3.1) | 2.6 (1.6–3.6) |
| Weighted No. | 22 | 7 | 17 | 20 | 5 | 7 | 78 |
| Methylsulfonylmethane (MSM) | 1.8 (0.3–3.4) | 2.9 (1.0–4.9) | 1.1 (0.2–2.0) | 3.1 (1.6–4.6) | 0.5 (0–1.3) | 3.1 (1.3–4.9) | 2.2 (1.5–2.9) |
| Weighted No. | 12 | 18 | 5 | 16 | 2 | 12 | 65 |
| Niacin | 1.5 (0.3–2.6) | 0.9 (0.1–1.7) | 2.7 (1.2–4.2) | 1.6 (0.3–3.0) | 3.1 (1.5–4.8) | 1.3 (0.1–2.6) | 1.7 (1.3–2.2) |
| Weighted No. | 9 | 6 | 14 | 9 | 10 | 5 | 52 |
| Saw palmetto | 2.6 (0.7–4.5) | NA | 5.1 (2.4–7.8) | NA | 2.2 (0.6–3.9) | NA | 1.7 (1.0–2.3) |
| Weighted No. | 17 | NA | 26 | NA | 7 | NA | 49 |
| Flax | 1.0 (0–2.3) | 1.2 (0.1–2.3) | 2.4 (0.5–4.3) | 2.8 (1.1–4.5) | 0.3 (0–0.8) | 0.7 (0–1.7) | 1.5 (0.8–2.2) |
| Weighted No. | 6 | 7 | 12 | 15 | 1 | 3 | 45 |
| Garlic | 1.1 (0.2–2.0) | 1.7 (0.8–2.6) | 2.8 (0.9–4.8) | 0.7 (0–1.4) | 1.6 (0.1–3.0) | 0.7 (0–1.8) | 1.4 (0.9–2.0) |
| Weighted No. | 7 | 10 | 14 | 4 | 5 | 3 | 43 |
| Coenzyme Q–10 | 0.5 (0–1.0) | 1.9 (0.7–3.1) | 2.0 (0.3–3.7) | 1.7 (0.5–2.9) | 1.4 (0.5–2.4) | 1.0 (0–2.1) | 1.4 (1.0–1.8) |
| Weighted No. | 3 | 11 | 10 | 9 | 4 | 4 | 42 |
| Ginkgo | 0.8 (0–1.9) | 0.8 (0–1.7) | 1.9 (0.4–3.3) | 2.5 (0.7–4.3) | 1.7 (0–3.3) | 1.2 (0–2.4) | 1.4 (0.9–2.0) |
| Weighted No. | 5 | 5 | 9 | 14 | 5 | 5 | 42 |
Abbreviations: CI, confidence interval; NA, not applicable.
>Estimates (numbers and percentages) weighted to account for differential probabilities of selection and differential nonresponse; CIs based on inversion of Wald tests constructed with the use of design-based SEs. Dietary supplements used by respondent as a single or multicomponent product.
Includes vitamin B12, vitamin B6, vitamin B-complex, or unspecified vitamin B.
Includes potassium chloride (available by prescription only), potassium acetate, potassium gluconate, and unspecified potassium.
Includes I-Caps, Preservision, and any medication recorded as an “eye vitamin” by interviewer.
Most Commonly Reported Medical Conditions and Comorbidity
Cardiovascular disease was the most commonly reported medical condition, and its prevalence was similar among men and women (Table 5). Women reported more comorbid conditions compared with men aged 57 through 64 years. For example, 37% (95% CI, 33.6%–40.9%) of men reported no comorbid conditions compared with 25% (95% CI, 20.6%–29.6%) of women in this age group. In addition, thyroid problems, arthritis, and asthma were more prevalent in women than in men across all age groups. A total of 26 respondents reported that a physician had told them that they had Alzheimer disease or some other form of dementia, but the interviewers considered them able to participate in the survey.
Table 5.
Weighted Prevalence Estimates of the Most Commonly Reported Medical Conditions and Comorbidity By Age and Gendera
| Medical Conditionb | Estimated Prevalence, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Aged 57–64 y (n = 1016) |
Aged 65–74 y (n = 1082) |
Aged 75–85 y (n = 878) |
Total (N = 2976) |
||||
| Men (n = 525) |
Women (n = 491) |
Men (n = 543) |
Women (n = 539) |
Men (n = 377) |
Women (n = 501) |
||
| Cardiovascular diseasec | 52.6 (47.2–58.1) | 51.1 (45.7–56.6) | 66.5 (62.8–70.1) | 57.8 (52.1–63.5) | 70.2 (64.2–76.2) | 70.3 (64.7–75.8) | 59.8 (57.2–63.3) |
| Weighted No. | 333 | 309 | 336 | 308 | 217 | 277 | 1780 |
| Arthritis | 37.2 (33.3–41.0) | 53.7 (49.3–58.1) | 44.8 (40.1–49.5) | 57.7 (53.6–61.9) | 54.6 (48.1–61.2) | 68.4 (63.9–73.0) | 51.4 (49.5–53.3) |
| Weighted No. | 235 | 325 | 226 | 307 | 169 | 270 | 1532 |
| Diabetes | 20.4 (16.5–24.3) | 17.9 (13.4–22.4) | 22.8 (18.6–27.0) | 20.3 (16.6–24.1) | 21.5 (17.1–25.9) | 15.7 (12.2–19.2) | 19.8 (17.7–21.8) |
| Weighted No. | 129 | 108 | 115 | 108 | 66 | 62 | 589 |
| Thyroid problems | 3.3 (1.7–4.9) | 21.9 (16.9–26.9) | 7.8 (4.7–10.9) | 27.4 (24.1–30.7) | 8.8 (5.5–12.1) | 23.8 (19.8–27.8) | 15.4 (13.7–17.2) |
| Weighted No. | 21 | 132 | 39 | 146 | 27 | 94 | 460 |
| Ulcers | 8.4 (6.11–10.6) | 14.1 (11.0–17.2) | 15.2 (11.5–18.9) | 15.0 (11.2–18.8) | 15.1 (11.5–18.7) | 14.8 (11.4–18.3) | 13.4 (12.1–14.8) |
| Weighted No. | 53 | 85 | 77 | 80 | 47 | 58 | 400 |
| COPD or emphysema | 6.4 (3.3–9.5) | 10.5 (7.1–13.9) | 12.4 (8.1–16.7) | 13.7 (10.5–16.9) | 14.4 (9.7–19.2) | 12.0 (8.8–15.1) | 11.1 (10.0–12.3) |
| Weighted No. | 41 | 63 | 63 | 73 | 45 | 47 | 332 |
| Asthma | 8.2 (5.5–10.9) | 14.9 (11.3–18.6) | 8.3 (5.7–10.9) | 11.1 (8.1–14.2) | 7.0 (4.2–9.8) | 9.2 (6.4–11.9) | 10.1 (9.0–11.2) |
| Weighted No. | 52 | 90 | 42 | 59 | 22 | 36 | 301 |
| Comorbidity indexd | |||||||
| 0 | 37.3 (33.6–40.9) | 25.1 (20.6–29.6) | 21.9 (18.2–25.6) | 21.5 (17.6–25.5) | 17.8 (14.1–21.4) | 16.8 (12.6–21.1) | 24.7 (22.9–26.4) |
| Weighted No. | 236 | 152 | 111 | 115 | 55 | 66 | 734 |
| 1–4 | 58.4 (54.5–62.3) | 69.4 (64.2–74.7) | 68.7 (64.9–72.6) | 72.9 (68.4–77.4) | 72.2 (67.6–76.8) | 76.2 (71.9–80.5) | 68.8 (66.8–70.8) |
| Weighted No. | 370 | 420 | 348 | 388 | 223 | 300 | 2049 |
| ≥5 | 4.3 (2.5–6.0) | 5.5 (2.8–8.2) | 9.4 (6.6–12.2) | 5.5 (3.5–7.6) | 10.0 (6.4–13.6) | 7.0 (4.4–9.5) | 6.6 (5.5–7.7) |
| Weighted No. | 27 | 33 | 47 | 30 | 31 | 27 | 196 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Estimates (numbers and percentages) weighted to account for differential probabilities of selection and differential nonresponse; CIs based on inversion of Wald tests constructed with the use of design-based SEs.
Conditions included are those of highest relevance to the most commonly used prescription and over-the-counter medications.
Includes respondents reporting ever being diagnosed with myocardial infarction, stroke, hypertension, and/or heart failure.
Range of possible scores, 0–10.
Concurrent Use of Medications
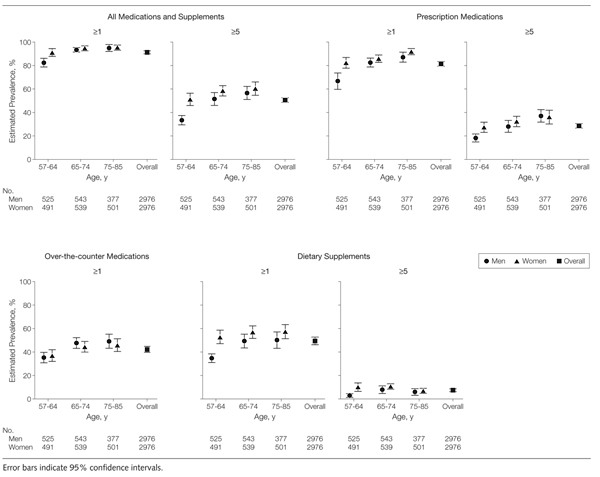
Overall, more than half of older adults used 5 or more prescription medications, over-the-counter medications, or dietary supplements. Figure 1 depicts this practice, stratified by respondents’ age and gender. For prescription medications, 29% (95% CI, 26.6%–30.6%) of all respondents used more than 5 medications. The prevalence of the use of 5 or more prescription medications increased steadily with age for both men and women and was overall significantly higher among women (P=.003), though this gender difference was observed only among the youngest 2 age groups. Only 3 respondents reported the use of 5 or more over-the-counter medications. However, nearly 1 in 8 older adults regularly used 5 or more dietary supplements.
Figure 1.
Weighted Prevalence Estimates of Prescription Medication, Over-the-counter Medication, and Dietary Supplement Use by Age and Gender
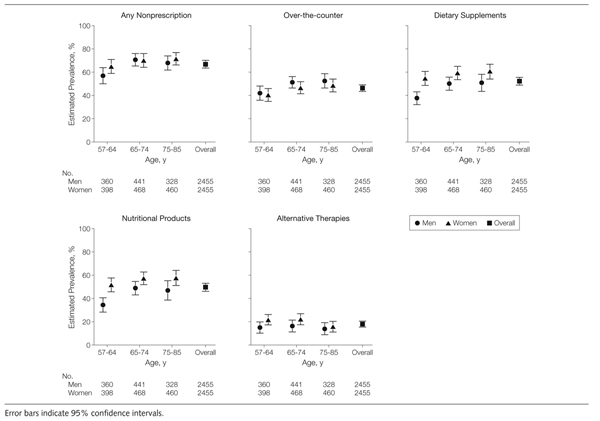
Figure 2 illustrates the prevalence of concurrent use of nonprescription therapies (those available without a prescription, regardless of whether a physician had prescribed them) among users of prescription medications by age and gender. Overall, 68% (95% CI, 64.8%–71.1%) of older adults using prescription medications were concurrently using over-the-counter medications, dietary supplements, or both. Across all age groups, men and women were equally likely to use prescription and nonprescription medications concurrently. However, men were more likely to concurrently use over-the-counter medications, whereas women were more likely to concurrently use dietary supplements. Sixty percent (95% CI, 54.0%–66.8%) of women in the oldest age group used prescription medications in combination with dietary supplements. Among prescription medication users, more women than men concurrently used nutritional products, alternative therapies, or both.
Figure 2.
Weighted Prevalence Estimates of Concurrent Use of Nonprescription Medications (Over-the-counter Medications and/or Dietary Supplements) Among Prescription Medication Users by Age and Gender
Potential Major Drug-Drug Interactions
A total of 46 potential drug-drug interactions were identified using Micromedex (TABLE 6). Among these, 11 were classified as potentially of major severity, 28 were classified as potentially of moderate severity, and 7 were classified as potentially of minor severity. Not one absolutely contraindicated drug-drug interaction was identified in the entire sample. Overall, 1 in 25 older adults (approximately 2.2 million) were at risk for a major potential drug-drug interaction. The rate of any major medication interaction increased with age for both men and women but was higher among men compared with women across all age groups. Prevalence of a major interaction was significantly greater among men than among women (P = .01). More than half of these major interactions involved the use of nonprescription therapies. In addition, nearly half involved the use of anticoagulants (eg, warfarin) or antiplatelet agents (eg, aspirin). Across all age groups, the concurrent use of aspirin and warfarin was significantly more common in men than women (P<.001). In addition, the concurrent use of niacin and atorvastatin was also significantly more common in men than in women (P=.03).
Table 6.
Potential Major Medication Interactions by Age and Gendera
| Medication Interactionb | Interactions, Weighted No. |
Potential Interaction Effect |
||||||
|---|---|---|---|---|---|---|---|---|
| Age 57–64 y (n = 1016) |
Age 65–74 y (n = 1082) |
Age 75–85 y (n = 878) |
Total (N = 2976) |
|||||
| Men (n = 525) |
Women (n = 491) |
Men (n = 543) |
Women (n = 539) |
Men (n = 377) |
Women (n = 501) |
|||
| Prescription-prescription | ||||||||
| Albuterol-atenolol | 0 | 1 | 1 | 0 | 1 | 1 | 5 | Decreased effectiveness |
| Albuterol-metoprolol | 0 | 1 | 1 | 1 | 2 | 1 | 6 | Decreased effectiveness |
| Warfarin-simvastatin | 5 | 2 | 4 | 3 | 7 | 5 | 25 | Increased risk of bleeding/rhabdomyolysis |
| Clopidogrel-warfarin | 0 | 0 | 0 | 1 | 1 | 1 | 3 | Increased risk of bleeding |
| Lisinopril-potassium | 0 | 8 | 5 | 5 | 9 | 6 | 33 | Increased risk of hyperkalemia |
| Nonprescription-prescription | ||||||||
| Aspirin-warfarinc | 7 | 0 | 7 | 1 | 11 | 2 | 27 | Increased risk of bleeding |
| Niacin-atorvastatind | 7 | 0 | 5 | 0 | 3 | 3 | 18 | Increased risk of myopathy or rhabdomyolysis |
| Garlic-warfarin | 0 | 0 | 0 | 0 | 1 | 0 | 1 | Increased risk of bleeding |
| Niacin-simvastatin | 1 | 1 | 4 | 2 | 1 | 0 | 10 | Increased risk of myopathy or rhabdomyolysis |
| Nonprescription-nonprescription | ||||||||
| Ginkgo-aspirin | 0 | 0 | 1 | 3 | 4 | 3 | 10 | Increased risk of bleeding |
| Any interaction, | 18 | 13 | 24 | 14 | 31 | 19 | 118 | |
| No. (% [95% CI])e | (2.9 [0.9–4.8]) | (2.1 [0.2–4.0]) | (4.7 [2.9–6.4]) | (2.6 [1.2–3.9]) | (10.1 [6.4–13.7]) | (4.8 [2.7–6.9]) | (4.0 [3.1–4.8]) | |
Percentages and numbers are weighted estimates to account for differential probabilities of selection and differential nonresponse.
Potential major medication interactions for the 20 most common prescription and over-the-counter medications and 20 most common dietary supplements.
Statistically significant (P< .001) difference between men and women.
Statistically significant (P=.03) difference between men and women.
Statistically significant (P= .01) difference between men and women.
COMMENT
Older adults are the biggest consumers of prescription and over-the-counter medications and dietary supplements and are most vulnerable to medication adverse effects and drug-drug interactions.4 This study uses directly observed medication information from a recent national probability sample of individuals in their homes to establish that the vast majority of older adults in the United States use prescription medications regularly, 1 in 3 uses 5 or more prescription medications regularly, and about half regularly use over-the-counter medications and dietary supplements. Thus, the use of prescription medications with over-the-counter medications or dietary supplements is common, particularly among the oldest age group and women. In 2005–2006, at least 1 in 25 older adults used a regimen posing a risk of a major potential drug-drug interaction; half of these potential interactions involved the use of nonprescription medications.
Our estimates of the prevalence of any medication use, as well as of the use of prescription medications, for men and women aged 65 through 74 and 75 to 85 years are similar to those reported among adults 65 years and older in the 2006 Slone survey,15 the most comparable population-based study of medication use in the United States. Compared with a decade prior,8 both we and the Slone researchers found that while the prevalence of overall medication use has remained stable among older adults, the rate of polypharmacy (ie, the use of ≥5 medications) has increased. Several factors have likely contributed to this increase in the rate of polypharmacy among older adults over the last decade. These include intensification of therapy for common chronic medical conditions (eg, diabetes, cardiovascular disease),25 increased access to medications because of policy changes (eg, Medicare Part D and assistance programs),1 and growth of the generic drug market.26
Our report also highlights substantial use of a number of newer drugs brought to the market during the last several years (eg, ezetimibe and clopidogrel) and a decrease in the use of conjugated estrogens coinciding with the widely publicized Women’s Health Initiative Study findings about health risks associated with use of menopausal hormone therapy by older women.27 We also find higher rates of use of dietary supplements among older adults as compared with earlier studies, including glucosamine, chondroitin, and omega-3 fish oils, as well as decreases in the use of Ginkgo biloba and garlic. This may be due to the increasing evidence supporting the benefits of glucosamine-chondroitin in the treatment of osteo-arthritis28 and of omega-3 fish oils in reducing cardiovascular events.29 Moreover, a decline in the use of G biloba and garlic may be due to the increased awareness of the potential adverse effects associated with their use.
Important gender differences in medication use among older adults include a significantly higher prevalence of the use of 5 or more prescription medications among women aged 57 through 64 years and a higher prevalence of dietary supplement use. This corresponds to the higher prevalence of diagnosed comorbid conditions among women as compared with men in this age group. Previous studies have shown that women use more prescription medications and dietary supplements than men across all age groups,3,8,14 but most have aggregated adults 65 years and older, therefore masking heterogeneity across older age groups and limiting cross-study comparisons. Although women were equally likely as men to have cardiovascular disease, they were significantly less likely than men to be using the most widely prescribed class of cholesterol-lowering drugs.30 Prevalence in the use of these lipid-lowering medications has increased in both men and women compared with previous studies,8 but previously documented gender disparities in primary and secondary prevention of cardiovascular disease persist as an important public health problem.31 The pattern we observe may indicate undertreatment of women at risk for a cardiovascular event. Alternatively, women may have been equally likely to be prescribed cardioprotective drugs but less likely than men to use them. The higher prevalence of hypothyroidism, osteoporosis, and menopausal use of hormone therapy among women in our study was expected because of the distributions of these conditions in the population and corroborates the internal validity of the NSHAP data set.11
We found that the potential for major drug-drug interactions extends beyond the concurrent use of prescription medications. Half of all potential major drug-drug interactions that we identified involved a nonprescription medication. Physicians are frequently unaware of their patients’ nonprescription medication use because they do not ask patients, patients do not report use of nonprescription medications, or both.14,32 With as many as 4%, or 2.2 million, of older adults in the United States affected, the economic and health consequences of these potential interactions are considerable. One recent report estimated that US adults older than 65 years make more than 175 000 emergency department visits annually for adverse drug events; commonly prescribed medications accounted for one-third of these events.33 Reassuringly, we found no cases of absolutely contraindicated drug-drug combinations. Our findings suggest that concurrent use of prescription and nonprescription medications in older adults remains a public health problem and could be an important focal point for further improvements in drug safety for seniors.
Our study has several limitations. First, methodological differences across studies may limit some cross-study comparisons. Second, virtually all therapeutic classes are underused by some populations and overused by others; our data do not allow for us to completely examine important questions for health policy and clinical care regarding the appropriateness of the regimens that we observe. For example, even in cases of a potential major drug-drug interaction, an individual’s physician may have prescribed the regimen, may be aware of the risks, and may be monitoring the patient appropriately. Third, we based our analyses of major medication interactions on Thomson Micromedex classifications; other methods of classification may lead to different estimates of the population prevalence of drug-drug interactions. No one method of classification is able to capture the entirety of clinical evidence to support a given drug’s safety, and we examined potential interactions, rather than actual patient harm. Despite this, Thomson Micromedex is a widely used clinical reference. Our method of classification would generally lead to underestimates of the potential risks associated with concurrent use of prescription and nonprescription therapies because the related drug safety literature, albeit increasing, is limited. Furthermore, because we identified interactions only among the 20 most common medications and dietary supplements and focus only on major interactions, our results underestimate the total risk for potential interactions.
CONCLUSIONS
Medications are a critical modality for prolongation of life and improved quality of life for many older adults. By establishing patterns of prescription and nonprescription medication use among older adults, these data may help support efforts to increase the safety and quality of pharmacotherapy for older adults. This is especially important, since in this sample of community-dwelling older adults in the United States, nearly 1 in 25 reported taking concurrent drugs with the potential for harm from serious drug-drug interactions.
Acknowledgments
Funding/Support: The National Social Life, Health and Aging Project (NSHAP) is supported by the National Institutes of Health, including the National Institute on Aging, the Office of Research on Women’s Health, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (5R01AG021487). NSHAP is also supported by NORC, whose staff was responsible for the data collection. The National Institutes of Health, National Institute on Aging University of Chicago—NORC Center on Demography and Economics of Aging Core on Biomarkers in Population-Based Health and Aging Research (5 P30 AG 012857) and the University of Chicago Program in Pharmaceutical Policy (David Meltzer, principal investigator) also supported the effort of Dr Qato and Mssrs Schumm and Johnson on this article with a pilot grant (Dr Lindau, principal investigator). The University of Chicago Program in Pharmaceutical Policy is supported by the Merck Foundation. Dr Alexander has career development awards from the Agency for Healthcare Research and Quality (K08 HS15699-01A1) and the Robert Wood Johnson Physician Faculty Scholars Program. Supplies were donated to the NSHAP study by OraSure, Sunbeam, A & D Medical/LifeSource, Wilmer Eye Institute at the Johns Hopkins Bloomberg School of Public Health, Schleicher & Schuell Bioscience, BioMerieux, Roche Diagnostics, Digene, and Richard Williams.
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; the analysis or interpretation of the data; or the preparation or final approval of the manuscript.
Additional Contributions: We gratefully acknowledge the research assistance of Andreea Mihai, who is a paid research assistant for Dr Lindau at the University of Chicago.
Footnotes
Reprints/E-prints reprints@ama-assn.org
Financial Disclosures: Dr Qato reported serving as a consultant for IMS Health. Dr Alexander reported serving as a consultant to AstraZeneca and receiving grant support from Pfizer and the Merck Foundation. No other authors reported disclosures.
REFERENCES
- 1.Catlin A, Cowan C, Hartman M, Heffler S National Health Expenditure Accounts Team. National health spending in 2006: a year of change for prescription drugs. Health Aff (Millwood) 2008;27(1):14–29. doi: 10.1377/hlthaff.27.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Hanlon JT, Fillenbaum GG, Ruby CM, Gray S, Bohannon A. Epidemiology of over-the-counter drug use in community dwelling elderly—United States perspective. Drugs Aging. 2001;18(2):123–131. doi: 10.2165/00002512-200118020-00005. [DOI] [PubMed] [Google Scholar]
- 3.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine (IOM) The Future of Drug Safety: Promoting and Protecting the Health of the Public. Washington, DC: IOM; 2006. [Google Scholar]
- 6. [Accessed September 2, 2008]; The Sentinel Initiative. a national strategy for monitoring medical product safety. US Food and Drug Administration Web site. http://www.fda.gov/oc/initiatives/advance/reports/report0508.html.
- 7.Chrischilles EA, Foley DJ, Wallace RB, et al. Use of medications by persons 65 and over—data from the Established Populations for Epidemiologic Studies of the Elderly. J Gerontol. 1992;47(5):M137–M144. doi: 10.1093/geronj/47.5.m137. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States—the Slone survey. JAMA. 2002;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 9.CDER drug and biologic approval reports. [Accessed September 3, 2008];US Food and Drug Administration Web site. http://www.fda.gov/cder/rdmt/default.htm.
- 10.Stagnitti MN. Statistical brief 180: the top five outpatient prescription drugs ranked by total expense for children, adults, and the elderly, 2004. [Accessibility verified November 20, 2008];Medical Expenditure Panel Survey Web site. 2007 http://www.meps.ahrq.gov/mepsweb/data_files/publications/st180/stat180.pdf.
- 11.Qato D, Schumm P, Johnson M, Lindau S. University of Chicago—NORC. [Accessibility verified November 20, 2008];The National Social Life, Health and Aging Project Technical Report on Medication Coding. http://biomarkers.uchicago.edu/pdfs/TR-PharmData.pdf.
- 12.Briesacher BA, Gurwitz JH, Soumerai SB. Patients at-risk for cost-related medication nonadherence: a review of the literature. J Gen Intern Med. 2007;22(6):864–871. doi: 10.1007/s11606-007-0180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmer GW, Lafferty WE, Tyree PT, Lind BK. Potential interactions between complementary/alternative products and conventional medicines in a Medicare population. Ann Pharmacother. 2007;41(10):1617–1624. doi: 10.1345/aph.1K221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardiner P, Graham RE, Legedza ATR, Eisenberg DM, Phillips RS. Factors associated with dietary supplement use among prescription medication users. Arch Intern Med. 2006;166(18):1968–1974. doi: 10.1001/archinte.166.18.1968. [DOI] [PubMed] [Google Scholar]
- 15. [Accessibility verified November 20, 2008];Patterns of medication use in the United States 2006: a report from the Slone Survey. http://www.bu.edu/slone/SloneSurvey/AnnualRpt/SloneSurvey-WebReport2006.pdf.
- 16.Lindau ST, Schumm P, Laumann EO, Levinson W, O’Muircheartaigh C, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lexicon Plus [database] [Accessibility verified November 20, 2008];Cerner Multum Inc Web site. 2008 http://www.multum.com/Lexicon.htm.
- 18.Micromedex Healthcare Series [Internet database] [Accessibility verified November 20, 2008];Thomson Reuters Healthcare Web site. http://www.micromedex.com/products/hcs/
- 19.Fillenbaum GG, Hanlon JT, Corder EH, Ziqubu-Page T, Wall WE, Brock D. Prescription and nonprescription drug use among black and white community-residing elderly. Am J Public Health. 1993;83(11):1577–1582. doi: 10.2105/ajph.83.11.1577. Jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaskin DJ, Briesacher BA, Limcangco R, Brigantti BL. Exploring racial and ethnic disparities in prescription drug spending and use among Medicare beneficiaries. Am J Geriatr Pharmacother. 2006;4(2):96–111. doi: 10.1016/j.amjopharm.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Poverty thresholds 2006. [Accessibility verified November 20, 2008];US Census Bureau Web site. http://www.census.gov/hhes/www/poverty/threshld/thresh06.html.
- 23.National Social Life, Health and Aging (NSHAP) Dataset. [Accessibility verified November 20, 2008];National Archive of Computerized Data on Aging (NACDA) Web site. http://www.icpsr.umich.edu/NACDA/news.html#nshap/
- 24.Stata [computer program]. Version 10. College Station, TX: StataCorp; 2007. [Google Scholar]
- 25.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Generics market expected to soar to $80 billion by 2008. [Accessed September 4, 2008];IMS Web site. http://www1.imshealth.com/ims/portal/front/articleC/0,2777,6599_41382706_52651243,00.html.
- 27.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 28.McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA. 2000;283(11):1469–1475. doi: 10.1001/jama.283.11.1469. [DOI] [PubMed] [Google Scholar]
- 29.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease [published correction appears in Circulation. 2003;107(3):512] Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 30.Commonly requested therapeutic class and product information. [Accessed September 12, 2008];IMS Web site. http://www1.imshealth.com/ims/portal/front/articleC/0,2777,6599_18731_63237611,00.html.
- 31.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298(13):1517–1524. doi: 10.1001/jama.298.13.1517. [DOI] [PubMed] [Google Scholar]
- 32.Hensrud DD, Engle DD, Scheitel SM. Underreporting the use of dietary supplements and nonprescription medications among patients undergoing a periodic health examination. Mayo Clin Proc. 1999;74(5):443–447. doi: 10.4065/74.5.443. [DOI] [PubMed] [Google Scholar]
- 33.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]