Abstract
Purpose
To determine the effects of imposed anisometropic retinal defocus on accommodation, ocular growth, and refractive state changes in marmosets.
Methods
Marmosets were raised with extended-wear soft contact lenses for an average duration of 10 wks beginning at an average age of 76 d. Experimental animals wore either a positive or negative contact lens over one eye and a plano lens or no lens over the other. Another group wore binocular lenses of equal magnitude but opposite sign. Untreated marmosets served as controls and three wore plano lenses monocularly. Cycloplegic refractive state, corneal curvature, and vitreous chamber depth were measured before, during, and after the period of lens wear. To investigate the accommodative response, the effective refractive state was measured through each anisometropic condition at varying accommodative stimuli positions using an infrared refractometer.
Results
Eye growth and refractive state are significantly correlated with the sign and power of the contact lens worn. The eyes of marmosets reared with monocular negative power lenses had longer vitreous chambers and were myopic relative to contralateral control eyes (p<0.01). Monocular positive power lenses produced a significant reduction in vitreous chamber depth and hyperopia relative to the contralateral control eyes (p<0.05). In marmosets reared binocularly with lenses of opposite sign, we found larger interocular differences in vitreous chamber depths and refractive state (p<0.001). Accommodation influences the defocus experienced through the lenses, however, the mean effective refractive state was still hyperopia in the negative-lens-treated eyes and myopia in the positive-lens-treated eyes.
Conclusions
Imposed anisometropia effectively alters marmoset eye growth and refractive state to compensate for the imposed defocus. The response to imposed hyperopia is larger and faster than the response to imposed myopia. The pattern of accommodation under imposed anisometropia produces effective refractive states that are consistent with the changes in eye growth and refractive state observed.
Keywords: accommodation, animal models, emmetropization, myopia, refractive error development
In a variety of species including chicks, tree shrews, macaques, guinea pigs, and even fish, imposing defocus with spectacle lenses has been shown to effectively alter eye growth and refractive state to compensate for the imposed defocus (for reviews see1-3). Imposing hyperopia with negative lenses results in increased axial length and a shift in refractive state that compensates for the imposed defocus, leaving the eye with axial myopia when the lens is removed. Imposing myopia with positive lenses results in decreased axial growth and compensatory refractive changes that leave the eye hyperopic when the lens is removed. These studies have been used as an experimental model of emmetropization and have been taken as evidence of the visual control of eye growth and development of refractive state. Studies with the common marmoset (Callithrix jacchus), a new world primate, have shown that imposing defocus with spectacle lenses4, 5 and extended wear contact lenses6, 7 is also effective in producing compensatory eye growth.
In the present study we extend the findings of those original studies in marmosets and establish monocular contact lens-rearing paradigms, including rearing marmosets with binocular contact lenses of opposite sign, that produce large amounts of anisometropia. In addition, we present data examining the way accommodation responds to the imposed defocus from the contact lenses under these conditions and consider how those responses may ultimately influence the growth response to the effective retinal defocus experienced over time.
In the presence of anisometropia, the binocular viewing of a near target produces unequal accommodative demands between the two eyes. There is very little known about how accommodation behaves under experimentally imposed anisometropic conditions. Although accommodation is usually thought of as consensual, small amounts of aniso-accommodation (<0.75D) have been described under lens-imposed anisometropia8. Hung et al.9, described the preferential near fixation pattern in rhesus monkeys reared with either plano/negative spectacle lens or plano/positive spectacle lens conditions and suggested from IR videoretinoscopy that the eye with lesser accommodative demand is used. Flitcroft et al.10 showed evidence that averaging of the accommodative demands between the two eyes during imposed anisometropia drove the accommodative response under dynamic conditions.
Whatever the pattern of accommodative response, it will affect the type and degree of retinal defocus being experienced over time. Understanding the accommodative responses to experimentally imposed visual defocus in animal models will help to understand the eye growth response and may shed light on how accommodation affects retinal defocus in humans during near work. Near work has been suspected to be a risk factor for the development of myopia (see for example11-18). The relationship of near visual behavior and myopia development, however, is complex. Recent attempts at systematically correlating the degree of myopia with the amount of reading reported by the subject were not significant19, 20, although significant independent associations between myopia, close reading distance, and continuous reading have been reported21.
Methods
Juvenile marmosets (n=28) were raised with extended wear soft contact lenses (Medlens Innovations, Front Royal, VA), removed daily overnight beginning at an average age of 76 d (range 41-101) for an average duration of 10 wks (range 6-17). Experimental animals wore either a positive (n=11) or negative (n=8) contact lens over one eye (±3 stepped up to ±5 D) and a plano contact lens or no lens over the other. Some experimental animals (n=9) wore binocular lenses of equal magnitude but opposite sign (±3 stepped up to ±5 D). Untreated marmosets (n=16) served as age matched controls, and three wore plano lenses monocularly. Lenses were fit to approximately 10% flatter than the corneal curvature measured with IR video keratometry. Cycloplegia was induced with 2 drops of 1% cyclopentolate 60 mins before measurements of refractive state, corneal curvature, and vitreous chamber depth were measured at 2-4 week intervals before, during, and after the period of lens wear (for details see22). All rearing and experimental procedures were approved by The New England College of Optometry Institutional Animal Care and Use Committee and confirm with the ARVO statement for animal use.
Accommodation in three untreated marmosets (refractive error range: +0.75D to -1.30D) was examined, first without lenses and then after being fit with the same combinations of contact lenses used to study the eye growth and refractive state response to extended periods of imposed defocus. Three anisometropic conditions were examined: (1) plano/+3 D, (2) plano/-3 D, (3) +3 D/-3 D. The lenses were worn for only the period of time needed to measure accommodative function (approximately 30-40 minutes). The marmosets were restrained in a custom-built primate chair and allowed to freely view a video (with sound) of marmosets from their home animal facility played on a small LCD video monitor (6.35 × 4.45 cm) presented at different positions. The LCD display was mounted on a movable platform that was positioned at six distances corresponding to 1.6, 2.0, 3.0, 4.0, 6.0 and 7.7 D near point demands several times during the measurement period. The marmoset and stimulus presentation system was contained within a black walled container that isolated the animal from external visual stimuli.
To investigate the accommodative response we used an infrared optometer (PowerRefractor, MultiChannel Systems, Tuebingen, Germany). The PowerRefractor was operated in continuous mode, which is capable of measuring refractive state binocularly along the vertical meridian at up to 25 Hz. However, the PowerRefractor did not consistently record data from both eyes simultaneously because of eye and head movements and because the pupil size of marmosets is close to the limit of the PowerRefractor (about 2.5 mm). As described elsewhere23, accommodative measurements for each monkey were compiled over several sessions for each of the accommodative stimuli tested. All data collected were filtered off-line to remove refractions when the gaze was more than 5 degrees from the center of the LCD display. For each eye, the data were averaged at each stimulus position and accommodative stimulus-response functions were calculated for each imposed anisometropic condition as well as with no lenses. Accommodative stimulus demands were calculated from the target distance, the monkey's cycloplegic refraction (measured on another day), and the power of the lens worn. For additional details on the methods used to measure accommodation see Troilo et al.23
Statistics
All statistical analyses were performed using Stata (College Station, TX) and KaleidaGraph data analysis and graphing software (Synergy Software, Reading, PA). ANOVA and post-hoc t-tests were used to examine interocular differences (treated–control). Unpaired t-tests were used to examine differences between data sets from different animals. The significance of correlations was tested using the Fisher Z transform.
Results
Contact lens rearing in marmosets with imposed anisometropic retinal defocus produced interocular changes in vitreous chamber depth and refractive state that effectively reduced the imposed defocus (Figures 1 & 2). Compared to the contralateral untreated eyes, at the end of the lens-rearing period monocular negative power contact lenses produced significantly greater vitreous chamber depth (experimental eye − control eye, mean±se: 0.170 ±0.028 mm, p<0.001) and myopia relative to the contralateral eye (-2.41 ±0.64 D, p<0.01). Monocular positive power lenses produced, on average, a significant reduction in vitreous chamber depth (-0.082 ±0.029 mm, p<0.05) and relative hyperopia (1.40 ±0.43 D, p<0.05). In marmosets reared binocularly with lenses of equal power but opposite sign, we found the largest interocular differences in vitreous chamber depths (negative lens–positive lens: 0.275 ±0.047 mm, p<0.001) and refractive state (-5.72 ±0.56 D, p<0.001).
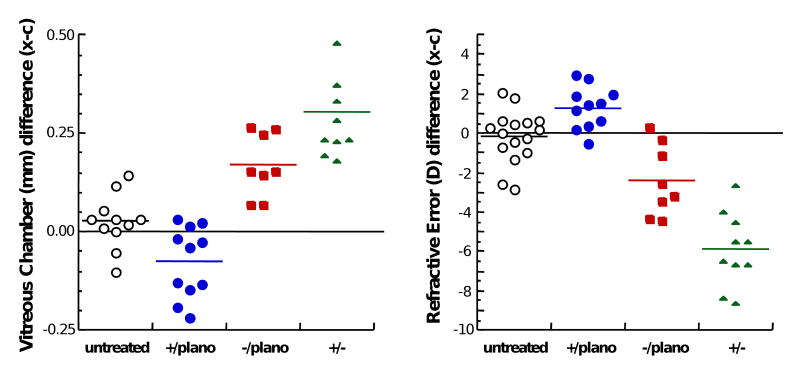
Figure 1.
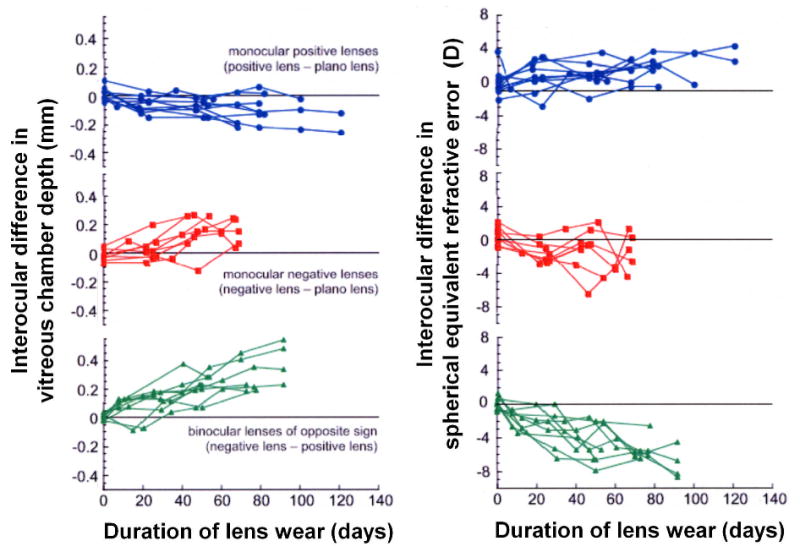
Development of interocular differences in vitreous chamber depth and refractive error in the monocular positive lens reared group (blue circles), the monocular negative lens reared group (red squares), and the binocular lenses of opposite sign group (green triangles).
Figure 2.
Contact lens imposed anisometropia induces changes in vitreous chamber depth (left) and refractive state (right). Data shown are at the end of the lens-rearing period. White circles represent interocular differences (right eye–left eye) from untreated age-matched marmosets. Blue circles show interocular differences (experimental–control) from marmosets treated monocularly with positive lenses. Red squares show interocular differences (experimental–control) from marmosets treated monocularly with negative lenses. Green triangles represent interocular differences (right eye–left eye) from marmosets reared binocularly with lenses of opposite sign.
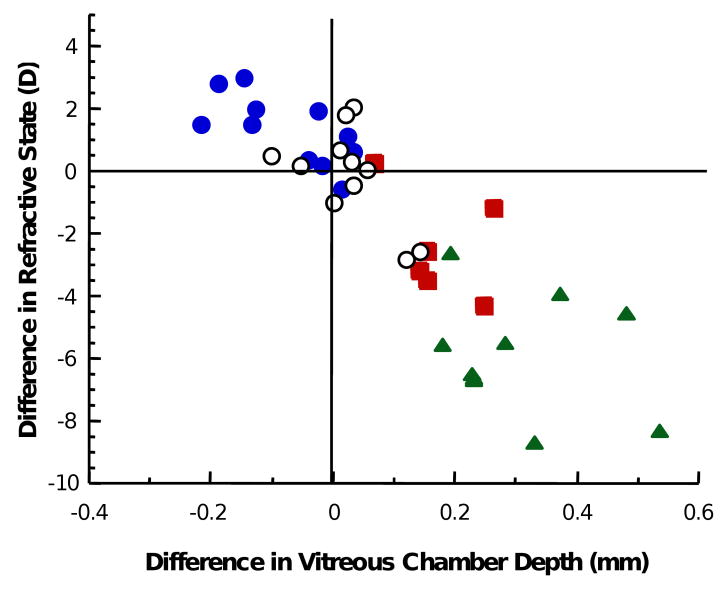
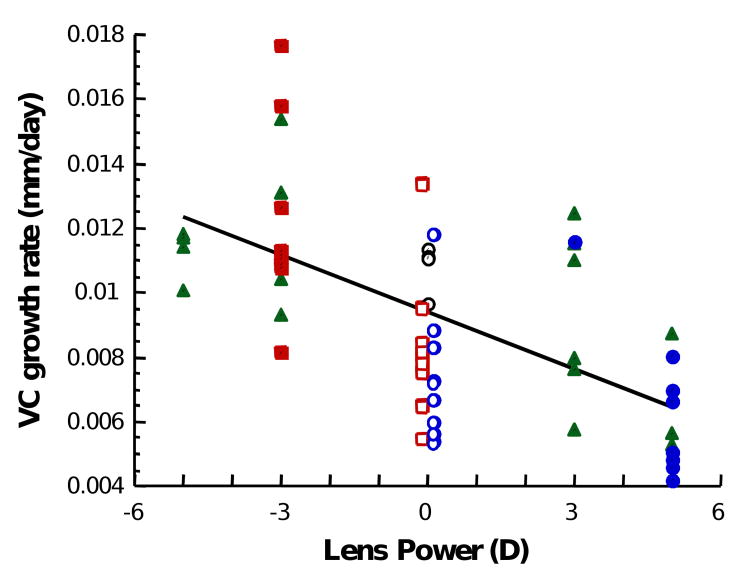
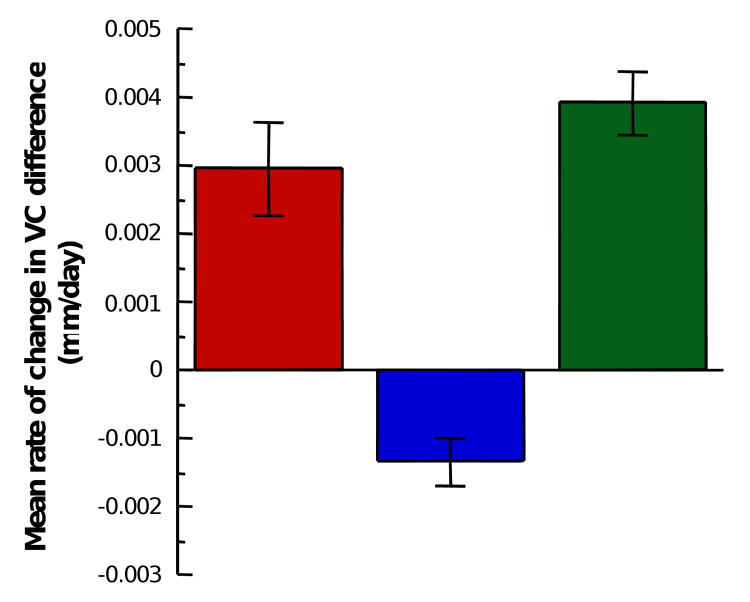
Considering all experimental groups together, the induced interocular difference in refractive state is significantly correlated with the difference in vitreous chamber depth (R=0.8622; p<0.01) indicating that the induced change in vitreous chamber depth accounts for approximately 74% of the observed difference in refractive state between the eyes (Figure 3). The growth rate of the vitreous chamber, measured between the last two measurements during the lens-rearing period, is significantly correlated (R=0.615; p<0.01) with the sign and power of the contact lens worn (Figure 4). The negative-lens-reared eyes grew faster than the positive lens reared eyes. The interocular change in vitreous chamber depth was faster in the monocular negative-lens-reared eyes compared to the monocular positive-lens-reared eyes and the interocular change was fastest in the group wearing binocular lenses of opposite sign (ANOVA, p<0.05, post-hoc t-tests, p<0.05).
Figure 3.
The interocular difference in refractive state is significantly correlated with the induced interocular difference in vitreous chamber depth. Blue circles indicate interocular differences from the monocular positive lens group, red squares indicate interocular differences from the monocular negative lens group, green triangles indicate the interocular differences between eyes binocularly reared with lenses of opposite sign. White circles indicate the interocular differences in the untreated eyes of control marmosets.
Figure 4.
The growth rate of the vitreous chamber is significantly correlated with the sign and power of the contact lenses. Blue circles represent monocular positive-lens-reared eyes (the blue-edged white circles indicate the contralateral control eyes). Red squares represent monocular negative-lens-reared eyes (the red-edged white squares indicate the contralateral control eyes). Black-edged white circles indicate the plano lens reared eyes in monocular plano-reared controls. Green triangles indicate the eyes reared binocularly with lenses of opposite sign.
The contralateral plano-reared control eyes in the monocular positive and negative lens rearing groups were examined to determine whether contact lens wear, regardless of the power of the lens, had any effect on eye growth and refraction. Comparing plano contact lens reared marmoset eyes to age-matched untreated marmosets showed that 10 weeks of contact lens wear did not significantly affect refractive state (plano vs untreated; mean±se; 0.25±0.42 vs 0.06±0.48 D; p=0.70), on-axis vitreous chamber depth (6.53±0.10 vs 6.39±0.12 mm; p=0.40), or radius of corneal curvature (3.45±0.02 vs 3.45±0.03; p=0.90). None of the experimental lens rearing procedures significantly altered corneal curvature; the interocular differences in corneal power were less than 5% of total corneal power.
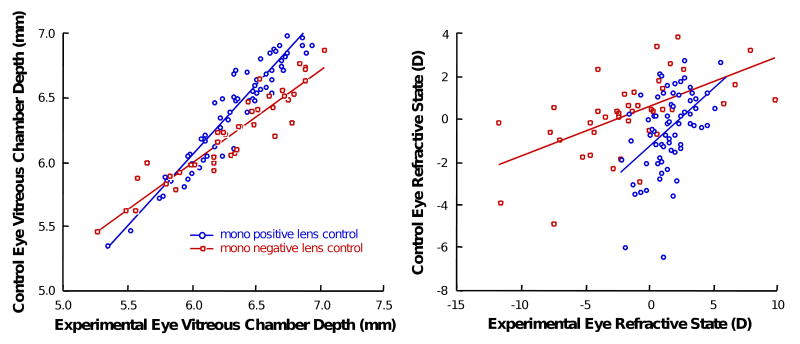
Contralateral eyes in the monocular lens-rearing paradigms, whether they wore plano lenses or not, showed a systematic change in growth and refraction that was related to the magnitude of change in the experimental eye (Figure 6).
Figure 6.
Control eye differences in monocular conditions. Data were obtained from repeated measures from all marmosets raised with either monocular positive lenses or monocular negative lenses. Blue-edged white circles indicate the control eyes from marmosets raised monocularly with positive lens. Red-edged white squares indicate the control eyes from marmosets raised monocularly with negative lens.
The contralateral eyes of the positive-lens-reared eyes compared to the negative-lens-reared eyes were significantly longer and the refractions more myopic (ANOVAs, p<0.05).
Accommodation through Contact Lens Imposed Anisometropia
The ability to freely accommodate to a fixed intermediate target (1 D) does not, on average, appreciably change the effective refractive state imposed by contact lenses. Figure 7 shows examples of the refractive state in marmosets measured either with and without binocular contact lenses of opposite sign (±3 D) when they are free to accommodate to a target fixed at 1 D. On average, the effective refractive state in the minus-lens-treated eye remains hyperopic although evidence of occasional episodes of accommodation to clear the lens imposed defocus are indicated by data points scattered below the average line.
Figure 7.
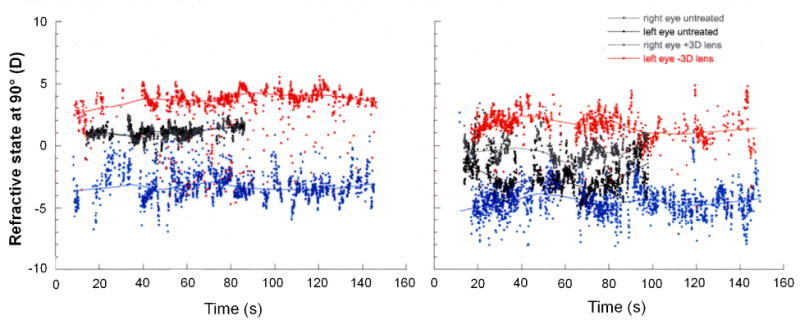
The effective refractive state in two untreated marmosets measured by IR videorefractometry shortly after receiving binocular contact lenses of opposite sign (±3D). The solid line is a running average through the refractions measured in the vertical meridian at 25 Hz (shown by the individual data points). The eye wearing the negative lens is shown in red and the eye wearing the positive lens is shown in blue. The marmosets were free to accommodate to a stimulus fixed at 1m. Relative to the refractions measured in the same eyes without the lenses (black and gray data), the average effective refractive state in the minus-lens-treated eye remains hyperopic although evidence of occasional accommodation to clear the imposed lens imposed defocus can be seen (red points scattered below the average line).
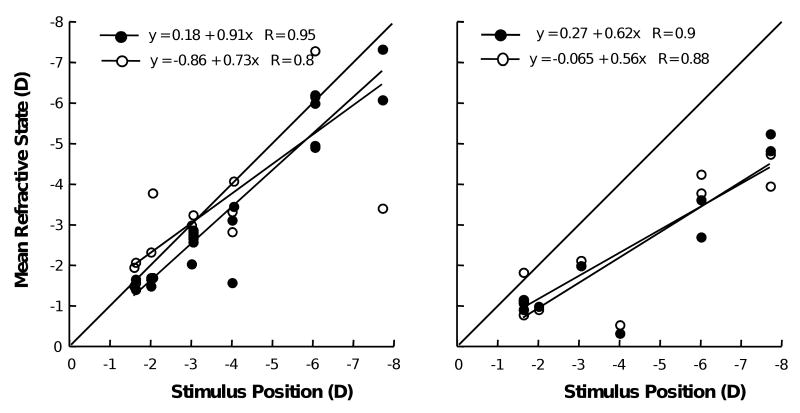
Marmosets measured without lenses demonstrate appropriate shifts in refractive state for changing stimulus positions, as shown by the stimulus-response functions (Figure 8). When lenses are worn for short periods by otherwise untreated marmosets, the binocular accommodative response to changing stimuli appears to depend on the combination of lenses worn (Figure 9).
Figure 8.
Two subjects, measured without lenses, demonstrating appropriate shifts in refractive state for changing accommodative stimulus positions as shown by the binocular stimulus-response for each. The right eye refractions are shown as black circles, left eye refractions are shown as white circles. Lines are best-fit linear regressions, the slopes are estimates of the accommodation stimulus-response gains. Each data point was obtained by taking the mean refraction state response measured during fixation on a stimulus located different distances from the marmoset.
Figure 9.
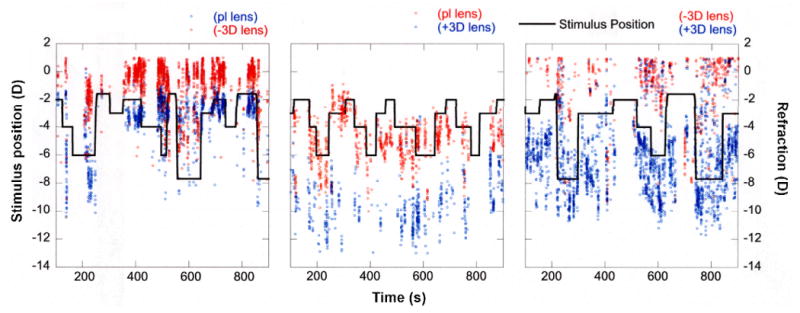
Examples of accommodative changes under three different lens-imposed anisometropic conditions (plano/-3D, plano/+3D, -3D/+3D). The solid black line indicates the accommodative stimulus. The accommodative response of the eye with relatively more imposed effective hyperopia is shown in red. The response of the eye with relatively more imposed effective myopia is shown in blue. Different strategies for focusing through the imposed anisometropia were employed. The relatively more myopic eye was better focused to the stimulus in plano/-3D and -3D/+3D conditions (left and right graphs respectively) but the relatively more hyperopic eye was better focused in the plano/+3D condition (middle graph).
Figure 9 shows examples of accommodative changes under the three different lens-imposed anisometropic conditions (plano/-3 D, plano/+3 D, -3 D/+3 D) used to alter eye growth and refractive state. We see evidence of different strategies for focusing through the imposed anisometropia. In the examples shown, the relatively more myopic eye was better focused to changing accommodative stimuli in the plano/-3 D and -3 D/+3 D conditions and the negative-lens-treated eyes remained relatively hyperopic. In the plano/+3 D lens condition the relatively more hyperopic eye was the better-focused eye and the plus-lens-reared eye was relatively myopic. Taken together, the data in Figures 8 and 9 indicate that even though marmosets are able to accommodate through the hyperopia imposed by the contact lenses used in this study, they do not consistently clear the lens-imposed defocus. The combinations of lenses worn produce patterns of binocular accommodation that maintain retinal defocus that are consistent with the changes in ocular growth and refractive state observed.
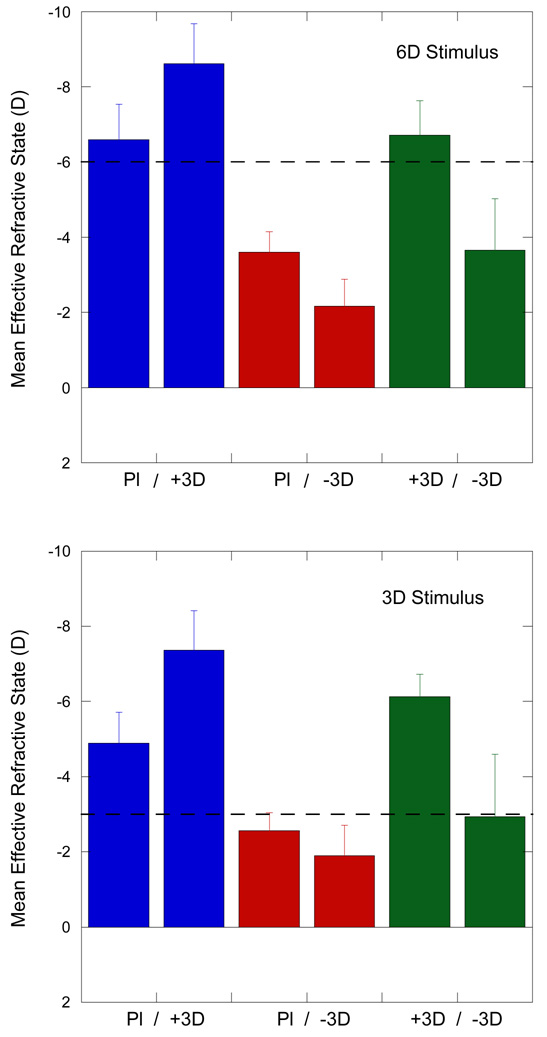
The average state of accommodation through the lenses, shown as the effective refractive states of both eyes from all animals examined at the 3 D and 6 D accommodative stimuli, also indicate different strategies for accommodating under lens-imposed anisometropia (Figure 10, ANOVA, p<0.01). In the plano/+3 D lens condition the eye wearing the plano lens was, on average, better focused to the stimulus while the contralateral eye with the +3 D was relatively myopic. In the +3 D/-3 D condition, the effectively more myopic eye wearing +3 D lens was better focused to the stimulus for the 6 D stimulus and the contralateral eye wearing the negative lens was relatively hyperopic. For the 3 D stimulus the eye wearing the -3D lens was better focused and the eye with the +3 D lens was relatively myopic. In the plano/-3 D lens condition, both eyes under-accommodated, particularly for the 6 D stimulus, and were relatively hyperopic, particularly the negative-lens-treated eye.
Figure 10.
The mean±se effective refractive states at the 3D (top) and 6D (bottom) accommodative stimulus show different strategies for accommodating under lens-imposed anisometropia (ANOVA, p<0.01). Conditions are shown indicated below the bars. In the plano/+3D lens condition (blue bars on left) the eye wearing the plano lens was better focused to the stimulus and the eye with the +3D was relatively myopic. In the +3D/-3D condition (green bars on right), the effectively more myopic eye wearing +3D lens was better focused to the 6D stimulus and the eye wearing the negative lens was relatively hyperopic. For the 3D stimulus, however, the eye wearing the -3D lens was better focused and the eye with the +3D lens was relatively myopic. In the plano/-3D lens condition (red bars in middle), both eyes under accommodated and were relatively hyperopic, but the negative lens treated eye was more so.
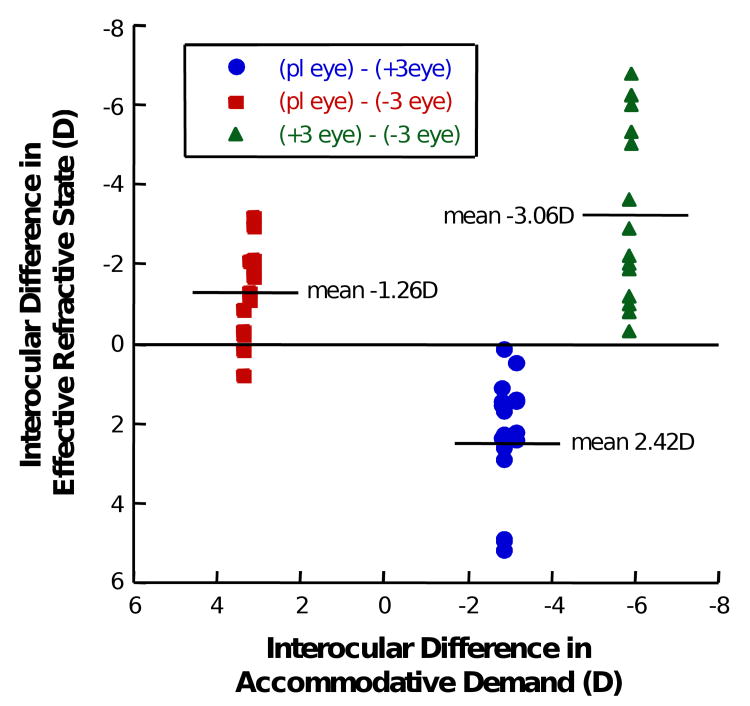
The mean interocular difference in the effective refractive state between the two eyes differed significantly between lens conditions during accommodation (Figure 11, ANOVA, P<0.01). Although the imposed anisometropic conditions created the expected differences in the accommodative demand between the two eyes (Figure 11, x-axis), the mean difference in the effective refractive state (Figure 11, y-axis) was, on average, less than the demand difference when a negative lens was worn: The mean difference in the response in the plano/-3 D condition and the -3 D/+3 D condition was about 50% less than the difference in the demand suggesting that the binocular accommodative response averages the demand under anisometropic conditions. However, the mean interocular difference in effective refractive state for the plano/+3 D condition was about what was expected (3 D) from the lenses.
Figure 11.
The mean interocular difference in accommodative demands and responses under the different lens imposed anisometropic conditions measured at all stimulus positions. Although the mean interocular difference in effective refractive state for the plano/+3D condition (blue circles) was about what was expected (3D) from the lenses, the mean difference in the response was about 50% less than the difference in the demand in the plano/-3D condition (red squares) and -3D/+3D condition (green triangles) supporting the notion that the binocular accommodative response averages the demand under anisometropic conditions.
Discussion
In this paper we describe the results of our contact lens rearing paradigm for studying the visual control of eye growth in the marmoset monkey, a new world primate that we have used in earlier studies of form deprivation myopia22-26. The results presented here confirm and extend results from an early study on the effects of contact lens rearing in marmoset monkeys7 and are consistent with other studies examining the effects of spectacle imposed retinal defocus on eye growth and refractive state in chicks27-29, primates9, 30, and other mammals31, 32. Imposing anisometropia in marmosets with monocular positive or negative power contact lenses, or with binocular contact lenses of opposite sign, results in compensatory changes of the refractive state by altering the rate of axial eye growth. Wearing soft contact lenses did not affect corneal power in any of the experimental groups used in this study. Moreover, in control experiments, plano contact lenses had no effect on corneal power, eye growth, or refractive state relative to untreated eyes.
There were significant shifts toward hyperopia in the positive-lens-treated eyes with imposed myopic defocus, but the negative-lens-imposed hyperopic defocus produced larger and faster changes in eye growth and shifts toward myopia. This may be due, in part, to small amounts of naturally occurring hyperopia in young marmosets, but earlier attempts at imposing larger amounts of myopic defocus did not produce larger compensatory responses5. The response to myopic defocus can, however, be extended if the defocus is stepped up gradually30, 32 and we increased positive lens power gradually to try to increase the effects we report here. We speculate that this response bias toward hyperopic defocus in the primate eye is because it is easier for the eye to effect a compensatory refractive change by increasing the rate of axial eye growth rather than what is needed to compensate for imposed myopic defocus either by reducing axial growth while equatorial growth increases (and reduces optical power by flattening the cornea and lens) or by reversing eye growth and actually reducing axial length. The choroid also contributes to a compensatory response to myopic defocus, but earlier studies indicate that the contribution in marmosets is small33. We did not see direct evidence supporting the existence of an axial growth mechanism that is reversible in the marmosets examined in this study, although several of the positive-lens-treated eyes did show periods where axial growth stopped over a period of weeks while the contralateral eye continued to grow (data not shown). In addition, our finding that rearing with binocular lenses of opposite sign produced generally larger interocular differences than seen in the monocular imposed defocus paradigms suggests that both mechanisms are capable of being activated independently in the two eyes.
There is, however, evidence of interocular effects when one eye is experimentally manipulated. We found in our monocular lens paradigms that the control eyes were affected slightly in such a way that control eyes for monocular positive-lens-treated marmosets were longer and relatively more myopic than the control eyes from the monocular negative-lens-treated marmosets. Control eye changes in monocular rearing paradigms (aphakia or form deprivation) have also been reported in macaques, but in the same direction as the effect in the experimental eye34.
Accommodation Under Imposed Anisometropia
Both accommodation and emmetropization use hyperopic defocus as a stimulus, therefore they must interact in some way so that accommodation does not reduce or eliminate the error signal for emmetropization. Several possibilities exist23): Emmetropization may use the residual hyperopic defocus from accommodative lag. It is also possible that the time constants for the accommodation and emmetropization controllers may differ sufficiently so that emmetropization is largely unaffected by normal accommodation behavior. However, the temporal pattern of accommodative behavior under real world conditions has only recently started to receive attention in order to determine how it actually affects retinal focus over time35. Moreover, the accommodative response through experimental lens-imposed defocus has never been adequately described. In this study we describe the binocular accommodative response under the same contact lens-imposed anisometropic conditions that we are using for our studies of experimental emmetropization, but before the eye growth responses are produced. This allows us to estimate the type and approximate amount of defocus being experienced by each eye under the lens-imposed anisometropic conditions.
Our results suggest that in primates reared under conditions of imposed anisometropia, the way the eyes accommodate and are used preferentially during near vision is affected. We observed that the effectively more myopic eye is preferred under near viewing conditions in most situations, but the effectively more hyperopic eye may be used in others. Imposing anisometropia with binocular contact lenses of opposite sign did not produce a consistent accommodative response; at the 6 D accommodative stimulus the effectively more myopic eye was better focused, but at the 3 D the effectively more hyperopic eye was better focused. The plano/+3 D condition resulted in over accommodation (although the plano eye was well-focused to the 6 D stimulus) and the plano/-3 D condition resulted in under accommodation at both stimulus positions. In general, the negative-lens-treated eyes were always hyperopic (with the exception of the +3 D/-3 D condition at the 3 D stimulus) and positive-lens-treated eyes were always myopic relative to the stimulus. In all situations, the eye contralateral to the better focused eye will have a larger accommodative error of the same sign of defocus that it experiences during distance vision. These observations, together with the observation that accommodation is not typically sustained through lens imposed hyperopia (see Figure 7), helps explain how compensatory shifts in eye growth and refractive state occur despite the ability to use accommodation to clear the hyperopia. Additional studies are needed to determine how longer periods of lens wear, including after the compensatory changes in eye growth and refractive state have taken place, affect the accommodative response.
Hung et al.9 described the preferential near fixation pattern in rhesus monkeys reared with either plano/negative or plano/positive spectacle lens conditions. Although the accommodative response was not quantified, their results suggested that the eye with less accommodative demand was preferred. Flitcroft et al.36 suggested a different mechanism. In that study, they examined the accommodative response to sinusoidal stimuli presented to the eyes in counterphase and found that in some cases the eye with less accommodative demand was used but in others the response was averaged for the demands between the two eyes. Our observations in marmosets support the idea that the different demands are averaged under certain conditions. In the plano/-3 D and the +3 D/-3 D conditions, the mean difference in the effective refractive state is only half of what would be expected from the interocular power of the lenses. However, the data shown are the average effective refractive states for each eye under a given accommodative stimulus and so we cannot distinguish whether the eyes are alternating fixation and accommodation, or whether aniso-accommodation is taking place, or both. Although we cannot rule out the possibility that aniso-accommodation may be involved, the amount needed to explain the reduced interocular difference in effective refractive estate (up to 3 D) is much more than what has been reported8.
Knowledge of how accommodation behaves under real world conditions during binocular viewing is important for understanding emmetropization and may have therapeutic significance concerning the development of myopia. Harb et al.35 reported that accommodation in myopes was more variable during reading than compared to emmetropes. In that study the subjects were not anisometropes and the effective refractive state was monitored in only one eye. Ip21 described the characteristics of dynamic accommodation responses between the dominant and the non-dominant eye in human subjects and found that the speed and response times of accommodation were faster in the dominant eye, although there was no difference in accommodation amplitude. The use of contact lens-induced anisometropia in humans (monovision), has been suggested as a potential therapy for the treatment of myopia37. In that study, Phillips hypothesized that making the non-dominant eye relatively myopic compared to the dominant eye (corrected for distance) would reduce the overall accommodation response at near and myopia progression in schoolchildren. However, the results of the study found that children preferred to use the distance-corrected dominant eye for accommodation, thus imposing relative myopia on the non-dominant eye. The rate of myopia progression in that eye was reduced, suggesting that the imposed myopic defocus slowed eye growth.
The results of our studies shed further light on the relationship of accommodation and the visual control of eye growth and refractive state. The data presented in this study show that accommodative behavior under the three anisometropia-producing lens conditions result in retinal defocus conditions that are consistent with the ocular growth and changes in refractive error observed in other marmosets reared for extended periods under the same lens conditions. The induced changes in eye growth and refractive state provide more evidence for the visual regulation of emmetropization in the primate eye, and our observations of accommodation under imposed anisometropia helps to explain how visual control of eye growth using retinal defocus as an error signal interacts with accommodation.
Figure 5.
The rate of change in the interocular difference in vitreous chamber for the three experimental groups was different. The red bar (left) indicates the mean interocular change in the monocular negative-lens-reared marmosets. The blue bar (middle) indicates the mean interocular change in the monocular positive-lens-reared marmosets. The green bar (right) indicates the mean interocular change in the marmosets raised with binocular lenses of opposite sign. Error bars show standard errors.
Acknowledgments
The authors are grateful to Chea-su Kee, Debora Nickla, Mark O'Conner, and Sanbrita Ghosh for their assistance with various parts of this study.
Support: NIH - EY11228 (DT)
References
- 1.Wildsoet CF. Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–90. [PubMed] [Google Scholar]
- 2.Smith EL., 3rd Spectacle lenses and emmetropization: the role of optical defocus in regulating ocular development. Optom Vis Sci. 1998;75:388–98. doi: 10.1097/00006324-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Res. 1999;39:189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 5.Troilo D, Nickla DL. Visual regulation of eye growth and refractive state in a new world primate. In: Thorn F, Troilo D, Gwiazda J, editors. Myopia 2000: Proceedings of the VIII International Conference on Myopia; Boston, MA. 2000. pp. 170–4. Myopia 2000, Inc. [Google Scholar]
- 6.Whatham AR, Judge SJ. Overnight lens removal avoids changes in refraction and eye growth produced by plano soft contact lenses in infant marmosets. Vision Res. 2001;41:257–65. doi: 10.1016/s0042-6989(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 7.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–73. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 8.Marran L, Schor CM. Lens induced aniso-accommodation. Vision Res. 1998;38:3601–19. doi: 10.1016/s0042-6989(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 9.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–5. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 10.Flitcroft DI. Accommodation and flicker: evidence of a role for temporal cues in accommodation control? Ophthalmic Physiol Opt. 1991;11:81–90. [PubMed] [Google Scholar]
- 11.Goldschmidt E. On the Etiology of Myopia: An Epidemiological Study. Copenhagen: Munksgaard; 1968. [PubMed] [Google Scholar]
- 12.Angle J, Wissmann DA. The epidemiology of myopia. Am J Epidemiol. 1980;111:220–8. doi: 10.1093/oxfordjournals.aje.a112889. [DOI] [PubMed] [Google Scholar]
- 13.Richler A, Bear JC. Refraction, nearwork and education. A population study in Newfoundland. Acta Ophthalmol (Copenh) 1980;58:468–78. doi: 10.1111/j.1755-3768.1980.tb05748.x. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101:405–7. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- 15.Curtin BJ. The Myopias: Basic Science and Clinical Management. Philadelphia: Harper & Row; 1985. [Google Scholar]
- 16.Sato T. The Cause and Prevention of School Myopia. Amsterdam: Excerpta Medica; 1993. [Google Scholar]
- 17.Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus. 1993;30:319–22. doi: 10.3928/0191-3913-19930901-12. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfield M. Accommodation and myopia. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Oxford: Butterworth-Heinemann; 1998. pp. 91–116. [Google Scholar]
- 19.Saw SM, Chua WH, Hong CY, Wu HM, Chan WY, Chia KS, Stone RA, Tan D. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. 2002;43:332–9. [PubMed] [Google Scholar]
- 20.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–40. [PubMed] [Google Scholar]
- 21.Ip JM, Saw SM, Rose KA, Morgan IG, Kifley A, Wang JJ, Mitchell P. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49:2903–10. doi: 10.1167/iovs.07-0804. [DOI] [PubMed] [Google Scholar]
- 22.Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005;46:1873–81. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007;47:1228–44. doi: 10.1016/j.visres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–24. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 25.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Invest Ophthalmol Vis Sci. 2000;41:2043–9. [PubMed] [Google Scholar]
- 26.Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47:1768–77. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 28.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–56. [PubMed] [Google Scholar]
- 29.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–94. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 30.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 31.McFadden S, Wallman J. Guinea pig eye growth compensates for spectacle lenses. Invest Ophthalmol Vis Sci. 1995;36:S758. [Google Scholar]
- 32.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- 33.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–58. [PubMed] [Google Scholar]
- 34.Bradley DV, Fernandes A, Boothe RG. The refractive development of untreated eyes of rhesus monkeys varies according to the treatment received by their fellow eyes. Vision Res. 1999;39:1749–57. doi: 10.1016/s0042-6989(98)00177-1. [DOI] [PubMed] [Google Scholar]
- 35.Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res. 2006;46:2581–92. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flitcroft DI, Judge SJ, Morley JW. Binocular interactions in accommodation control: effects of anisometropic stimuli. J Neurosci. 1992;12:188–203. doi: 10.1523/JNEUROSCI.12-01-00188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips JR. Monovision slows juvenile myopia progression unilaterally. Br J Ophthalmol. 2005;89:1196–200. doi: 10.1136/bjo.2004.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]