Abstract
The ability of a species to reproduce successfully requires the careful orchestration of developmental processes during critical time points, particularly the late embryonic and early postnatal periods. This article begins with a brief presentation of the evidence for how gonadal steroid hormones exert these imprinting effects upon the morphology of sexually differentiated hypothalamic brain regions, the mechanisms underlying these effects, and their implications in adulthood. Then, I review the evidence that aberrant exposure to hormonally-active substances such as exogenous endocrine-disrupting chemicals (EDCs), may result in improper hypothalamic programming, thereby decreasing reproductive success in adulthood. The field of endocrine disruption has shed new light on the discipline of basic reproductive neuroendocrinology through studies on how early life exposures to EDCs may alter gene expression via non-genomic, epigenetic mechanisms, including DNA methylation and histone acetylation. Importantly, these effects may be transmitted to future generations if the germline is affected via transgenerational, epigenetic actions. By understanding the mechanisms by which natural hormones and xenobiotics affect reproductive neuroendocrine systems, we will gain a better understanding of normal developmental processes, as well as to develop the potential ability to intervene when development is disrupted.
Keywords: imprinting, neuroendocrinology, endocrine disruption, hypothalamus, preoptic area, fetal basis of adult disease, sexual dimorphism, reproduction, epigenetic
1. Introduction to development of reproductive neuroendocrine circuits
In vertebrates, the ability to attain reproductive competence in adulthood involves the organization of a complex, steroid-sensitive network in hypothalamic-preoptic-limbic brain regions during critical developmental windows. This process includes the establishment of the hypothalamic neural network of gonadotropin-releasing hormone (GnRH) cells, together with their regulatory inputs from other neuronal and glial cells in the brain [60], that enable feedback effects of steroid hormones on pulsatile GnRH release, and the preovulatory GnRH/LH surge in females. The anatomical development of this steroid-sensitive hypothalamic network occurs early in life, typically the late embryonic and early postnatal period in mammals, and its organization is key to the attainment and activation of appropriate reproductive functions in adulthood. Importantly, this same early developmental period is also a critical period for sexual differentiation of hypothalamic-limbic neural networks that must be organized perinatally to enable proper behavioral activation in adulthood.
During mammalian development, the fetal organism is exposed to its own gonadal hormones, placental steroids, and maternal hormones that may cross the placental barrier [168, 30, 56]. There are sex differences in exposures to androgens and estrogens that appear to underlie normal reproductive neuroendocrine development [85, 164, 146]. Aberrations in these developmental patterns in females can cause masculinization (acquisition of a male-typical trait) or defeminization (loss of a female-typical trait), and in males, may cause feminization or demasculinization (comparably defined). The fetal testis in males is steroidogenically active and produces substantial levels of testosterone that are necessary for the masculinization of the reproductive tract, the genitalia, and the brain. These actions may be exerted directly upon ARs, either by testosterone or its metabolite, 5-dihydrotestosterone, synthesized by the 5α-reductase enzyme. In addition, testosterone is aromatized to estradiol by the p450 aromatase enzyme, thereby enabling fetal estradiol to exert actions on ERs. Both testosterone and estradiol are necessary for normal sexual differentiation of the male brain [98]. In developing females, although it has been proposed that the fetal ovary is relatively quiescent compared to the male testis, it is important to note that androgens and estrogens are measurable in the late embryonic period in female rats [13, 70]. However, sexual differentiation of the female and male brain still differs due in part to alpha-fetoprotein, which protects the brain from effects of maternal estrogens [10, 42]. Alpha-fetoprotein knockout mouse females are masculinized and defeminized in brain and behavior [10], providing further support for a role for estrogens in the masculinization of the brain. Additional differences in brain development may result from even subtle differences in the timing of hormone exposures between the sexes, as the mammalian brain is exquisitely sensitive to hormones in late embryonic and early postnatal time periods, and even small differences may exert large effects.
As epigenetic effects have been defined as “the study of the mechanisms of temporal and spatial control of gene activity during the development of complex organisms” [77], this concept is highly applicable to the field of developmental reproductive neuroendocrinology.
2. Morphological effects of neonatal steroid imprinting in two sexually dimorphic brain regions
The consequences of perinatal exposure to hormones include permanent alterations in the morphology of the brain in a sex-typical manner, which in turn correlate with adult sexual behaviors. There are numerous sexually dimorphic brain nuclei [18] and here I have selected to discuss two representative regions that are strongly implicated as being neonatally imprinted and subsequently controlling sex-typical physiology and behaviors in the rat model. These two brain regions, the anteroventral periventricular nucleus (AVPV), and the sexually dimorphic nucleus of the preoptic area (SDN-POA), undergo sexually dimorphic development due to the influence of endogenous hormones, and can be manipulated in size (and presumably function) by castration or administration of exogenous hormones. Finally, these brain regions are targets of permanent epigenetic imprinting effects of endocrine-disrupting chemicals, as described later in this paper.
2.1 Anteroventral periventricular nucleus (AVPV)
The AVPV is a small preoptic brain region that is abundant in nuclear hormone receptors such as ERα [25], ERβ [108, 26], AR [143], and PR [122] in a sexually dimorphic manner (ERα [27], ERβ [108], PR [122]). The AVPV of rats is larger in female than male rodents [18, 43, 150], due to influences of both estradiol and testosterone during the early postnatal (and possibly prenatal) periods [43, 150, 142]. The AVPV is essential to the control of preovulatory GnRH/LH release in female rats [167]. Consistent with this role, a subset of neurons arising from the AVPV directly innervates GnRH perikarya [117, 71], suggesting an anatomical pathway for the mediation of positive feedback effects of steroids. Although less is known in males about the function of the AVPV, male rats that are stressed neonatally have larger (feminized) AVPV volumes and are less likely to ejaculate, suggesting that the AVPV size correlates inversely with masculine sexual behavior [127].
Notably, the AVPV is only a part of the neural circuitry controlling preovulatory GnRH/LH release and other reproductive functions, as it is interconnected with other key regions regulating reproductive neuroendocrine function, including (but not limited to) inputs from medial amygdala and the bed nucleus of the stria terminalis, and outputs to the organum vasculosum of the lamina terminalis, parvocellular compartment of the paraventricular nucleus, and other hypothalamic nuclei [117, 71]. These projections too may be sexually dimorphic. Thus, the AVPV appears to be a key node in the regulation of sexually dimorphic reproductive neuroendocrine physiology, and a “hot topic” for current research in effects of hormonal imprinting.
2.2 Sexually dimorphic nucleus of the preoptic area (SDN-POA)
A second preoptic brain region that is neonatally imprinted by steroid hormone exposure is the SDN-POA [66], which is approximately 5-fold larger in male than female rats [67, 68, 137]. The medial preoptic nucleus, which contains the SDN-POA, also expresses nuclear hormone receptors, making these regions direct targets of hormone imprinting via ERα [25], ERβ [93], AR [97] and PR [123]. Figure 1 shows representative photomicrographs from two young adult male Sprague-Dawley rats at the SDN-POA level, which exemplify the relative density and expression of ERα and AR. The developing SDN-POA size, like that of the AVPV, is organized by endogenous gonadal steroid hormones [66]. More specifically, testosterone given to fetal rats on gestational days 18, 19 or 20 (but not earlier in gestation) or on postnatal days 2, 3, 4 or 5 resulted in a larger SDN-POA of females (i.e., masculinized; [125, 126]), and deprivation of endogenous hormones via neonatal castration (day 1) in male rats caused the SDN-POA to be smaller, suggesting a feminizing or demasculinizing event [81].
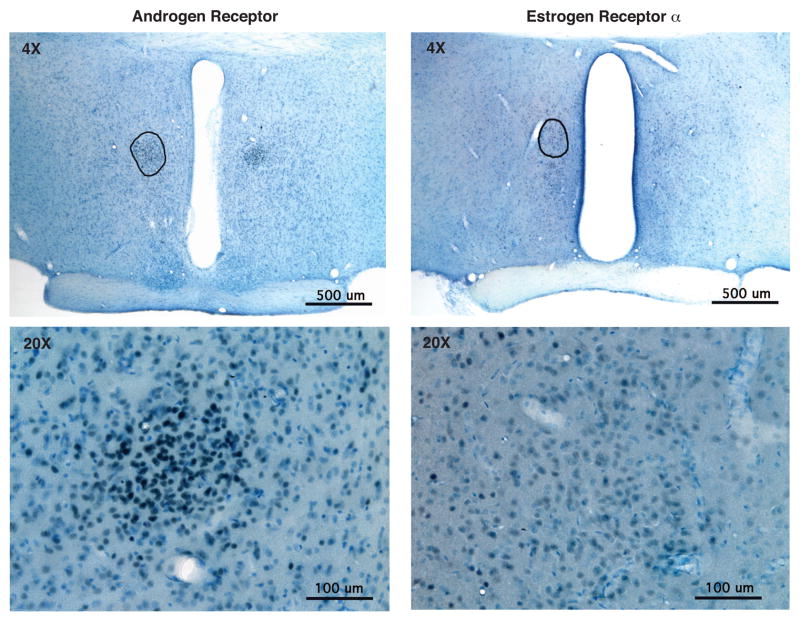
Figure 1.
Photomicrographs of androgen receptor (left) and estrogen receptor α (right) of representative young adult (4 month) sexually-experienced male Sprague-Dawley rats. Rats were deeply anesthetized, perfused with 4% paraformaldehyde, and their brains sectioned at 40 μm. Immunohistochemistry was performed using a polyclonal rabbit antibody to ERα (Upstate C1355, 1:20,000) or AR (PG21, 1:2000, provided by Dr. Gail Prins; [120]). The level of the SDN-POA is circled at 4X magnification (top panels), based on Nissl staining and comparison to Gorski et al. [68]. Bottom panels show higher magnification (20X) micrographs of the same regions. Results show abundant expression of the two nuclear hormone receptors in SDN-POA of male rat brains. Note that AR labeling is darker and more intense than ERα labeling, but both are expressed in cell nuclei throughout the SDN-POA. (Photomicrographs from D. Wu and A.C. Gore, unpublished).
A clear causal relationship between the SDN-POA and function remains elusive, despite correlations between SDN-POA size or integrity on masculine behaviors in rats. Small lesions to this region did not impair copulatory behavior, although larger preoptic lesions (which spared the AVPV) decreased male copulatory patterns in that study [8]. Another group reported that SDN-POA lesions in male rats decreased the percentage of animals that ejaculated in a mating test, and increased latencies to mount, intromit, or ejaculate [41]. Although an exact homolog of the SDN-POA may not exist in other species, there is evidence for sexual dimorphism of the anterior hypothalamus and preoptic area in other species that have been studied [152, 22, 111, 2], making the SDN-POA of the rat a valuable comparative model.
2.3. Mechanisms for hormone effects on AVPV and SDN-POA morphology
At least one of the mechanisms by which steroid hormones cause changes in hypothalamic morphology is apoptosis, or programmed cell death [52]. When steroid hormones bind to their receptors within target cells that express pro- or anti-apoptotic genes, they activate or inhibit genes that stimulate or inhibit apoptosis, and hence target the neurons for survival or death. There are sex differences in expression of pro- and anti-apoptotic genes in the POA. For example, bcl-2 (an anti-apoptotic protein) expression is higher in male than female rats on postnatal day 8, consistent with the overall larger POA size of male rats [78]. A subsequent study from the Hsu group also showed specificity of this phenomenon to the SDN-POA, for which bcl-2 expression was higher in neonatal (postnatal day 2) males than females [79]. Davis et al. found that the number of apoptotic cells in the SDN-POA exhibited differential developmental profiles between the sexes, peaking at postnatal day 6 in males and later (between days 7–10) in the females [40]. Yoshida et al. [170] reported that the total number of apoptotic cells in the developing periventricular preoptic area (roughly equivalent to the AVPV) was higher in male than female rats, consistent with the smaller AVPV size of males due to greater cell death. Another study confirmed this result, showing that in the AVPV, the number of apoptotic cells is greater in male than female rats in late embryonic/early postnatal development [150, 170].
With regard to the steroid regulation of apoptosis, it is notable that neonatal androgens maintain a larger SDN-POA size through inhibition of apoptosis, whereas in the AVPV, neonatal androgens promote apoptosis, resulting in a smaller male AVPV (reviewed in [158]). These differences may be due to a differential balance in the expression of members of the anti-apoptotic family of Bcl-2 genes, and those in the pro-apoptotic family of Bax family genes. Male and female mice with mutations to these genes have significant changes in neuron numbers in the AVPV and SDN-POA, and there are natural sex differences in the expression of apoptotic genes in wildtype animals [172, 53, 157]. More specifically, deletion of Bax (pro-apoptotic) abolished sex differences in AVPV cell numbers [53], and overexpression of Bcl-2 (anti-apoptotic) had a similar effect [172].
3. Physiological and behavioral outcomes of neonatal steroid imprinting
The functional significance of hypothalamic programming during fetal and early postnatal life is the acquisition of the ability to exhibit appropriate physiological and behavioral responses that enable successful reproduction in adulthood. The discipline of reproductive neuroendocrinology was among the first to recognize that the prenatal environment plays a key role in determining adult physiology and behavior in mammals (see [46, 109] for examples). Although beyond the scope of this article, studies of non-mammalian species have been very important in defining the pathways by which exogenous hormones, which may be applied to eggshells or added to water of fish, amphibians, reptiles and birds, result in inappropriate adult sexual behaviors [24, 58]. Here, I will briefly review the evidence for, and the mechanisms by which, neonatal imprinting of hypothalamic circuits results in altered reproductive neuroendocrine physiology and behavior in mammals.
3.1. Fetal imprinting and the GnRH circuitry
Despite the obvious differences of spermatogenesis in males, and oogenesis and follicular development in females, surprisingly, most features of the hypothalamic-pituitary-gonadal axis of the two sexes are quite similar. One of the fundamental differences in reproductive neuroendocrine control between the sexes is the reproductive cycle in spontaneously ovulating females such as rats and primates (estrous or menstrual cycles, respectively) and the preovulatory GnRH/LH surge in response to positive steroid feedback. For information on species with reflex ovulation (e.g., ferret, rabbit, cat), I refer readers to Bakker and Baum [9].
With respect to the ability to generate a preovulatory GnRH/LH surge, there is a critical window ending at about postnatal day 5 in rats during which the brain is permanently imprinted by hormones, disruption of which prevents the ability to exhibit a surge (reviewed in [14]). Importantly, not all adult behaviors are modified by neonatal manipulations, supporting the specificity of this phenomenon to reproductive neuroendocrine systems. For example, treatment of early postnatal female Long-Evans rats with testosterone had no effect on maternal behavior in adulthood [121]. In addition, in male rats, early postnatal castration followed by treatment in adulthood with sequential estradiol and progesterone (at concentrations that induce the LH surge in female rats) did not induce an LH surge [106, 163]. Thus, the male brain is prenatally defeminized by steroid hormones in such a manner as to prevent the adult occurrence of GnRH/LH surges [163].
The preovulatory GnRH/LH surge of spontaneous ovulating females is not the only manifestation of sexually dimorphic function in the HPG axis, although it is certainly one of the most robust. Nevertheless, other pathways controlling hypothalamic GnRH neurons are also organized in a sexually dimorphic manner that is influenced by steroid imprinting. One example is the co-expression of the neuropeptide galanin in GnRH cells. Female rats have approximately five-fold higher co-expression of the galanin and GnRH neuropeptides within the same neurons than do male rats, and respond to hormone treatment in adulthood with increased colocalization [102]. Although male rats do not normally respond to hormone treatment with increased co-expression of galanin and GnRH, neonatal castration of male rats, followed by hormone treatment in adulthood, enables males to manifest the up-regulation of galanin and GnRH co-expression [102]. These findings by Merchenthaler et al. suggest a sexually differentiated pathway for GnRH regulation that is active in female rats, but is unmasked in neonatally castrated male rats.
Another player in the sexually dimorphic imprinted neural circuitry controlling GnRH release is the kisspeptin system. This relatively newly discovered neuropeptide system has been the subject of considerable attention in the past few years, as the ligand KiSS-1 and its receptor GPR54, are obligatory for the control of GnRH function (reviewed in [147, 139]). Significant research shows that expression of KiSS-1 and GPR54 are sexually dimorphic, with most studies focusing on the AVPV as a key control center of the preovulatory GnRH/LH surge, and the arcuate nucleus as a potential target for negative feedback of the hypothalamic-pituitary-gonadal axis by steroid hormones [147]. In the context of neural imprinting, when newborn male pups were given a single injection of estradiol on day 1, and hypothalamic gene expression was measured in adulthood at day 60, there was a significant decrease in KiSS-1 mRNA and serum LH, although GnRH and GPR54 mRNA did not differ from controls [105]. Although these data merit further investigation, they implicate the kisspeptin system as part of the imprinted hypothalamic network that controls reproductive neuroendocrine function.
3.2. Role of prostaglandin E2 (PGE2) in steroid imprinting
Some effects of steroid hormones on the developing hypothalamus are mediated by the PGE2 system. As reported in 2004 by McCarthy et al. [3, 156], blockade of PGE2 on days 1 and 2 of life in male rats impaired adult masculine behaviors, and activation of PGE2 in females at that same age masculinized adult behavior. PGE2 did not defeminize behaviors, however, as feminine sexual and maternal behaviors were not suppressed in the PGE2 treated females, and newborn males in which PGE2 was blocked did not exhibit feminine behaviors in adulthood [156]. Interestingly, these experimental manipulations did not alter SDN-POA size [156] suggesting that this region’s volume is related to defeminization (which did not occur in this study) rather than masculinization. These results are important because they provide novel insights into the mechanisms for brain sexual differentiation and shed light on discriminating between pathways involved in masculinization and defeminization of the brain.
3.3. Developmental expression and sexual dimorphism of histone H1 expression in hypothalamus
Evidence from the field of effects of endocrine-disrupting chemicals on reproductive systems, discussed in detail below, shows that imprinting mechanisms may include modifications to factors that regulate gene expression. For example, promoters may be methylated at the cytosine of CpG islands with a net effect of suppressing gene expression, and conversely, demethylation enables genes to be expressed [69, 76]. Histone modifications involve post-translational regulation of the histone tail by acetylation, a process usually associated with activation of gene transcription [45]. This field is relevant to endocrine disruption but has not yet been applied systematically to basic neuroendocrine research. Nevertheless, such effects of steroids on the developing hypothalamus are highly likely to underlie some of the effects of neonatal imprinting of brain and behavior. To my knowledge only one study has directly addressed this question. Garcia-Segura et al. [55] analyzed the expression and hormone regulation of histone H1°, a neural-specific H1-like protein. They reported that H1° is distributed throughout the nervous system, it is located primarily in neuronal nuclei, and it is developmentally regulated, increasing from birth through adulthood. In the arcuate nucleus of the hypothalamus, they found that females have higher expression of H1° than males, a sex difference that was reversed by neonatal androgenization of females by testosterone given on the day of birth [55]. This paper is important because it shows that histone H1° may be a target for hypothalamic imprinting by steroid hormones.
3.4. Fetal imprinting and the maturation of adult sexual behaviors
It is notable that whereas the critical period of brain sexual differentiation occurs during late embryonic and early postnatal life, the manifestation of most of these effects does not occur (“activated”) until much later in life, beginning in puberty, when the gonads begin to produce large amounts of steroid hormones. In the absence of fetal imprinting, the pubertal activation of sex-specific mating behaviors never occurs [43, 116]. Furthermore, not only is puberty important for activational effects of steroid hormones on neonatally imprinted circuits [12, 153, 16, 116, 119], but the brain may be sensitive to the organizational effects of steroid hormones during this developmental stage [119, 129, 39, 144]. Matsumoto and Arai showed substantial neural development, as manifested by neurite outgrowth, in the hypothalamus of pubertal animals, and further, that this process was stimulated by exogenous hormone exposure [96, 7]. Recent work by Sisk et al. shows that brain masculinization/defeminization continues via pubertal exposure to steroid hormones [138]. Administration of exogenous hormones such as estradiol and testosterone to pubertal rodents disrupted the progression of puberty [110], suggesting that the timing of exposure to hormones is key, again supporting the theme of puberty as a critical developmental window.
4. The developmental basis of adult disease and dysfunction
The first part of this article has presented evidence for clear imprinting effects of hormones on brain and behavior, and some of the neural targets, cellular mechanisms (e.g., apoptosis), and mediators (e.g., steroid hormone receptors, neurotransmitter systems, PGE2) for this process. The second part of this article discusses the concept of the developmental basis of adult disease (often referred to as the “fetal basis of adult disease” when specific to gestational exposures), and introduces the field of endocrine disruption. This discipline is beginning to inform basic research in, and an understanding of, neuroendocrine development. The developmental basis of adult disease was originally described in the context of how maternal nutrition or undernutrition predisposed a developing fetus to metabolic disorders much later in life (reviewed in [11]).
Several important discoveries have emerged from these initial observations. First, the latency between fetal insult and the manifestation of dysfunction can be extremely long; in the case of humans the development of diseases such as cancer, metabolic disorders, and infertility, can be decades. This concept may not be surprising to neuroendocrinologists who have long appreciated the influence of fetal hormones on adult physiology and behavior, but it was a breakthrough for endocrinology and toxicology as a whole. Second, the individual must be considered as an important variable, as different individuals will respond to the same fetal insult (e.g. undernutrition, smoking, environmental toxicants) in very unique ways due to disparities in their genomes. In other words, the gene by environment interaction must be considered as a predisposing factor for the development of dysfunction [45]. Third, the effects of fetal exposures may be manifested not only on the exposed individual but potentially on his/her offspring, and on future generations, either due to overt mutation as is the case for high-dose toxic exposures, or to epigenetic modifications including “heritable changes in gene expression that occur without a change in DNA sequence” [45], including, but not limited to, DNA methylation and histone acetylation [69]. It is important to keep in mind that mitotic epigenetic modifications (e.g. effects on somatic cells) are fundamentally different from meiotic epigenetic modifications (e.g., germline effects) (see [35] for review). Such changes to the epigenome are observed for lower-level exposures to environmental factors, as opposed to overt mutations caused by high dose exposures.
Epigenetic changes to an individual’s genes may be transmitted to the offspring through the germline through effects on ova or sperm (reviewed in [82]), or through behaviors such as how a mother behaves towards her offspring ([161, 29, 45]; see [35] for further discussion of these different avenues of epigenetic modifications). This latter concept of the epigenetic transmission of a trait through the modification of the infant’s genes by maternal behavior has been studied extensively in the area of stress regulation, stress responsiveness, and the hypothalamic-pituitary-adrenal axis, but is yet to be studied in any depth for the hypothalamic-pituitary-gonadal axis. The former concept of germline transmission of an epigenetic trait has been most clearly demonstrated in the field of reproductive toxicology, in which Skinner et al. showed that prenatal exposure to endocrine-disrupting chemicals causes changes in DNA methylation that were manifested as the latent development of male infertility, reproductive cancers, and other dyfunctions, and that this phenotype is transmitted through the male germline across multiple generations [5, 6]. Furthermore, these animals were also behaviorally modified across generations. A recent collaborative report by Crews, Gore, Skinner et al. showed that reproductive behaviors such as partner preference, and attractiveness of individuals to a conspecific mate, were modified in the third generation of descendants of animals exposed fetally to endocrine disruptors [36]. This is strong evidence that neonatal imprinting may epigenetically modify neuroendocrine genes that control reproduction. However, the identity of the genes that have been modified to affect these behaviors is not yet known.
5. Endocrine disruption and developing neuroendocrine systems
The recognition that endogenous hormones shape brain morphology and permanently program neuroendocrine brain function and behavior, and that these pathways can be disrupted via hormonal manipulations during the critical period of brain sexual differentiation, has led to the investigation of effects of xenobiotics, particularly xenoestrogens and xenoandrogens, on neuroendocrine development. These environmental substances can include industrial chemicals, pesticides, fungicides, plasticizers, and even heavy metals and natural plant products such as phytoestrogens. What these compounds have in common is the ability, albeit via different mechanisms, to perturb natural hormonal systems and processes. In many, although not all cases, effects are exerted through actions on nuclear hormone receptors such as ERs (in the case of xenoestrogens) or ARs (in the case of xenoandrogens), whereby exogenous substances act as hormone agonists or antagonists. However, the mechanism for xenobiotic disruption extends to other processes involved in steroid-sensitive pathways such as actions upon enzymes that synthesize or degrade hormones; steroid coregulatory factors; membrane hormone receptors; neurotransmitter receptors, and beyond (reviewed in [62]). Considering that endocrine-disrupting chemical (EDC) exposure is ubiquitous [23] and that exposures often occur during critical developmental times, including from maternal-fetal transfer or via lactation in mammals [4, 94], the discipline of endocrine disruption has considerable relevance to neuroendocrine function.
The US Environmental Protection Agency (EPA)’s definition of an endocrine-disrupting chemical is: “an exogenous agent that interferes with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood born hormones that are present in the body and are responsible for homeostasis reproduction and developmental process.” In addition, endogenous agents also should be included in this definition, as a developing fetus’ own gonads; the gonads of a neighboring twin; the placenta; or maternal hormones (e.g. in polycystic ovarian syndrome or overactivity of the maternal adrenal gland), can expose the developing organism to natural hormones but in an inappropriate manner, such as at the wrong critical window, the wrong dose, or at a gender-inappropriate level [109, 46]. Thus, I include as endocrine disruptors endogenous hormones that when produced outside the normal range may exert some aberration in endocrine or reproductive systems, particularly when occurrence takes place during critical developmental windows.
There are a number of key issues in endocrine disruption that are relevant to neuroendocrine imprinting, several of which have already been introduced. 1) The developmental (fetal) basis of adult disease: The developing fetus or infant is much more sensitive to endocrine-disrupting compounds than the adult; moreover, effects early in life may not be manifested until adulthood [63]. 2) Extremely low-dose exposures may exert significant effects on a developing organism, particularly if the exposure occurs during critical developmental periods [165, 140, 15, 91]. Furthermore, effects are often manifested via non-traditional dose-response curves, similar to actions of steroid and nuclear receptor hormones [165, 63]. 3) Environmental exposures generally occur in complex mixtures, as a contaminated environment is rarely affected by a single toxicant, and the effects may occur by more than one mechanism [15]. 4) Exposures may be acute (e.g., toxic spills or contamination; [47]) but more often they are chronic. 5) Transgenerational, epigenetic effects may be exerted by endocrine disruptors [5, 6, 36]. 6) Mechanisms of endocrine disruption are complex and include not only direct actions on nuclear hormone receptors, but also membrane steroid receptors, non-steroid receptors (e.g., neurotransmitter receptors), enzymes involved in synthesis/degradation of hormones, and other mechanisms that control levels or activity of hormones (reviewed in [63, 1]).
6. Morphological effects of fetal endocrine-disrupting chemicals (EDCs) on the hypothalamus
Several recent studies have demonstrated direct effects of endocrine disruptors on hypothalamic neural circuitry, hypothalamic morphology, the phenotype of hypothalamic cells, and expression of steroid hormone receptors [141, 115, 134, 113]. For illustrative purposes, the AVPV and SDN-POA will again be the focus of discussion, as the morphology of brain regions are particularly well studied in the endocrine disruption field. As will become apparent, the bulk of the literature demonstrates that pre- or early postnatal exposure to endocrine-disrupting chemicals has permanent effects upon the developing hypothalamus, beginning with morphological changes, and extending to disruptions in physiology and behavior.
6.1. Effects of perinatal EDCs on the AVPV and SDN-POA morphology
Several classes of EDCs have been shown to change AVPV and SDN-POA volume and cellular phenotype, although results have not always been consistent due to different EDC choices, different timing of exposure, and sex and species differences (reviewed in [44, 158]). Nevertheless, the bulk of the literature supports effects of neonatal EDCs on the morphology of sexually dimorphic brain regions, consistent with permanent imprinting effects, and in most case due to estrogenic or androgenic actions. The largest literature is for phytoestrogens. Effects of early exposure to genistein and resveratrol on the volumes of the AVPV [115, 73] and SDN-POA [49, 50, 73, 145, 88, 136] have been shown. Maternal resveratrol consumption resulted in larger AVPV and smaller SDN-POA volumes in adult male rats, with no effect on these endpoints in females [73]. Early postnatal exposure to genistein increased SDN-POA volume in female (but not male) rats [88, 49]. Presumably these effects are due to the actions of phytoestrogens on estrogen receptors, possibly as anti-estrogens [87]. It should be noted that Masutomi et al. did not report any effects of neontal genistein and xenoestrogens (methoxychlor, phthalates) on SDN-POA volume in early adulthood [95]; differences in results may be attributable to the age of analyses, and doses and modes of administration of the EDCs.
Other classes of xenobiotics can alter the AVPV and/or SDN-POA volume following prenatal or early postnatal exposure, with most research in this arena focusing on the SDN-POA. Ikeda et al. [80] showed that prenatal dioxin exposure decreased SDN-POA volume of male rats but did not influence this parameter in female rats, suggesting a demasculinizing effect. The estrogenic pharmaceutical diethylstilbestrol (DES) also organizes the developing hypothalamus: the SDN-POA region was increased in size in the female rat but was not affected in male littermates, indicating a masculinizing effect [169]. Prenatal ethinyl estradiol, the most common estrogen in oral steroid contraceptives, decreased SDN-POA volume of males but had no effect in females, suggesting a demasculinizing effect [141]. Again, not all studies confirm effects of prenatal xenoestrogens on SDN-POA sexual differentiation. Kubo et al. [86] did not find effects of resveratrol, DES or bisphenol A on SDN-POA volume, although they reported effects in a different brain region, the locus coeruleus. Again, differences among studies are likely to be due to differential timing in the exposures to, or doses of, the EDCs.
Not only do EDCs affect sexually dimorphic regional volumes, but they affect the phenotypic expression of hormone and neurotransmitter receptors that are expressed in these and other hypothalamic-preoptic regions. Early postnatal exposure of rat pups to genistein or bisphenol A decreased both the number and the percentage of cells in the AVPV that coexpressed tyrosine hydroxylase (an enzyme involved in dopamine synthesis) and ERα in the AVPV of female rats; this effect was seen just for number (not percentage) of double-labeled cells in males, suggesting that female rats are more sensitive to this early EDC exposure [113]. Similar results were reported in mice by Rubin et al. [130], who showed that the sex difference in AVPV size, and tyrosine hydroxylase expression in the AVPV, were diminished by prenatal bisphenol A, primarily due to effects in the females. Prenatal treatment of pregnant dams from days 15–19 of gestation with the PCB mixture Aroclor 1254 resulted in higher mRNA levels of 5-alpha reductase mRNA (the enzyme that converts testosterone to dihydrotestosterone), and lower levels of androgen receptor mRNA, in the hypothalamus of female but not male embryos on gestational day 20 [33]. This same laboratory also showed that prenatal Aroclor 1254 stimulated levels of arylhydrocarbon receptor (AhR) protein in hypothalamus of male but not female rats [118]. A study evaluating effects of prenatal ethinyl estradiol showed a significant decrease in mRNA levels of the GABA transporter-1, with a greater effect in females than males [141]. Collectively, these studies show sex differences in the EDC-responsiveness of hypothalamic molecules involved in mediating hormone signals.
A frequent target of study in endocrine disruption work is the ERβ, the expression of which is not only sexually dimorphic [108], but whose expression is affected in the adult hypothalamus by prenatal exposure to EDCs. Effects on hypothalamic ERβ expression have been shown for a phytoestrogen (daidzein; [124]) an organochlorine pesticide (methoxychlor; [154], and PCBs [134]. Takagi et al. [154] reported female-specific effects of exposure to ethinyl estradiol and methoxychlor from gestational day 15 through postnatal day 10 on ERβ mRNA levels in the medial POA of the P10 offspring. A lifelong phytoestrogen rich diet resulted in significantly fewer ERβ-, but not ERα-expressing neurons in the AVPV of male, but not female rats [21]. As the ERβ gene is subject to DNA methylation, as shown for mammary carcinoma cells [128] (albeit not yet shown for the brain) this may explain how EDCs may modify its gene expression. The ERα promoter, specifically the exon 1b region (ERα1b) that regulates gene transcription of the ERα gene, is modified in the preoptic area in infancy by maternal care via DNA methylation ([29]; see also article in this issue by Champagne [28]). Although not studied for reproductive neuroendocrine physiology, this is another interesting target for future research.
6.2. Apoptotic mechanisms for EDC effects on AVPV and SDN-POA morphology
As discussed earlier, normal developmental apoptosis determines the size and phenotype of sexually dimorphic brain nuclei through a hormone-dependent mechanism. Just as this apoptotic mechanism can be disrupted by early life exposures to abnormal timing in exposures, or inappropriate doses of exogenous hormones such as androgens and estrogens, it can also be permanently altered by EDCs that act upon hormonally-sensitive pathways (reviewed in [158, 44]).
Although the AVPV of male rats fed a soy isoflavone-rich diet throughout their lifetime was not different in size from rats fed a phytoestrogen-free diet, the number of apoptotic neurons (assessed through TUNEL labeling) was higher in the soy diet group [21]. There was a concomitant decrease in ERβ, but not ERα, cell number in the soy group from that study. In accord with that finding, expression of apoptotic genes was decreased in the medial basal hypothalamus of soy-fed rats [20]. Although these data are somewhat difficult to interpret with respect to sexual differentiation, as the exposure to soy was life-long, it suggests effects of phytoestrogens on programmed cell death.
6.3. Effects of EDCs on hypothalamic GnRH neurons
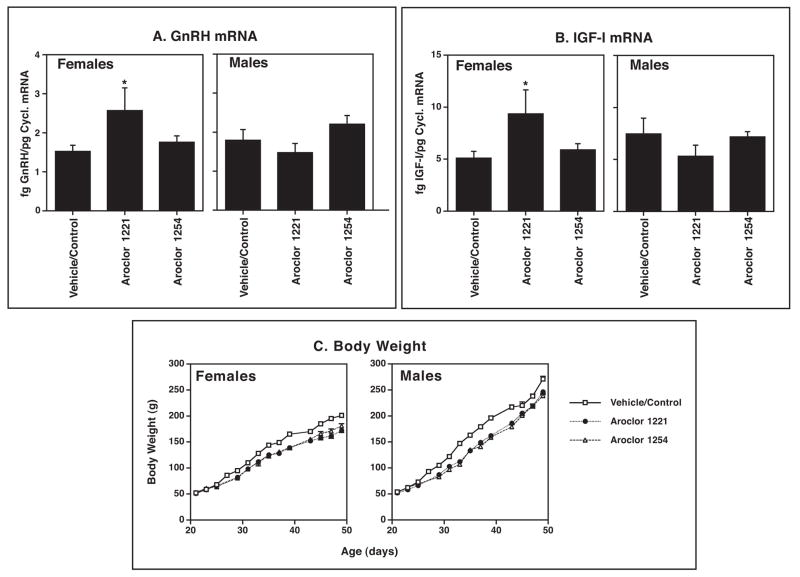
Because GnRH neurons are the primary driving force upon the hypothalamic-pituitary-gonadal axis, they represent a logical target for investigation in the arena of endocrine disruption [59]. Although relatively few studies have been performed, most show effects of endocrine disrupting chemicals, both in vitro and in vivo, on GnRH functional properties. In the hypothalamic GT1-7 GnRH cell line [100], polychlorinated biphenyls [65] and organochlorine pesticides such as methoxychlor and chlorpyrifos [61] have significant effects on GnRH gene expression, GnRH peptide release, and GT1-7 cell morphology. GnRH neurons in vivo are also affected by phytoestrogens such as coumestrol [99, 171] and industrial contaminants, fungicides, and pesticides [17, 59], as manifested by alterations in pulsatile GnRH/LH release, GnRH gene expression, and GnRH cell numbers. For example, in Figure 2 a single exposure of developing fetal rats in my laboratory to low levels of an estrogenic mixture of PCBs, Aroclor 1221, resulted in significantly elevated GnRH mRNA levels in adulthood in females, but not males. Bisenius et al. [17] reported a significant decrease in numbers of GnRH neurons in both male and female rabbits treated with vinclozolin, an effect which was limited to the rostral organum vasculosum of the lamina terminalis (the most rostral part of the hypothalamus). It remains to be determined whether these effects are manifested as altered sexually dimorphic GnRH release such as the preovulatory GnRH surge. This is an important area of research that merits future investigation.
Figure 2.
Pregnant Sprague-Dawley rats (N=3 per treatment) were either uninjected (control) or given a single s.c. injection on gestational day 16 of 0.1 ml of vehicle (DMSO) or one of two PCB mixtures, Aroclor 1221 (A1221) or Aroclor 1254 (A1254), each at 1 mg/kg. After birth, pups were monitored for progression of reproductive development, weighed every other day, and euthanized at 50 days of age (early adulthood). The POA-anterior hypothalamus was dissected, RNA extracted, and mRNA levels of GnRH and IGF-1 were quantified by RNase protection assay (all methods are described in [37, 38, 64]. Results are shown with the control and DMSO vehicle groups combined, as these groups did not differ from one another. Significant effects of A1221, but not A1254 were found for female rats (p < 0.05). Males were unaffected by treatment. Results suggest that some PCB congeners (A1221) but not others (A1254) affect neuroendocrine gene expression in a sex-specific manner. (C) Body weights of rats were monitored every other day. Beginning shortly after weaning (day 21), rats treated prenatally with A1221 and A1254 were significantly smaller than control/vehicle rats. It should be noted that litter sizes and sex ratios did not differ among groups. (A.C. Gore, unpublished data).
6.4. Other hypothalamic neurotransmitter and neurotrophic factor targets of EDCs
A complex neural and glial circuitry regulates hypothalamic-pituitary-gonadal function through actions upon the GnRH neural system (reviewed in [92]). Any or all of the neurotransmitters and neurotrophic factors that control GnRH neurons are potential targets for endocrine disruption. There are many reports showing neurotransmitter actions of EDCs in the brain, with consequences for cognitive function, although few studies have investigated this mechanism specifically for neuroendocrine systems. Nevertheless, the same neurotransmitters that regulate GnRH neurons are disrupted by EDCs [104, 90], suggesting that GnRH neurons may be indirect targets of EDC actions on neurotransmitter receptors. Neurotrophic factors may also play a similar role, and I would like to propose the hypothalamic insulin-like growth factor-1 (IGF-1) system as a target for neuroendocrine disruption. My laboratory and others have shown that IGF-1 regulates GnRH release and gene expression, and that this is subject to developmental regulation [74, 75, 37]. The IGF-1 system is also steroid sensitive [75, 48, 101]. Moreover, GnRH neurons co-express both IGF-1 and IGF-1 receptors [37, 38] suggesting the possibility of autocrine regulation of cells co-expressing GnRH and IGF-1 via the IGF-1 receptor. The IGF-1 receptor is a target of gene regulation by the phytoestrogen, daidzein [124]. Interestingly, IGF-1 can stimulate histone H3 and H4 acetylation in the nervous system, and a causal relationship was established between overexpression of IGF-1 in cortex and hippocampus (hypothalamus was not studied) and this epigenetic process [151]. In neural cells lines IGF-1 also regulates the activity of methionine synthase, an enzyme that converts homocysteine to methyionine, which may serve as a methyl donor [159]. Moreover, in the same study by Waly et al. [159] IGF-1 was able to increase global methylation in the neural cell line. Thus, IGF-1 has the capacity to activate DNA methylation and/or acetylation in neural tissues, and changes in the expression or level of IGF-1 protein would affect its ability to modify epigenetic processes.
Endocrine-disrupting chemicals may regulate hypothalamic gene expression of IGF-1 mRNA, together with GnRH mRNA described above. A single low-dose treatment of pregnant rats with PCBs did not alter the timing of puberty, as measured by vaginal opening in females and preputial separation in males, but resulted in significantly elevated concentrations of IGF-1 mRNA (Figure 2). These effects were specific to females, as mRNA levels were unaltered in male rats. In addition, beginning shortly after weaning, body weights of both male and female PCB-treated rats were significantly smaller than vehicle/control rats, and this was maintained until the age of euthanasia at 50 days. The body weight effect could not be attributed to differences in litter size or composition, which did not vary among groups (data not shown). Therefore, my preliminary data indicate that a single prenatal exposure to PCBs may affect reproductive neuroendocrine gene expression in a sex-typical manner. It should be noted that only A1221 but not A1254 had this effect, probably due to differences in the physical and chemical properties of the PCB mixtures [54].
7. Physiological and behavioral outcomes of neonatal EDC imprinting
Because neonatal EDCs affect the same circuits, brain regions, and hormone-sensitive pathways and receptors as do endogenous hormones, it is not surprising that early EDC exposure is manifested as compromised adult sexual behaviors. Such effects have been documented for several classes of EDCs across a range of species. Although only mammalian studies are discussed herein, non-mammalian vertebrate models have been extremely informative for showing causal links between exposures to EDCs and reproductive behavioral impairments (e.g., reviewed in [112]). Clemens’ group has reported consistent deleterious effects of prenatal EDCs on feminine mating behaviors in rats [31, 32, 160]. My laboratory reported evidence that acute prenatal exposure to low doses of PCBs had permanent consequences on paced mating behavior in adult females [148]. As the ability of a female to control the pace of mating enhances her reproductive success [34] we believe that these data are relevant to non-laboratory animals, including wildlife. Some of the behavioral effects exerted by prenatal PCBs in our laboratory exhibited an inverted-U or U-shaped dose-response curve [148]. This is important because hormones and EDCs sometimes exert non-linear dose-response curves due to the range of mechanisms acted upon by these substances, as discussed above and reviewed in Gore et al. [63]. Aberrations in mating behavior of female rats have also been shown for phytoestrogens such as soy, reported by Patisaul et al. [114]. Early postnatal treatment with relatively high-dose coumestrol also inhibited lordosis behavior in adulthood [84], and postnatal treatment with genistein reduced the lordosis quotient [83]. Moniz et al. [103] reported that perinatal exposure (day 18 of gestation and postnatal days 1 through 5) to the pyrethroid insecticide fenvalerate significantly reduced the lordosis quotient in female rats as adults. Thus, neonatal EDC effects on neuroendocrine systems have functional outcomes relevant to reproductive success in female mammals.
Male mammals have also been studied in the context of endocrine disruption of reproductive behaviors, although the literature in this arena is more limited (see [44] for review). Early postnatal coumestrol given to developing male rats reduced aspects of their sexual behavior including numbers of mounts and ejaculations, and the latency to first mount and ejaculation [166]. Maternal resveratrol exposure affected sexual behavior of the male offspring, with decreased mount frequencies reported [73]. The PCB mixture A1254 given to neonates resulted in a decrease in the number of virgin males who mated in their first mating trial [133]. A single dose of PCB126 (but not PCB77) given to pregnant rats in mid-gestation resulted in an increased number of mounts with intromissions in the adult male offspring [51]. This result is surprising as it is the only one, to my knowledge, that shows increased masculine sexual activities; it is possible that this PCB compound acts through another mechanism, possibly as an androgen or estrogen receptor agonist, to exert this action.
8. Transgenerational effects of endocrine-disrupting chemicals on neuroendocrine systems
The ability of EDCs to influence not only the exposed individuals but also subsequent generations of offspring has been shown for reproductive systems. As already discussed, Skinner et al. reported that fetal EDCs cause epigenetic modifications, specifically differences in DNA methylation, that are passed to up to four generations of offspring via the male germline [5]. The best evidence for both the fetal basis of adult disease together with transgenerational effects in humans is the case of the pharmaceutical, diethylstilbestrol (DES). The DES story is important because it is one of the few examples in humans of a direct causal relationship between fetal exposure and the latent development of disease and dysfunction much later in life. The female offspring of pregnant women taking DES grew up to exhibit a relatively high incidence of reproductive tract abnormalities and rare clear cell cervicovaginal cancers (reviewed in [57]). Women exposed as fetuses to DES continue to have long-term reproductive health changes such as infertility and an earlier age at menopause [72, 57]. Male offspring also have a small but significantly higher likelihood of reproductive tract malformations such as hypospadias [19], although the overall reproductive impact of DES on males is less than that in females. A recent epidemiologic study of “DES granddaughters” reported subtle differences in menstrual cycles and a possible association with fewer live births than descendants of unexposed women [155], suggesting the potential for transgenerational effects. This area of research merits additional investigation and follow up, because animal models support transgenerational effects of DES on reproductive systems (reviewed in [107]). For example, gene expression of lactoferrin, an estrogen-regulated gene in the uterus, was up-regulated in both F1 female mice (perinatally exposed to DES) and their F2 offspring [107]. The mechanism for such an effect may involve epigenetic modifications, although this has not been reported specifically for lactoferrin. Other animal models show that neonatal DES treatment resulted in demethylation of the immediate early gene c-fos in the mouse uterus [89]. In epididymis of male mice, DES given on postnatal days 1–5 altered expression of DNA methyltransferase genes that are involved in the regulation of DNA methylation status [135]. Ruden et al. have proposed a model whereby DES alters the interactions of estrogen receptor, the chaperone protein Hsp90, and chromatin-mediated Wnt signaling involved in developmental patterning, and which may contribute to the transgenerational phenotype [132, 131].
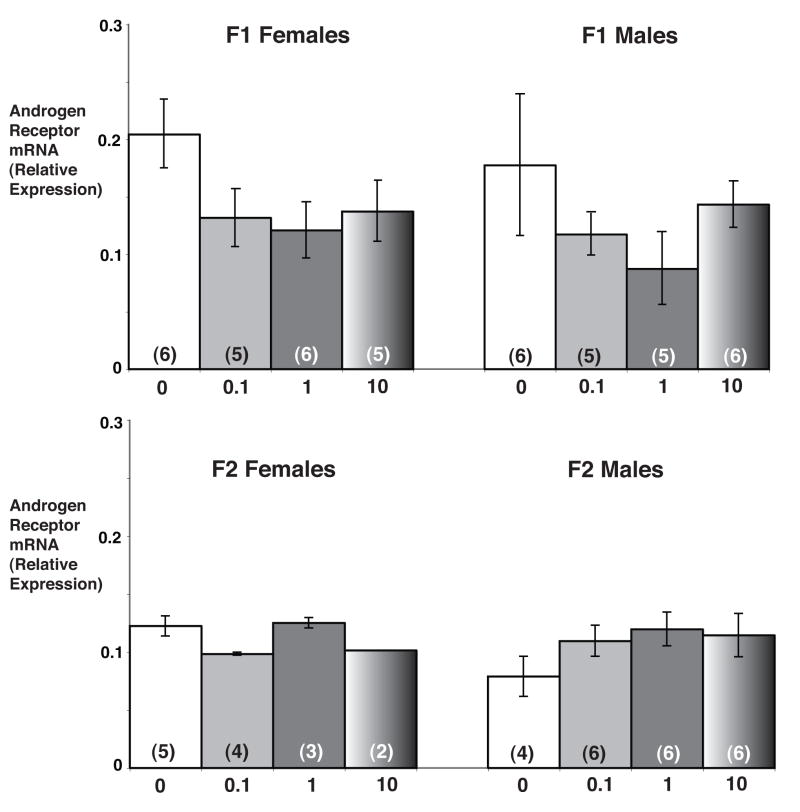
My laboratory is beginning to investigate transgenerational effects of prenatal EDCs on reproductive neuroendocrine function, using the model of PCBs. We previously showed that late embryonic (gestational days 16 and/or 18) exposures to the estrogenic PCB mixture, Aroclor 1221 (A1221), decreased expression of ERβ in the AVPV of adult female rats [134], stimulated GnRH mRNA levels in the preoptic area (see Figure 2 and [59]); and altered female paced mating behavior in adulthood [148]. We have recently extended some of our A1221 studies to a second generation. In this experiment, pregnant female rats were injected with low doses of A1221 twice during late gestation, on embryonic days 16 and 18 (see [148] for experimental details). Some F1 pups were euthanized on postnatal day (P) 1 for gene expression analysis of the POA. Other pups were allowed to mature to investigate effects on adult reproductive functions and mating behaviors. After delivery, some F2 pups were euthanized on P1 for gene expression measurements; others matured to enable us to monitor reproductive cycles in adulthood. The preliminary data from this two-generational study show that F2 adult females have altered cyclic patterns of hormone release, including suppressed progesterone and LH concentrations on proestrus [149]. Recently, we evaluated gene expression of neuroendocrine genes in the POAs of P1 male and female pups from both the F1 and F2 generations (D.M. Walker, R.M. Steinberg, A.C. Gore, unpublished). Genes measured in this region were GnRH, ERα, ERβ, AR and PR. We observed effects of A1221 on all of the genes in both generations of rats. Preliminary data for AR mRNA levels, quantified by real-time PCR, are shown (Figure 3). In the F1 generation, AR mRNA levels in the POA were decreased in both male and female P1 pups treated with A1221 as fetuses. In the F2 generation, AR mRNA levels were consistently elevated in the male but not the female offspring of A1221 treated F1 rats. These data need to be interpreted cautiously because of the small sample sizes, but they suggest direct hypothalamic-preoptic actions of perinatal PCB exposure in two generations of rats, with the likelihood of epigenetic modifications to factors involved in the control of neuroendocrine gene expression.
Figure 3.
Androgen receptor (AR) mRNA profiles in the POA of postnatal day 1 (P1) rat pups from two generations. Pregnant dams (F0) were treated with Aroclor (A) 1221 on gestational days 16 and 18. POAs were collected from F1 male and female rat pups on P1. Some F1 females were allowed to mature, mate, and give birth to an F2 generation. POAs were collected on P1 from these F2 male and female pups. Numbers below the bars refer to the original treatment of the F0 dam. Numbers within bars in parentheses refer to the number of POAs from individual rats assays by real-time PCR. These preliminary data for mRNA levels of AR suggest potential effects of A1221 in both male and female P1 pups in the F1 generation, and effects in the P1 male pups in the F2 generation. Notably, patterns of gene expression are different in males of the two generations. See [148, 149] for more detailed methodology. (D.M. Walker, R.M. Steinberg, A.C. Gore, unpublished data).
Together, these studies indicate that perinatal EDCs impact reproductive neuroendocrine function at several levels. They alter morphology of sexually dimorphic brain regions; they affect the phenotype of cells in the hypothalamus; they have detrimental actions on the neuroendocrine circuits, including GnRH neurons and their regulatory inputs; and they have functional outcomes on reproductive behaviors. Moreover, there are transgenerational effects of EDCs on reproductive systems that may include neuroendocrine targets.
9. Endocrine disruption, reproductive neuroendocrinology, and future directions
The field of endocrine disruption has contributed in two important ways to expanding knowledge about reproductive neuroendocrinology. First, the concept that perinatal hormones have permanent imprinting effects on the hypothalamus, manifested early on as morphological sex differences in the brain, and manifested much later on as physiological and behavioral differences between the sexes, forms the foundation of the “developmental basis of adult disease.” Although researchers in EDCs were not the first to make this discovery, their research has called much broader attention to the importance of critical periods of development, particular the perinatal stage, to a wide range of disciplines, including toxicology, cancer biology, psychiatric disease, and general endocrinology (not just reproduction). Moreover, researchers in endocrine disruption are beginning to focus their research efforts on understanding the underlying mechanism(s) by which early hormonal programming exerts its effects. In particular, how DNA and gene expression are modified, not by overt mutations, but rather by alterations in factors that control gene expression such as methylation status or histone acetylation, is a key concept that can be applied back to basic reproductive neuroendocrinology. This cross-fertilization among diverse disciplines in the biological sciences has resulted in significant new discoveries in the past few years.
Second, recent studies are showing that when gene expression is modulated through molecular epigenetic modifications such as histone acetylation and DNA methylation, these effects may be expressed in subsequent generations. This concept of transgenerational epigenetic effects of EDCs stems from reproductive toxicology, but it is now being extended to other disciplines including reproductive neuroendocrinology. Such observations raise concerns about permanent effects of exposures to environmental toxicants, not just to exposed individuals but also to future generations. Despite our best efforts to clean up contaminated sites, or to avoid the use of known or suspected EDCs, it may be too late if the epigenome has been affected in a manner that can be transmitted to offspring (e.g., see [36]). By understanding the neuroendocrine consequences of early EDC exposures, and whether effects are passed on by a transgenerational, epigenetic mechanism, we will be able to determine when, and how, we may be able to intervene. Recent evidence showing that factors that alter histone acetylation may break the cycle of epigenetic transmission of a trait is very encouraging [162], with broad implications for mitigating effects of environmental contamination.
Acknowledgments
I would like to acknowledge two graduate students in my laboratory, Sarah Dickerson and Deena Walker, for discussions about many of the topics covered in this article, and for their keen insights and ideas. Preliminary data in the figures were provided by present and former graduate students Di Wu (photomicrographs of the SDN-POA in Figure 2) and Deena Walker and Rebecca Steinberg (AR mRNA levels in Figure 3). My research programs on reproductive neuroendocrine systems have been generously supported by the NIH (AG028051, AG16765, ES12272, ES07784) and the NSF (IBN-0334221).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler SR. Cellular mechanisms of endocrine disruption. In: Gore AC, editor. Endocrine-disrupting Chemicals: From Basic Research to Clinical Practice. Humana Press; New Jersey: 2007. pp. 135–174. [Google Scholar]
- 2.Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol Behav. 2007;90:438–449. doi: 10.1016/j.physbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nature Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 4.Ando M, Saito H, Wakisaka I. Transfer of polychlorinated biphenyls (PCBs) to newborn infants through the placenta and mothers’ milk. Arch Environ Contam Toxicol. 1985;14:51–57. doi: 10.1007/BF01055761. [DOI] [PubMed] [Google Scholar]
- 5.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 7.Arai Y, Matsumoto A. Synapse formation of the hypothalamic arcuate nucleus during post-natal development in the female rat and its modification by neonatal estrogen treatment. Psychoneuroendocrinology. 1978;3:31–45. doi: 10.1016/0306-4530(78)90039-2. [DOI] [PubMed] [Google Scholar]
- 8.Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–54. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 9.Bakker J, Baum MJ. Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol. 2000;21:220–262. doi: 10.1006/frne.2000.0198. [DOI] [PubMed] [Google Scholar]
- 10.Bakker J, DeMees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 11.Barker DJP. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- 12.Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- 13.Baum MJ, Woutersen PJA, Slob AK. Sex difference in whole-body androgen content in rats on fetal days 18 and 19 without evidence that androgen passes from males to females. Biol Reprod. 1991;44:747–751. doi: 10.1095/biolreprod44.5.747. [DOI] [PubMed] [Google Scholar]
- 14.Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell Mol Neurobiol. 1997;17:699–715. doi: 10.1023/a:1022542221535. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron JM, Crews D, McLachlan JA. PCBs as environmental estrogens: Turtle sex determination as a biomarker of environmental contamination. Environ Health Perspec. 1994;102:780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: Fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- 17.Bisenius ES, Veeramachaneni DNR, Sammonds GE, Tobet S. Sex differences and the development of the rabbit brain: Effects of vinclozolin. Biol Reprod. 2006;75:469–476. doi: 10.1095/biolreprod.106.052795. [DOI] [PubMed] [Google Scholar]
- 18.Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982;212:118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- 19.Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, de Gier RPE, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Human Reprod. 2006;21:666–669. doi: 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- 20.Bu L, Lephart ED. Soy isoflavones modulate the expression of BAD and neuron-specific beta III tubulin in male rat brain. Neurosci Lett. 2005;385:153–157. doi: 10.1016/j.neulet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 21.Bu L, Lephart ED. AVPV neurons containing estrogen receptor-beta in adult male rats are influenced by soy isoflavones. BMC Neurosci. 2007;8:1–12. doi: 10.1186/1471-2202-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byne W, Bleier R. Medial preoptic sexual dimorphisms in the guinea pig. I. An investigation of their hormonal dependence. J Neurosci. 1987;7:2688–2696. doi: 10.1523/JNEUROSCI.07-09-02688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calafat AM, Needham LL. Human exposures and body burdens of endocrine-disrupting chemicals. In: Gore AC, editor. Endocrine-disrupting Chemicals: From Basic Research to Clinical Practice. Humana Press; New Jersey: 2007. pp. 253–268. [Google Scholar]
- 24.Carere C, Balthazart J. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol Metab. 2007;18:73–80. doi: 10.1016/j.tem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ERα) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- 26.Chakraborty TR, Ng L, Gore AC. Age-related changes in estrogen receptor β in rat hypothalamus: A quantitative analysis. Endocrinology. 2003;144:4164–4171. doi: 10.1210/en.2003-0052. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor α in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med. 2005;230:49–56. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- 28.F.A. Champagne this issue
- 29.Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 30.Chan SWC, Leathem JH. Placental steroidogenesis in the rat: Progesterone production by tissue of the basal zone. Endocrinology. 1975;96:298–303. doi: 10.1210/endo-96-2-298. [DOI] [PubMed] [Google Scholar]
- 31.Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol. 1999;62:664–670. doi: 10.1007/s001289900925. [DOI] [PubMed] [Google Scholar]
- 32.Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- 33.Colciago A, Negri-Cesi P, Pravettoni A, Mornati O, Casati L, Celotti F. Prenatal Aroclor 1254 exposure and brain sexual differentiation: Effect on the expression of testosterone metabolizing enzymes and androgen receptors in the hypothalamus of male and female rats. Reprod Toxicol. 2006;22:738–745. doi: 10.1016/j.reprotox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Coopersmith C, Erskine MS. Influence of paced mating and number of intromissions on fertility in the laboratory rat. J Reprod Fertil. 1994;102:451–458. doi: 10.1530/jrf.0.1020451. [DOI] [PubMed] [Google Scholar]
- 35.D. Crews, This issue.
- 36.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: Relationship to GnRH neurons. Endocrinology. 2003;144:2034–2045. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- 38.Daftary SS, Gore AC. The hypothalamic insulin-like growth factor receptor (IGF-1R), and its relationship to GnRH neurons during postnatal development. J Neuroendocrinol. 2004;16:160–169. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- 39.Davis EC, Shryne JE, Gorski RA. A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinol. 1995;62:579–585. doi: 10.1159/000127053. [DOI] [PubMed] [Google Scholar]
- 40.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 41.DeJonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, Van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 42.De Mees C, Laes JF, Bakker J, Smitz J, Hennuy B, Van Vooren P, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein controls female fertility and prenatal development of the gonadotropin-releasing hormone pathway through an antiestrogenic action. Mol Cell Biol. 2006;26:2012–2018. doi: 10.1128/MCB.26.5.2012-2018.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeVries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 4. Academic Press; New York: 2002. pp. 137–192. [Google Scholar]
- 44.Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 45.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007 doi: 10.1007/s11154-007-9046-0. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- 48.Etgen AM, Gonzalez-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Faber KA, Hughes CL., Jr The effect of neonatal exposure to diethylstilbestrol, genistein, and zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol Reprod. 1991;45:649–653. doi: 10.1095/biolreprod45.4.649. [DOI] [PubMed] [Google Scholar]
- 50.Faber KA, Hughes CL., Jr Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotropin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod Toxicol. 1993;7:35–39. doi: 10.1016/0890-6238(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 51.Faqi AS, Dalsenter PR, Merker HJ, Chahoud I. Effects on developmental landmarks and reproductive capability of 3,3′,4,4′-tetrachlorobiphenyl and 3,3′,4,4′,5-pentachlorobiphenyl in offspring of rats exposed during pregnancy. Hum Exp Toxicol. 1998;17:365–372. doi: 10.1177/096032719801700702. [DOI] [PubMed] [Google Scholar]
- 52.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frame GM. A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns. 2. Semi-quantitative Aroclor congener distributions. Fresenius J Anal Chem. 1997;357:714–722. [Google Scholar]
- 55.Garcia-Segura LM, Luquin S, Martinez P, Casas MT, Suau P. Differential expression and gonadal hormone regulation of histone H1° in the developing and adult rat brain. Devel Brain Res. 1993;73:63–70. doi: 10.1016/0165-3806(93)90046-d. [DOI] [PubMed] [Google Scholar]
- 56.Gibori G, Sridaran R. Sites of androgen and estradiol production in the second half of pregnancy in the rat. Biol Reprod. 1981;24:249–256. doi: 10.1095/biolreprod24.2.249. [DOI] [PubMed] [Google Scholar]
- 57.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: A review of the long-term health effects. Ann Int Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 58.Godwin J, Crews D. Hormones, brain and behavior in reptiles. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. New York: Academic Press; 2002. pp. 545–585. [Google Scholar]
- 59.Gore AC. Environmental toxicant effects on neuroendocrine function. Endocrine. 2001;14:235–246. doi: 10.1385/ENDO:14:2:235. [DOI] [PubMed] [Google Scholar]
- 60.Gore AC. GnRH: The Master Molecular of Reproduction. Kluwer Academic Publishers; Boston: 2002. [Google Scholar]
- 61.Gore AC. Organochlorine pesticides directly regulate gonadotropin-releasing hormone gene expression and biosynthesis in the GT1-7 hypothalamic cell line. Mol Cell Endocrinol. 2002;192:157–170. doi: 10.1016/s0303-7207(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 62.Gore AC. Introduction to endocrine-disrupting chemicals. In: Gore AC, editor. Endocrine-disrupting Chemicals: From Basic Research to Clinical Practice. Humana Press; New Jersey: 2007. pp. 3–8. [Google Scholar]
- 63.Gore AC, Heindel JJ, Zoeller RT. Endocrine disruption for endocrinologists (and others) Endocrinology. 2006;147:s1–3. doi: 10.1210/en.2005-1367. [DOI] [PubMed] [Google Scholar]
- 64.Gore AC, Roberts JL. Regulation of gonadotropin-releasing hormone gene expression by the excitatory amino acids kainic acid and N-methyl-D, L-aspartate in the male rat. Endocrinology. 1994;134:2026–2031. doi: 10.1210/endo.134.5.8156903. [DOI] [PubMed] [Google Scholar]
- 65.Gore AC, Wu TJ, Oung T, Lee JB, Woller MJ. A novel mechanism for endocrine-disrupting effects of polychlorinated biphenyls: Direct effects on gonadotropin-releasing hormone (GnRH) neurons. J Neuroendocrinol. 2002;14:814–823. doi: 10.1046/j.1365-2826.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 66.Gorski RA. Hypothalamic imprinting by gonadal steroid hormones. Adv Exp Med Biol. 2002;511:57–70. doi: 10.1007/978-1-4615-0621-8_5. [DOI] [PubMed] [Google Scholar]
- 67.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 68.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 69.Guerrero-Bosagna C, Valladares L. Endocrine disruptors, epigenetically induced changes, and transgenerational transmission of characters and epigenetic states. In: Gore AC, editor. Endocrine-disrupting Chemicals: From Basic Research to Clinical Practice. Humana Press; New Jersey: 2007. pp. 175–189. [Google Scholar]
- 70.Habert R, Picon R. Testosterone, dihydrotestosterone and estradiol-17β levels in maternal and fetal plasma and in fetal testes in the rat. J Steroid Biochem. 1984;21:193–198. doi: 10.1016/0022-4731(84)90383-2. [DOI] [PubMed] [Google Scholar]
- 71.Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol. 2006;494:190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- 72.Hatch EE, Troisi R, Wise LA, Hyer M, Palmer JR, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Adam E, Noller KL, Herbst AL, Robboy S, Hartge P, Hoover RN. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am J Epidemiol. 2006;164:682–688. doi: 10.1093/aje/kwj257. [DOI] [PubMed] [Google Scholar]
- 73.Henry LA, Witt DM. Effects of neonatal resveratrol exposure on adult male and female reproductive physiology and behavior. Dev Neurosci. 2006;28:186–195. doi: 10.1159/000091916. [DOI] [PubMed] [Google Scholar]
- 74.Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor I: a possible metabolic signal involved in the regulation of female puberty. Neuroendocrinol. 1991;54:420–423. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- 75.Hiney JK, Srivastava V, Dearth RK, Dees WL. Influence of estradiol on insulin-like growth factor-1-induced luteinizing hormone secretion. Brain Res. 2004;1013:91–97. doi: 10.1016/j.brainres.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 76.Ho SM, Tang WY. Techniques used in studies of epigenome dysregulation due to aberrant DNA methylation: An emphasis on fetal-based adult diseases. Reprod Toxicol. 2007;23:267–282. doi: 10.1016/j.reprotox.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holliday R. Mechanisms for the control of gene activity during development. Biol Rev. 1990;65:431–471. doi: 10.1111/j.1469-185x.1990.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 78.Hsu C, Hsieh YL, Yang RC, Hsu HK. Blockage of N-methyl-D-aspartate receptors decreases testosterone levels and enhances postnatal neuronal apoptosis in the preoptic area of male rats. Neuroendocrinology. 2000;71:301–307. doi: 10.1159/000054550. [DOI] [PubMed] [Google Scholar]
- 79.Hsu HK, Shao PL, Tsai KL, Shih HC, Lee TY, Hsu C. Gene regulation by NMDA receptor activation in the SDN-POA neurons of male rats during sexual development. J Mol Endocrinol. 2006;34:433–445. doi: 10.1677/jme.1.01601. [DOI] [PubMed] [Google Scholar]
- 80.Ikeda M, Mitsui T, Setani K, Tamura M, Kakeyama M, Sone H, Tohyama C, Tomita T. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats disrupts brain sexual differentiation. Toxicol Appl Pharmacol. 2005;205:98–105. doi: 10.1016/j.taap.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Jacobson CD, Csernus VJ, Shryne JE, Gorski RA. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci. 1981;1:1142–1147. doi: 10.1523/JNEUROSCI.01-10-01142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Rev Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, Yamanouchi K. Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm Behav. 2003;44:140–145. doi: 10.1016/s0018-506x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 84.Kouki T, Okamoto M, Wada S, Kishitake M, Yamanouchi K. Suppressive effect of neonatal treatment with a phytoestrogen, coumestrol, on lordosis and estrous cycle in female rats. Brain Res Bull. 2005;64:449–454. doi: 10.1016/j.brainresbull.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 85.Krohmer RW, Baum MJ. Effect of sex, intrauterine position and androgen manipulation on the development of brain aromatase activity in fetal ferrets. J Neuroendocrinol. 1989;1:265–271. doi: 10.1111/j.1365-2826.1989.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 86.Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 87.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 88.Lewis RW, Brooks N, Milburn GM, Soames A, Stone S, Hall M, Ashby J. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- 89.Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinogenesis. 2003;38:78–74. doi: 10.1002/mc.10147. [DOI] [PubMed] [Google Scholar]
- 90.Lyng GD, Snyder-Keller A, Seegal RF. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol Sci. 2007;97:128–139. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- 91.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 92.Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn PM, editor. Handbook of Models for Human Aging. Academic Press/Elsevier; Burlington, MA: 2006. pp. 533–552. [Google Scholar]
- 93.Mann PE, Babb JA. Neural steroid hormone receptor gene expression in pregnant rats. Brain Res Mol Brain Res. 2005;142:39–46. doi: 10.1016/j.molbrainres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 94.Masuda Y, Kawaga R, Kuroki H, Kuratsune M, Yoshimura T, Taki I, Kusuda M, Yamashita F, Hayashi M. Transfer of polychlorinated biphenyls from mothers to foetuses and infants. Food Cosmet Toxicol. 1978;16:543–546. doi: 10.1016/s0015-6264(78)80221-1. [DOI] [PubMed] [Google Scholar]
- 95.Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–70. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto A, Arai Y. Precocious puberty and synaptogenesis in the hypothalamic arcuate nucleus in pregnant mare serum gonadotropin (PMSG) treated immature female rats. Brain Res. 1977;129:375–378. doi: 10.1016/0006-8993(77)90019-1. [DOI] [PubMed] [Google Scholar]
- 97.McAbee MD, DonCarlos LL. Regulation of androgen receptor messenger ribonucleic acid expression in the developing rat forebrain. Endocrinology. 1999;140:1807–1814. doi: 10.1210/endo.140.4.6632. [DOI] [PubMed] [Google Scholar]
- 98.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 99.McGarvey C, Cates PS, Brooks NA, Swanson IA, Milligan SR, Coen CW, O’Byrne KT. Phytoestrogens and gonadotropin-releasing hormone pulse generator activity and pituitary luteinizing hormone release in the rat. Endocrinology. 2001;142:1202–1208. doi: 10.1210/endo.142.3.8015. [DOI] [PubMed] [Google Scholar]
- 100.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 101.Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front Neuroendocrinol. 2006;27:391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 102.Merchenthaler I, Lennard DE, Lopez FJ, Negro-Vilar A. Neonatal imprinting predetermines the sexually dimorphic, estrogen-dependent expression of galanin in luteinizing hormone-releasing hormone neurons. Proc Natl Acad Sci USA. 1993;90:10479–10483. doi: 10.1073/pnas.90.22.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moniz AC, Cruz-Casallas PE, Salzgeber SA, Varoli FMF, Spinosa HS, Bernardi MM. Behavioral and endocrine changes induced by perinatal fenvalerate exposure in female rats. Neurotoxicol Teratol. 2005;27:609–614. doi: 10.1016/j.ntt.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Morse DC, Seegal RF, Borsch KO, Brouwer A. Long-term alterations in regional brain serotonin metabolism following maternal polychlorinated biphenyl exposure in the rat. Neurotoxicology. 1996;17:631–638. [PubMed] [Google Scholar]
- 105.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 106.Neill JD. Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology. 1972;90:1154–1159. doi: 10.1210/endo-90-5-1154. [DOI] [PubMed] [Google Scholar]
- 107.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–S17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 108.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci USA. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Padula AM. The freemartin syndrome: an update. Anim Reprod Sci. 2005;87:93–109. doi: 10.1016/j.anireprosci.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 110.Pak TR, Lynch GR, Ziegler DM, Lunden JB, Tsai PS. Disruption of pubertal onset by exogenous testosterone and estrogen in two species of rodents. Am J Physiol Endocrinol Metab. 2003;284:E206–E212. doi: 10.1152/ajpendo.00352.2002. [DOI] [PubMed] [Google Scholar]
- 111.Panzica GC, Viglietti-Panzica C, Calacagni M, Anselmetti GC, Schumacher M, Balthazart J. Sexual differentiation and hormonal control of the sexually dimorphic medial preoptic nucleus of the quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- 112.Panzica GC, Viglietti-Panzica C, Ottinger MA. Introduction: neurobiological impact of environmental estrogens. Brain Res Bull. 2005;65:187–191. doi: 10.1016/j.brainresbull.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Patisaul HB. Neonatal genistein or bisphenol A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm Behav. 2004;45:270–277. doi: 10.1016/j.yhbeh.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–392. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 117.Polston EK, Simerly RB. Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. J Comp Neurol. 2006;495:122–132. doi: 10.1002/cne.20874. [DOI] [PubMed] [Google Scholar]
- 118.Pravettoni A, Colciago A, Negri-Cesi P, Villa S, Celotti F. Ontogenetic development, sexual differentiation, and effects of Aroclor 1254 exposure on expression of the arylhydrocarbon receptor and of the arylhydrocarbon receptor nuclear translocator in the rat hypothalamus. Reprod Toxicol. 2005;20:521–530. doi: 10.1016/j.reprotox.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- 120.Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- 121.Quadagno DM, McCullough J, Kan-Hwa Ho G, Spevak AM. Neonatal gonadal hormones: Effect on maternal and sexual behavior in the female rat. Physiol Behav. 1973;11:251–254. doi: 10.1016/0031-9384(73)90357-0. [DOI] [PubMed] [Google Scholar]
- 122.Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- 123.Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol. 2007;504:42–56. doi: 10.1002/cne.21427. [DOI] [PubMed] [Google Scholar]
- 124.Ren MQ, Kuhn G, Wegner J, Nurnberg G, Chen J, Ender K. Feeding daidzein to late pregnant sows influences the estrogen receptor beta and type I insulin-like growth factor receptor mRNA expression in newborn piglets. J Endocrinol. 2001;170:129–135. doi: 10.1677/joe.0.1700129. [DOI] [PubMed] [Google Scholar]
- 125.Rhees R, Shryne J, Gorski R. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990a;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- 126.Rhees R, Shryne J, Gorski R. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Dev Brain Res. 1990b;52:17–23. doi: 10.1016/0165-3806(90)90217-m. [DOI] [PubMed] [Google Scholar]
- 127.Rhees RW, Al-Saleh HN, Kinghorn EW, Fleming DE, Lephart ED. Relationship between sexual behavior and sexually dimorphic structures in the anterior hypothalamus in control and prenatally stressed male rats. Brain Res Bull. 1999;3:193–199. doi: 10.1016/s0361-9230(99)00191-4. [DOI] [PubMed] [Google Scholar]
- 128.Rody A, Holtrich U, Solbach C, Kourtis K, von Minckwitz G, Engels K, Kissler S, Gätje R, Karn T, Kaufmann M. Methylation of estrogen receptor beta promoter correlates with loss of ER-beta expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer. 2005;12:903–916. doi: 10.1677/erc.1.01088. [DOI] [PubMed] [Google Scholar]
- 129.Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 130.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:2681–2691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 131.DM. Ruden, D.C. Jamison, D.R. Zeeberg, M.D. Garfinkel, J.N. Weinstein, P. Rasoli, X. Lu, this issue
- 132.Ruden DM, Ziao L, Garfinkel MD, Lu X. Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstilbestrol on uterine development and cancer. Hum Mol Genet. 2005;14:R149–R155. doi: 10.1093/hmg/ddi103. [DOI] [PubMed] [Google Scholar]
- 133.Sager DB. Effect of postnatal exposure to polychlorinated biphenyls on adult male reproductive function. Environ Res. 1983;31:76–94. doi: 10.1016/0013-9351(83)90063-4. [DOI] [PubMed] [Google Scholar]
- 134.Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls (PCBs) on female reproductive development and estrogen receptor β expression. Environ Health Perspect. 2003;111:1278–1282. doi: 10.1289/ehp.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters the expression of DNA methyltransferases and methylation of genomic DNA in the epididymis of mice. Endocrine J. 2006;53:331–337. doi: 10.1507/endocrj.k06-009. [DOI] [PubMed] [Google Scholar]
- 136.Scallet AC, Divine RL, Newbold RR, Delclos KB. Increased volume of the calbindin D28k-labeled sexually dimorphic hypothalamus in genistein and nonylphenol-treated male rats. Toxicol Sci. 2004;82:570–576. doi: 10.1093/toxsci/kfh297. [DOI] [PubMed] [Google Scholar]
- 137.Scallet AC, Meredith JM. Quantitative three-dimensional reconstruction: Feasibility for studies of sexually dimorphic hypothalamic development in rats. Neurotoxicol Teratol. 2002;24:81–85. doi: 10.1016/s0892-0362(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 138.Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Seminara SB. Mechanisms of disease: the first kiss – a crucial role for kisspeptin-1 and its receptor, G-protein-coupled receptor 54, in puberty and reproduction. Nat Clin Pract Endocrinol Metab. 2006;2:328–334. doi: 10.1038/ncpendmet0139. [DOI] [PubMed] [Google Scholar]
- 140.Sheehan DM, Willingham E, Gaylor D, Bergeron JM, Crews D. No threshold dose for estradiol-induced sex reversal of turtle embryos: How little is too much? Environ Health Perspec. 1999;107:155–159. doi: 10.1289/ehp.99107155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shibutani M, Masutomi N, Uneyama C, Abe N, Takagi H, Lee KY, Hirose M. Down-regulation of GAT-1 mRNA expression in the microdissected hypothalamic medial preoptic area of rat offspring exposed maternally to ethinylestradiol. Toxicology. 2005;208:35–48. doi: 10.1016/j.tox.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 142.Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- 143.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 144.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 145.Slikker W, Jr, Scallet AC, Doerge DR, Ferguson SA. Gender-based differences in rats after chronic dietary exposure to genistein. Int J Toxicol. 2001;20:175–179. doi: 10.1080/109158101317097764. [DOI] [PubMed] [Google Scholar]
- 146.Slob AK, Ooms MP, Vreeburg JTM. Prenatal and early postnatal sex differences in plasma and gonadal testosterone and plasma luteinizing hormone in female and male rats. J Endocrinol. 1980;87:81–87. doi: 10.1677/joe.0.0870081. [DOI] [PubMed] [Google Scholar]
- 147.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 148.Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Steinberg RM, Walker DM, Gore AC. Prenatal PCB exposure results in altered development and sexual behaviors in female rats; Proceedings of the Endocrine Society Forum on Endocrine-Disrupting Chemicals; San Diego, CA. 2005. (Abstract) [Google Scholar]
- 150.Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in related effects of androgen in prenatal rats. Neurosci Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- 151.Sun LY, D’Ercole AJ. Insulin-like growth factor-I stimulates histone H3 and H4 acetylation in the brain in vivo. Endocrinology. 2006;147:5480–5490. doi: 10.1210/en.2006-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- 153.Swanson HE, van der Werff ten Bosch JJ. The “early androgen” syndrome: differences in response to pre-natal and post-natal administration of various doses of testosterone propionate in female and male rats. Acta Endocrinologica. 1964;47:37–50. [PubMed] [Google Scholar]
- 154.Takagi H, Shibutani M, Lee KY, Masutomi N, Fujita H, Inoue K, Mitsumori K, Hirose M. Impact of maternal dietary exposure to endocrine-acting chemicals on progesterone receptor expression in microdissected hypothalamic medial preoptic areas of rat offspring. Toxicol Appl Pharmacol. 2005;208:127–136. doi: 10.1016/j.taap.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 155.Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, Kaufman R, Adam E, Strohsnitter W, Noller K, Herbst AL, Gibson-Chambers J, Hartge P, Hoover RN. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES) Am J Epidemiol. 2006;35:862–868. doi: 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- 156.Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: A point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 157.Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- 158.Walker DM, Gore AC. Endocrine-disrupting chemicals and the brain. In: Gore AC, editor. Endocrine-disrupting Chemicals: From Basic Research to Clinical Practice. Humana Press; New Jersey: 2007. pp. 63–109. [Google Scholar]
- 159.Waly M, Olteanu H, Banerjee R, Choi SW, Mason JB, Parker BS, Sukumar S, Shim S, Sharma A, Benzecry JM, Power-Chamitsky VA, Deth RC. Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiat. 2004;9:358–370. doi: 10.1038/sj.mp.4001476. [DOI] [PubMed] [Google Scholar]
- 160.Wang XQ, Fang J, Nunez AA, Clemens LG. Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol Behav. 2002;75:689–696. doi: 10.1016/s0031-9384(02)00673-x. [DOI] [PubMed] [Google Scholar]
- 161.Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 162.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weiland NG, Barraclough CA. Neonatal castration of male and female rats affects luteinizing hormone responses to estrogen during adulthood. Biol Reprod. 1984;31:942–949. doi: 10.1095/biolreprod31.5.942. [DOI] [PubMed] [Google Scholar]
- 164.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 165.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Whitten PL, Lewis C, Russell E, Naftolin F. Phytoestrogen influences on the development of behavior and gonadotropin function. Proc Soc Exp Biol Med. 1995;208:82–86. doi: 10.3181/00379727-208-43836. [DOI] [PubMed] [Google Scholar]
- 167.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotrophin secretion in the female rat. Neuroendocrinol. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 168.Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: The interplay between placenta and fetal brain. J Soc Gynecol Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 169.Yamamoto M, Shirai M, Tamura A, Kobayashi T, Kohara S, Murakami M, Arishima K. Effects of maternal exposure to a low dose of diethylstilbestrol on sexual dimorphic nucleus volume and male reproductive system in rat offspring. J Toxicol Sci. 2005;30:7–18. doi: 10.2131/jts.30.7. [DOI] [PubMed] [Google Scholar]
- 170.Yoshida M, Yuri K, Kizaki Z, Sawada T, Kawata M. The distributions of apoptotic cells in the medial preoptic areas of male and female neonatal rats. Neurosci Res. 2000;36:1–7. doi: 10.1016/s0168-0102(99)00100-5. [DOI] [PubMed] [Google Scholar]
- 171.Zhao R, Wang Y, Zhou Y, Ni Y, Lu L, Grossmann R, Chen J. Dietary daidzein influences laying performance of ducks (Anas platyrhynchos) and early post-hatch growth of their hatchlings by modulating gene expression. Comp Biochem Physiol A Mol Integr Physiol. 2004;138:459–66. doi: 10.1016/j.cbpb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 172.Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG. Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci. 2003;23:2357–2362. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]