Abstract
We report a patient harboring a novel homozygous mutation of c.604T>G (p.F202V) in POMT2. He showed delayed psychomotor development but acquired the ability to walk at the age of 3 years and 10 months. His brain MRI was normal. No ocular abnormalities were seen. Biopsied skeletal muscle revealed markedly decreased but still detectable glycosylated forms of alpha-dystroglycan (α-DG). Our results indicate that mutations in POMT2 can cause a wide spectrum of clinical phenotypes as observed in other genes associated with alpha-dystroglycanopathy. Presence of small amounts of partly glycosylated α-DG may have a role in reducing the clinical symptoms of alpha-dystroglycanopathy.
Keywords: POMT2, alpha-dystroglycan, alpha-dystroglycanopathy, congenital muscular dystrophy, limb girdle muscular dystrophy, brain MRI
1. Introduction
Alpha-dystroglycan (α-DG) is a surface membrane protein that links extracellular basal lamina and intracellular cytoskeleton. α-DG is a highly glycosylated protein mainly composed of unique O-mannosyl glycans. Reduced/altered glycosylation of α-DG causes a wide variety of muscular dystrophies including Walker-Warburg syndrome (WWS), muscle-eye-brain disease (MEB), Fukuyama type congenital muscular dystrophy (FCMD), congenital muscular dystrophies type 1C and type 1D, and limb girdle muscular dystrophies (LGMD) type 2I, 2K to 2N. They are collectively called alpha-dystroglycanopathies (α-DGP). So far, 6 causative genes for α-DGP have been identified including protein-O-mannosyl transferase 1 and 2 (POMT1 and POMT2), protein O-mannose beta-1,2-N-acetylglucosaminyltransferase (POMGnT1), fukutin (FKTN), fukutin-related protein (FKRP), and acetylglucosaminyl transferase-like protein (LARGE). Here we report a mild congenital muscular dystrophy patient associated with a novel homozygous mutation in POMT2.
2. Case report
A 4-year-old Japanese boy, the only child from healthy consanguineous parents, was delivered uneventfully at full term. During few days after birth, he was low spirited and showed sucking weakness. Floppiness was not prominent but serum CK levels were markedly elevated up to 33,000 IU/l (normal ≤70). His condition was improved within 2 weeks, but serum CK levels were persistently higher than 1,000 IU/l. His motor milestones were delayed and he could control his head at 5 months of age. At 6-month-old, he could not sit without support, and muscle weakness and atrophy were noticed in lower limbs. Deep tendon reflexes were normal. No high arched palate or macroglossia were seen. Enjoji Scale of Infant Analytical Development (ESID) at his age of 7 months revealed mild delay in body movement (developmental age was 4 months, expression of language: 5 months), and his DQ was 83. Brain computed tomography (CT) revealed no definite abnormalities. Nerve conduction study was normal. His motor functions developed gradually and he was able to walk without support at 3 years and 10 months old. Gowers’ sign was positive. Mild calf hypertrophy was seen with no joint contractures (Fig. 1A). Deep tendon reflexes were normal except for diminished Achilles tendon reflexes. ESID performed at his age of 3 years and 11 months showed general developmental delay (body movement: 15 months, hand movement: 24 months, activity of daily living: 27 months, personal relations: 24 months, expression of language: 18 months, and comprehension of language: 24 months), and his DQ was 47. Brain magnetic resonance imaging at 4 years and one month old revealed no notable anomaly or cortical dysplasia (Fig. 1B). Detailed ophthalmological examinations revealed no abnormalities. No cardiac involvement was detected by chest X-ray, electrocardiogram and echocardiography.
Fig. 1.
(A) The patient can stand and walk with no support. Minimal calf hypertrophy is seen.
(B) T2 weighted brain magnetic resonance imaging shows no obvious brain anomaly, cortical dysplasia, or white matter changes.
(C) Sequence analysis of POMT2 revealed a homozygous mutation at c.604T>G in exon 5.
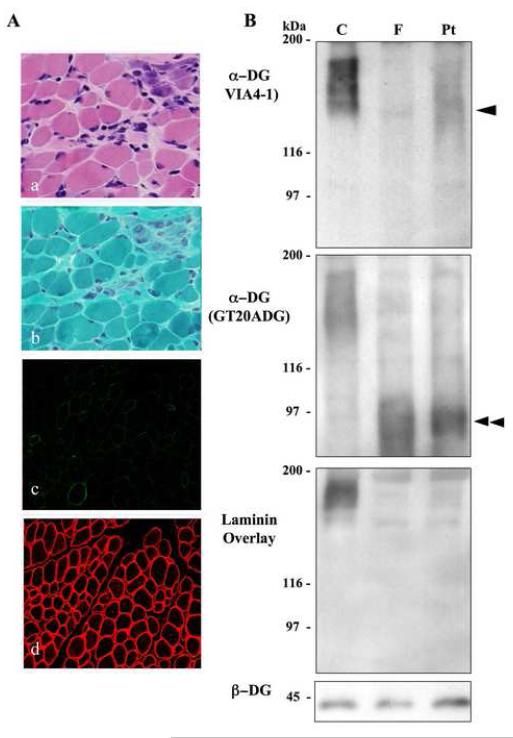
Muscle biopsy taken at 7 months of age with informed consent showed dystrophic changes with scattered necrotic and regenerating fibers and mild endomysial fibrosis (Fig. 2A). No inflammatory changes were seen. On immunohistochemistry, glycosylated forms of α-DG detected by VIA4-1 antibody (Upstate Biotechnology, NY) was markedly reduced in the sarcolemma, while immunoreactions for the core region of α-DG using GT20ADG antibody [1] (data not shown) and for β-DG (43DAG1/8D5; Novocastra Laboratories, UK) was well preserved (Fig. 2A). On immunoblotting analysis, faint, broad band of around 140 kDa in size was detected by VIA4-1, whereas GT20ADG recognized a band of around 90 kDa in size. Laminin overlay assay showed barely detectable binding product (Fig. 2B). These results suggested altered glycosylation of α-DG in the muscle.
Fig. 2.
(A) Histological analysis. On Hematoxylin and eosin (a) and modified Gomori-trichrime (b) staining, variation in fiber size and scattered necrotic and regenerating fibers are seen. Immunohistochemical analysis using antibodies VIA4-1 (c), which recognize heavily glycosylated form of α-dystroglycan (α—DG), showed greatly reduced sarcolemmal staining in patient, but well-preserved immunoreactivities of β-DG (d) is seen. Bar = 50m.
(B) Immunoblotting analysis. Immunoblotting analysis using antibodies of VIA4-1, GT20ADG for α-dystroglycan (α-DG) and laminin overlay assay are performed using skeletal muscle from control (C), Fukuyama-type congenital muscular dystrophy (FCMD; F), and the patient (Pt). VIA4-1 recognizes a broad band about 156 kDa in size in control, and approximately 90kDa in FCMD. In the patient muscle, reduced in size and amount compared with control was observed. GT20ADG revealed bands at approximately 90 kDa in both the patient and FCMD muscles. Laminin overlay assay shows barely detectable band in both the patient and FCMD.
We performed mutation screening in all six causative genes for α-DGP. Genomic DNA was extracted from peripheral lymphocytes using standard technique after informed consent. Primer sequences we used are available on request. All exons and their franking intronic regions were directly sequenced by ABI PRISM 3100 (PE Applied Biosystems, CA). We identified a homozygous missense mutation of c.604T>G (p.F202V) in exon 5 of POMT2 (Fig. 1C), which is not described in previous publications [3-8] and the mutation database (http://www.dmd.nl/).
The protein O-mannosyltransferase (POMT) activity was measured as previously described [2]. Mutant POMT2 (F202V) co-expressed with POMT1 in COS cells showed barely detectable POMT activity (data not shown).
3. Discussion
POMT2 is the gene encoding an enzyme for protein O-mannosylation, and it is required to form a complex with POMT1 for the enzyme activity [2]. Recently, some patients with mutations in POMT2 have been reported [3-8]. Most patients showed floppiness at birth, delayed psychomotor development, congenital muscular dystrophy, and severe mental retardation with or without ocular involvement. Brain anomalies are prominent including hydrocephalus, lissencephaly, agenesis of the corpus callosum, fusion of the hemispheres, and cerebellar hypoplasia [3-5]. In contrast, the patient reported here shows milder clinical features. Although his psychomotor milestones were delayed, he achieved independent ambulation with no marked brain malformation and ocular involvement. His clinical phenotype was intermediate between congenital muscular dystrophy and limb girdle muscular dystrophy. Milder clinical features with mutations in POMT2 have been recently reported and designated as limb girdle muscular dystrophy type 2N [6, 7]. Mutations in POMT2 can cause wide spectrum of clinical phenotypes from Walker-Warburg syndrome to limb girdle muscular dystrophy (LGMD), as demonstrated in patients with FKRP, FKTN, or POMT1 mutations.
Pathological changes of skeletal muscle also showed mild dystrophic changes consistent with clinical findings. Clinical and pathological severity may not be always correlated to the molecular mass of α-DG [9]. However, some clinically milder patients with α-DG detected by the VIA4-1 antibody [10] Preservation of partly glycosylated forms of α-DG could contribute to the milder clinical phenotype of this patient.
Acknowledgement
We thank Dr. S. Shalaby (National Institute of Neuroscience, NCNP) for reviewing the manuscript. K.P. Campbell is an investigator of the Howard Hughes Medical Institute. This study was supported by the Research on Health Sciences focusing on Drug Innovation from the Japanese Health Sciences Foundation, the Research on Psychiatric and Neurological Diseases and Mental Health of Health and Labor Sciences Research Grants, the Research Grant, for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare, the Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, and the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant 1 U54 NS053672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kim DS, Hayashi YK, Matsumoto H, Ogawa M, Noguchi S, Murakami N, et al. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in alpha-DG. Neurology. 2004;62:1009–11. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- [2].Akasaka-Manya K, Manya H, Nakajima A, Kawakita M, Endo T. Physical and functional association of human protein O-mannosyltransferases 1 and 2. J Biol Chem. 2006;281:19339–45. doi: 10.1074/jbc.M601091200. [DOI] [PubMed] [Google Scholar]
- [3].van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L, et al. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42:907–12. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mercuri E, D’Amico A, Tessa A, Berardinelli A, Pane M, Messina S, et al. POMT2 mutation in a patient with ’MEB-like’ phenotype. Neuromuscul Disord. 2006;16:446–8. doi: 10.1016/j.nmd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- [5].Yanagisawa A, Bouchet C, Van den Bergh PY, Cuisset JM, Viollet L, Leturcq F, et al. New POMT2 mutations causing congenital muscular dystrophy: identification of a founder mutation. Neurology. 2007;69:1254–60. doi: 10.1212/01.wnl.0000268489.60809.c4. [DOI] [PubMed] [Google Scholar]
- [6].Biancheri R, Falace A, Tessa A, Pedemonte M, Scapolan S, Cassandrini D, et al. POMT2 gene mutation in limb-girdle muscular dystrophy with inflammatory changes. Biochem Biophys Res Commun. 2007;363:1033–7. doi: 10.1016/j.bbrc.2007.09.066. [DOI] [PubMed] [Google Scholar]
- [7].Godfrey C, Clement E, Mein R, Brockington M, Smith J, Talim B, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130:2725–35. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- [8].Peat RA, Smith JM, Compton AG, Baker NL, Pace RA, Burkin DJ, et al. The diagnosis and etiology of congenital muscular dystrophy. Neurology. 2007 doi: 10.1212/01.wnl.0000284605.27654.5a. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [9].Matsumoto H, Hayashi YK, Kim DS, Ogawa M, Murakami T, Noguchi S, et al. Congenital muscular dystrophy with glycosylation defects of alpha-dystroglycan in Japan. Neuromuscul Disord. 2005;15:342–8. doi: 10.1016/j.nmd.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [10].Murakami T, Hayashi YK, Noguchi S, Ogawa M, Nonaka I, Tanabe Y, et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann Neurol. 2006;60:597–602. doi: 10.1002/ana.20973. [DOI] [PubMed] [Google Scholar]