Abstract
Background
Telemonitoring, the use of communication technology to monitor clinical status, is gaining attention as a strategy to improve the care of patients with heart failure. A system of frequent monitoring could alert clinicians to early heart failure decompensation, providing the opportunity for intervention before patients become severely ill and require hospitalization. Moreover, patients’ participation in a daily monitoring program could have a favorable effect on their health behaviors. The literature on telemonitoring for heart failure, however, is quite limited.
Methods
Telemonitoring to Improve Heart Failure Outcomes (Tele-HF), is a randomized, controlled, trial designed to compare an automated, daily symptom and self-reported weight monitoring intervention with usual care in reducing (all-cause) hospital readmissions and mortality among patients recently hospitalized with decompensated heart failure. The intervention will be implemented and all outcomes will be assessed over a six-month period. The purpose of the intervention is to collect information about symptoms, clinical status and weight and to engage participants in their own self-care. Participants are recruited from general cardiology, heart failure specialty, and primary care practices across the United States.
Conclusions
The results of this study may inform future policy decisions regarding implementation of telemonitoring in treatment of heart failure.
Keywords: heart failure, disease management, self-care
Introduction
Telemonitoring, the use of communication technology to monitor clinical status, is gaining attention as a novel disease management strategy to improve the care of patients with chronic illness 1. By collecting clinical data without the need for face-to-face contact, telemonitoring can efficiently enhance communication between patients and medical personnel and has the potential to improve outcomes through early intervention. Telemonitoring holds particular promise for patients with heart failure, who may experience deterioration in their health status with an increase in weight and symptoms over a period of days and weeks before presenting to medical attention and requiring hospitalization2. A system of frequent monitoring could alert clinicians to the early signs and symptoms of decompensation, providing the opportunity for intervention before patients become severely ill and require hospitalization. Moreover, patients’ participation in communicating information about their weight and health status on a daily basis could have a favorable effect on their health behaviors, including adherence to medical recommendations.
In the last five years, several studies based on remote monitoring of HF patients have been conducted. The literature on telemonitoring for heart failure, however, is limited in several important respects. First, many studies were small and conducted at a single center with selected study populations 3, 4. Second, there are few studies of automated telemonitoring. Most studies have been based on monitoring by nurse via live one-on-one telephone calls with patients using management strategies that were complex and difficult to replicate in many real world practice settings 5, 6. Finally, many studies have included biometric monitoring 7, 8 (i.e., blood pressure, electrocardiography). The effect of symptom and self-reported weight monitoring, obviating the need for devices in patients’ homes, has received less examination.
We have therefore designed a large scale, randomized controlled trial, funded by the National Heart, Blood and Lung Institute (Clinical Trials registration number NCT00303212), to test the effect of automated telemonitoring, consisting of symptom and self-reported weight monitoring, on reducing re-hospitalizations and mortality among patients recently hospitalized for heart failure. The purpose of this paper is to describe the design and intervention of this clinical trial.
Methods
Objectives
The primary objective of this study is to determine the effect of automated symptom and self-reported weight monitoring compared with usual care on the combined endpoint of all cause hospitalization and mortality in patients recently hospitalized for heart failure. The intervention will be implemented and all outcomes will be assessed over a six-month period. The secondary objectives are to determine the impact of the intervention on the following endpoints: rate of hospital readmission for HF; number of office visits with the clinician receiving information from the telemonitoring system; time to readmission or death; cost of inpatient and outpatient medical care; health status; patients’ satisfaction with care; and patients’ reported confidence in their self-management of HF.
Design
The Telemonitoring to Reduce Hospitalizations for Heart Failure Patients (Tele-HF) is a randomized controlled trial to compare automated symptom and self-reported weight monitoring with usual care. Outcomes are evaluated in a blinded manner by a central endpoint committee. In accordance with the American Heart Association’s taxonomy for disease management 9, the key features of this study are outlined in Table 1 and discussed in greater detail below. The institutional committee on human research at Yale University has approved this study protocol.
Table 1.
Taxonomy of Tele-HF study
| Study population | Recently hospitalized (within 30 days of enrollment) for HF |
| Intervention recipient | Patients and healthcare providers |
| Intervention content | Symptom and self-reported weight monitoring |
| Delivery personnel | Automated monitoring with review by nurses and physicians |
| Method of communication | Telephone |
| Intensity | Daily monitoring for six months |
| Environment | Patient’s homes |
| Clinical outcomes | Primary outcome: all-cause rehospitalization and mortality Seconary outcomes: rate of hospital readmission for HF; number of office visits; time to readmission or death; cost of inpatient and outpatient medical care; health status; patients’ satisfaction with care; and patients’ self-management of HF |
Site Network
Study participants are recruited from cardiology and internal medicine practices across the United States. Practices were selected to enhance generalizability of the study’s results, with particular attention to geographic, racial, and socio-economic diversity in patients. Additionally, a mix of practice types, including primary care, general cardiology, and heart failure specialty, have been included to represent the spectrum of practice settings in which heart failure patients currently receive care.
These practice sites are responsible for recruiting participants, reviewing and managing information from the telemonitoring system. A nurse from each site is designated “site coordinator” and has primary responsibility for overseeing execution of the study protocol at that site.
Study Population
The study participants are patients discharged from a heart failure hospitalization within 30 days of enrollment into the study. For the identification of heart failure hospitalizations a primary admitting diagnosis of heart failure, supported by physician notes, chest x-ray findings and medication use, is required.
Exclusion criteria are: age less than 18 years; long-term nursing home residence, as these patients are less likely to be involved in their own care; residence in a correctional facility; inability to speak either English or Spanish; irreversible medical conditions likely to affect 6 month survival or ability to participate in the study protocol; Folstein Mini-Mental State Exam10 score less than 20; no access to a telephone line; currently scheduled for cardiac transplant, left ventricular assist device or valvular surgery; and planned coronary artery bypass grafting or percutaneous coronary intervention within 90 days of randomization.
Randomization
Randomization is centralized and performed by telephone. Randomization is stratified by study site, and force randomized within each study site in blocks of 20 (10 intervention, 10 control), to ensure a balance across study arms within each site. The randomization sequence is developed by the coordinating center using a computer random-number generator. The sequence is unknown to the attending cardiologists and nurses. The study nurses call the coordinating center when they enroll a new patient; the coordinating center personnel then assigns the new patient to intervention or control group according to the randomization list for that study site (Figure).
Figure.
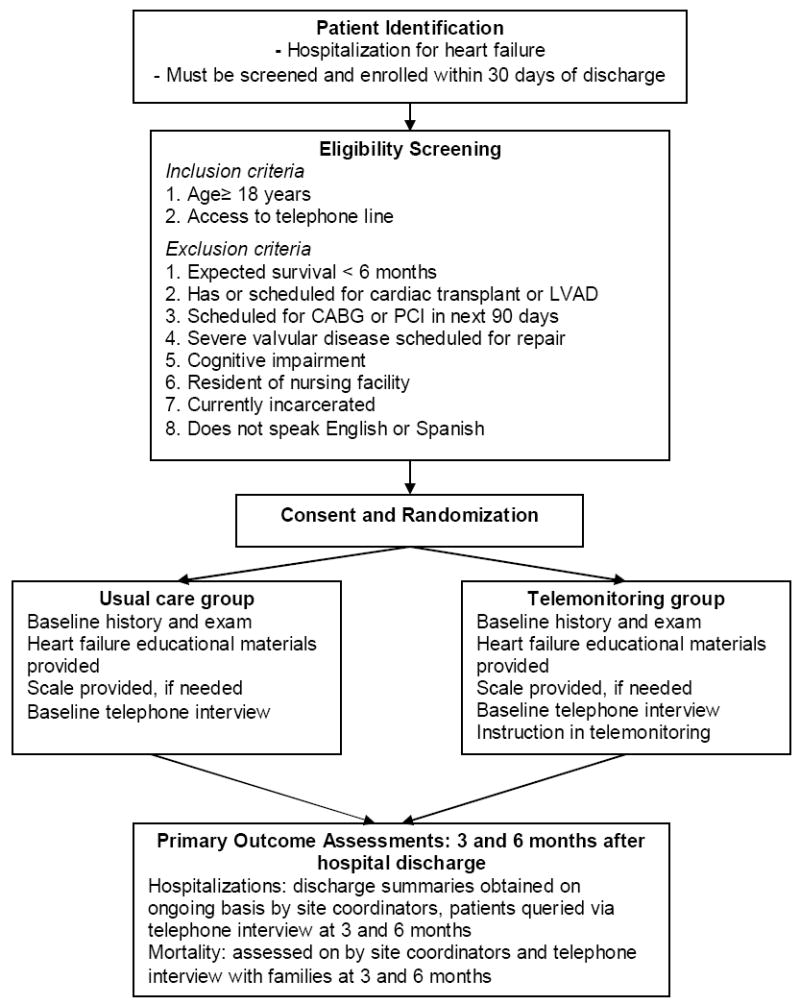
Flow diagram.
Intervention
Participants in the intervention group are instructed to make a daily toll-free call to an automated telemonitoring system being provided by Pharos Innovations® (Chicago, IL) for 6 months. Study participants are instructed on use of the system during study enrollment and are given a telephone number for technical support. On each call participants hear a pre-recorded voice that conveys a series of questions about symptoms and daily weight (Table 2). If participants miss a daily call, they receive an automated reminder call from the telemonitoring service to encourage adherence. The purpose of the intervention is to collect daily information about symptoms, clinical status and weight and to engage participants in their own self-care through daily reporting of these items. No formal patient education is provided as part of the intervention as the goal is to examine the effect of intensive symptom and weight monitoring alone. Participants in the study are instructed that the telemonitoring system is not to be used for urgent/emergent matters and information obtained may not be reviewed for several days (e.g., a holiday week-end).
Table 2.
Content of Telemonitoring Program
| Domain | Question | Frequency | Variance Trigger |
|---|---|---|---|
| HF Status | Have you felt more short of breath in the last day? | Daily | “Yes” |
| HF Status | Have you noticed more swelling in the last day? | Daily | “Yes” |
| HF Status | Did you wake up short of breath last night? | Daily | “Yes” |
| HF Status | Did you sleep in a chair, or prop up with pillows more than usual last night? | Daily | “Yes” |
| HF Status | Have you had any lightheadedness or dizziness in the last day? | Daily | “Yes” |
| Weight | Enter morning weight | Daily | More than 3 lbs above or below first weight entered |
| General health | Today, would you say that your health is Excellent, Very Good, Good, Fair or Poor? | Daily | 2 category worsening |
| General health | Compared to yesterday, would you say that you are feeling about the same, better, worse, or much worse? | Daily | “Much worse” |
| Depressive symptoms (PHQ-214) | Over the last 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless? Would you say “Not at all,” “Several days,” “More than half the days,” or “Nearly everyday? | Monthly | “Yes” |
| Depressive symptoms (PHQ-2) | Over the last 2 weeks, how often have you been bothered by feeling little interest or pleasure in doing things? Would you say “Not at all,” “Several days,” “More than half the days,” or “Nearly everyday”? | Monthly | “Yes” |
Abbreviations used in this table: HF, heart failure
Information from the telemonitoring system is automatically downloaded to a secure Internet site for review by clinicians at each practice site. The data is displayed in such a way that participants’ with “variances” (see Table 2 for triggers of variances) are displayed at the top of the screen, flagging the clinician’s attention. Clinicians (typically nurses) are instructed to review information on a daily basis, except holidays and week-ends. If a variance is noted, the protocol is for clinicians to call participants to confirm that the reported information is correct and obtain more information if needed. Clinicians are able to modify the acceptable range of responses to each question, thereby modifying what would trigger a variance. This approach helps avoid repeated variances on a patient who, for example, persistently reports dizziness. Clinicians are instructed to respond to telemonitoring data using clinical judgment as they would if the information was obtained during routine clinical care. They may offer advice to the patient (e.g. modify diet, increase diuretic dose or adhere to medications); consult with the physicians in their practice site; advise an urgent clinic or emergency department visit; or refer the patient to another specialist, as appropriate. Clinicians are instructed to record details (directly into the Internet site) of any interventions they implement as a result of information from the telemonitoring. Practice sites do not receive protocols directing clinical management of participants as part of the study intervention.
Realizing that there are a variety of mechanisms by which telemonitoring may have an effect (as outlined in Table 3), information is being collected on all participants (usual care and intervention groups) at baseline and follow-up time points to understand which of these factors are most relevant. Using contemporary, well-validated measures, this information will allow investigation into the mechanism by which the intervention is effective. Patient-related factors which may be relevant to the intervention’s effect include improved confidence in heart failure self-care, greater adherence to medications and diet, and decreased social isolation. Clinician-related factors include intensification of medications (i.e., increasing doses of existing medications or addition of new medications), diagnosis and treatment of comorbid conditions, and patient education. Because of greater contact with participants receiving telemonitoring, clinicians may also refer these participants for cardiac device placement more frequently.
Table 3.
Possible mediators of telemonitoring effect on outcomes
| Mediator | Instrument |
|---|---|
| Patient-related | |
| Improved confidence in self-care | SCHFI15 |
| Improved adherence to diet, medications | Morisky16 |
| Decreased social isolation | ESSI17 |
| Clinician-related | |
| Intensification of medications | N/A |
| Diagnosis and treatment of comorbid conditions | N/A |
| Patient education | N/A |
| Placement of cardiac devices | N/A |
| Systems-related | |
| Improved systems of care delivery | N/A |
Usual Care: guideline based care
Participants assigned to usual care are treated by the attending physician in the usual manner and in accordance with the American College of Cardiology/American Heart Association Guidelines for the management of heart failure 11. Clinciains are encouraged to discuss these Guidelines with the participants before enrollment.
Data Collection Schedule
At the time each patient is enrolled into the study (usual care and intervention groups), the site coordinator at each local site performs a baseline history and physical examination, provides basic heart failure educational material (from the American Heart Association) and scales (if participants do not already own one) to measure body weight. In addition, all participants undergo a detailed baseline interview via telephone by staff from the central coordinating site.
Outcomes are assessed at 3 and 6 months after enrollment into the study. Participants are interviewed by telephone at these time points to assess health and psychosocial status, self-care skills, satisfaction and healthcare utilization. Additionally, site coordinators monitor for hospitalizations and obtain discharge summaries on an ongoing basis. We will evaluate the cost-effectiveness of the telemonitoring intervention, incorporating costs associated with hospitalizations, outpatient visits, emergency department visits and home care services.
Quality Assurance
In order to ensure adherence with the protocol, a Project Manager (from the central coordinating site) visits each site before any participants are enrolled. During this site visit, the protocol is reviewed and the local site coordinator demonstrates proficiency in executing the protocol, including identifying potential study participants and using the telemonitoring web site. All data collected by the site coordinators is reviewed for completeness by the Project Manager before participants are discharged from the protocol. All information obtained in follow-up interviews (at 3 and 6 months) is entered into an electronic database, with automated checks for data accuracy and completeness.
Compensation
Although study participants are not compensated for participation, sites receive $300 for each patient enrolled in usual care and $900 for each patient enrolled in telemonitoring. The higher reimbursement for participants assigned to the intervention reflects the greater effort required by sites, specifically to monitor daily responses and to follow up on variances.
Statistical Analysis
All randomized participants will be included in analyses with an intention to treat principle. All participants will be followed up until the end of the study, with long-term follow-up (up to 2 years) being planned. An independent Events Review Committee will assess and classify the primary and secondary end point events in a centralized and blinded manner.
Statistical Hypothesis of the sample
The primary end point is all-cause mortality or hospitalizations. To estimate the sample size, we considered an alpha error of .05 for the final test and a power of 90%. The annual incidence of the primary end point was estimated in 30% for the control group and 22.5% for the intervention group (i.e., a 25% relative risk reduction). Thus 1,640 participants were needed (820 in each group), with a follow-up period of 6 months. For sample size calculations, we also anticipated 10% of patient would dropout during the 6-month follow-up period.
Primary endpoint analysis
We will summarize the characteristics of participants by study arm and test for differences in age, HF severity, and other patient attributes between the study groups to assure the success of the randomization. In the event that there are significant differences, these will be accounted for through statistical adjustment in the outcome analyses.
We will test the primary hypothesis by comparing a dichotomous variable, indicating at least 1 hospital readmission for any cause or death during the 6 months or death following enrollment, across the 2 study arms. Whether the proportion of participants with this variable differs will be tested using Chi- Square tests, stratified on practice site.
In addition, to account for any imbalance in baseline characteristics, and to improve precision of estimates, we will test the primary hypothesis using a logistic regression model. This model will use the primary outcome as the dependent variable and include any baseline characteristics (including presence of comorbid conditions and duration of heart failure) that differ across study groups, as well as intervention group, as independent variables. Although we considered a time-to-event analysis, the logistic model was ultimately chosen due to concern that the intervention may (by giving participants more frequent contact with caregivers) actually cause a delay in hospital readmission.
A Data Safety Monitoring Board (DSMB) convenes bi-annually (with additional meetings as needed) to review enrollment, adverse events and effectiveness of the intervention. The DSMB has the capacity to terminate the trial in the event of poor enrollment, safety concerns, or overwhelming evidence of benefit. Members include two biostatisticians, and two general internists. A Scientific Advisory Board (SAB), comprised of prominent scientists with strong interest and expertise in this field, functions as an independent group that is charged with critically reviewing all aspects of the trial and advising the PI on new scientific developments that may affect the design or conduct of the trial. Removed from the day-to-day operation of the trial, the SAB is charged with providing a frank appraisal of the overall success of the Operations Committee in achieving its operational objectives related to recruitment, data quality, and safety.
Discussion
The Tele-HF study has been designed to rigorously test the effect of an intervention consisting of simple, automated, telephone-based symptom and weight monitoring. Although we have chosen to use a commercially available telemonitoring system (the Pharos Tel-Assurance system), this study is designed to test a concept rather than a specific product. In accordance with that objective, the full content of the telemonitoring intervention, including responses that trigger variances, has been made explicit.
The Weight Monitoring in Heart Failure (WHARF) trial by Golderg et al is the only major, published study to date of a technology-based strategy to provide daily monitoring of symptoms and body weight in patients with heart failure. This study included only 288 patients recently hospitalized for heart failure and followed in 16 specialized heart failure centers. No differences in the primary outcome of rehospitalization rates was observed, but there was a 56% reduction in mortality (P<0.003) in the telemonitoring group. Aside from being underpowered, there are other important issues to consider in interpreting the results of the WHARF trial. The information from telemonitoring appliances was reviewed by nurses employed by the telemonitoring company, with reports sent to patients’ own physicians only in the event of specific levels of change in weight or symptoms. This lack of involvement in day-to-day monitoring of the patients by clinical practices is a notable difference from Tele-HF, which is firmly embedded in the offices of the managing physicians by design. Overall, the study provided useful information but did not resolve the issue of the effectiveness of telemonitoring.
The Tele-HF study is occurring at a time of great interest in management strategies to improve health outcomes and reduce cost. Of note, the Centers for Medicare and Medicaid Services (CMS) is implementing widespread demonstration projects that mandate the use of remote monitoring technologies to enable the exchange of pertinent clinical information, such as vital signs and symptoms 12. Up to 300,000 participants with heart failure, complex diabetes mellitus, and chronic obstructive pulmonary disease will be enrolled, and the interventions may eventually be implemented nationally. While the demonstration projects will certainly provide valuable information about a range of monitoring systems in participants with several chronic diseases, the Tele-HF study supplements these projects in several important ways. The CMS projects are randomizing participants based on HF billing codes (not confirmed diagnoses) and will not have baseline data on all participants. They are not designed as rigorous research projects to test the effect of a telemonitoring strategy and lack many of the design features that are included in this proposal (e.g., clinically relevant inclusion/exclusion criteria and measurement of baseline clinical and health status in all participants).
Many disease management programs to date remain a “black box” with little clarify about the active ingredients. This trial will be uniquely positioned to identify which factors (e.g., improved patient self-management or adherence, intensification of therapy by clinicians, etc) are most relevant in the effect of telemonitoring. This information is relevant for the design of future disease management efforts, including remote monitoring programs.
In designing this trial, we faced several central challenges. First, due to potential variation in practice patterns across sites, we decided to stratify randomization by site. While this stratification raises the possibility of contamination in the usual care group, this would bias our results to the null. Second, as our goal was to test the real-world effectiveness of telemonitoring, we chose not to dictate management of information obtained from the monitoring system. Rather, we encourage clinicians to react to information from the telemonitoring system as they would in the course of usual clinical care. Finally, we decided to have clinicians from participants’ usual source of cardiac care review and manage information from the telemonitoring system. This approach is in contrast with the systems being widely used by several major, private telemonitoring companies, in which the information is reviewed and managed by anonymous care providers at the company, with “alerts” sent to participants’ physicians when deemed appropriate by the company’s guidelines. Involving the clinical providers in reviewing and managing the data can result in more integrated delivery of care, while strengthening the relationship between the patient and clinician, rather than working around it.
Although the evidence suggests that comprehensive discharge planning with postdischarge support for patients hospitalized with HF improves health outcomes 13, there remains uncertainty as to the ideal approach. Considerable heterogeneity exists in the content, intensity and delivery of disease management programs being implemented in practice settings in efforts to improve care for patients with chronic disease. This randomized clinical trial tests an approach that isolates telephone-based symptom and weight monitoring for heart failure patients. The results of this mutli-center, randomized clinical trial can provide useful information in future policy decisions about the implementation of telemonitoring for patients with HF. Since this intervention is automated, it can be delivered in a consistent manner in a variety of practice settings, with standardized content and frequency. If the intervention in this study proves effective, it could be implemented in a variety of practice settings, without a need for specialized devices in patients’ homes. Furthermore, information about the cost-effectiveness of disease management programs is currently quite scarce. In the course of this study, data (including inpatient, outpatient and home services charges) are being collected to allow for cost-effectiveness analyses, which can then inform reimbursement if widespread implementation of this intervention is warranted.
Acknowledgments
The authors gratefully acknowledge members of the Scientific Advisory Board, including Drs. Barbara Riegel, Stan Kaufman, Marvin Konstam, Simon Stewart, and Michael W. Rich, for their expert guidance in the design of this trial.
We would also like to thank members of the DSMB, including Drs. Jeph Herrin, Darren DeWalt, and James Dziura, for their careful attention to the conduct of this trial, ensure that the highest ethical and scientific standards are adhered to in the conduct of this trial.
Finally, we would like to thank Dr. Lawton Cooper, whose scientific and programmatic oversight have been invaluable.
This work is supported by the National Heart, Lung, and Blood Institute, R01 HL080228
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaudhry SI, Phillips CO, Stewart SS, Riegel B, Jerant AF, Krumholz HM. Telemonitoring in Patients with Heart Failure: A Systematic Review. Journal of Cardiac Failure. 2006 doi: 10.1016/j.cardfail.2006.09.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of Weight Change Preceding Hospitalization for Heart Failure. Submitted. 2006 doi: 10.1161/CIRCULATIONAHA.107.690768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunagan WC, Littenberg B, Ewald GA, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005 Jun;11(5):358–365. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Cordisco ME, Benjaminovitz A, Hammond K, Mancini D. Use of telemonitoring to decrease the rate of hospitalization in patients with severe congestive heart failure. Am J Cardiol. 1999 Oct 1;84(7):860–862. A868. doi: 10.1016/s0002-9149(99)00452-x. [DOI] [PubMed] [Google Scholar]
- 5.Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Archives of Internal Medicine. 2002;162(6):705–712. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 6.DeBusk RF, Miller NH, Parker KM, et al. Care management for low-risk patients with heart failure: a randomized, controlled trial. Ann Intern Med. 2004 Oct 19;141(8):606–613. doi: 10.7326/0003-4819-141-8-200410190-00008. [DOI] [PubMed] [Google Scholar]
- 7.Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Archives of Internal Medicine. 2003;163(3):347–352. doi: 10.1001/archinte.163.3.347. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005 May 17;45(10):1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Krumholz HM, Currie PM, Riegel B, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006 Sep 26;114(13):1432–1445. doi: 10.1161/CIRCULATIONAHA.106.177322. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 12.Super N. Medicare’s Chronic Care Improvement Pilot Program: What Is Its Potential? National Health Policy Forum. 2004;(797) [PubMed] [Google Scholar]
- 13.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004 Mar 17;291(11):1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003 Nov;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 15.Riegel B, Carlson B, Glaser D. Development and testing of a clinical tool measuring self-management of heart failure. Heart & Lung: Journal of Acute & Critical Care. 2000;29(1):4–15. doi: 10.1016/s0147-9563(00)90033-5. [DOI] [PubMed] [Google Scholar]
- 16.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986 Jan;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Seeman TE, Berkman LF. Structural characteristics of social networks and their relationship with social support in the elderly: who provides support. Social Science & Medicine. 1988;26(7):737–749. doi: 10.1016/0277-9536(88)90065-2. [DOI] [PubMed] [Google Scholar]


