Abstract
We have previously shown that immunization with SIV-, SHIV-, or HA (influenza hemagglutinin)-virus-like particles (VLPs) elicits a strong humoral immune response in mice. However, little is known about the action VLPs exert on immune effector cells, including B cells. In this study, we found that all three types of VLPs could directly bind and activate B cells in vitro. VLPs stimulated the proliferation of B220+IgM+CD43−CD5− B2 cells and their differentiation to plasma cells that preferentially produce IgG2a antibodies. Up-regulation of Blimp-1, XBP-1, IRF4, and AID genes, which are responsible for class switch recombination and somatic hypermutation, was observed in VLPs-activated B2 cells. Stimulation of naïve splenocytes with VLPs led to high expression of IL-12, RANTES and MIP, the cytokine milieu that favors B cell differentiation into IgG2a secreting cells. VLP immunization of C57BL/6 mice corroborated our in vitro data showing enlarged germinal centers and expanded conventional B2 cells, but no enlarged marginal zone B1 cells, in the spleen. Enhanced antigen-specific plasma cell formation, antibody production, and IgG2a class switching were found in VLPs-immunized groups. The current study details the VLPs and B cell interactions which result in preferential IgG2a antibody production following VLP vaccination.
Keywords: Virus-like particle vaccine, B2 cells, B cell differentiation, IgG2a
1. Introduction
Virus-like particles (VLPs) represent a highly attractive vaccine approach due to its unique structural, immunogenic, and safety properties (Doan et al., 2005). We have previously shown that SIV Gag based VLPs including SIV VLPs (SIV Gag only), SHIV VLPs (SIV Gag plus HIV Env), and chimeric HA/SIV VLPs (SIV Gag plus influenza hemagglutinin) have the ability to elicit strong humoral and cellular immune responses in mouse models (Guo et al., 2003; Yao et al., 2002; Yao et al., 2004). The binding and activation of dendritic cells (DC) by different VLPs have been reported in many studies (Bosio et al., 2004; Lenz et al., 2001; Tsunetsugu-Yokota et al., 2003; Zhang et al., 2004). However, the effect of VLPs on other immune cells such as B cells is largely understudied. Elucidation of the details of B cell activation, B cell subset involvement, differentiation, class switch recombination (CSR), somatic hypermutation (SHM), gene regulation, cytokine production profiles and Ag-specific antibody production properties by VLP stimulation would certainly furnish important information for the development of a new generation of VLPs-based anti-viral or anti-tumor vaccines.
B cells are a master lymphocyte population that generates a humoral immune response. Based on surface markers and physiological functions, B cells can be divided into two obviously different subsets: B1 cells and B2 cells (Hardy and Hayakawa, 2001). B1 cells are typically identified by the expression of CD5 and CD43 and are also characterized by high expression of IgM. The B1 subset responds poorly to protein antigens and undergoes less CSR and SHM, resulting in the production of lower affinity antibodies with broad epitope recognition (Berland and Wortis, 2002; Fagarasan and Honjo, 2000). In contrast, the B2 subset is involved in adaptive humoral immunity with functions such as germinal center formation, memory B cell production, and long-lived plasma cell generation at later stages (Goldsby et al., 2000). During B2 cell activation in an adaptive humoral immune response, thymus dependent (TD) antigen is recognized through the B cell receptor (BCR), and subsequently processed and expressed in the context of MHC class II (MHC II). B2 cells become activated and up-regulate the expression of surface markers such as CD69, CD40, CD86, and MHC II for activating CD4+ T helper cells, which in turn provide positive signals for further B2 cell activation. Therefore, necessary secondary proliferation and differentiation signals can be provided by the encounter with T helper cells (Mitchison, 2004). The formation of T-B cell conjugates leads not only to the directional release of cytokines essential for B cell differentiation, but also to the up-regulation of CD40L on the surface of the T helper cell which then interacts with CD40 on the surface of B cells providing an essential signal for B2 cell function. Th1 cells promote B2 cell differentiation into plasma cells that produce predominant IgG2a antibody, which has been shown to be of great importance for the development of vaccines against viral infections, whereas Th2 cells induce the production of IgG1 antibodies (Nimmerjahn and Ravetch, 2006).
After encountering TD antigen and receiving T cell’s help, it is mostly B2 cells that form or enter the germinal center microenvironment and undergo SHM, CSR, and competitive affinity selection to subsequently differentiate into either plasma or memory B cells (Shapiro-Shelef and Calame, 2005). SHM and CSR play essential roles in the development of high affinity antibodies, which mainly take place in germinal centers and are critical for vaccine efficiency (Blank et al., 1972; Hangartner et al., 2006). Activation-induced cytidine deaminase (AID) has been recognized as an essential enzyme for the deamination of cytosine to uracil during SHM (Larson and Maizels, 2004). This enzyme is also related to CSR through different functional domains (Shinkura et al., 2004). During plasma cell development, a series of transcription activators and repressors become activated to drive the phenotypic changes required by the cell. The transcriptional repressor, B-lymphocyte-induced maturation protein-1 (Blimp-1) initiates a cascade of gene regulation which includes the suppression of genes required for the identity of mature and germinal center B cells and allows the expression of a subset of genes involved in the plasma differentiation pathway and immunoglobulin secretory pathway (Calame, 2001). X-box binding protein-1 (XBP-1) is absolutely required in plasmacytic differentiation and acts downstream of Blimp-1 (Calame, 2001). Another important transcriptional activator is the interferon regulatory factor 4 (IRF4), which appears to play an important role during the developmental stage when a B2 cell commits to plasma cell differentiation (Calame et al., 2003).
It is well-known that B cells can be efficiently activated by antigens with a highly ordered, repetitive structure, which can very effectively cross-link B cell surface antibodies (BCR), resulting in a strong activation response (Bachmann and Zinkernagel, 1997). An example of such a repetitive structure is the membrane protein or virion capsid of most viruses. VLPs resemble native virions and thus conserve the repetitive antigenic array making them ideal carriers for foreign antigens which can be used for vaccination (Lechner et al., 2002). However, there is little information on the role of VLPs in different B cell subsets and the mechanisms of B cell activation and differentiation upon VLP treatment. In this study, we analyzed the ability of VLPs to bind and activate naïve B cells and determined which B cell subset is activated by VLP treatment in vitro. We also studied the proliferation, plasma cell formation, antibody production, and class switching in VLPs-stimulated B cells. The expression of SHM and CSR related transcription factors in VLPs-treated B cells was also analyzed. Using in vitro cultures, we measured the cytokines released from VLPs-stimulated splenocytes that may affect the B cell response. In addition, we showed that VLP vaccination induced an adaptive humoral immune response including the formation of germinal centers, the generation of plasma cells, antibody production and antibody class switching in vivo.
2. Materials and methods
2.1. Production of SIV-, SHIV- and chimeric HA/SIV VLPs
VLPs were generated using recombinant baculoviruses and purified as reported previously (Guo et al., 2003; Yao et al., 2000). Briefly, for the production of SIV VLPs, Sf9 cells grown in fetal calf serum free media were infected with recombinant baculovirus (rBV) expressing SIVmac239 gag at an m.o.i. of 2; for the production of SHIV VLPs, in addition to using an SIV gag rBV at an m.o.i. of 2, cells were co-infected with a rBV expressing HIV envt (cytoplasmic domain truncation mutant of Env) at an m.o.i. of 10; for the production of chimeric HA/SIV VLPs, the cells were co-infected with a rBV HA at an m.o.i. of 5. After 3 days of infection, the cells were collected and centrifuged. The supernatant was filtered using a 0.45 μM filter system and VLPs were then pelleted at 300,000g for 1 h at 4°C. VLPs were then resuspended in phosphate-buffered saline (PBS) and purified through a 20–60% continuous sucrose gradient at 100,000 × g for 16 h at 4°C. The band corresponding to the virus-like particles was collected and dialyzed against PBS using a 10,000 MW cutoff membrane cassette (Pierce Biotechnology, Rockford, IL). The VLPs were then pelleted at 300,000 × g for 1 h at 4°C and resuspended overnight in PBS. To determine Env and HA protein incorporation in our VLPs, Western blot analysis was performed using primary antibodies against HIV Env and against influenza A/PR8 virus. A Bio-Rad protein assay system was used to determine the total protein concentration of VLPs and the endotoxin level was quantitated using Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, MA) and was controlled at less than 0.0041 μg/ml.
2.2. VLP Binding assay
To label the VLPs, 100 μg of VLPs in PBS were mixed with 1.5 μg of octadecyle rhodamine B (R18), a lipid soluble red fluorescent probe. The mixture of VLPs and R18 dye was incubated in the dark for 1 h at RT with constant stirring. As a negative control, we incubated R18 with bovine serum albumin (BSA). There was no incorporation of the dye to BSA. The mixture was then washed twice with 30 ml of PBS followed by ultracentrifugation for 2 h at 120,000 × g.
To perform the binding assay, murine splenocytes were isolated from whole spleen and a total of 106 cells were incubated with 20 μg of R18 labeled SIV, SHIV, or HA/SIV VLPs in 100 μl of PBS supplemented with 10% calf serum (CS) and 0.08% NaN3 for at least 4 h at 4°C. For dose-dependent VLP binding assay, a dose range from 0.1 – 20 μg VLPs was added. For the unlabeled VLP competitive binding assay, 20 μg of total VLPs were used but with different ratios of labeled and unlabeled VLPs. Any unbound VLPs were removed by washing twice with PBS and splenocytes were then incubated with FITC conjugated anti-CD19 or anti-CD3e antibodies to stain B cells and T cells, respectively, for 30 min on ice. After thorough washing, the cells were collected and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA).
2.3. Flow Cytometry
Single cells were resuspended in PBS with 3% CS and 0.08% NaN3. A total of 1 × 106 cells were added to each well of a V-bottom micro-titer plate. Fluorescent dye conjugated primary antibodies were diluted in 50 μl of PBS with 3% CS and 0.08% NaN3 and dispensed to each well after Fc blockage. To assess the activation of B cells, we used anti-B220, anti-CD69, and anti-CD86 antibodies. To determine which B cell subset was stimulated by VLPs, we stained for anti-B220, anti-CD5, anti-CD43, and anti-IgM antibodies. The cells were then incubated in the dark for 30 min on an ice bath. Following this incubation period, 200 μl of PBS with 0.08% NaN3 were added to each well and washed three times. The plates were then centrifuged at 350 × g for 3 min followed by decanting. The cell pellet was resuspended in 300 μl of PBS with 0.08% NaN3 and run on a FACSCalibur (Becton Dickinson, San Jose, CA).
2.4. Naïve B2 cell isolation and purification
Naïve CD43−CD5− B2 cells were isolated by using a modified untouched B2 cell isolation kit (Miltenyi Biotec Inc., Auburn, CA, USA). This system consists of an indirect magnetic labeling system that isolates untouched resting B cells from the total splenocytes population. Briefly, single-cell suspensions of splenocytes were labeled with biotin conjugated anti-CD43, anti-CD5, and anti-CD4 antibodies for 30 minutes at 4°C. The cells were then washed twice to remove any unbound primary antibody and after centrifugation the cell pellet was resuspended in labeling buffer (90 μl for every 107 cells). Streptavidin MicroBeads (10 μl) were added to the mix and incubated for 30 min at 4°C. Cells were then washed and centrifuged at 350 × g for 4 min. The cells were resuspended in separation buffer and applied to the pre-conditioned LD column which was placed on a magnetic holder of a suitable MACS Separator. The wash-through, which corresponds to unlabelled naïve B cells, was collected. The purity of CD43−CD5− B2 cells is above 92%.
2.5. Proliferation assay
A total of 2 × 105 splenocytes or 105 purified B cells/T cells from naïve C57BL/6 mice in RPMI 1640 media supplemented with 10% FBS were incubated with SIV-, SHIV-, or HA/SIV VLPs at a final concentration of 50 μg/ml for 48 h at 37°C. As a positive control, we incubated the splenocytes with anti-CD40 Ab (10 μg/ml) and LPS (5 μg/ml). Incubation with PBS served as a negative control. To determine whether the stimulation of splenocytes was due to VLPs and not caused by residual endotoxin in our preparations, we incubated the cells as stated above in the presence or absence of polymyxin B for 3 days. The cell proliferation assay was done similarly as described before (Li et al., 2008). Briefly, 1 μCi of 3[H]thymidine (Perkin-Elmer, Boston, MA) was added 16 h before harvesting. The cells were harvested onto glass-fiber filters using a Filtermate Harvester (Packard, Merion, CT). 3[H]thymidine uptake was measured by using a TOPCOUNT (Packard) liquid scintillation counter. The mean and standard deviation were calculated from triplicate wells. The stimulation indexes were determined by calculating the ratio of VLPs-stimulated cpm to the control cpm.
2.6. Bio-Plex cytokine assay
After stimulation of splenocytes with different VLPs, following the protocol for the proliferation assay, supernatants were collected and the cytokine/chemokine levels were measured by using a Bio-plex multiplex assay as previously described (Bharadwaj et al., 2007). The multi-panel assay allows for the quantification of most B cell related cytokines/chemokines simultaneously. In short, 50 μl of pre-mixed beads coated with target capture antibodies were transferred to a 96-well filter plate (approximately 5000 beads per well for each cytokine/chemokine). Beads were washed three times and 50 μl of either pre-mixed standards or supernatants diluted at a ratio of 1:4 with cell media were added to each well. The plate was incubated for 30 min at RT followed by washing and addition of 50 μl of premixed biotinylated detection antibodies to each well. The plate was incubated at RT for 10 min, and after subsequent washing, detection was performed by staining with streptavidin-phycoerythrin (PE). The plate was incubated at RT for 10 min, and after washing, the beads were resuspended in 125 μl of Bio-plex assay buffer. The bound beads were read on the Bio-Plex suspension array system, and data were analyzed using Bio-Plex Manager software. The lowest level of detection for all the cytokines measured was 3.2 pg/ml.
2.7. Real time RT-PCR
Total RNA was extracted from VLPs-stimulated B cells 4 days after incubation using RNAqueous-4PCR kit (Ambion Inc., Austin, TX) according to the manufacturer’s instructions. For real time PCR the following primers were used: mBlimp forward 5′-CATGGAGGACGCTGATATGAC-3′ reverse 5′-ATGCCTCGGCTTGAACAGAAG-3′, mIRF4 forward 5′-TCCGACAGTGGTTGATCGAC-3′ reverse 5′-CCTCACGATTGTAGTCCTGCTT-3′, mXBP forward 5′-AGCAGCAAGTGGTGGATTTG-3′ reverse 5′-CCAAGCGTGTTCTTAACTCCT-3′, mAID forward 5′-GCCACCTTCGCAACAAGTCT-3′ reverse 5′-CCGGGCACAGTCATAGCAC-3′. Murine β-Actin or GAPDH were included as a house keeping control gene. The mRNA levels for these differentiation markers were analyzed using an iCycler system (Bio-Rad Laboratories, Hercules, CA, USA) as previously described. In short, real-time PCR was carried using the SYBR supermix kit (Bio-Rad). The PCR mixture included the following: iQ SYBR Green supermix, diluted cDNA templates and 100 nM of each primer in a total volume of 25 μl. The reaction was run for 40 cycles at 95 °C for 20 s and 60 °C for 1 min. To determine the PCR specificity the melting curve data was collected. cDNA samples were run in triplicate, and as a negative control, the sample without reverse-transcriptase (RT) mRNA was included. The mRNA level for each sample was normalized to the mRNA levels of our control house keeping genes. The amount of PCR product was measured by using the threshold cycle (Ct) values and the 2^-dd Ct was used to present the fold-change in expression of a gene.
2.8. Immunization
C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were immunized once with 100 μg of either SIV-, SHIV- or HA/SIV VLPs by intraperitoneal injection. Blood samples were collected from tail veins at days 7 and 12 post-inoculation. After clotting and centrifugation, murine serum was separated and collected. Murine spleens were harvested at day 12 post-immunization and single-cell suspensions from whole splenocytes were prepared.
2.9. ELISPOT assay
For the ELISPOT assay we used an immuno-PVDF membrane on a filter plate (Millipore Corporation, Billerica, MA) as previously described (Guo et al., 2003; Yao et al., 2004). Each well was coated with 50 μl of SIV antigen (optimal concentration for SIV VLPs were 10 μg/ml in PBS) and incubated overnight at 4°C. After washing, 200 μl of PBS supplemented with 10% CS was added to each well followed by overnight incubation at 4°C. Splenocytes were resuspended in complete RPMI 1640 media and adjusted to a concentration of 106 cells/ml. A total of 2.5 × 105 cells were then added to each well followed by incubation at 37°C for 2 h in a humidified 5% CO2 incubator. The cell suspensions were decanted and the filter plate was thoroughly washed with PBS followed by two more washes with distilled water and vigorous shaking to remove all splenocytes. PBS (200 μl) with 10% CS was then added to each well followed by overnight incubation at 4°C. We then added 100 μl of HRP or AP conjugated antibody diluted to the optimal concentration in PBS supplemented with 10% CS. After incubating at RT for 1 h, the plates were washed three times with PBS containing 0.05% Tween 20 (PBS-T) followed by another washing step with Tris-HCl (pH8.5). Alkaline phosphatase substrate, (BCIP®/NBT-Blue Liquid Substrate System for Membranes, Sigma-Aldrich Inc., St. Louis, MO), (100 μl) was added to each well and the plates were incubated at RT for 15 min or until spots could be seen using a stereoscope. After washing three times with PBS-T and once with acetate buffer (pH5.0), 100 μl of soluble HRP substrate, (3-amino-9-ethylcarbazole substrate set, BD Biosciences, San Jose, CA), was added to each well and incubated at RT for 1 h. Pictures were taken and spots scored using a light microscope at low magnification.
2.10. ELISA assay
Mice were immunized with different VLPs and the serum was collected at days 7 and 12 post-immunization. Specific antibody levels in the blood serum were determined by using ELISA assay as previously described (Guo et al., 2003; Yao et al., 2004). Briefly, 96 well micro-titer plates were coated with either SIV VLPs or inactive Influenza A PR8 at a concentration of 10 μg/ml in PBS and incubated overnight at 4°C. The plates were then blocked in PBS supplemented with 10% CS at RT for 1 h. After three washes with PBS containing 0.05% Tween 20 (PBS-T), 5-fold serially diluted (beginning at 1:100) sera was added to the plates and incubated at RT for 2h. The plates were washed three times with PBS-T and treated with goat anti-mouse IgG1-, IgG2a-, IgG-, or IgM-HRP conjugated antibodies. The plates were washed three times with PBS-T, and ABTS substrate solution (2,2′-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid, Sigma-Aldrich Inc., St. Louis, MO) was added to each well. The enzymatic reaction was stopped by adding SDS solution and ODs were read at 405 nm (reference at 490 nm) in an ELISA reader (EL800; Bio-Tek Instruments, Winooski, VT). Data was analyzed and concentrations were determined by comparing the reading for the experimental samples with the standard curves. Results are given as the arithmetic mean ± SD.
2.11. Statistical analysis
Antibody production data were expressed as the mean ± standard deviation (SD). Comparisons between different VLPs and PBS stimulation were analyzed by using the Student’s t-test. A P value < 0.05 was considered statistically significant.
3. Results
3.1. VLPs directly bind to and activate naïve B cells but not T cells in vitro
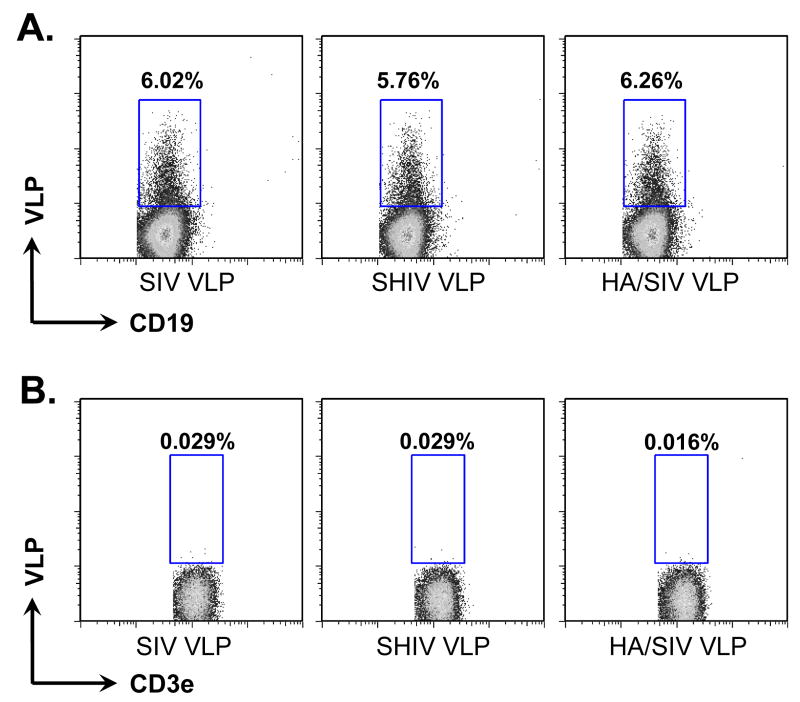
We have previously generated and characterized three different types of VLPs, which include SIV- (only SIV Gag), SHIV- (SIV Gag plus HIV envelope), or chimeric influenza HA/SIV- (SIV Gag plus influenza hemoagglutinin) VLPs (Guo et al., 2003; Yao et al., 2000). These VLPs were shown to be strong immunogens in mouse models leading to high titers of HIV-specific and HA-specific antibodies (Guo et al., 2003; Yao et al., 2002; Yao et al., 2004). In order to determine whether VLPs have a direct interaction with B cells, naïve mouse splenocytes were incubated with these VLPs in vitro. We studied the binding of the VLPs to either naïve CD19+ B cells or CD3e+ T cells by flow cytometry. As shown in Fig. 1, all three VLPs were able to bind to naïve CD19+ B cells (Fig. 1A) but not to CD3e+ T cells in vitro (Fig. 1B). Since mouse cells have no human CD4 molecule for HIV Env protein to bind, VLPs will not bind to T cells. The percentage of VLPs-bound B cells showed no obvious differences among three VLP groups. The mean fluorescent intensity (MFI), which is correlated with binding efficiency between VLPs and cells, was also similar among the three groups. There was only a slightly higher B cell binding capacity for the HA/SIV VLPs when compared with the SIV- and SHIV VLPs (Fig. 1A). The immunofluorescence pictures of different bindings of VLPs to B and T cells were shown in supplementary Fig. S1.
Fig 1.
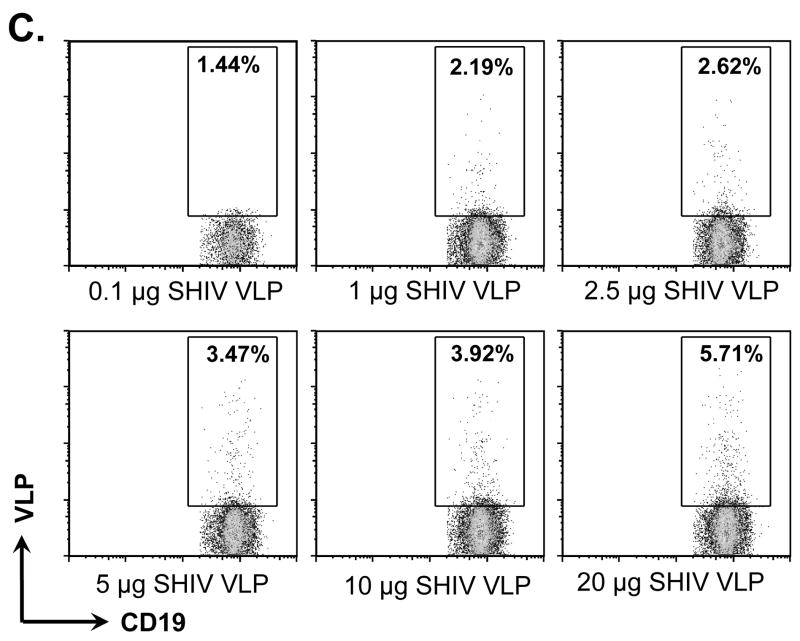
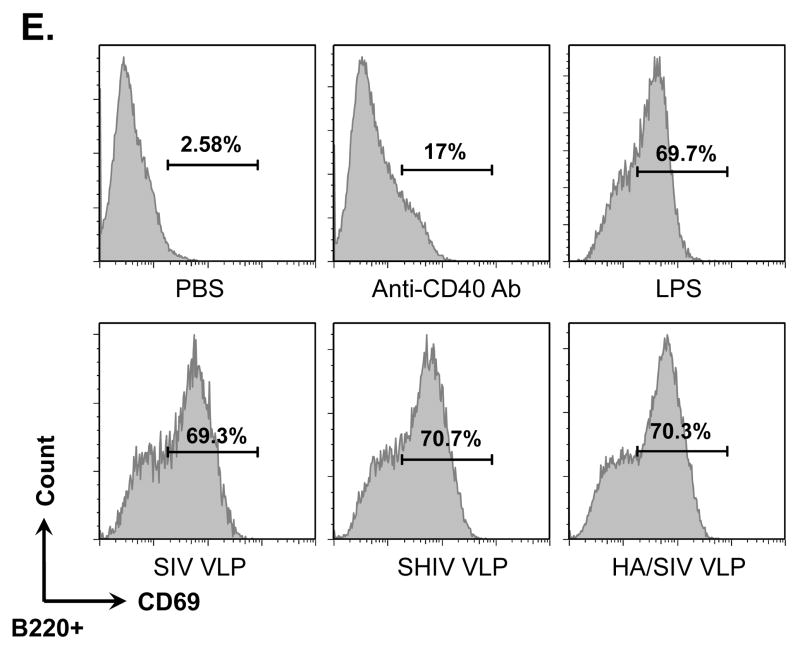
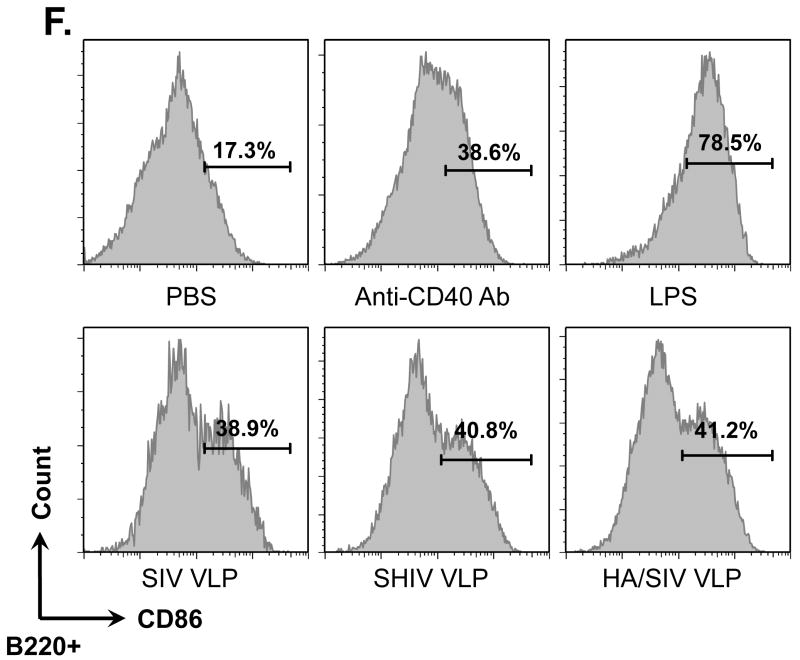
VLPs bound to and activated naïve B cells but not T cells. Naïve B6 mouse splenocytes (single cell suspension) were incubated with different VLPs (20 μg/ml) at 4°C for 6 h or overnight. B cells were then stained with anti-CD19-FITC conjugated antibody. T cells were stained with anti-CD3e-FITC conjugated antibody. VLPs were pre-labeled with R18 red fluorescent dye. A) Percentage of CD19+ B cells binding to R18 labeled VLPs. B) Percentage of CD3e+ T cells binding to R18 labeled VLPs. C). Dose-dependent binding of VLPs to CD19+ B cells. D). Competition of unlabeled VLPs with labeled VLPs binding to CD19+ B cells. E) and F) VLPs stimulating B cell activation. Mouse splenocytes (single cell suspension) were treated with SIV-VLPs, SHIV-VLPs or HA/SIV-VLPs at a final concentration of 20 μg/ml for 48 h. PBS treatment served as a negative control. Incubation with anti-CD40 Ab (10 μg/ml) and LPS (10 μg/ml) served as positive controls. B cell activation markers CD69 (E) and CD86 (F) were stained and analyzed by flow cytometry. Representative examples of B cell activation figures were shown from at least three repeated experiments of splenocytes from different mice.
To determine whether the binding between VLPs and B cells is really specific and not just a result of stickiness of B cells but not T cells, a dose-dependent binding and unlabelled VLP competition assays were performed. As shown in Fig. 1C, addition of increasing amounts of VLPs from 0.1 – 20 μg SHIV VLPs resulted in increased binding of VLPs to B cells from 1.44% to 5.71%, indicating a dose-dependent effect of the binding. Furthermore, as shown in Fig. 1D, increasing amounts of unlabeled VLPs decreased binding of labeled VLPs from 6.79% to 2.2%, indicating competition binding of unlabeled VLPs with labeled VLPs. Therefore, VLP binding to CD19+ B cells is VLP specific.
To study any possible activation of naïve B cells induced by VLPs, the expression of the activation markers CD69 and CD86 on CD19+ B cells after treatment with SIV-, SHIV- or HA/SIV VLPs was determined (Fig. 1, E and F). Similarly to the positive controls which were incubated with anti-CD40 Ab or the potent B cell mitogen LPS, incubation of the splenocytes with all three VLPs led to the up-regulation of CD69 to almost identical levels as those observed with LPS and to the up-regulation of CD86hi to the levels obtained with anti-CD40 Ab.
3.2. VLPs mainly stimulate naïve, conventional B220+IgM+CD43 −CD5− B2 cells but not B220+IgM+CD43+ B1 cells in vitro
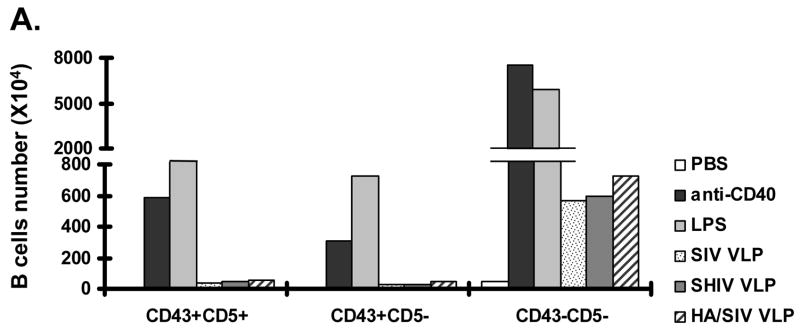
Since VLPs can bind to and activate naïve B cells, it is important to determine which particular B cell subset (B1 or B2 subset) is activated. This would confer a better understanding on the possible mechanisms by which VLPs induce B cell activation and it could also implicate the subsequent immune response generated from VLP stimulation. As demonstrated in Fig. 2A, among B220+IgM+ B cells, the total number of conventional B2 cells (CD43−CD5−) were increased upon the treatment with SIV-, SHIV-, and HA/SIV VLP, compared with PBS cells (negative controls), although these numbers were lower than those in LPS or anti-CD40 Ab treated cells (negative controls). The B1 cell subpopulation including B1a cells (B220+IgM+CD43+CD5+) and B1b cells (B220+IgM+CD43+CD5−) remained relatively unchanged when the cells were treated with VLPs. Thus, these results show that VLPs predominantly stimulate the conventional B220+IgM+CD43−CD5− B2 cells to expand in vitro.
Fig 2.
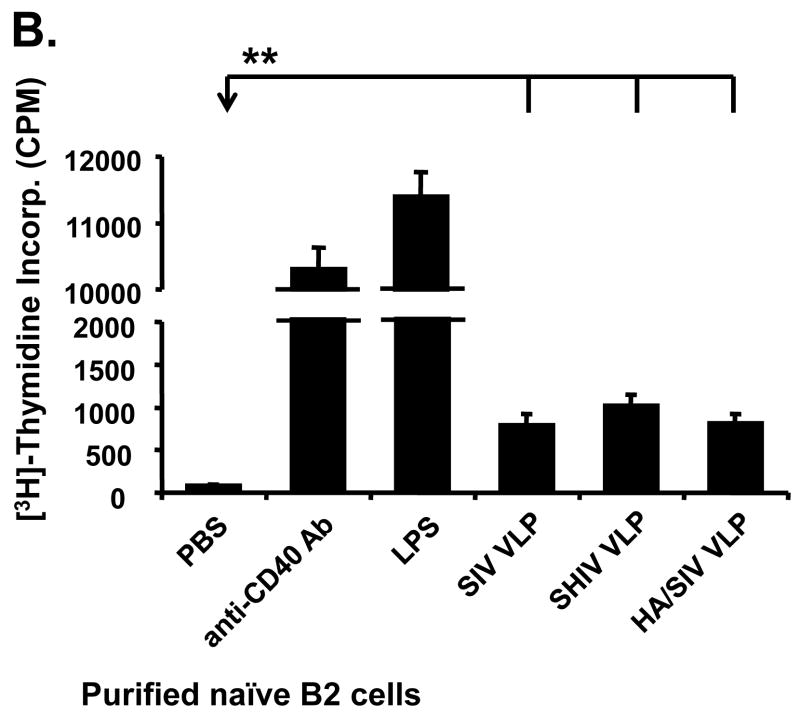
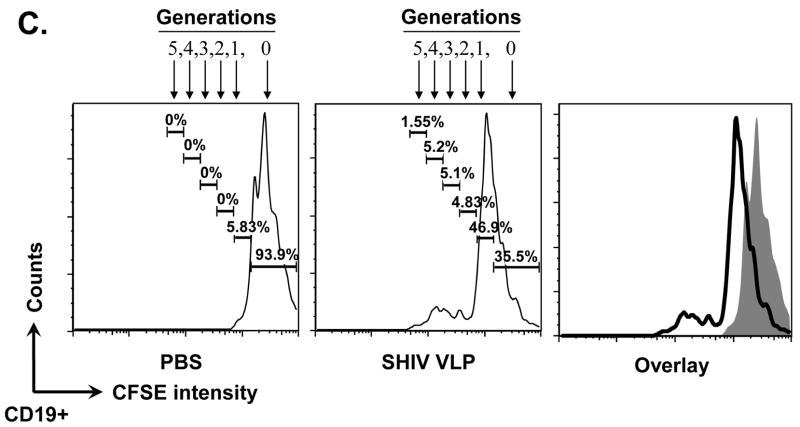
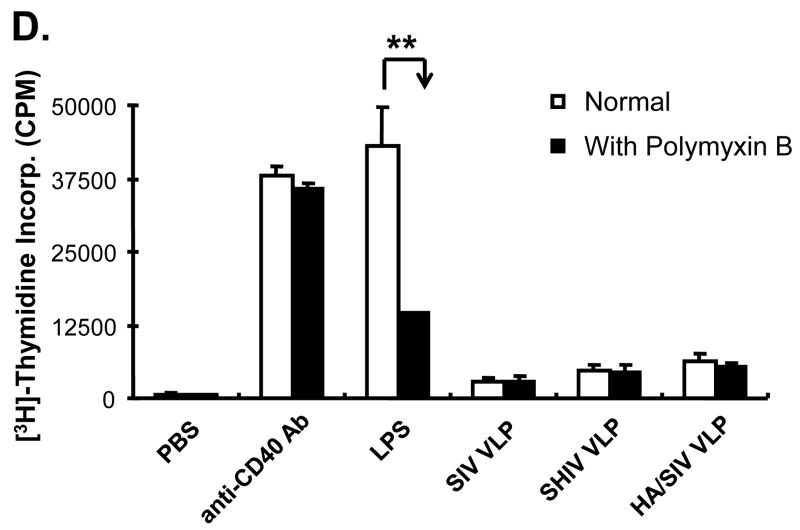
VLPs mainly stimulate the conventional B2 B cell subset expansion in vitro. Mouse splenocytes (single cell suspension) were treated with SIV-VLPs, SHIV-VLPs or HA/SIV-VLPs at a final concentration of 20 μg/ml for 48 h. PBS treatment served as a negative control. Incubation with anti-CD40 Ab (10 μg/ml) and LPS (10 μg/ml) served as positive controls. Anti-B220, CD5, CD43, and IgM Abs were stained and analyzed by using corresponding labeled antibodies for flow cytometry. A) B cell number changed upon different VLP treatments. Lymphocytes were first gated based on B220 and CD43 staining. Then two B220+ B populations were sub-gated based on CD5 and IgM staining to distinguish B220+IgM+CD43−CD5− conventional B2 cells, B220+IgM+CD43+CD5+ B1a B cells, and B220+IgM+CD43+CD5− B1b B cells. Cell numbers were converted by calculating out the percentage of positive cells in the total cell population. Representative examples of B cell number changes were shown from at least three repeated experiments of splenocytes from different mice. B) VLPs stimulated the purified naïve CD43−CD5− B2 B cell proliferation but not T cell proliferation. Naïve CD43−CD5− B2 B cells were purified by using magnetic beads and biotin labeled anti-CD43, CD5, and CD4 Abs. VLPs (20 mu;g/ml) were added to the purified B2 B cells for 3 days and [3H] labeled thymidine was added to the culture media 16 h before harvesting the plate. C). CFSE-labeled B cell proliferation. Cells were gated on CD19+ B cells. X-axis represents CFSE intensity, Y-axis represents the cell counts. Five generations of proliferation were measured. For the overlay, the dark line represents B cells stimulated with SHIV VLPs, solid peak represents B cells stimulated with PBS. D) Stimulation of splenocytes by VLPs was different from the effect exerted by LPS. Splenocytes were stimulated with different VLPs, LPS, and anti-CD40 Ab in the presence or absence of polymyxin B (approximately 10:1 to LPS molar) for 3 days and [3H] labeled thymidine was subsequently added to the culture media 16 h before harvesting the plate. Representative examples were shown from at least three repeated experiments of splenocytes from different mice. (Statistically significances are shown, ** indicate p< 0.01.)
Actual B cell proliferation was measured by [3H]-thymidine incorporation after incubating the purified naïve B2 cells for three days with VLPs. As shown in Fig. 2B, incubation with SIV-, SHIV-or HA/SIV VLPs induced the proliferation of purified B2 cells, albeit to a lower level than LPS or anti-CD40 Ab treatments, which are nonspecific broad B cell mitogens. To further study whether the cell proliferation is achieved by B cells which bind to VLPs, B cells were labeled with CFSE and then stimulated with PBS or VLPs, the number of divisions and the proportion of cells dividing in response to stimuli at later time points were assessed. Fig. 2C shows the histogram of cell proliferation after CFSE labeling with different stimulations. After SHIV VLP stimulation, there was only a small portion of B cells divided vigorously vs. PBS control, indicating proliferation may be initiated by the small B cell population that interacts with VLPs. All these data demonstrate that VLPs are not only capable of binding to B cells, but also capable of inducing activation and proliferation of naïve B2 cells in vitro. Thus, VLPs may induce a humoral immune response through the specific binding and stimulation of naïve B2 cells.
It can be argued that VLP activation of B2 cells may be caused by the presence of endotoxin, which can be carried over from the production of VLPs. Traces of endotoxin in the VLP preparation would then be able to broadly activate non-specific B cells by interacting with toll-like receptor 4 (TLR4) on their surface. To show that activation of B2 cells is due to the VLPs themselves and not to any presence of endotoxin, splenocytes were stimulated with VLPs, LPS, or anti-CD40 Ab in the presence or absence of polymyxin B (PMB), which is an antibiotic that binds to and inactivates endotoxin. As shown in Fig. 2D, the proliferation of the purified naïve B2 cells was greatly reduced when cells were incubated with LPS in the presence of PMB. This, however, was not the case for cells treated with VLPs and anti-CD40 Ab which showed no significant difference when they were incubated in the presence or absence of PMB. This indicates that the activation of B2 cells by VLPs is not due to the presence of trace amounts of endotoxin in the VLP preparation but rather to direct interaction of the VLPs with the B cells.
3.3. VLPs stimulate a unique cytokine profile in vitro
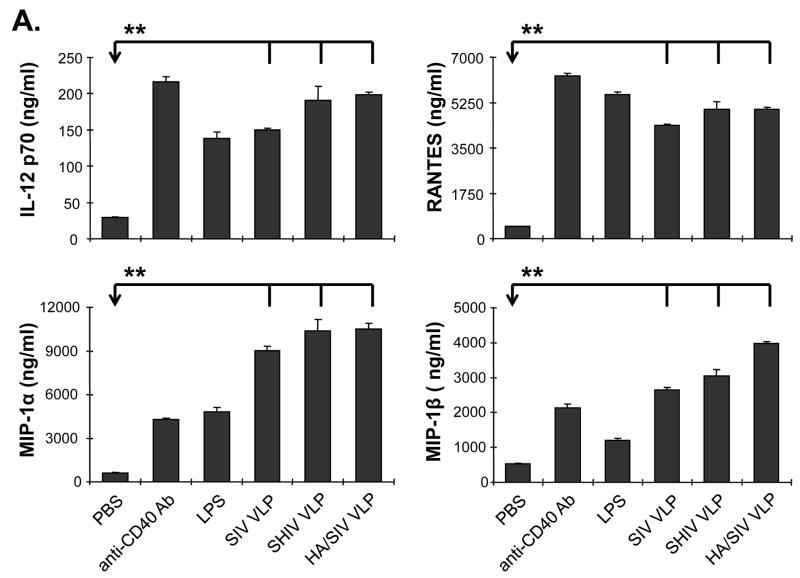
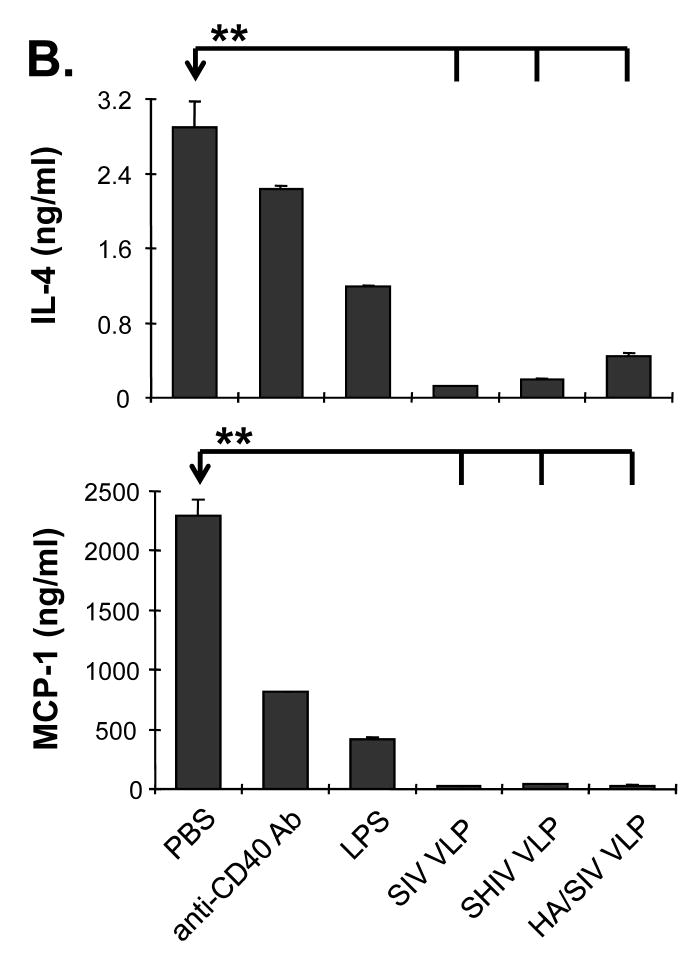
In order to determine which cytokines are stimulated by VLP treatment to promote B cell functions, naïve mouse splenocytes were stimulated with VLPs and the supernatant was collected to determine cytokine expression profiles. We found that stimulation with VLPs led to an elevated expression of IL-12, MIP-1α, MIP-1β, and RANTES, with a downregulation of IL-4 and MCP-1 (Fig. 3A and B). The elevated levels of IL-2, RANTES, MIP-1α and MIP-1β cytokine milieu upon VLP treatment favor IgG2a antibody generation. Levels of IL-4 and MCP-1 which favor IgG1 antibody production were greatly reduced upon VLP treatment. These data indicate that VLP stimulation favors IgG2a class-switch recombination. ELISPOT results further confirmed that there was an increase in the number of IFN-γ, but not IL-4 secreting cells upon VLP treatment (supplementary Fig. S2 and S3). These results suggest that VLP vaccination induces a unique cytokine/chemokine profile, which promotes B cell differentiation and IgG2a class-switch recombination in vitro.
Fig 3.
Cytokines and chemokines produced by VLPs-stimulated lymphocytes. Naïve mouse splenocytes (single cell suspension) were stimulated with SIV-VLPs, SHIV-VLPs or HA/SIV-VLPs at a final concentration of 20 μg/ml for 48 h. PBS treatment was used as a negative control. Incubation with anti-CD40 Ab (10 μg/ml) and LPS (10 μg/ml) were used as positive controls. The supernatant was collected and the various cytokine levels in the different VLPs-stimulated media were detected by using a Bio-Plex assay. A). Production of cytokine IL-12 and chemokines RANTES, MIP-1α and MIP-1β. B). Production of cytokine IL-4 and chemokine MCP-1 production. (Statistically significances are shown, ** indicate p< 0.01.)
3.4. VLPs stimulate B cell differentiation, plasma cell formation, specific antibody production, and class-switch recombination in vitro
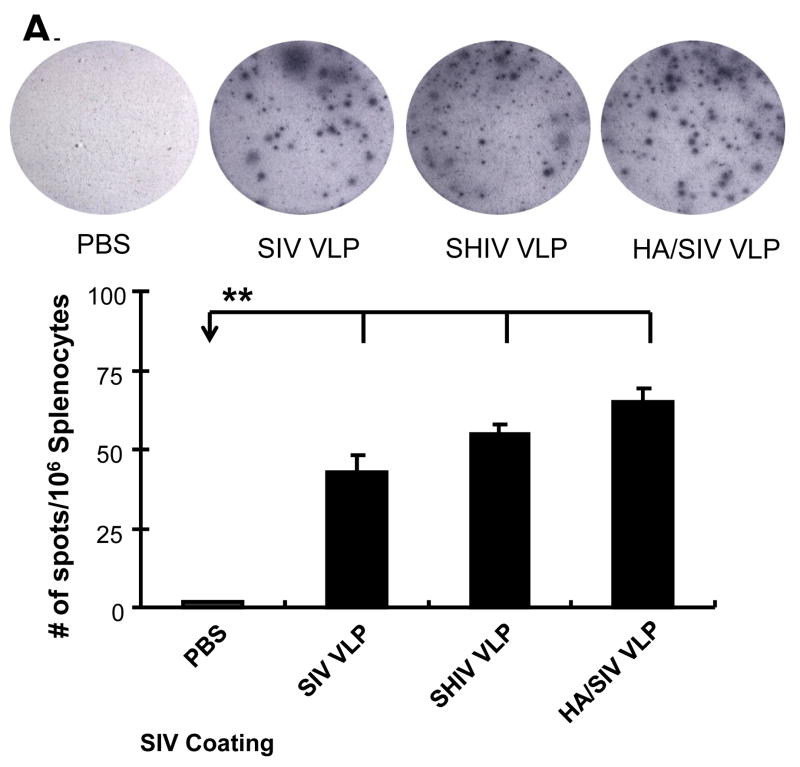
After an initial exposure to TD antigens, early activated B cells need cognate contact with activated T helper cells and cytokine signals in order to differentiate into plasma cells. Without additional stimulatory signals, B cells can proliferate, but may not differentiate efficiently into antibody producing cells or undergo CSR and SHM. In order to determine whether VLPs could induce the right signaling for B cells to differentiate into plasma cells, mouse splenocytes were initially incubated with VLPs and then transferred to SIV-VLPs coated PVDF filter plates. Incubation of mouse splenocytes with VLPs led to a marked increase in the number of spots as represented by focal regions of differentiated plasma cells among which, HA/SIV VLPs led to the highest level of antibody producing cells (Fig. 4A).
Fig 4.
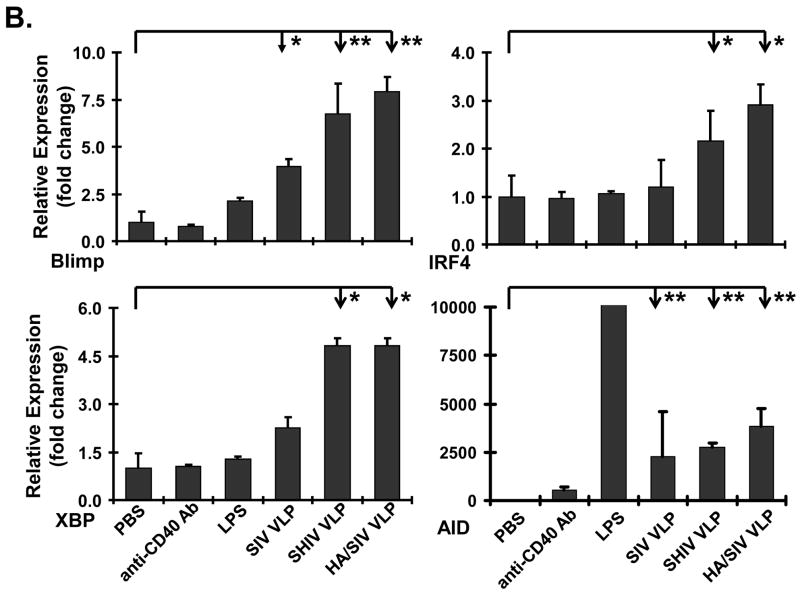
VLPs stimulate B cells to differentiate into plasma cells and antibody IgG2a class switching in vitro. A) VLPs stimulated B cells to differentiate into antibody producing cells. After treatment with VLPs for 48 h, splenocytes were transferred to a SIV VLPs-coated PVDF filter plate. After a 3 h incubation period at 37°C, the plate was harvested to detect antibody producing cells by ELISPOT assay. B) VLPs stimulated B cells to express plasma cell differentiation and class switching molecules, Blimp-1, IRF4, XBP, and AID. The VLPs-stimulated splenocytes were collected after day 4 and B cells were isolated. The mRNA was extracted for real-time RT-PCR analysis. C) VLPs stimulated antibody production. The supernatant from the VLPs-treated splenocytes was collected after day 6. Different specific antibody levels (both IgM and IgG) to SIV VLPs and inactivated PR8 influenza virus were measured by ELISA. Different specific antibody levels (both IgG1 and IgG2a) to SIV VLPs were measured by ELISA. Representative examples were shown from at least three repeated experiments of splenocytes from different mice. (Statistically significances are shown, * indicates p<0.05 and ** indicate p< 0.01.)
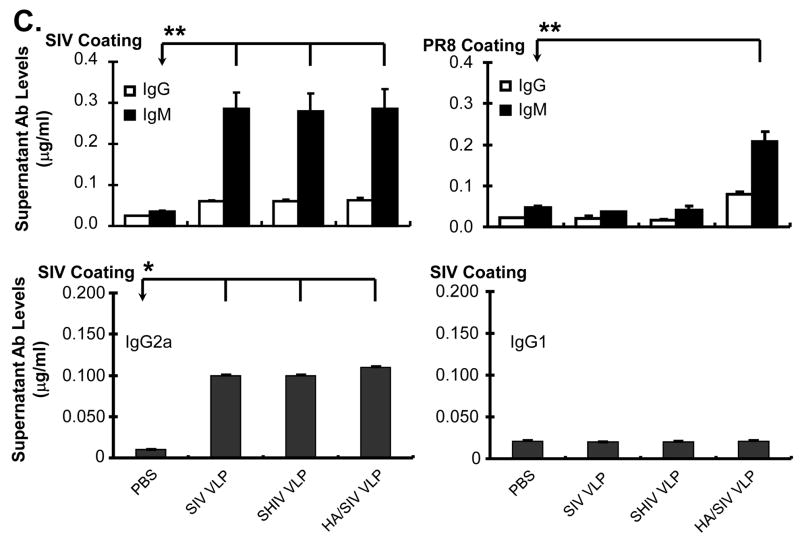
To confirm the ELISPOT results which showed differentiation of B cells into plasma cells upon VLP stimulation, real-time RT-PCR analysis from VLP-stimulated splenocytes was performed. As shown in Fig. 4B, the level of Blimp-1 and XBP-1 mRNA was significantly increased upon VLP treatment compared with the controls. The mRNA level for the transcription factor IRF-4 was also increased upon stimulation with VLPs as was the mRNA level for AID. Again, these results suggest that VLPs have the ability to stimulate B cell differentiation in vitro. The specific levels of antibody production upon plasma cell differentiation were then determined. The supernatant from VLPs-treated splenocytes was collected at day 6 post-stimulation and both IgM and IgG antibody levels to SIV VLPs or inactivated PR8 influenza virus were detected by ELISA. For Ab production against SIV VLPs, all three VLPs induced high levels of antibody including both IgM and IgG (Fig. 4C). Compared with the PBS control, the increased antibody levels were statistically significant (for IgG, p<0.05, for IgM, p<0.01). For Ab production against influenza viral protein, only HA/SIV VLPs significantly stimulated IgG antibody production against inactivated influenza virus (p<0.01). A clear preferential production of IgG2a antibody relative to IgG1 was also observed (Fig. 4C). Statistically significant amount of IgG2a production was observed in all three VLPs (p<0.01). The results show that upon B cell differentiation into plasma cells, there is a specific production of IgM and IgG2a antibodies as expected for a VLPs-stimulated humoral immune response in vitro.
3.5. VLPs induce a strong humoral immune response with large germinal center formation and plasma cell transformation in vivo
To further study B cell functions elicited by VLP vaccination in vivo, VLPs-induced primary humoral responses were assessed. In the murine spleen, there are two vital functional zones involved with B cells. Marginal zones are located at the periphery and consist mostly of B1 cells. B cells in marginal zones have been found to highly express CD21 but not CD23. As shown in Fig. 5A, when compared with the PBS control (8.06%), the percentage of CD21hiCD23− marginal zone B cells in SIV-, SHIV-, and HA/SIV VLP vaccinated groups (5.64%, 5.77% and 5.89%) was decreased. This suggests that VLP vaccination does not stimulate the expansion of marginal zone B1 cells.
Fig. 5.
VLP immunization predominantly stimulates conventional B2 B cells, but not B1 cells, to expand and promotes germinal center reaction and plasma cell formation in the murine spleen. Mice were immunized (i.p.) with different VLP antigens (100 μg/mouse). At day 12 post immunization, splenocytes were isolated and stained for marginal zone B cells population and germinal center. A) The marginal zone B cells. Splenocytes were stained with anti-B220, CD21, and CD23 Abs. The marginal zone B cells were demonstrated by CD21hiCD23lo cells in the B220+ pre-gated B cells. B) Total numbers of B220+IgM+CD43−CD5− conventional B2 cells vs. B220+IgM+CD43+CD5+ B1a B cells and B220+IgM+CD43+CD5− B1b B cells in the primed murine spleen. Lymphocytes were gated as same as in vitro and the number of sub-tupes of splenic B cells were shown. C) The germinal center B cells reaction. Splenocytes were stained with anti-B220 and fas (high expression in germinal center B cells) Abs and GL-7 ab (Mono-clonal antibody, specific to germinal center B cells). The anti-fas and GL-7 double positive germinal center B cells were shown in the B220+ pre-gated B cells. D) The plasma cell formation in the VLPs-primed murine spleen. Splenocytes (single cell suspention) were transferred to a SIV VLPs-coated PVDF filter plate. After a 3 h incubation period at 37°C, the plate was harvested to detect antibody producing cells. The plasma cells were counted and showed as Mean ± SD. These representative data were shown from three repeated experiments and at least three mice in each group. (Statistically significances are shown, * indicates p<0.05 and ** indicate p< 0.01.)
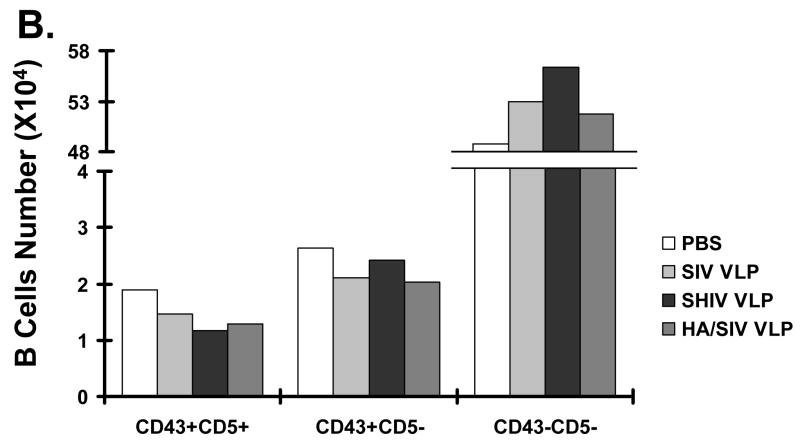
To elucidate general responses of B1 cells in the murine spleen, CD5 and CD43 expression was also analyzed. As shown in Fig. 5B, the B1 cell subpopulation in the murine spleen which includes CD5+ B1a cells and CD5− B1b cells, remained relatively unaltered after VLP immunization (SIV VLPs 3.86%, SHIV VLPs 3.97%, and HA/SIV VLPs 3.72%, vs. PBS control 4.96%). Consistent with our data obtained from in vitro VLP treatment, the results indicate that VLP vaccination does not induce the expansion of B1 cells.
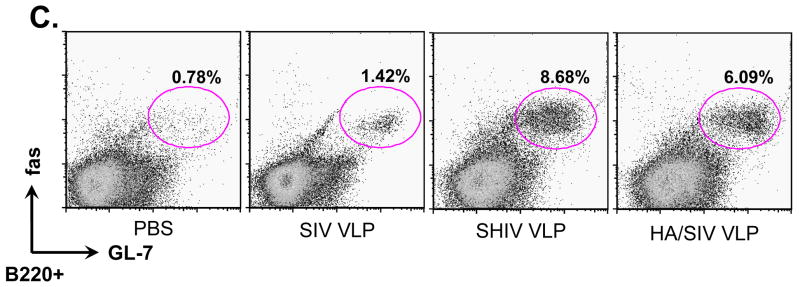
The development of humoral immunity is characterized by germinal center formation which is necessary to produce long-term protective antibody responses by the generation of long-lived plasma cells and memory B cells. To address the capacity of VLP antigens to induce germinal center formation, we stained germinal center B cells by using anti-GL-7 (specific to B cells inside germinal center) and anti-fas (high expression in B cells inside germinal center) antibodies. As shown in Fig 5C, compared with the PBS control, the percentage of B220+GL-7+fas+B cells in germinal centers after SHIV- and HA/SIV VLP vaccination was significantly increased (8.68% and 6.09%, respectively, versus 0.78%. p<0.001). There was also a slight but statistically significant increase in B220+GL-7+fas+ B cells inside germinal centers for the SIV VLP group (1.42% vs. 0.78%, p<0.05). The population of B220+ and GL-7+ B cells was also analyzed by immunofluoresence staining of splenic tissue (supplementary Fig. S4). We observed large germinal centers with B cells positive for both GL-7 (green) and B220+ (red) in the spleens from SHIV- and HA/SIV VLPs-immunized mice. There were fewer germinal centers in the SIV VLP group. The GL-7+ germinal centers were not found in the spleen from control mice immunized with PBS. These data suggests that VLPs have the ability of presenting its TD antigens to generate an adaptive humoral immune response leading to the formation of large germinal centers.
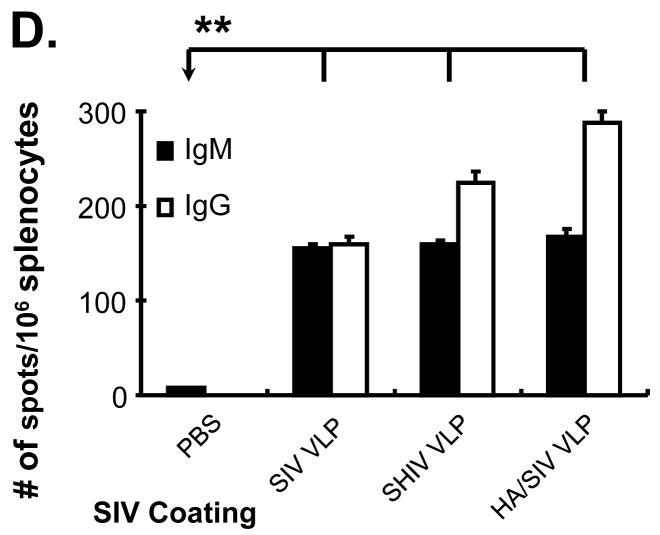
Knowing from our in vitro results that VLPs were capable of activating B cells and stimulating their differentiation into plasma cells, we next wanted to determine the capacity of VLPs-stimulated B cells to differentiate into antibody producing plasma cells following VLP immunization. Splenocytes from VLPs-immunized mice showed elevated levels of both specific IgM and IgG secreting cells relative to splenocytes from control mice (Fig. 5D). Splenocytes from HA/SIV VLP immunized mice produced the highest level of IgG compared with the other VLP immunized mice (Supplementary Fig. S5). These data suggest that VLP vaccination can stimulate B cell differentiation into plasma cells as well as class-switch recombination.
3.6. VLP immunization induces IgG2a antibody class-switch in vivo
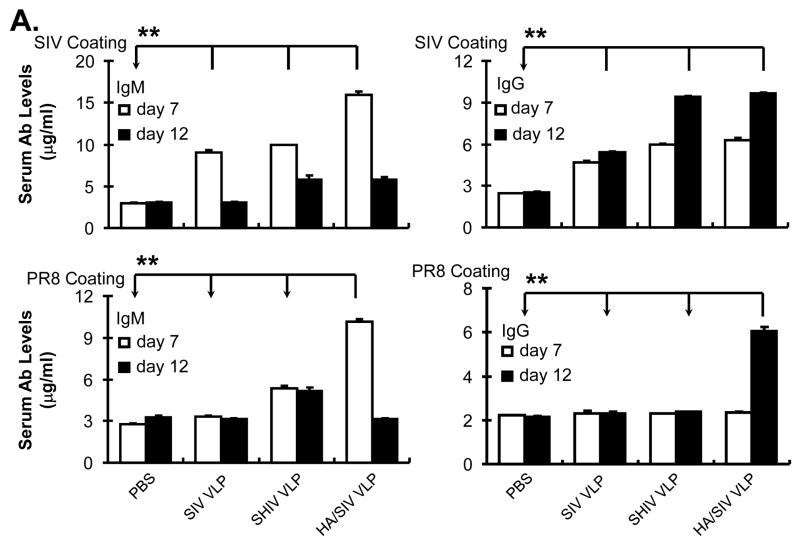
In order to determine the specificity of the IgM and IgG antibodies, serum was collected from primed mice at days 7 and 12 post-immunization. Specific anti-SIV or anti-PR8 influenza virus IgM and IgG antibody levels were determined by ELISA. Since all VLPs contain the SIV backbone proteins, they should all induce antibodies against antigenic determinants on the SIV surface, and only HA/SIV VLPs should induce antibodies specific for influenza hemagglutinin, which is recognized by anti-PR8 influenza hemagglutinin antibodies. As shown in Fig. 6A, immunization with the various VLPs leads to the production of SIV specific IgM and IgG antibodies, anti-SIV IgG showed an increase at day 12 relative to day 7 post immunization. For anti-PR8 influenza hemagglutinin antibodies, mice immunized with HA/SIV VLPs showed a marked increase in the levels of anti-PR8 influenza hemagglutinin IgG at day 12.
Fig 6.
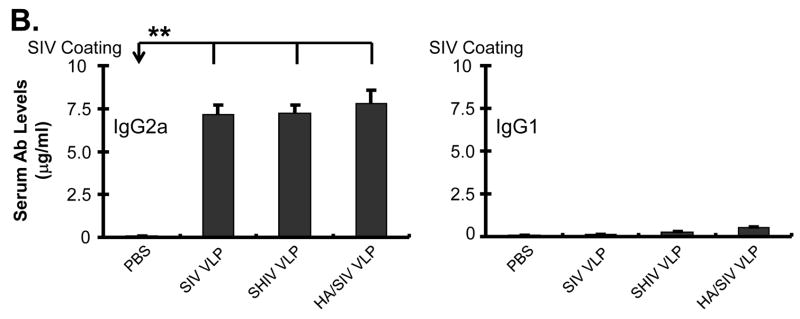
VLP immunization stimulates specific antibody production and IgG2a class switching in vivo. Mice were immunized (i.p.) with different VLP antigens (100 μg/mouse). At day 7 and 12 post immunization, blood was collected from murine tail veins and then separated for serum. A) SIV VLPs and PR8 specific antibody production in VLPs-immunized mice. Specific anti-SIV VLPs or anti-PR8 IgM and IgG antibodies were determined by ELISA assay. Chromogenic substrate ABTS was used, and the data were captured as O.D. value at 405 nm wavelength after reaction was stopped by SDS. The linear range of O.D. values was pre-titrated to be within 1:500~2500 of diluted serum. Dilution of 1:1000 was then used to compare OD values from different treatments, and the results were shown as Mean ± SD. B) IgG2a class switch in VLPs-immunized animals. Specific anti-SIV VLP IgG1 and IgG2a antibodies were determined by ELISA assay as above. These representative data were shown from three repeated experiments and at least three mice each group. (Statistically significances are shown, * indicates p<0.05 and ** indicate p< 0.01.)
Since our in vitro data shows antibody class switching to IgG2a upon VLP stimulation and our in vivo results demonstrate production of specific anti-SIV IgG antibodies, we determined whether VLP vaccination also induced class-switch recombination to IgG2a antibodies. The ELISA results showed that VLP vaccination induced the production of IgG2a but not IgG1 antibodies (Fig. 6B). These results suggest that VLP vaccination through intra-peritoneal injection is capable of inducing class-switch recombination to IgG2a antibody production.
4. Discussion
Our previous reports showed that VLPs were capable of efficiently inducing cellular and humoral immune responses. Besides SIV VLPs, many other VLPs like rotavirus VLPs, human papilloma VLPs (HPV VLPs) and HIV VLPs have also been shown to have the capacity of inducing humoral immunity (Deml et al., 1997; Fromantin et al., 2001; Liu et al., 1998). VLPs can effectively be captured by APCs to drive or maybe directly elicit adaptive B cell responses (Da Silva et al., 2007). Nevertheless, very little is known about the immune mechanism of B cells in enveloped VLP vaccination. In this study, we demonstrated that SIV-, SHIV-, and HA/SIV-VLPs could directly bind and interact with naïve B cells in vitro, inducing the expression of activation markers CD69 and CD86 and expansion of the conventional B2 cells with a B220+IgM+CD43−CD5− phenotype. The expansion and activation of B cells may be induced by direct VLP interaction and subsequent cytokine stimulation. These VLPs were capable of stimulating naïve B2 cell differentiation, plasma cell formation, specific-antibody production and IgG2a class switching both in vitro and in vivo.
In the VLP binding to B cells assay, we found that only about 6% of the B cells were bound by dye labeled VLPs after overnight incubation. We believe that those B cells were specific to the VLP antigens. Firstly, it is estimated that there is less than one epitope-specific B cell per million naïve B cells in the body. There are countless antigenic proteins and epitopes in nature for B cells to recognize and keep host survival from microbe invasion. Therefore, antigen-specific B cells are rare, and the 6% only accounts for B cells specifically binding to VLPs since VLPs are pseudo-virion particles, which contain many different epitopes. Secondly, we speculate that those B cells were VLP antigen specific, and this binding was through the BCR. Although B cells also have pattern recognition receptors like TLR and Fc receptors, B cells recognize antigen specifically only through the BCR. If VLP binding to B cells was through TLR, the binding frequency would be much higher, and all B cells would be bound by dye labeled VLPs. We tried to use anti-IgM and anti-IgD antibodies to neutralize VLP binding to B cells. However, it is difficult to obtain polyclonal antibodies that are specific to antigen binding domains of IgM and IgD. B cells recognizing different antigens is dependant on these specific domains. The sequence and antigenicity of the domain should be varied in different B cells. We found that using anti-IgM whole molecules could only reduce 20% of binding (data not shown). Since no polyclonal anti-IgD antibodies available, it is difficult to totally block the VLP binding. In the current study, partial blocking of VLP binding to B cells can be achieved by anti-IgM antibody, indicating the binding of VLPs to the B cells may through BCR. In addition, we showed that there was a large proportion of B cells which upregulate the activation markers CD69 and CD86; however, only 6% of B cells showed specific VLP binding. Since B cells can be activated not only upon direct antigen stimulation through the BCR complex, but also upon the presence of cytokines, small portion of Ag binding B cells activate and subsequently secrete cytokines, which further help larger population of B cells activation and differentiation. Therefore, in our in vitro results, activated B cells may include not only B cells that directly interacted with VLPs but also B cells that were indirectly activated by cytokines. Those cytokines may be initially produced by B cells that were specific to VLP antigen and then expand B cells indirectly. The limitation in our in vitro system is that it is difficult to distinguish those B cells that were indirectly activated by cytokines. However, these data suggest that VLPs have a capacity to drive B cell proliferation, an important step in B cell immunity.
In this study, an important observation is the ability of VLPs to stimulate B220+IgM+CD43−CD5− B2 cells relative to the B1 cell subset. B1 cells play a critical role in T helper cell-independent innate humoral immune responses and are responsible for the production of most non-immune serum IgM (natural antibodies), and their receptors show poly-specificity which results in the production of low affinity antibodies for a variety of antigens (Hayakawa and Hardy, 2000; Montecino-Rodriguez and Dorshkind, 2006). The B2 B cell subpopulation, on the other hand, is capable of undergoing thorough somatic hypermutation to produce specific high affinity antibodies and to develop long-lived plasma and memory cells. Activation of this B cell subset becomes increasingly important for vaccination purposes where high affinity antibodies and memory cells are desired to provide serological protection against an infectious pathogen. A previous study using human papillomavirus type 16 (HPV16) L1 VLPs showed that these VLPs induced the activation of naïve B cells with an increase in the B1 cell subpopulation (Yang et al., 2004). B cell activation was shown to be mediated by non-specific TLR4- and MyD88-dependent signaling and led to a CD4+ T cell independent humoral immune response. Such a discrepancy can be attributed to the nature of the VLPs utilized. HPV16 L1 VLP is a non-enveloped VLP while the VLPs used in our study contain a membrane envelope. Several non-enveloped viruses like rotavirus (Franco and Greenberg, 1997) and polyomavirus (Szomolanyi-Tsuda et al., 2001) have been shown to generate T helper cell-independent neutralizing antibodies and it is not surprising that a non-enveloped VLP will behave in a similar manner. Another study showed that viral particles driven from RNA phage induced marginal zone B1 cells, which was clono-typically heterogeneous (Gatto et al., 2007). There is an opinion that a B1 cell response with natural antibodies is suitable for acutely cytopathic viruses like influenza A, but chronic, poorly or non-cytopathic viruses preferably require a B2 cell response with the production of high affinity matured antibodies (Hangartner et al., 2006).
In an adaptive immune response, cytokines are known to be a critical factor in determining the modulation B2 cell activation, proliferation, and differentiation which includes CSR and SHM (Acosta-Rodriguez et al., 2007). Many cytokines are found to be involved in the humoral response by directly or indirectly driving the different functional fates of activated B cells (Schijns et al., 1994). Incubation of naïve splenocyte single cell suspensions with VLPs resulted in the production of a cytokine profile characterized by IL-12 as well as chemokines such as RANTES, MIP-1α and MIP-1β. RANTES, also known as CCL5, is a potent chemoattractant for memory T cells and monocytes, and is an important pro-inflammatory chemokine linked to the function of cytotoxic T lymphocytes (Appay et al., 2000; Schall, 1991). Macrophage inflammatory protein 1 alpha (MIP-1α) known as CCL3 and macrophage inflammatory protein 1 beta (MIP-1β) known as CCL4 have been shown to promote the development of IFN-γ producing cells and induce Th1 helper cell differentiation (Luther and Cyster, 2001). On the other hand, the level of IL-4, a characteristic cytokine which inhibits pre-Th cells from entering the Th1 pathway was significantly down-regulated. Furthermore, the concentration of the chemokine monocyte chemoattractant protein-1 (MCP-1), which stimulates IL-4 production, was also greatly reduced. Incubation of naïve mouse splenocytes with VLPs results in the production of cytokines that predominantly promote B cell differentiation into IgG2a producing cells. In Fig. 3, we showed several cytokine production results. A Bio-plex assay was used to determine cytokine concentrations in VLPs-stimulated lymphocytes supernatants. Those cytokines may include not only secretion from VLPs-primed B cells but also from VLPs-primed B cells that are co-stimulated by T helper cells. However, B cells may not react to VLP stimulation alone in in vivo situation. Other accessory lymphocytes like T cells may help mount the B cell response, which also includes secreted cytokines. Therefore, in Fig. 3, we only try to address which cytokines affect the B cell response, like IgG2a class switching upon VLP treatment, but not to uncover their source. This cytokine production pattern was confirmed to be correlated in our in vivo and in vitro antibody data.
When naïve B2 cells become activated in vivo, they get trapped in the T-cell zone of lymphoid tissues where they encounter armed T helper cells, which provide secondary signals for further activation or differentiation of B2 cells into antibody secreting cells. Recognition of peptide:MHC class II complexes on B cells can trigger T helper cells to up-regulate the expression of cell surface molecules like CD40L and secrete related cytokines, which in turn play an essential role in germinal center formation where class switch recombination and antibody affinity maturation may take place (Banchereau et al., 1994; Quezada et al., 2004). The specificity of an antibody response is dependent on both variable domains (VH and VL) but the effecter function is determined by the isotype of the antibody heavy-chain C region (Nimmerjahn and Ravetch, 2005). Cell-associated signals like CD40-CD40L are necessary to initiate antibody isotype switching but the prevailing cytokine environment plays the major role as a regulator and determinant of class switch recombination. The antibody classes in activated B2 cells may be switched to IgG2a, IgG1 or others subclass from both IgM and IgD, which are finally decided by the interaction strength of various predominant cytokines (Snapper and Paul, 1987). Among the IgG subclasses, IgG2a and 2b are considered to be the most potent inducers of effecter responses (Markine-Goriaynoff and Coutelier, 2002). Each IgG subclass has a different affinity for either activating (FcγRs) or inhibitory (FcγRIIB) Fc receptors (FcRs) (Nimmerjahn and Ravetch, 2005; Ravetch and Bolland, 2001). The differences in the ratio of activating-to-inhibitory receptor binding (A/I ratio) determine the in vivo activity of the antibody. IgG2a has been shown to have the highest A/I ratio displaying the best biological outcomes. In our studies we have shown that incubation of mouse splenocytes with our VLPs led to the production of antibody secreting cells. Furthermore, there is a class switching in vitro to the IgG2a subclass, confirming an anti-virus oriented immune response and help. Real-time PCR analysis of VLPs-treated B cells revealed an increase in the mRNA levels of essential genes required for isotype switching and differentiation into antibody producing plasma cells like the regulator of terminal B cell differentiation Blimp-1 as well as the transcription factors XBP and IRF4 upon VLP treatments. AID is a regulator of antibody diversification and is involved in the initiation of somatic hyper-mutation, gene conversion and class-switch recombination (Sohail et al., 2003). Its levels were also increased upon incubation of splenocytes with VLPs, confirming the presence of T cell help to induce secondary antibody diversification on B cells.
Intra-peritoneal immunization of B6 mice with our different VLPs confirmed that our in vitro results translated to an in vivo setting. Flow cytometry analysis showed that there was no increase in marginal zone B cells and the percentage of two types of B1 cells was not affected. Germinal center formation, characteristic of TD antigens, was extensively observed in the murine spleen following VLP vaccination. Our ELISPOT assay confirmed that our VLPs were highly efficient in inducing the activation and differentiation of B cells into antibody producing plasma cells. The IgM levels were increased by day 7 and then gradually declined to the basal levels at which time the IgG antibodies were increased substantially as expected. The specificity of antibody production was shown by detection of anti-HA (PR8) IgM and IgG antibodies in the serum of mice immunized with the chimeric HA/SIV- VLPs. The class switching induced by VLP immunization followed a cytokines profile with dramatically increased the levels of IgG2a but not IgG1.
In this study, we have found that enveloped VLPs can directly bind and activate B cells. The majority of the responding B cell population was conventional B2 cells, but not B1a or B1b cells. Their direct interaction with APCs (previously shown) and indirect interaction with T cells results in the induction of a featured cytokine profile that leads to B cell differentiation into IgG2a producing cells. These results are of great interest for the use of such particles as vaccine agents against pathogens in order to induce a favorable B cell response with the development IgG2a antibodies.
Supplementary Material
Acknowledgments
The authors would like to thank Mr. Christian Marin-Muller for editing this manuscript. This work was supported in part by National Institutes of Health (NIH) Research Grants DE15543 and AT003094 (Q. Yao).
Abbreviations
- VLP
virus-like particle
- SIV
simian immunodeficient virus
- SHIV
simian-human immunodeficient virus
- CSR
class switch recombination
- SHM
somatic hypermutation
- Blimp-1
B-lymphocyte-induced maturation protein-1
- IRF4
interferon regulatory factor 4
- AID
Activation-induced cytidine deaminase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Merino MC, Montes CL, Motran CC, Gruppi A. Cytokines and chemokines shaping the B-cell compartment. Cytokine Growth Factor Rev. 2007;18:73–83. doi: 10.1016/j.cytogfr.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Cerundolo V, McMichael A, Czaplewski L, Rowland-Jones S. RANTES activates antigen-specific cytotoxic T lymphocytes in a mitogen-like manner through cell surface aggregation. Int Immunol. 2000;12:1173–1182. doi: 10.1093/intimm/12.8.1173. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67:5479–5488. doi: 10.1158/0008-5472.CAN-06-3963. [DOI] [PubMed] [Google Scholar]
- Blank SE, Leslie GA, Clem LW. Antibody affinity and valence in viral neutralization. J Immunol. 1972;108:665–673. [PubMed] [Google Scholar]
- Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, Bavari S. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Calame KL. Plasma cells: finding new light at the end of B cell development. Nat Immunol. 2001;2:1103–1108. doi: 10.1038/ni1201-1103. [DOI] [PubMed] [Google Scholar]
- Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- Da Silva DM, Fausch SC, Verbeek JS, Kast WM. Uptake of human papillomavirus virus-like particles by dendritic cells is mediated by Fcgamma receptors and contributes to acquisition of T cell immunity. J Immunol. 2007;178:7587–7597. doi: 10.4049/jimmunol.178.12.7587. [DOI] [PubMed] [Google Scholar]
- Deml L, Kratochwil G, Osterrieder N, Knuchel R, Wolf H, Wagner R. Increased incorporation of chimeric human immunodeficiency virus type 1 gp120 proteins into Pr55gag virus-like particles by an Epstein-Barr virus gp220/350-derived transmembrane domain. Virology. 1997;235:10–25. doi: 10.1006/viro.1997.8669. [DOI] [PubMed] [Google Scholar]
- Doan LX, Li M, Chen C, Yao Q. Virus-like particles as HIV-1 vaccines. Rev Med Virol. 2005;15:75–88. doi: 10.1002/rmv.449. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- Fromantin C, Jamot B, Cohen J, Piroth L, Pothier P, Kohli E. Rotavirus 2/6 virus-like particles administered intranasally in mice, with or without the mucosal adjuvants cholera toxin and Escherichia coli heat-labile toxin, induce a Th1/Th2-like immune response. J Virol. 2001;75:11010–11016. doi: 10.1128/JVI.75.22.11010-11016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto D, Bauer M, Martin SW, Bachmann MF. Heterogeneous antibody repertoire of marginal zone B cells specific for virus-like particles. Microbes Infect. 2007;9:391–399. doi: 10.1016/j.micinf.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Goldsby R, Kindt T, Osborne B. Kuby Immunology 2000 [Google Scholar]
- Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313:502–513. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12:346–353. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- Larson ED, Maizels N. Transcription-coupled mutagenesis by the DNA deaminase AID. Genome Biol. 2004;5:211. doi: 10.1186/gb-2004-5-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner F, Jegerlehner A, Tissot AC, Maurer P, Sebbel P, Renner WA, Jennings GT, Bachmann MF. Virus-like particles as a modular system for novel vaccines. Intervirology. 2002;45:212–217. doi: 10.1159/000067912. [DOI] [PubMed] [Google Scholar]
- Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Abdul-Jabbar I, Qi YM, Frazer IH, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- Markine-Goriaynoff D, Coutelier JP. Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants. J Virol. 2002;76:432–435. doi: 10.1128/JVI.76.1.432-435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison NA. T-cell-B-cell cooperation. Nat Rev Immunol. 2004;4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Schall TJ. Biology of the RANTES/SIS cytokine family. Cytokine. 1991;3:165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Schijns VE, Claassen IJ, Vermeulen AA, Horzinek MC, Osterhaus AD. Modulation of antiviral immune responses by exogenous cytokines: effects of tumour necrosis factor-alpha, interleukin-1 alpha, interleukin-2 and interferon-gamma on the immunogenicity of an inactivated rabies vaccine. J Gen Virol. 1994;75 (Pt 1):55–63. doi: 10.1099/0022-1317-75-1-55. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- Shinkura R, Ito S, Begum NA, Nagaoka H, Muramatsu M, Kinoshita K, Sakakibara Y, Hijikata H, Honjo T. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat Immunol. 2004;5:707–712. doi: 10.1038/ni1086. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Sohail A, Klapacz J, Samaranayake M, Ullah A, Bhagwat AS. Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations. Nucleic Acids Res. 2003;31:2990–2994. doi: 10.1093/nar/gkg464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szomolanyi-Tsuda E, Brien JD, Dorgan JE, Garcea RL, Woodland RT, Welsh RM. Antiviral T-cell-independent type 2 antibody responses induced in vivo in the absence of T and NK cells. Virology. 2001;280:160–168. doi: 10.1006/viro.2000.0766. [DOI] [PubMed] [Google Scholar]
- Tsunetsugu-Yokota Y, Morikawa Y, Isogai M, Kawana-Tachikawa A, Odawara T, Nakamura T, Grassi F, Autran B, Iwamoto A. Yeast-derived human immunodeficiency virus type 1 p55(gag) virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8(+) T cells by cross-presentation of DCs. J Virol. 2003;77:10250–10259. doi: 10.1128/JVI.77.19.10250-10259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Murillo FM, Cui H, Blosser R, Uematsu S, Takeda K, Akira S, Viscidi RP, Roden RB. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J Virol. 2004;78:11152–11160. doi: 10.1128/JVI.78.20.11152-11160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Kuhlmann FM, Eller R, Compans RW, Chen C. Production and characterization of simian--human immunodeficiency virus-like particles. AIDS Res Hum Retroviruses. 2000;16:227–236. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- Yao Q, Vuong V, Li M, Compans RW. Intranasal immunization with SIV virus-like particles (VLPs) elicits systemic and mucosal immunity. Vaccine. 2002;20:2537–2545. doi: 10.1016/s0264-410x(02)00160-3. [DOI] [PubMed] [Google Scholar]
- Yao Q, Zhang R, Guo L, Li M, Chen C. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J Immunol. 2004;173:1951–1958. doi: 10.4049/jimmunol.173.3.1951. [DOI] [PubMed] [Google Scholar]
- Zhang R, Li M, Chen C, Yao Q. SHIV virus-like particles bind and activate human dendritic cells. Vaccine. 2004;23:139–147. doi: 10.1016/j.vaccine.2004.05.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.