Abstract
Theoretical models suggest that symptoms of schizophrenia may be due to a dysfunctional modulatory system associated with the cerebellum. Although it has long been known that the cerebellum plays a critical role in associative learning and motor timing, recent evidence suggests that it also plays a role in nonmotor psychological processes. Indeed, cerebellar anomalies in schizophrenia have been linked to cognitive dysfunction and poor long-term outcome. To test the hypothesis that schizophrenia is associated with cerebellar dysfunction, cerebellar-dependent, delay eye-blink conditioning was examined in 62 individuals with schizophrenia and 62 age-matched non-psychiatric comparison subjects. The conditioned stimulus was a 400 ms tone, which co-terminated with a 50 ms unconditioned stimulus air puff. A subset of participants (25 with schizophrenia and 29 controls) also completed the Wechsler Abbreviated Scale of Intelligence. Participants with schizophrenia exhibited lower rates of eye-blink conditioning, including earlier (less adaptively timed) conditioned response latencies. Cognitive functioning was correlated with the rate of conditioned responsing in the non-psychiatric comparison subjects but not among those with schizophrenia, and the magnitude of these correlations significantly differed between groups. These findings are consistent with models of schizophrenia in which disruptions within the cortico-cerebellar-thalamic-cortical (CCTC) brain circuit are postulated to underlie the cognitive fragmentation that characterizes the disorder.
1. Introduction
Accumulating theoretical and empirical evidence suggests that schizophrenia may be associated with a fundamental disturbance in the timing of neural processes (Andreasen et al., 1998; Friston, 1998; Green & Nuechterlein, 1999; Tononi & Edelman, 2000; Bressler, 2003; Paulus & Braff, 2003; Andreasen & Pierson, 2008). This putative deficit in the temporal coordination of information processing in the brain, sometimes referred to as cognitive dysmetria (Andreasen et al., 1998; Andreasen and Pierson, 2008), may lead to disturbances of consciousness as well as poor coordination of perceptual, affective, cognitive, and motor processes.
Although the cerebellum has traditionally been viewed as primarily responsible for the coordination of voluntary movement, gait, and posture, compelling evidence is accumulating that it also may play a role in a wide variety of psychological functions–including cognitive and affective processes (e.g., Ivry & Keele, 1989; Leiner et al., 1993; Schmahmann & Sherman, 1998; Schmahmann, 2001a, 2001b; Katz & Steinmetz, 2002; Schmahmann, 2004). The cerebellum is an especially important target of study in schizophrenia because abnormalities in a cortico-cerebellar-thalamic-cortical (CCTC) brain circuit are a possible source of anomalies in the fluidity of behavior across time in the disorder (Andreasen et al., 1998; Andreasen & Pierson, 2008). A variety of cerebellar structural anomalies have been observed in schizophrenia (e.g., Ivry & Keele, 1989; Leiner et al., 1993; Schmahmann & Sherman, 1998; Schmahmann, 2001a, 2001b; Katz & Steinmetz, 2002), some of which have been associated with cognitive deficits, clinical symptoms, and outcomes (Nopoulos et al., 1999; Wassink et al., 1999; Ichimiya et al., 2001; Ho et al., 2004; Okugawa et al., 2006), providing further support for the cognitive dysmetria theory of schizophrenia (Andreasen, 1999). However, results to the contrary have also been reported (Wang et al., 2003).
Although results from these structural studies offer support for the theory that cerebellar abnormalities contribute to cognitive dysmetria in schizophrenia (Andreasen et al., 1999; Andreasen & Pierson, 2008), very little is known about the functional integrity of the cerebellum in the disorder nor is much known about neural mechanisms that may provide valuable insight into the observed functional deficits. The evidence that schizophrenia is associated with cerebellar abnormalities is especially interesting given that the cerebellum appears to play a fundamental role in the timing of neural processes associated with not only response timing (e.g., Ivry et al., 1988; Ivry & Keele, 1989; Fiala et al., 1996; Steinmetz, 2000; Spencer et al., 2003; Steinmetz, 2004), but also perceptual, and cognitive functioning (e.g., Ivry & Keele, 1989; Leiner, Leiner, & Dow, 1991; Katz & Stenmetz, 2002). For example, the cerebellum has been implicated in a variety of cognitive domains, including working memory and executive control, inner speech, attention, mental imagery, and emotion (see Baillieux et al., 2008, for review). Moreover, patients with cerebellar lesions show deficits in timing tasks (Ivry et al., 198; Ivry & Keele, 1989) and sometimes exhibit symptoms that are remarkably similar to those seen in schizophrenia, including impaired visuospatial memory, blunted affect or disinhibited, contextually inappropriate behavior, impaired executive function, and inattention (Schmahmann & Sherman, 1997,1998).
The potential importance of the cortico-cerebellar-thalamic-cortical circuit (CCTCC) in schizophrenia is further underscored by the fact that feedback and feedforward loops are widely known to connect the cerebellum with areas of the brain implicated in the disorder, including the thalamus, limbic system (Anand et al., 1959; Snider et al., 1976), and prefrontal cortex (Schmahmann & Pandya, 1995). Taken together, these findings and the connectivity of the cerebellum with brain areas affected in schizophrenia implicate cerebellar dysfunction in schizophrenia. However, despite the evidence supporting the CCTCC model of schizophrenia, the specific functional abnormalities of the cerebellum in schizophrenia are unclear. This lack of knowledge was an impetus for this study of cerebellar-dependent eye-blink conditioning (EBC) in schizophrenia.
The neural circuitry associated with EBC is distinct and well characterized. While structures besides the cerebellum modulate CR acquisition and performance in the delay version of EBC (e.g., the amygdala, septum, and hippocampus; Christian & Thompson, 2003), only the cerebellum is critical for performance in short interval, delay classical EBC (Steinmetz, 2004; Lavond et al., 1993; Kim & Thompson, 1997; Christian & Thompson, 2003). Furthermore, the magnitude of conditioning is related to the morphology and volume of the cerebellum in humans (Woodruff-Pak et al., 2000) and, thus, could be altered by structural anomalies observed in schizophrenia. Consistent with the theoretical and empirical evidence of a role for the cerebellum in neural timing and cognitive, perceptual, and affective processes, functional abnormalities in cerebellar-mediated EBC have been reported in psychiatric disorders with cognitive, perceptual, and affective symptoms, including bipolar disorder (Bolbecker et al., 2008) and schizophrenia (Spain, 1966; Sears et al., 2000; Hofer et al., 2001; Marenco et al., 2003; Brown et al., 2005). However, the specific nature of delay EBC findings in schizophrenia have been contradictory in the smaller patient groups studied to date. Impaired acquisition has been reported (Hofer et al., 2001; Brown et al., 2005) while other studies have reported no differences (Marenco et al., 2003) or facilitated conditioning (Spain, 1966; Sears et al., 2000). In addition, both longer (Marenco et al., 2003) and shorter (Brown et al., 2005) response onset and peak latencies have been reported. Because these conflicting results may be due to insufficient sample sizes or methodological differences, this study was undertaken to further characterize EBC in schizophrenia.
Given that the cerebellum is critical for acquisition and timing of EBC (Steinmetz, 2004) and the cognitive dysmetria model posits that the cerebellar abnormalities are related to cognitive deficits in schizophrenia (Andreasen et al., 1998; Andreasen & Pierson, 2008), EBC deficits may be associated with cognitive performance in schizophrenia. In healthy people, cerebellar volume is positively correlated with both intelligence (Andreasen et al., 1993; Paradiso et al., 1997) and the magnitude of eye-blink conditioning (Woodruff-Pak et al., 2000). However, to our knowledge, neuropsychological correlates of EBC in schizophrenia have not been explored. Hence, a secondary goal of this study was to examine the relationship between intelligence and EBC in schizophrenia.
The present paper reports findings from a large sample of schizophrenia patients (N=62) and age-matched controls (N=62). The major hypothesis, based on empirical and theoretical evidence, was that the schizophrenia group would manifest impaired learning (i.e. fewer conditioned responses) and abnormally timed conditioned responses (i.e. earlier response latencies). In addition, a subset of participants underwent neuropsychological testing. We predicted that cognitive performance would be positively correlated with conditioned response acquisition.
2. Methods
2.1. Participants
Participants were 62 individuals (23 women) with DSM-IV schizophrenia and 62 age-matched non-psychiatric healthy controls (32 women). Controls were matched to a corresponding schizophrenia participant if their ages were within 2 years of each other. Diagnostic status was determined using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) sections for mood disorders, psychotic disorders, and substance abuse disorders, and chart review. Symptom severity was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). A subgroup of patients (N=29) and controls (N=25) were administered the two-subtest form of the Wechsler Abbreviated Scale of Intelligence (WASI). This version includes Matrix Reasoning and Vocabulary and yields an estimate of full-scale IQ. All assessments were administered within a week of eye-blink conditioning. The study procedures were approved by the University’s Human Subjects Institutional Review Board and written informed consent was obtained from all participants.
As expected, given the age-matching procedure, the mean age of schizophrenia participants (39.82 yrs, SD=9.54) did not differ from controls (39.85 yrs, SD=9.99), t(122)=0.02, p=0.99. Inclusion criteria were completion of grade school level education, normal or corrected to normal hearing and vision, no history of cardiovascular or neurological disease, and no history of head injury that resulted in loss of consciousness. Participants who met criteria for substance dependency within three months prior to testing were not considered for the study.
Twelve individuals with schizophrenia were off psychotropic medications (antipsychotics, mood stabilizers, and anti-depressants) at the time of testing. All of the remaining 50 schizophrenia participants were on antipsychotics (34 atypical, 7 typical, 9 both), 18 were on extrapyramidal medications, 9 were on mood stabilizers, 18 were on extrapyramidal medications, 17 were on SSRI or tricyclic antidepressants, and 9 were on mood stabilizers.
2.2. Eye-blink Conditioning Procedure
Participants completed a single-cue tone delay eye-blink conditioning task. The conditioned stimulus (CS) was a 400 ms, 1000 Hz (80 dB SPL) tone, which, on paired trials, co-terminated with a 50 ms air puff, the unconditioned stimulus (US). Subjects were presented with 8 US alone trials (ITI=15 s), followed by 10 blocks of conditioning trials (mean ITI=15 s; range=10 to 20 s). Each trial block consisted of 9 CS/US paired trials and 1 CS-alone trial. The extinction phase consisted of 25 randomly presented CS alone and 25 US alone trials (mean ITI=15 s; range=10–20 s). To maintain the participants’ attention throughout the experiment, neutral photographs selected from the International Affective Picture System (Lang & Greenwald, 1988) were presented (2 s duration) between each trial and participants rated the pleasantness of the images by pressing a response pad button. In addition, participants were observed via a closed circuit monitor to ensure that their eyes remained open. The experiment was briefly suspended if signs of fatigue were observed so that the examiner could interact with the participant.
2.3. Procedure
Bipolar eletromyographic (EMG) electrodes (4mm Ag/Ag-Cl), one placed 1 cm below the eyelid and centered with the pupil and the other place 1 cm below the lateral cantus, were used to record eye-blinks from the orbicularis palpebrarum muscle of the left eye. A ground electrode was placed on the forehead. The left eye was presented with a US air puff (50 ms, 10 lbs psi at source) delivered via copper tubing (fused to the rim of lens-less glasses) connected to a regulator delivering air via plastic tubing (120″). The CS tone was delivered via ear inserts (E-A-RLINK – Aearo Company Auditory Systems). EMG recordings were made continuously (2.5 KHz A/D rate; high pass filter=1 Hz; low pass filter=500 Hz; gain=1000) throughout the experiment and stored offline.
2.4. Data Analysis
Continuous data files for each subject were divided into 1086 ms epochs starting 500 ms prior to CS onset. After a 10 Hz (6 dB/octave) high pass filter was applied, the data were rectified and smoothed using a 41-point Gaussian weighted moving average. Data were entered into DataMunch, a Matlab computer program purpose written for eye-blink conditioning data analysis (unpublished data by King DAT and Tracy J, available upon request from Hetrick WP) was used for further eye-blink conditioning analysis. Alpha responses, which are reflexive, non-associative orienting EMG responses to the tone CS, were assessed between 25 and 100 ms after CS onset. On a subject-by-subject basis, responses were recorded as blinks if the amplitude exceeded five standard deviations above the baseline (baseline window for each trial=125 ms prior to CS onset). Conditioned responses were recorded if the blink occurred between 100 and 350 ms after CS onset, which corresponded to a period beginning 250 ms before the onset of the US. The onset latency was calculated as the point in time where the conditioned response is exceeds 0.5 standard deviations from the baseline. The peak latency is the time point for the maximal value for that conditioned response. Spontaneous blinks occurring within a window from 75 ms prior to CS presentation to 25 ms following CS onset relegated the trial as a bad trial and it was excluded from further analysis. There were no significant differences between groups on number of bad trials.
The effects of Group (schizophrenia, controls) and Block (10) were evaluated for all eye-blink conditioning measures using 2×10 repeated measures ANOVAs. Bad trials were used as a covariate in CR analyses after Marenco et al. (2003) but this did not alter the reported results.
Results of the major dependent variables are reported with their corresponding effect sizes in the form of partial eta2 (ηP2): small effect sizes are less than .06; moderate effect sizes range from .06 to .14; large effect sizes are greater than .14 (Cohen, 1973).
Associations between EBC primary dependent variables and WASI scores were assessed using bivariate correlations. The resulting Pearson’s r coefficients for each group were then transformed to z-scores using Fischer’s transformation. These values were then transformed into a standardized score in order to assess whether the magnitude of the correlation differed between groups.
3. Results
3.1. Characterization of raw EMG data
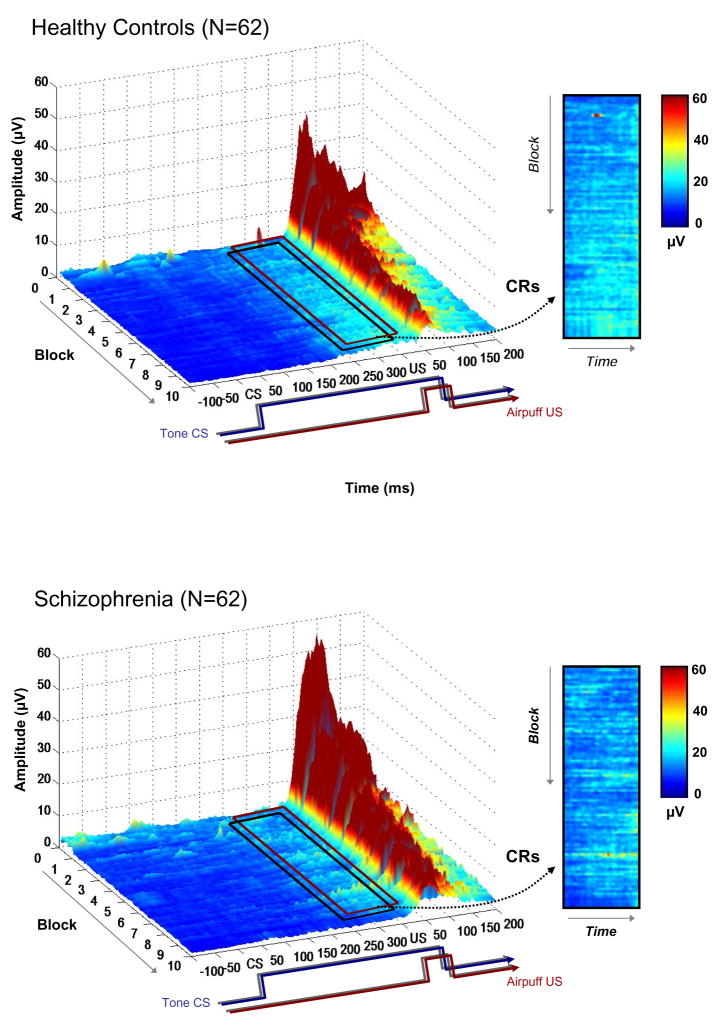
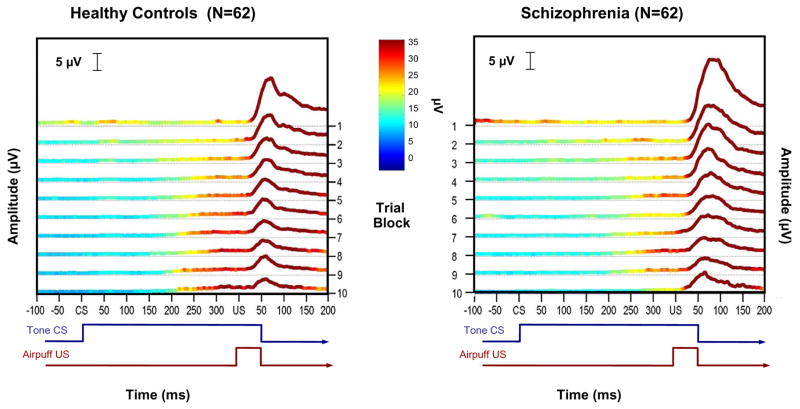
The grand averaged EMG data from all paired conditioning trials are plotted by group in Figure 1. A temporal schematic of the relationship between the tone CS and air puff US is shown below the x-axis. The boxed inset contains the CR window, which is followed in the main figure by a prominent deflection; this large amplitude deflection is the unconditioned blink response to the air puff. These averaged raw data illustrate that schizophrenia was associated with less EMG activity in the conditioned response window compared to the healthy control group, as indicated by the increased light blue and green regions in the boxed inset for the controls relative to the schizophrenia group. Comparing the insets, it is apparent that in the final blocks of the experiment control subjects exhibited CR activity on more trials than the schizophrenia group, and that this activity occurred on most of these later trials and was greater in amplitude (i.e., lighter blue and green areas) compared to individuals with schizophrenia. Moreover, these higher amplitude areas were aggregated towards the onset of the US in the control group, indicating that this was when peak eyelid closure tended to occur. This feature is notably absent from the data from the participants with schizophrenia. These observations are further emphasized in Figure 2, which averages the CRs within each of the 10 trial blocks together into a single trace for each block, making CR activity more apparent. However, it should be noted that grand averaged representations of the data in Figures 1 and 2 differ from the quantitative results described below because the latter are based on information extracted on a trial-by-trial basis within subjects. Temporal features of the CRs derived from the quantitative, subject-by-subject analyses are graphically illustrated in Figure 3b, where it can clearly be seen that peak CR latencies remain short in schizophrenia as the task progresses. In contrast, the peak CR latencies for the healthy controls become longer and are timed to immediately precede the onset of the US air puff. Table 1 shows the means and standard deviations for important CR and UR variables for the schizophrenia and control groups.
Figure 1.
Grand averaged trial-by-trial EMG data for all trials for the healthy control (upper panel) and schizophrenia (lower panel) subjects. The 400 ms conditioned stimulus (CS) co-terminates with the 50 ms (10psi) air puff. Both groups developed conditioned responses (CRs) as the experiment progressed, as can be seen in the period just prior to unconditioned stimulus (US) onset (indicated by the boxed inset), but the schizophrenia group exhibited fewer and more poorly timed CRs compared to controls.
Figure 2.
Block averages derived from the grand averaged trial-by-trial EMG data shown. Single traces represent CR activity for each of the 10 trial blocks, with data from the healthy control participants shown in the left panel and data from the schizophrenia group in the right panel. CR amplitude and timing are more robust and consistent by the end of the experiment in the control group as compared to the schizophrenia group.
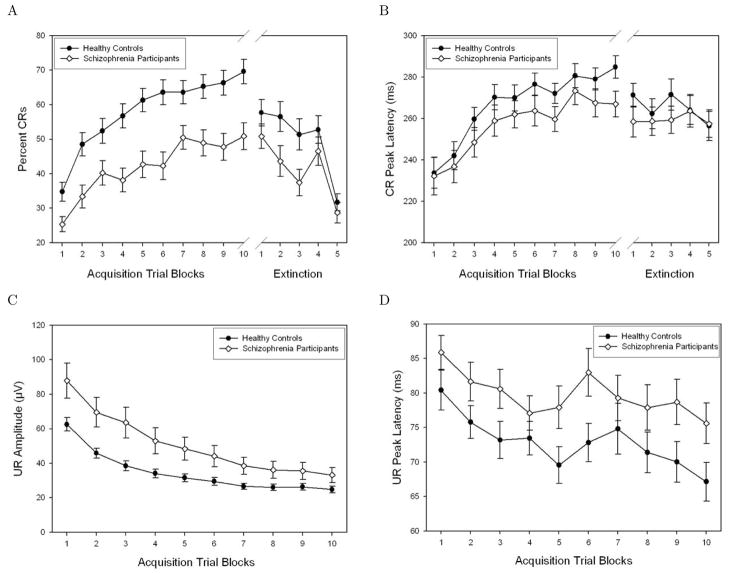
Figure 3.
Mean ± SE across blocks for CR and UR primary dependent variables. (A) Comparison of Percent CRs for schizophrenia and healthy control groups. Both groups showed evidence of learning, as indicated by an increase in CR incidence as the experiment progressed, but the schizophrenia group had fewer CRs throughout the experiment. (B) Comparison of CR Peak Latency for schizophrenia group compared to healthy controls. Schizophrenia patients had faster CR latencies than healthy controls, indicating less adaptively timed responses. (C) Comparison of UR peak amplitude for healthy control and schizophrenia groups. Amplitude decreased for both groups as the experiment progressed, but the schizophrenia group had larger amplitudes compared to the healthy control group. (D) UR peak latency across blocks in schizophrenia and healthy control groups. UR latency decreased for both groups with experience, but the schizophrenia group had significantly slower UR latencies.
Table 1.
Means and standard deviations for healthy control and schizophrenia groups on CR and UR variables.
| Number of Participants | Percent CRs | CR Peak Latency (ms) | UR Peak Amplitude (μV) | UR Peak Latency (ms) | |
|---|---|---|---|---|---|
| Healthy Controls | N=62 | 58.22 (26.85) | 266.86 (46.16) | 34.60 (18.89) | 72.86 (22.19) |
| Schizophrenia | N=62 | 42.02 (27.67) | 256.98 (56.07) | 51.00 (52.69) | 79.76 (23.51) |
3.2. Baseline UR Amplitude
In order to rule out blink performance differences between groups as a source of differences in percentage of CRs, responses to 8 US-alone stimuli presented prior to the conditioning phase of the procedure were analyzed. The average peak UR amplitudes were higher in the schizophrenia group compared to healthy controls (t(122)=−2.21, p=0.03), suggesting that differences in CR rates between groups are unlikely to be due to deficits in sensorimotor processing or motor responding in the schizophrenia group.
3.3. Bad Trials Analysis
The bad trial window exists to exclude trials where EMG activity is increased immediately before (−75 ms) and shortly after CS onset (+25 ms). If a subject exhibits EMG blink activity during this interval, it is questionable whether a conditioned response can be emitted immediately thereafter. That is, spontaneous blinks occurring immediately prior to and following CS onset (i.e., in the “bad trial” window), may interfere with the subsequent execution of a conditioned response. Blinks recorded in the “bad trial” window are considered spontaneous blinks because they occur too early in reference to CS onset to be considered either tone-related or conditioning-related. Accordingly, the number of “bad trials” can be used as a rough index of spontaneous blink rate. The average number of “bad trials” rejected from analysis did not differ between groups, t(122)=−1.494, p=0.14.
3.4. Acquisition
3.4.1. Conditioned Responses
3.4.1.1. Percent CRs
Examination of the difference in CR acquisition between the schizophrenia and control groups indicated that although both groups showed learning as evidenced by increased CR incidence across successive blocks, the schizophrenia group had consistently poorer performance relative to the control group (Figure 3a). In accordance with these observations, there was a main effect of both Block, F(9)=18.21, p<0.001, ηp2=0.59, and Group (schizophrenia vs. controls), F(1)=17.47; p<0.001, ηp2=0.13. The Group × Block interaction was not significant.
3.4.1.2. CR peak latency
As learning occurs across the conditioning session, the timing of the CR should shift to more precisely approximate the onset of the air puff; hence, CRs which occur shortly after CS onset are considered less adaptive compared to CRs occurring later and immediately prior to US onset. Results showed that CR peak latency increased across blocks for both groups, F(9)=6.79, p<0.001, ηp2=0.35, but the schizophrenia group’s latencies were consistently shorter, F(1)=4.18, p<0.05, ηp2=0.03 (see Figure 3b). The Block × Group interaction was not statistically significant.
3.4.1.3. CR amplitude
Overall, the amplitude of CRs increased as the experiment progressed, F(9)=2.69, p<0.01, ηp2=0.18. The groups did not differ in amplitude.
3.4.2 Unconditioned Responses
3.4.2.1. UR peak amplitude
UR amplitude decreased as the experiment progressed when all participants were considered together, F(9)=12.73, p<0.001, ηp2=0.50, an effect readily observed in Figure 3c. Schizophrenia patients had significantly higher UR amplitudes compared to controls, F(1)=6.38, p<0.05, ηp2=0.05. The Group × Block interaction was not significant.
3.4.2.2. UR peak latency
UR peak latency decreased as the experiment progressed, main effect of block: F(9)=5.94, p<0.001, ηp2=0.32. The schizophrenia group had significantly slower UR latencies, F(1)=4.34, p<0.05, ηp2=0.03, but there was no Block × Group interaction (see Figure 3d).
3.5. Extinction
3.5.1. Conditioned Responses
Data were available for extinction trial for 54 schizophrenia patients and their age matched controls. This sample size was reduced because the remaining patients in the schizophrenia sample did not undergo extinction but instead experienced a shift in the interstimulus interval (ISI), and those data will be reported in a separate study.
3.5.1.1. Percent CRs
The number of CRs declined as the extinction trials progressed, F(4)=19.31, p<0.001, ηp2=0.43. Schizophrenia patients had a fewer CRs during the extinction phase, a difference that was significant at the trend level, F(1)=3.74, p=0.06 (ηp2=0.03). However, there was no interaction between block and group, which suggests that the groups did not differ in their rate of extinction (Figure 3a). However, comparing percent CRs during the 2 different learning conditions of the experiment using a 2 (acquisition, extinction) × 2 (Healthy Controls, Schizophrenia) repeated measures ANOVA yielded main effects of Condition, F(1)=8.85, p<0.01 (ηp2=0.08), and of Group, F(1)=9.15, p<0.01, (ηp2=0.08), as well as an interaction, F(1)=5.76, p<0.05, (ηp2=0.05),
3.5.1.2. CR peak latency
There was no main effect of Block or Group and no Group × Block interaction (Figure 3b).
3.5.1.3. CR peak amplitude
CR amplitude decreased throughout the extinction phase, F(4)=6.31, p<0.001 (ηp2=0.19), but there was no difference between groups and no Group × Block interaction.
3.6. Clinical and Medication Status
Averages for dependent variables in the 10-block acquisition and 5-block extinction phases were computed to explore their relationships with clinical and medication status using bivariate correlational analyses. There were no significant correlations between PANSS (positive, negative, or general scales) and any averages of EBC dependent variables either during the acquisition or extinction phase. Likewise, chlorpromazine equivalent dosages were not correlated with EBC dependent variables. Moreover, when unmedicated schizophrenia patients (N=13) were compared with age-matched controls on %CRs, there were main effects of Block, F(9)=4.069, p<0.01, ηp2=.70, and Group, F(1)=10.96, p=0.01, ηp2=0.31. For peak latency, there was also a main effect of block, F(9)=4.31, p<0.01, ηp2=0.71, and of Group, F(1)=4.92, p<0.05, ηp2=0.17. It should be noted that the effect sizes in these comparisons were greater in this unmedicated sample than for the entire sample.
3.7. Correlations between neuropsychological and EBC variables
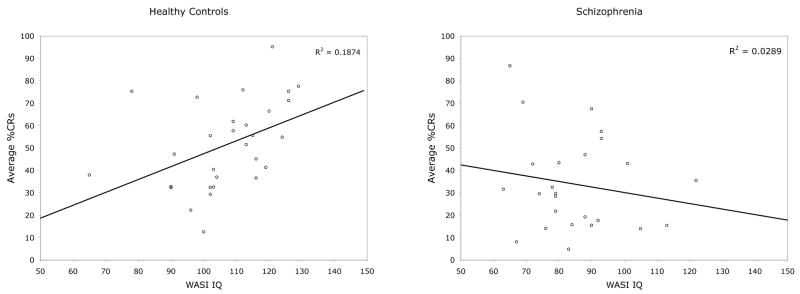
The schizophrenia and control groups who underwent cognitive testing did not differ on age, t(54)=−0.123, p=0.90. As inspection of Figure 4 suggests, average %CRs was statistically significantly correlated with the WASI intelligence quotient in non-psychiatric comparison participants (r(29)=0.43, p<0.05) but not in schizophrenia (r(25)= −0.185, p=NS). Similarly, the Vocabulary subscale showed a positive correlation with conditioned responses in controls (r=0.42, p<0.05), but was not significantly associated with %CRs in schizophrenia (r=−.244, p=NS). The Matrix Reasoning subscale was not significant for either group. Fisher’s r to z transforms were computed so that the statistical difference between the correlations within each group could be evaluated. The IQ-%CRs correlations were significantly different (z=2.19, p<.05), but the Vocabulary-%CRs correlations did not reach significance (z=1.75, p=NS).
Figure 4.
Scatter plots showing the relationships between IQ and conditioning in healthy controls (left) and in schizophrenia (right).
4. Discussion
The current findings are notable because of the clear impairment in cerebellar-dependent eye-blink conditioning in the schizophrenia group and the large sample size. Impairment in the functional integrity of the cerebellum and associated brainstem circuits that support eye-blink conditioning was indicated by two primary findings. First, the schizophrenia group produced significantly fewer conditioned responses; second, when conditioned responses were produced, they were timed unusually early compared with the onset of the US air puff. These findings are consistent with models in which cerebellar dysfunction may contribute to poor temporal coordination of cognitive and perceptual processing in schizophrenia (Andreasen et al., 1999; Andreasen & Pierson, 2008).
On the basis of an extensive animal and human literature, the observed conditioning deficits in schizophrenia implicate anomalies in both the cerebellar cortex and deep interpositus nucleus, and, perhaps, the function of the Purkinje cells that project from the cortex to the deep nuclei of the cerebellum. Animal studies have shown that the critical CS-US association occurs in the anterior interpositus nucleus ( Yeo et al., 1985; Steinmetz et al., 1992; Lavond, 2002), one of the deep cerebellar nuclei. The cortex of the cerebellum is thought to control the expression of conditioned responses and modulate both their timing and amplitude (Garcia & Mauk, 1998; Steinmetz, 2000; Steinmetz et al., 2002). The anterior lobe, through Purkinje cells projecting to the interpositus, appears to play a critical role in response timing, delaying the onset of conditioned responses until just prior to the US onset (Garcia et al., 1999; Perrett et al., 1993). For example, anterior lobe lesions in rabbits caused previously acquired responses to occur at a fixed, short latency (i.e., non-adaptive latency; Perrett et al., 1993). Findings from these animal studies are supported by studies in humans suggesting that abnormalities in cerebellar structure are associated with eye-blink conditioning abnormalities (Gerwig et al. 2005; Edwards et al., 2008).
The observed impairment of cerebellar function is consistent with previous reports of neurochemical and structural cerebellar anomalies in schizophrenia. For example, decreased density and size of Purkinje cells has been reported in schizophrenia (Reyes & Gordon, 1981; Tran et al. 1998). Post-mortem studies of schizophrenia have shown an up-regulation of cerebellar extracellular signal-regulated kinase (ERK), a protein involved in synaptic development, dendritic growth, and cell death (Kyosseva, 2004). The overactivation of this protein may play a role in neurodevelopmental brain abnormalities in schizophrenia. Further, significant changes in synaptic protein expression in the cerebellum of patients with schizophrenia have been reported (Eastwood et al., 2001). In addition, Reelin, a protein that modulates synaptic plasticity in adulthood and embryonic cortical cell migration, has also been widely reported to be decreased in the cerebellum of patients with schizophrenia (Impagnatiello et al., 1998; Fatemi et al., 2005). Interestingly, recent evidence suggests that peripheral administration of the neuropeptide secretin, which is endogenously released in the cerebellum (Lee et al., 2005), ameliorates eyeblink conditioning abnormalities in schizophrenia (Bolbecker et al., 2009). This finding suggests secretin be deficient in schizophrenia and may have therapeutic implications for improving cerebellar function.
The schizophrenia group produced fewer conditioned eyeblink responses across the acquisition phase and, given the central role of the cerebellum in delay eyeblink conditioning, it seems likely that this deficit is related to abnormal cerebellar function. However, the absence of a group by block statistical interaction raised the possibility that the origin of these deficits may be non-cerebellar. For example, the schizophrenia group may have had a preexisting deficit in the performance of blinks rather than a cerebellar-mediated eyeblink conditioning deficit per se. Two findings from the present study further strengthen the inference of a cerebellar source for the observed deficits by ruling out such a preexisting eye-blink performance deficit. First, the schizophrenia group did not differ from the healthy controls in the performance of unconditioned blink responses to solitary air puffs presented before the conditioning phase. In fact, there was a trend for larger amplitude blinks in the patient group. This result suggests that motor response characteristics in the schizophrenia group were not responsible for the observed conditioned response deficits. Moreover, an analysis of the number of bad trials, an index of spontaneous blink rate, indicated that there was no difference between groups. These findings argue against a pre-conditioning blink performance deficit in schizophrenia that could have accounted for the observed conditioning abnormalities and strengthen the interpretation that the observed findings suggest cerebellar dysfunction in the disorder. Evidence that structural impairments in the cerebellum are associated with schizophrenia (Nopoulos et al., 1999; Wassink et al., 1999; Ichimiya et al., 2001) and that patients with cerebellar lesions sometimes exhibit symptoms associated with schizophrenia (Schmahman & Sherman, 1997, 1998; Schmahmann, 2004) further substantiate these conclusions.
While it seems likely that the observed deficits in EBC are related to a failure in patients with schizophrenia to acquire a cerebellar-mediated conditioned response, alternative explanations for the observed findings exist. For example, although schizophrenia patients demonstrated intact motor responses by producing unconditioned blink responses, one possibility is that the conditioned response signal is incorrectly relayed to brainstem nuclei receiving output from the cerebellum in schizophrenia, (e.g., red nucleus), resulting in a performance deficit rather than an acquisition, or learning, deficit.
To our knowledge, this is the first study to report an association between measures of intellectual functioning and EBC in healthy people or lack of such a relationship in schizophrenia. In the subset of participants who underwent cognitive testing, the non-psychiatric controls showed a positive correlation between conditioned response acquisition and full-scale IQ as well as verbal IQ, but schizophrenia patients showed no significant correlation on either of these measures. Our finding of a significant relationship between cognitive measures and conditioning in healthy people is in contrast with earlier reports that found no relationship (Sears et al., 1994; Franks & Franks, 1962). No statistical results are provided in Sears et al., but it is not unlikely that small sample size (N=11) may have contributed to the difference in results. Franks and Franks (1962) tested 80 healthy participants and found no relationship, but again no statistics are provided, so the exact nature of their findings are unclear. Methodological differences in both IQ testing and EBC procedures may have contributed to the discrepancy in results between this early study and the results reported here.
Given the non-significant correlation in our schizophrenia group, it may be that cognitive ability and EBC are only related in healthy populations. Consistent with this interpretation, CB volume is linked with IQ in healthy people (Andreasen et al., 1993; Paradiso et al., 1997). Interestingly, a report by Hermann et al. (2004) found that cerebellar volume is correlated with eye-blink conditioning in healthy controls, (as has been previously reported by Woodruff-Pak et al., 2000), but not in temporal lobe epilepsy.
The relationships between cognitive function and EBC reported here as well as between cerebellar volume and conditioning reported elsewhere (Woodruff-Pak et al., 2000; Hermann et al., 2004) are consistent with theoretical evidence that the cerebellum is associated with higher cognitive functions (Leiner et al., 1993; Katz & Steinmetz, 2002; Schmahmann, 2004; Baillieux et al., 2008). The reason for a lack of association between cognitive measures and EBC in schizophrenia is unclear. While it may be due to a range restriction in %CRs, examination of Figure 4 suggests this is not the case. Instead, cerebellar deficits, indicated by impaired EBC in schizophrenia in the present study, may indicate altered functional relationships between brain regions contributing to the CCTCC and therefore changed relationships with respect to task performance. This interpretation is consistent with a study by Schlosser et al. (2003) reporting both abnormal connectivity in the CCTCC and poorer performance in schizophrenia relative to healthy controls during a working memory task. The schizophrenia group had reduced connectivity in prefrontal-cerebellar and cerebellar-thalamic limbs of the CCTCC and enhanced connectivity in the thalamo-cortical limb on a working memory task, which was interpreted as supporting the cognitive dysmetria model of schizophrenia (Andreasen, 1999; Andreasen & Pierson, 2008).
The conclusions reached from the present study are inferential and more work will be necessary to further explore cerebellar involvement in EBC in schizophrenia. Imaging studies would be especially helpful in illuminating cerebellar activity in schizophrenia compared with healthy people. In addition, further research will be necessary to disentangle the extent to which schizophrenia is associated with cerebellar-mediated conditioned response acquisition versus pure production deficits. For example, EBC procedures that place a premium on temporal components of this task would be informative in further investigations of cerebellar dysfunction in schizophrenia. One such task, the interstimulus interval (ISI) shift, is believed to depend more on cerebellar cortex. Therefore, if cerebellar deficits are indeed responsible for the present results, schizophrenia patients should show a more pronounced ISI shift deficit—irrespective of basal levels of CR production—since the ability to shift conditioned responses to the new ISI depends on cerebellar cortex. Hence, although the schizophrenia group’s basal rate of responding could be low in the ISI shift task if there is a problem in the red nucleus, this task would have a higher probability of uncovering a cerebellar cortical deficit if it exists.
The majority of patients in this sample were on psychotropic medications and, although attempts were made to control for this statistically, the effects of medication are not completely known. However, analyses of the subset of patients who were unmedicated during testing revealed impaired acquisition and timing in the schizophrenia group, as was observed in the larger sample, but with even larger effect sizes. This is consistent with findings of poorer EBC in unmedicated patients with bipolar disorder (Bolbecker et al., 2008).
In summary, theoretical and empirical findings suggest that cerebellar abnormalities may result in temporal processing deficits in other functional domains over which the cerebellum exerts modulatory influence. For example, the striking invariance of cerebellar circuitry suggests that it performs similar operations on its neuronal inputs regardless of their source (Katz & Steinmetz, 2002; Schmahmann, 2004; Schutter & Van Honk, 2005). Findings of topographic projections organized in bidirectional information streams connecting the cerebellum with motor, prefrontal, and posterior parietal corte (Schmahmann, 2004) have led to suggestions that the cerebellum integrates information from different functional domains (Katz & Steinmetz, 2002; Schmahmann, 2004; Schutter & Van Honk, 2005). This integration may provide the moment-by-moment context for behavioral output, binding together perceptual, cognitive, emotional, and motivational information from disparate brain regions (Schutter & Van Honk, 2005). Given such a model, timing deficits revealed in one domain, such as motor timing in EBC, may indicate or covary with abnormalities in temporal coordination in other domains, as predicted in the cognitive dysmetria model of cerebellar involvement in schizophrenia (Andreasen & Pierson, 2008). In this interpretation, the deficits in motor timing reported here may be indicative of abnormal temporal integration of in cognitive, affective, and perceptual domains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand B, Malhotra C, Singh B, Dua A. Cerebellar projections to limbic system. J Neurophysiol. 1959;22(4):451–457. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, 2nd, O’Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150(1):130–4. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 1999. 1999;56(9):781–7. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–8. doi: 10.1016/j.biopsych.2008.01.003. Epub 2008 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillieux H, Smet HJ, Paquier PF, De Deyn PP, Mariën P. Cerebellar neurocognition: Insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110(8):763–73. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Bolbecker AR, Mehta C, Johannesen J, Edwards CR, Tracy JA, O’Donnell BF, Shekhar A, Nurnberger JI, Hetrick WP. Eye-blink conditioning anomalies in bipolar disorder suggest cerebellar dysfunction. Bipolar Disord. 11(1):19–32. doi: 10.1111/j.1399-5618.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- Bolbecker AR, Hetrick WP, Johannesen JK, O’Donnell BF, Steinmetz JE, Shekhar AS. Secretin effects on cerebellar-dependent motor learning in schizophrenia. Am J Psychiatry. doi: 10.1176/appi.ajp.2008.08040597. Epub 2009 Feb 17. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Cortical coordination dynamics and the disorganization syndrome in schizophrenia. Neuropsychopharmacology. 2003;28(Suppl 1):35–39. doi: 10.1038/sj.npp.1300145. [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, O’Donnell BF, Steinmetz JE, Hetrick WP. Eye-blink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn. 2005;58(1):94–108. doi: 10.1016/j.bandc.2004.09.011. Epub 2005 Jan 4. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10(6):427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in communication science. Hum Comm Res. 1973;28:473–490. [Google Scholar]
- Cromwell RL, Palk BE, Foshee JG. Studies in activity level. V. The relationships among eyelid conditioning, intelligence, activity level, and age. Am J Ment Defic. 1961;65:744–8. [PubMed] [Google Scholar]
- Eastwood SL, Cotter D, Harrison PJ. Cerebellar synaptic protein expression in schizophrenia. Neurosci. 2001;105(1):219–229. doi: 10.1016/s0306-4522(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Edwards CR, Newman S, Bismark A, Skosnik PD, O’Donnell BF, Shekhar A, Steinmetz JE, Hetrick WP. Cerebellum volume and eye-blink conditioning in schizophrenia. Psychiatry Res. 2008;162(3):185–94. doi: 10.1016/j.pscychresns.2007.06.001. Epub 2008 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72(2–3):109–22. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Grossberg S, Bullock D. Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye-blink response. J Neurosci. 1996;16(11):3760–3774. doi: 10.1523/JNEUROSCI.16-11-03760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37(4–5):471–80. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci. 1999;19(24):10940–7. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D. Timing of conditioned eye-blink responses is impaired in cerebellar patients. J Neurosci. 2005;25(15):3919–31. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: Timing is of the essence. Arch Gen Psychiatry. 1999;56(11):1007–1008. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Sears L, Hansen R, Bayless K, Rutecki P, Dow C. Cerebellar atrophy in temporal lobe epilepsy affects procedural memory. Neurology. 2004;63(11):2129–31. doi: 10.1212/01.wnl.0000145774.89754.0c. [DOI] [PubMed] [Google Scholar]
- Ho B, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naïve schizophrenia patients: Clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55(12):1146–1153. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51(2–3):127–36. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naïve schizophrenia. Biol Psychiatry. 2001;49(1):20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95(26):15718–23. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73(1):167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing of functions of the cerebellum. J Cogn Neurosci. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Katz DB, Steinmetz JE. Psychological functions of the cerebellum. Behav Cogn Neurosci Rev. 2002;1(3):229–241. doi: 10.1177/1534582302001003004. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20(4):177–81. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Kyosseva SV. The role of the extracellular signal-regulated kinase pathway in cerebellar abnormalities in schizophrenia. Cerebellum. 2004;3(2):94–99. doi: 10.1080/14734220410029164. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK. The International Affective Picture System standardization procedure and initial group results for affective judgements: Technical Report 1A. The Center for Research in Psychophysiology, University of Florida; Gainseville, FL: 1988. [Google Scholar]
- Lavond DG. Role of the nuclei in eye-blink conditioning. Ann N Y Acad Sci. 2002;978(1):93–105. doi: 10.1111/j.1749-6632.2002.tb07558.x. [DOI] [PubMed] [Google Scholar]
- Lavond DG. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–42. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Lee M, Chen L, Chow BKC, Yung WH. Endogenous release and multiple actions of secretin in the rat cerebellum. J Neurosci. 2005;134(2):377–386. doi: 10.1016/j.neuroscience.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebrocerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44(2):113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16(11):444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Cintron C, Yurgelun-Todd DA. Morphometry of individual cerebellar lobules in schizophrenia. Am J Psychiatry. 2001;158(6):952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR, Scheurs BG. Single-cue delay and trace classical conditioning in schizophrenia. Biol Psychiatry. 2003;53(5):390–402. doi: 10.1016/s0006-3223(02)01506-8. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439–441. [PubMed] [Google Scholar]
- Nopoulos PC, Ceilly JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: Evidence in support of the cognitive dysmetria concept. Biol Psychiatry. 1999;46(5):703–711. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Minami T, Takase K, Sugimoto T, Saito Y, Yoshimura M, Kinoshita T. Neural disorganization in the superior cerebellar peduncle and cognitive abnormality in patients with schizophrenia: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1408–12. doi: 10.1016/j.pnpbp.2006.05.014. Epub 2006. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, O’Leary DS, Arndt S, Robinson RG. Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10(1):1–8. [PubMed] [Google Scholar]
- Paulus MP, Braff DL. Chaos and schizophrenia: does the method fit the madness? Biol. Psychiatry. 2003;53(1):3–11. doi: 10.1016/s0006-3223(02)01701-8. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13(4):1708–18. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes MG, Gordon A. Cerebellar vermis in schizophrenia. Lancet. 1981;2(8248):700–701. doi: 10.1016/s0140-6736(81)91039-4. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Prefontal cortex projections to the basilar pons: Implications for the cerebellar contribution to higher function. Neurosci Letters. 1995;199(3):175–178. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. Cerebellar cognitive affective syndrome. Int Rev Neurobiol. 1997;41:433–40. doi: 10.1016/s0074-7742(08)60363-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatry. 2001a;13:247–260. [Google Scholar]
- Schmahmann JD. The cerebellar cognitive affective syndrome: clinical correlations of the dysmetria of thought hypothesis. Int Rev Psychiatry. 2001b;13:313–322. [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19(3):751–63. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290–4. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Sears LL, Finn PR, Steinmetz JE. Abnormal classical eye-blink conditioning in autism. J Autism Dev Disord. 1994;24(6):737–51. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eye-blink conditioning. Biol Psychiatry. 2000;48(3):204–9. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Snider R, Maiti A, Snider S. Cerebellar pathways to ventral midbrain and nigra. Exp Neurol. 1976;53(3):714–28. doi: 10.1016/0014-4886(76)90150-3. [DOI] [PubMed] [Google Scholar]
- Spain B. Eyelid conditioning and arousal in schizophrenic and normal subjects. J Abnorm Psychol. 1966;71(4):260–66. doi: 10.1037/h0023596. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300(5624):1437–9. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Ivkovich D, Logan CG, Thompson RF. Disruption of classical eyelid conditioning after cerebellar lesions: damage to a memory trace system or a simple performance deficit? J Neurosci. 1992;12(11):4403–26. doi: 10.1523/JNEUROSCI.12-11-04403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE. Brain substrates of classical eye-blink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110(1–2):13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Kim J, Thompson RF. Biological models of associative learning. In: Gallagher M, Nelson R, editors. Handbook of Psychology. Vol. 3. New York: Wiley; 2002. pp. 499–541. [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Rev. 2000;31(2–3):391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155(9):1288–1290. doi: 10.1176/ajp.155.9.1288. [DOI] [PubMed] [Google Scholar]
- Wang F, Sun Z, Du X, Wang X, Cong Z, Zhang H, Zhang D, Hong N. A diffusion tensor imaging study of middle and superior cerebellar peduncle in male patients with schizophrenia. Neurosci Lett. 2003;348(3):135–8. doi: 10.1016/s0304-3940(03)00589-5. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Andreasen NC, Nopoulos P, Faum M. Cerebellar morphology as a predictor of symptoms and psychosocial outcome in schizophrenia. Biol Psychiatry. 1999;45(1):41–48. doi: 10.1016/s0006-3223(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, Lemieux SK. Cerebellar volume in humans related to magnitude of classical conditioning. Neuroreport. 2000;11(3):609–15. doi: 10.1097/00001756-200002280-00035. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 1985;60(1):99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]