Abstract
We investigated 0.01–0.08HZ low-frequency fluctuations of BOLD-fMRI signals in the face and object responsive regions during the resting state and during face or object viewing tasks. By comparing the effects of the face-responsive regions of interest with those of the object-responsive regions of interest, we observed a distributed cortical network of face perception during the resting state among posterior fusiform gyrus, inferior occipital gyrus, and superior temporal sulcus. This network was also significantly activated during the face perception task. The face perception task also activated additional areas in the frontal and parietal regions. Our results suggest that the “core” but not the “extended” network for face processing is already in some form of activation during the resting state. A possible function of the resting state face perception network is perhaps to prepare the brain to process faces that individuals are highly likely to encounter in their environment.
Keywords: resting state, face processing, core and extended networks, fMRI
Introduction
Faces play a paramount role in our everyday social interactions with others. Extensive research has shown that some regions of the brain are preferentially responsive to human faces, such as fusiform gyrus, inferior occipital gyrus, superior temporal sulcus, amygdala, and some part of the frontal and parietal lobe [1–3]. Haxby et al. has proposed that these regions are part of a distributed neural system for face perception in which different brain regions play different roles. He further divided these regions into a “core” system for face processing that include fusiform gyrus, inferior occipital gyrus, superior temporal sulcus and an extended system that goes beyond the occipital-temporal cortex [4].
The theory of distributed cortical network for face perception has been supported by some recent studies [5–9], especially the functional [5, 6] and effective connectivity [7–9] analysis with blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI). However, these studies all required participants to perform visual tasks of face processing [10]. It is unclear as to whether the face perception network is only activated during face processing tasks or is intrinsically organized and already in some form of activation without explicit processing tasks or external stimulations.
In recent years, a set of widely distributed resting-state networks have been identified by using spontaneous low-frequency fluctuations in BOLD-fMRI [11–19], with its frequency limited between 0.01 and 0.08HZ. In the absence of external stimuli or goal-directed mental tasks, these spontaneous fluctuations in BOLD signals are a reflection of intrinsic resting-state activities, corresponding to functional connectivities among anatomically related discrete regions. Among these resting-state networks, some regions in the visual cortex show strong intra-regional activation and inter-regional connectivity [17–19].
The aim of our study was to compare the low-frequency fluctuations of the distributed face-perception network during the resting state and during a face or object perception task. By using 0.01–0.08HZ low-frequency spontaneous fluctuations of BOLD-fMRI signals, we aimed to test the hypothesis that due to faces’ important role in our daily interactions with others, there might exist an intrinsically organized low-frequency network for face perception during the resting state. This network might involve the known face responsive brain regions, and this network might overlap with the network during the face perception task.
Methods
Subjects and procedures
Sixteen healthy participants (8 females, age: 20–27 years) with normal vision participated in this study after giving their informed consent. The study was approved by the research ethics committee at the Tiantan Hospital.
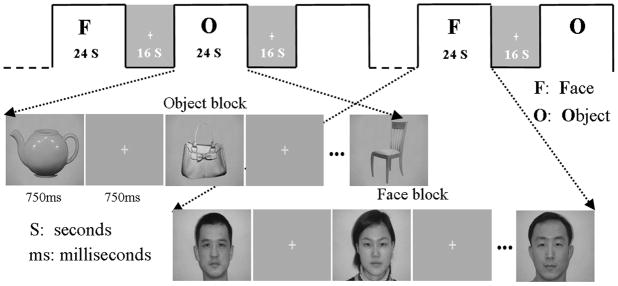
The experiment consisted of 3 phases. In the first phase, a resting scan was obtained. Participants who were unaware of the exact experimental design were instructed to lie with their eyes closed, thinking of nothing in particular. In the second phase, participants participated in a passive viewing task where they were instructed to pay close attention to a sequence of images of faces or common objects appearing on a screen. This task was used to obtain data about the networks involved in face or object perception. The third phase involved a localizer task. The localizer task was modeled after the common method used in the face processing studies [2][20] to obtain face- or object-responsive regions of interests (ROIs) for analyzing the data from the first two tasks. The localizer task was identical to the passive viewing task except that there were occasional one-back matching trials (two trials per block) in which the preceding stimulus was the same as the subsequent stimulus. In this task, participants were instructed to press a button when they saw two identical pictures appearing in a row to ascertain the participant to be attentive.
In the passive viewing and localizer tasks, visual stimuli consisted of alternating epochs of face/non-face object blocks and fixation “+”. Face stimuli were male and female Chinese faces and non-face objects were common objects such as bag, chair, and car. The resting-state scan lasted 6 minutes, the passive viewing task scan and the localizer each lasted 5 minutes and 32 seconds.
Data acquisition
MR imaging was carried out using 3.0 T MR Scanner (Siemens Trio Tim). Functional images were collected axially using a T2*-weighted gradient-echo echo planar imaging (EPI) sequence (TR/TE=2000/30 ms; 32 slices, 4mm sickness; matrix=64×64) covering the whole brain with a resolution of 3.75×3.75mm. Structural images were acquired with a three-dimensional enhanced fast gradient-echo sequence with a thickness of 1mm and a resolution of 1×1mm.
Data analysis
Preprocessing
Preprocessing was performed on each participant’s scan data using SPM5 software (www.fil.ion.ucl.ac.uk/spm/). The first three volumes of each fMRI scan were discarded. Scans were slice timing corrected, spatially realigned, normalized into the standard MNI atlas space according to the segmented grey images; re-sampled to 2mm cube voxels, smoothed with a full width of 6mm at half maximum.
Identification of ROIs based on the Localizer Task
For each participant’s localizer scan, a standard general linear model analysis was conducted with SPM5’s restricted maximum likelihood (ReML) estimation. Each participant’s face responsive regions were identified by contrasting the face block with the common object block (threshold p=.0001, the same level of alpha as many previous studies [2][20]). We also defined each participant’s object responsive regions by contrasting the object block with the face block (p=0.0001). Each individual’s strongest active regions in occipital-temporal cortex were selected as regions of interest (ROI) for further analysis.
Resting state and passive viewing task scan data analysis
Two steps were taken. First, we conducted low-frequency fluctuation tests to detect the existence of the significant effects of 0.01–0.08HZ low-frequency fluctuations during the resting state. The same tests were performed for the face and object viewing tasks. The 0.01–0.08HZ low-frequency fluctuations of BOLD signals in the resting state scan and in the face and object viewing task scans were modeled within the framework of general linear model analysis. In SPM5, the 0.01–0.08 HZ band pass filter can be implemented by using a set of discrete cosine transform (DCT) basis functions. For the fMRI scan containing 177 volumes with the time interval of TR=2, a set of discrete cosine bases containing 121 regressors were acquired across the span of the frequency between 0.01~0.08HZ. We performed F-test to compare the effect of this band frequency with that of 0~0.25HZ which contained 177 cosine basis set (threshold p=.001). The selection of this comparison frequency band was based on the Nyquist’s Sampling Theorem whereby the upper band frequency equal to 1/(TR*2) and thus .25HZ. Significant results would suggest that there existed 0.01–0.08HZ low-frequency fluctuations in the face- and object-responsive regions during the two types of scans.
Second, each participant’s resting scan and passive viewing scan were preprocessed in the following manner: (1) global proportional scaling was performed to yield whole brain intensity value of 1000; (2) drifts were de-trended by second-order polynomial detrending; (3) six parameters of head motion, signals in ventricular regions and signals extracted from white matter were removed by linear regression; (4) discrete cosine transform domain(DCT) band-pass filter (0.01–0.08Hz) was performed to retain the low frequency signals only. After data preprocessing, for each participant, the signal intensity in the face ROI was extracted by averaging the time courses of all voxels in the face ROI. The same procedure was performed to obtain the signal intensity in the object ROI. These time dependent signal intensity data were then used as predictors (regressors) to perform whole brain functional connectivity magnetic resonance imaging (fcMRI) analysis within the framework of general linear model analysis. Using the GLM model, we examined the effect of the signal intensity in the face ROI, and the effect of the signal intensity in the object ROI. Further, we examined the effect of the signal intensity contrast between the face ROI and the object ROI (Face minus Object and Object minus Face). As a result, four statistical parametric t-test maps were obtained for each participant. Finally, multi-participant maps were generated by performing a random effect group analysis with the corrected (FDR) threshold of p=.01.
Results
Localizer results
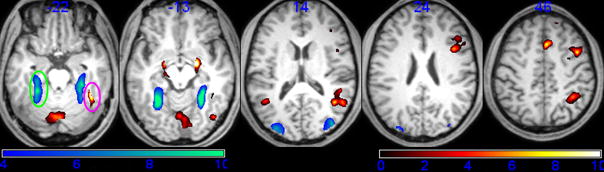
Localizer results showed that regions with greater face than object responsiveness were localized in the right lateral posterior fusiform gyrus, the right inferior occipital gyrus, the bilateral superior temporal sulcus, the inferior parietal lobe, the middle frontal gyrus, the inferior frontal gyrus, the right anterior cingulate gyrus, and the amygdale (p<0.0001, uncorrected; contingent voxels>20; see Fig. 2). Object responsive regions were localized in the medial posterior fusiform gyrus, and the lateral occipital gyrus (p<0.0001, uncorrected; contingent voxels>20; Fig. 2). Among these face- and object- responsive regions, regions in the right lateral posterior fusiform gyrus in 15 of 19 participants were significantly more active for faces than for objects; regions in the left medial posterior fusiform gyrus in 14 of 19 participants showed significantly more activation for objects than for faces. The MNI coordinates of these two regions (face: [40,−50,−20] object: [−30,−40,−18]) were in line with previous studies about the locale of the fusiform face area (FFA) and that of the object responsive region, respectively (Fig. 2). Thus, these two regions for each individual were selected as regions of interest for whole brain functional connectivity magnetic resonance imaging (fcMRI) analysis.
Figure 2.
Localization of the face and object responsive regions with the face responsive regions of interest in cyan circle and the object related regions of interests in purple circle.
Resting state results
First, low-frequency fluctuation tests revealed that, for each participant’s resting scan, there existed significant 0.01–0.08HZ low-frequency fluctuations in widespread areas throughout the brain including occipital-temporal visual regions, inferior parietal lobe and frontal areas (p<0.001, uncorrected).
Second, the low-frequency fcMRI analysis revealed that in the resting state the activities in the face ROI showed widespread low-frequency correlations with most of the occipital-temporal cortex, especially significant in parts of the primary visual cortex and the bilateral fusiform gyrus (p<0.001, FDR corrected). Activities in the object ROI showed widespread low-frequency correlations with the activities in the occipital-temporal cortex and parts of the frontal cortex (p<0.001, FDR corrected).
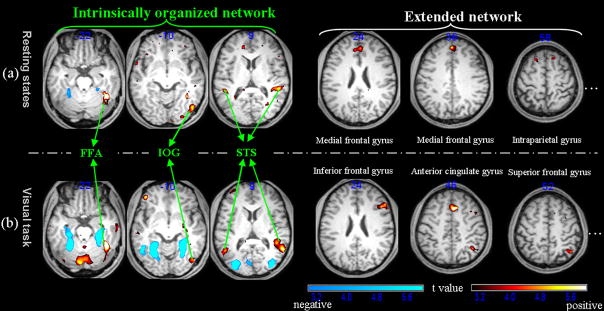
In addition, the contrast between the effect of the face ROI and that of the object ROI showed significantly greater face ROI related activations in several regions of the brain. These areas included the right lateral posterior fusiform gyrus, the right inferior occipital gyrus, the bilateral superior temporal sulcus, the medial/superior frontal gyrus, and the posterior cerebellum (p<0.01, contingent voxels>20, FDR corrected; Figure 3a). In contrast, only the medial posterior fusiform gyrus showed greater object ROI related activities than face ROI related activities.
Figure 3.
Contrasts between the face ROI related activities and the object ROI related activities in the resting state and the passive viewing tasks (warm colors: face>object; cold colors: object>face). The left panel shows the common network between the resting state and the passive viewing task; the right panel shows that differences between the resting state and the passive viewing tasks (the labels for the significantly activated regions greater than 20 voxels are also shown).
Passive viewing task results
First, similar to the resting state results, in the face passive viewing task, low-frequency fluctuation tests revealed that there also existed significant 0.01–0.08HZ low-frequency fluctuations throughout the brain (p<0.001, uncorrected). These finding suggest that in both the resting state and the face viewing task, regions involved in the face perception network contain significantly strong low-frequency fluctuations.
Second, the low-frequency fcMRI analysis revealed that during the passive viewing task the activities in the face ROI and the object ROI showed widespread low-frequency correlations with most of the occipital-temporal cortex, the inferior parietal gyrus, and the lateral frontal regions (p<0.001, FDR corrected).
Further, the contrasts between the face ROI related activities and the object related activities revealed significantly greater face ROI related activities in the lateral posterior fusiform gyrus, the inferior occipital gyrus, the bilateral superior temporal sulcus, the inferior/medial frontal gyrus, the intra-parietal gyrus, and the medial cerebellum (p<0.01, contingent voxels>20, FDR corrected; Figure 3b, Table 1(2)). In contrast, only the bilateral medial posterior fusiform gyrus and the middle occipital gyrus showed greater object ROI related activities than face ROI related activities.
Table 1.
Activation peaks in resting states (1) and passive viewing task (2) by comparing the effect of face responsive regions of interest with that of object responsive regions of interest
| (1) | |||||
|---|---|---|---|---|---|
| region | MNI coordinates |
cluster size | t value | ||
| x | y | z | |||
| Right hemisphere | |||||
| Posterior fusiform gyrus | 40 | −48 | −20 | 140 | 7.33 |
| Inferior occipital gyrus | 46 | −74 | −10 | 36 | 3.77 |
| Superior temporal sulcus | 52 | −56 | 10 | 81 | 5.15 |
| Superior frontal gyrus | 14 | 24 | 57 | 38 | 3.62 |
| 6 | −22 | 70 | 61 | 4.56 | |
| Medial frontal gyurs | 0 | 44 | 36 | 310 | 5.8 |
| 0 | 58 | 24 | 32 | 3.76 | |
| Left hemisphere | |||||
| Superior temporal sulcus | −50 | −54 | 8 | 22 | 4.9 |
| Superior frontal gyrus | −14 | 26 | 56 | 27 | 3.41 |
| −6 | −24 | 72 | 33 | 4.12 | |
| Posterior cerebellum | −34 | −80 | −28 | 97 | 4.36 |
| (2) | |||||
| region | MNI coordinates |
cluster size | t value | ||
| x | y | z | |||
|
| |||||
| Right hemisphere | |||||
| Posterior fusiform gyrus | 40 | −50 | −20 | 189 | 7.96 |
| Inferior occipital gyrus | 44 | −76 | −12 | 47 | 4.12 |
| Superior temporal sulcus | 58 | −54 | 10 | 92 | 5.04 |
| Middle frontal gyrus | 48 | 12 | 44 | 29 | 3.57 |
| Inferior frontal gyrus | 50 | 24 | 24 | 63 | 4.87 |
| Anterior cingulate gyrus | 8 | 20 | 46 | 74 | 5.3 |
| Intraparietal gyrus | 38 | −56 | 52 | 47 | 3.61 |
| Insula | 32 | 22 | −4 | 79 | 5.81 |
| Left hemisphere | |||||
| Superior temporal sulcus | −46 | −54 | 12 | 55 | 4.37 |
| Insula | −38 | 20 | −2 | 53 | 5.35 |
| Medial cerebellum | −6 | −80 | −30 | 127 | 5.1 |
When the results from the resting state scan and the face passive viewing task scan were compared (Figures 3a&b), the coordinates of the peak activation for the face ROI related activities in the occipital-temporal cortex for the two scans differed less than 6mm at the individual level (Mean=4.2mm, SD=3.1mm) and less than 10mm at the group level (Mean=6.4, SD=6.8).
Discussion
Our results suggest the existence of an intrinsically organized low-frequency network for face perception in human occipito-temporal cortex, which consists of three regions: the lateral posterior fusiform gyrus, the inferior occipital gyrus, and the superior temporal sulcus. These three regions are part of the “core” face processing system in Haxby’s model [4]. The location and inter-regional connectivity within the three regions changed little during the resting state and the face perception task. In contrast, several parietal and frontal regions showed altered connectivity from the resting state to the face perception task, suggesting the modulation of the “extended” face processing network by external face stimuli.
Many previous studies have found that, in the absence of external visual stimuli, the sensory cortex shows consistent spontaneous fluctuations [17–19]. Our low-frequency fluctuation testing results are consistent with these findings: by removing the high-frequency physiological noise and low-frequency drifts, we also observed that BOLD signals in the frequency range of 0.01–0.08HZ fluctuated significantly in the occipito-temporal cortex. Further, fcMRI analysis demonstrated that in the resting state the right posterior fusiform gyrus (often referred to as “fusiform face area” or FFA) had significant correlations with the occipito-temporal regions including the primary visual cortex, the fusiform gyrus, and the temporal lobe. These significant correlations also existed in symmetrical “mirror” locations across hemispheres such as the bilateral fusiform gyri and the bilateral inferior occipital gyri.
More importantly, we found that by subtracting the effect of the object responsive regions of interest from the effect of the face responsive regions of interest during the resting state, a distributed resting network was observed in the whole brain for face perception but not for common object perception. This resting state network for face perception included the posterior fusiform gyurs, the inferior occipital gyrus, the superior temporal sulci as well as the medial frontal gyrus and the intraparietal gyrus, and the posterior cerebellum. The locations of the first three active regions overlapped with the face responsive regions in the extrastriate visual cortex activated in the face perception task, which are part of Haxby’s core face processing system. According to Haxby’s theory [4] and Ishai’s subsequent studies [7–9, 21–23], face perception is mediated by a distributed cortical network. The core system of the network consists of three regions in extrastriate visual cortex: inferior occipital gyrus, fusiform face area, and superior temporal sulcus. Previous support to the model of the distributed face-perception network was mainly derived from studies that used external face stimuli and required participants to perform face perception tasks. Our results suggest that without external stimuli, the three occipital-temporal regions contain similar low-frequency neural activities, suggesting that the three regions may have an intrinsically organized network during the resting state for face processing. The existence of such network may reflect the fact that faces are not only the visual stimuli that we tend to have the highest processing expertise [24] but also the most common visual stimuli we tend to encounter in our everyday social life. Thus, this intrinsically organized face resting state network may prepare the visual system to anticipate to process faces, the most socially significant category of visual stimuli.
To further investigate the modulation of the low-frequency network, similar methods were used to obtain active regions in the face-object passive viewing task scans. In the object task, only the bilateral medial posterior fusiform gyrus and bilateral middle occipital gyrus were activated greater for objects than for faces. In contrast, in the face perception task, significant activation for faces than objects appeared in the posterior fusiform gyrus, the inferior occipital gyrus, the superior temporal sulcus, the middle frontal gyrus, the inferior frontal gyrus, and the medial cerebellum. The active regions in the occipital-temporal cortex suggest that the intrinsically organized network for face perception does exist in low-frequency BOLD signals and is not dependent on external face stimuli. The fact that the middle frontal gyrus and the inferior frontal gyrus activated in the face perception task but not in the resting state suggest that these regions might have been temporarily recruited to participate in active face perception.
The medial and superior frontal gyrus activated in the resting states but not in the face perception task may suggest the brain’s expectation of face input during the resting state. Indeed, there is a large body of evidence that medial frontal gyrus (MFG) play a significant role in face processing. For example, MFG has been often implicated in processing of familiar faces, for which a face ‘template’ is presumably more readily available[25], top-down generation of face-related contextual information[26], and processing of affective face[27] and ambiguous face[24] whereby high level cognitive interpretations are needed.
It is unclear as to why cerebellum was part of the resting state network for face perception as well as why the locations of cerebellum activation during the resting state were different from those during the face perception task. Future studies will need to explore the specific role of these active regions within the face perception network under different states.
Conclusions
In the present study, we investigated the 0.01–0.08HZ low-frequency fluctuations of BOLD fMRI signals in face responsive and object responsive regions during the resting state and the perception of faces and objects. We found an intrinsically organized low-frequency network for face perception in the occipital-temoporal cortex which involves areas consistent with Haxby’s core network for face processing, which is altered to include the extended face processing network when individuals are performing a face perception task. Our results suggest that the “core” but not the “extended” network for face processing is already in some form of activation during the resting state. A possible function of this network is perhaps to prepare the brain to process faces that individuals are highly likely to encounter in their environment.
Figure 1.
Visual stimuli used in the passive viewing task and the localizer task.
Acknowledgments
This paper is supported by the Project for the National Basic Research Program of China (973) under Grant No.2006CB705700, Changjiang Scholars and Innovative Research Team in University under Grant No.IRT0645, CAS Hundred Talents Program, CAS scientific research equipment develop program (YZ0642,YZ200766),863 program under Grant No. 2006AA04Z216, the Joint Research Fund for Overseas Chinese Young Scholars under Grant No.30528027, the National Natural Science Foundation of China under Grant No. 30672690, 30600151, 30500131, 60532050, 60621001, Beijing Natural Science Fund under Grant No. 4071003, and NIH R01 HD046526
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanwisher N, Yovel G. The Fusiform Face Area: A cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 4.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 5.Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 6.Bokde ALW, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, et al. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- 7.Ishai A. Let’s face it: it’s a cortical network. NeuroImage. 2008;40:415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Fairhall SL, Ishai A. Effective Connectivity within the Distributed Cortical Network for Face Perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Liu J, Huber DE, Rieth CA, Tian J, Lee K. Detecting faces in pure noise images: a functional MRI study on top-down perception. NeuroReport. 2008;19:229–233. doi: 10.1097/WNR.0b013e3282f49083. [DOI] [PubMed] [Google Scholar]
- 11.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 12.Lowe MJ, Mock BJ, Sorenson JA. Functional Connectivity in Single and Multislice Echoplanar Imaging Using Resting-State Fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 13.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Peter F. Spontaneous Low-Frequency BOLD Signal Fluctuations: An fMRI Investigation of the Resting-State Default Mode of Brain Function Hypothesis. Hum Brain Map. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Jiang T, Yu C, Tian L, Li J, Liu Y, et al. Spontaneous activity associated with primary visual cortex: a resting-state FMRI study. Cereb Cortex. 2008;18:697–704. doi: 10.1093/cercor/bhm105. [DOI] [PubMed] [Google Scholar]
- 19.Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. NeuroImage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N. reponse properties of the human fusiform face area. Cogn Neuropsychol. 2000;17:257–279. doi: 10.1080/026432900380607. [DOI] [PubMed] [Google Scholar]
- 21.Ishai A. Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Ishai A, Haxby JV, Ungerleider LG. Visual imagery of famous faces: effects of memory and attention revealed by fMRI. Neuroimage. 2002;17:1729–1741. doi: 10.1006/nimg.2002.1330. [DOI] [PubMed] [Google Scholar]
- 23.Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci U S A. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia. 2007;45:163–173. doi: 10.1016/j.neuropsychologia.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- 26.Mechelli A, Price CJ, Friston KJ, Ishai A. Where Bottom-up Meets Top-down: Neuronal Interactions during Perception and Imagery. Cereb Cortex. 2004;14:1256–1265. doi: 10.1093/cercor/bhh087. [DOI] [PubMed] [Google Scholar]
- 27.Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]