Abstract
γ-Glutamyl transpeptidase (GGT) plays key roles in glutathione homeostasis and metabolism of glutathione S-conjugates. Rat GGT is transcribed via five tandemly arranged promoters into seven transcripts. The transcription of mRNAV is controlled by promoter 5. Previously we found that GGT mRNAV-2 was responsible for the induction of GGT in rat alveolar epithelial cells by 4-hydroxynonenal (HNE). In the current study, the underlying mechanism was investigated. Reporter deletion and mutation analysis demonstrated that an electrophile-response element (EpRE) in the proximal region of GGT promoter 5 (GP5) was responsible for the basal- and HNE-induced promoter activity. Gel-shift assays showed an increased binding activity of GP5 EpRE after HNE exposure. The nuclear content of NF-E2-related factor 2 (Nrf2) was significantly increased by HNE. The recruitment of Nrf2 to GP5 EpRE after HNE treatment was demonstrated by supershift and chromatin immunoprecipitation assays. The tissue expression pattern of GGT mRNA V was previously unknown. Using polymerase chain reaction, we found that GGT mRNAV-2 was expressed in many tissues in rat. Taken together, GGT mRNAV-2 is widely expressed in rat tissues and its basal and HNE-induced expression is mediated through EpRE/Nrf2 signaling.
Keywords: γ-Glutamyl transpeptidase, 4-Hydroxy-2-nonenal, Glutathione, EpRE, Nrf2, Tissue distribution, Free radicals
Introduction
γ-Glutamyl transpeptidase (γ-glutamyl transferase, GGT), an enzyme located on the outer surface of the plasma membrane, catalyzes the transfer of the γ-glutamyl moiety from glutathione (GSH), glutathione S-conjugates, or other γ-glutamyl compounds to γ-glutamyl acceptors such as amino acids, dipeptides, or H2O. GGT plays key roles in the maintenance of glutathione homeostasis [1–7] and metabolism of biomolecules such as leukotriene C4 and xenobiotics following their conjugation with GSH [8].
Many substances, especially those that generate reactive oxygen/nitrogen species (ROS/RNS) and/or perturb the redox balance, can increase GGT expression in various cells and tissues. These substances include aflatoxin B1 [9], NO2 [10], redox cycling quinones [11–14], and hyperoxia [15]. Induction of GGT by oxidative stress facilitates GSH turnover, de novo GSH synthesis, and the metabolism and detoxification of GSH conjugates and therefore is an important adaptive response that protects cells from oxidative and xenobiotic injury. However, the mechanism underlying the induction of GGT in response to oxidative stress remains unclear.
We recently reported that GGT could be induced by 4-hydroxynonenal (HNE). HNE is a major α, β-unsaturated aldehyde end product derived from peroxidation of ω-6 polyunsaturated fatty acids such as linoleic acid and arachidonic acid. The concentration of HNE is markedly increased under conditions of oxidative stress [16–22]. The biologic significance of HNE was first recognized to be as a secondary toxicant that was responsible for the “action at a distance”-damage caused by free radicals [23,24] and as a biomarker of oxidative stress [25]. Now it is clear that HNE can function as a signaling molecule at physiological concentrations by activating various signal transduction pathways and inducing expression of many genes, including phase II and antioxidant genes [26]. Thus, we use HNE as a specific agent although it also serves as a model compound to investigate the mechanism of expression of oxidative stress-induced genes, such as GGT.
Rat GGT has a single copy of the gene, the expression of which is regulated by five tandemly arranged promoters that generate seven transcripts with different 5′-untranslated regions (5′-UTR) but the same protein coding sequence [27]. Of the seven types of rat GGT mRNAs, several have been found to be increased in response to oxidative stress, including mRNA II and IV in the initial segment of epididymis [28] and types I and III in whole lung [10,29]. GGT mRNA V was first identified in undifferentiated rat hepatoma cells, and its transcription was regulated by the most distal promoter—promoter 5. With alternate splicing, two types of mRNA V, V-1 and V-2, are generated by GGT promoter 5 (GP5) [30]. Compared to V-1, V-2 does not have the 252 nucleotides (nt) corresponding to the second exon for the mRNA IV and V-1. Previously, we reported that mRNA V-2 was one of the major types of GGT and was induced by 4-hydroxynonenal in rat lung epithelial cells (L2 cells) [31]. The underlying mechanism of its induction by HNE, however, remains unclear. Furthermore, the tissue distribution of mRNA V has not been previously described.
The rat GP5 contains several putative consensus transcription factor-binding sequences, including 12-O-tetradecanoyalphorbol-13 acetate-response elements (TRE; also called the AP-1-binding element) and an electrophile-response element (EpRE; also called antioxidant-response element, or ARE). Both TRE and EpRE are involved in the redox regulation of many genes in response to ROS/electrophiles [32–35]. Nonetheless, which cis-acting element is involved in HNE-induced expression of the GGT promoter 5 is unknown. Therefore, in this study we investigated the cis elements required for HNE induction of GGT mRNA V-2 expression. By using reporter deletion/mutation strategies, we show that the EpRE sequence in the proximal region of GP5 is required for the basal and HNE-induced gene expression. We also demonstrated that NF-E2-related factor 2 (Nrf2), the redox sensitive transcription factors involved in the induction of many EpRE-mediated antioxidant and phase II genes [36,37], is activated by HNE and its recruitment to GP5 EpRE was significantly increased upon HNE exposure. Furthermore, we found that GGT mRNA V-2 is widely expressed in various rat tissues, suggesting the potential role of EpRE/Nrf2 signaling in oxidative stress-mediated GGT induction in these tissues.
Methods and materials
Chemicals and reagents
Unless otherwise noted, all chemicals were from Sigma (St. Louis, MO). HNE was purchased from Cayman Chemical (Ann Arbor, MI). Antibodies for Western Blotting and supershift EMSA were from Santa Cruz (Santa Cruz, CA). Rat tissue RNA samples and DNA-free were from Ambion (Austin, TX); TaqMan reverse transcription reagent and SYBR Green PCR Master Mix were from Applied Biosystems (Foster City, CA). pGL3 luciferase reporter vectors, competent cells, gel-shift assay kit, luciferase activity assay kit and restriction enzymes were from Promega (Madison, WI). FuGENE 6 transfection reagent was from Roche (Indianapolis, IN). M-PER mammalian protein extraction reagent and NE-PER nuclear extraction reagents were from Pierce (Rockford, IL). QuickChange site-directed mutagenesis kit was from Stratagene (La Jolla, CA). The ChIP (chromatin immunoprecipitation) assay kit was from Upstate Cell Signaling Solutions (Lake Placid, NY). All chemicals used were at least analytical grade.
Cell culture and treatments
L2 cells (from the American Type Culture Collection) were cultured in F-12K medium (Life Technologies, Grand Island, NY) supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified incubator containing 5% CO2 at 37°C.
HNE was dissolved in ethanol, and the final concentration of ethanol in the medium was 0.05%. L2 cells were treated at about 90% confluence. Cells were rinsed with cold PBS before being harvested with rubber policemen.
GGT mRNA assay
Total RNA was extracted using TRIzol reagent and treated with DNA-free reagent according to manufacturers’ protocols. DNA-free RNA samples were reverse-transcribed using the TaqMan reverse transcription system and real-time polymerase chain reactions (PCR) were run with a Cepheid 1.2 real-time PCR machine. Briefly, 5 µl of reverse transcription reaction product was added to PCR tube containing 12.5 µl SYBR Green PCR Master Mix and the primer pair specific for GGT mRNA V. The total PCR was 25 µl. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control (25 µl PCR: 2.5 µl reverse transcription reaction product, 12.5 µl SYBR-Green PCR Master Mix, primers, and water). The primer pair for GAPDH is forward 5′-ACCCCCAATGTATCCGTTGT-3′, reverse 5′-TACTCCTTGGAGGCCATGT-3′; GGT mRNA V primers are forward 5′-GAAACGGTGGGGGAACTGAGAC-3′, reverse 5′-ACACTCTGCGGAAACCCACA-3′. β-Actin primer pairs are forward 5′-CGTAAAGACCTCTATGCCAACA-3′ and reverse 5′ TAGGAGCCAGGGCAGTAATC-3′. The specificity of PCR product of GGT mRNA V-2 was confirmed by DNA sequencing.
Plasmids
DNA fragments of GGT promoter 5 were amplified by using primer pairs with a specific nuclease digestion site (underlined), and DNA from L2 cells was used as the PCR template. The reverse primer for all DNA fragments is 5′-GCTAGATCTTGTCTTTGTGCTACTG-3′ (BglII), and the forward primers are as follows: GP5 (−645/+18), 5′-CTACGCGTGGATGGATAGATAGATA-3′ (MluI); GP5 (−421/+18), 5′ CTACGCGTTAGGAAGTGATGGTT-3′ (MluI); GP5 (−355/+18), 5′-GCACAGCTACGCGTCCCACATGCTTCCTTCT-3′ (MluI); GP5 (−305/+18), 5′-GCACAGCTACGCGTCTGAGCGAAGCCTACTA-3′ (MluI); GP5 (−255/+18), 5′-GCACAGCTACGCGTGGAAAACCCAACAATC-3′ (MluI); GP5 (−152/+18), 5′-GCACAGCTACGCGTCCTTGCATGCCCTCTA-3′ (MluI); GP5 (−99/+18), 5′-GCACAGCTACGCGTACCAGATGCTCCCACTCT 3′ (MluI); GP5 (−55/+18), 5′-GCACAGCTACGCGTAGGGGCTGAGTAAACAT-3′ (MluI). The GP5-Luc plasmids were constructed by ligating the MluI-BglII fragments to pGL3 basic or promoter luciferase reporter vectors (Promega), and the DNA sequence of constructs was confirmed by DNA sequencing. GP5 luciferase reporters containing GP5 EpRE were made by inserting PCR-amplified double-stranded oligonucleotides into pGL3 promoter luciferase reporter vector after digestion with specific enzymes. The oligonucleotides were (underline shows nuclease digestion site, and lower case nucleotides were mutated ) wild-type, forward 5′-CCATCTCCTACGCGTACCCACAATGACACAGCAAGAAAGCCT-3′ (MluI) and reverse 5′-CCATCTCGCTAGATCTAGGCTTTCTTGCTGTGTCATTGTGGGTAC (BglII); GP5 EpRE M1-Luc, forward 5′-CCATCTCCTACGCGTACCCACAAgGACACAGCAAGAAAGCCT-3′ (MluI) and reverse 5′-CCATCTCGCTAGATCTAGGCTTTCTTGCTGTGTCcTTGTGGGTAC (BglII); GP5 EpRE M2-Luc, forward 5′-CCATCTCCTACGCGTACCCACAATGACACATAAAGAAAGCCT-3′ (MluI) and reverse 5′-CCATCTCGCTAGATCTAGGCTTTCTTtaTGTGTCATTGTGGGTAC (BglII).
Site-directed mutagenesis of GGT-Luciferase vector
The site-directed mutagenesis of GGT-Luciferase vector used GP5 (−645/+18)-Luc as the template and followed the protocol provided with the QuikChange site-directed mutagenesis kit (Stratagene). The forward primers (reverse not shown) containing the desired mutations are GP5 (−645/+18)-Luc M1, 5′-GTACCCACAAgGACACAGCAAGAAAGCCT-3′ and M2, 5′-GTACCCACAATGACACAtaAAGAAAGCCT-3′ (lower case nucleotides were mutated). Briefly, the mutagenic oligonicleotide primer pairs were added into 50 µl of reaction mixture containing template, and the reaction was amplified in a thermal cycler for 12 cycles. Then 1 µl of DpnI restriction enzyme was added into the reaction, mixed and incubated at 37°C for 1 h to digest the parental dsDNA. One microliter of DpnI-treated DNA was used to transform XL1-Blue competent cells. Clones were selected, and mutagenesis was confirmed by DNA sequencing.
Transfection procedure and assay of Luciferase and β-galactosidase activity
Cells (70–80% confluence) were transfected with plasmids by using FuGENE 6 transfection reagent (Roche), and β-galactosidase plasmid (1/10 of total amount of plasmids) was cotransfected as an internal control. Twelve hours after transfection, the medium was replaced; 24 h later, the cells were treated with/without HNE. The cell pellet was lysed with M-PER mammalian protein extraction reagent (Pierce) and centrifuged at 12,500g for 5 min. The supernatant was then used for determination of the activity of luciferase and β-galactosidase.
To determine the β-galactosidase activity, 25 µl of the supernatant was added to a reaction mixture containing 300 µM 4-methyllumbelliferyl β-d-galactoside. After the mixture was incubated at room temperature for 20 min with shaking, β-galactosidase activity was determined in a fluorescence microplate reader at an excitation wavelength of 360 nm and an emission wavelength of 450 nm.
For the luciferase assay, a luciferase assay kit (Promega) was used. Briefly, 20 µl of cell lysate (see above for preparation) was added to the reaction mixture provided in the kit, and the luciferase activity was determined in a luminometor. The final luciferase activity was normalized with the activity of cotransfected β-galactosidase.
Western blotting
Western blotting was performed as described previously [38]. Briefly, protein was extracted, and 25 µg protein was heated for 15 min at 95°C in a 2× loading buffer containing SDS (Tris base, pH 6.5, glycerol, DTT, and pyronin Y), electrophoresed under denaturing conditions on a 10% Trisglycine acrylamide gel (Invitrogen, Carlsbad, CA), and then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore, Bedford, MA). Membranes were blocked with 5% fat-free milk at room temperature for 1 h, and then incubated overnight at 4°C with appropriate primary antibody in 5% milk in Tris-buffered saline (TBS). After being washed with Tris-buffered saline containing 0.05% Tween 20 (TTBS), the membrane was incubated with appropriate secondary antibody at room temperature for 2 h. After TTBS washing, the membrane was treated with an enhanced chemiluminescence (ECL Plus; Amersham, Arlington Heights, IL) reagent mixture for 5 min. The target bands were imaged on a Kodak Image Station 2000R.
Electrophoretic mobility-shift assay (EMSA)
EMSA was performed as described previously [35]. Briefly, nuclear extracts were prepared from L2 cells treated with or without HNE by using NE-PER nuclear extraction reagent. An aliquot of 8 µg nuclear extract was preincubated in a gel-shift-binding reaction containing 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 4 mM DTT, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5), and 0.2 µg poly(dI-dC) at room temperature for 10 min before γ-[32P]ATP end-labeled double-stranded oligonucleotides were added; then samples were incubated for an additional 20 min at room temperature before electrophoresis. For specificity analysis, 100-fold molar excesses of unlabeled oligonucleotide competitors were added and preincubated for 10 min before radiolabeled oligonucleotides were added. The gel-shift reaction was electrophoresed on 6% DNA retardation gel (Invitrogen) and dried. The gels were then scanned with the Cyclone Storage Phosphor System, and the total counts were quantified with OptiQuant image analysis software. The forward oligonucleotides used for EMSA were as follows: EpRE of GP5, 5′-GTACCCACAATGACACAGCAAGAAAGCCT-3′; M1 of GP5 EpRE, 5′-GTACCCACAAgGACACAGCAAGAAAGCCT-3′; M2 of GP5 EpRE, 5′GTACCCACAATGACACAtaAAGAAAGCCT3′.
In the supershift assay, after the addition of γ-[32P]ATP-labeled oligonucleotides, the mixture was incubated for 20 min at room temperature before the antibody against Nrf2 was added. Then the reaction was incubated at room temperature for 1 h before being electrophoresed. An antibody to the p65 component of nuclear factor κB was used to demonstrate that the supershift was not a result of nonspecific interaction.
ChIP assay
ChIP assays were performed following a protocol provided with the kit (Upstate Cell Signaling Solutions). Briefly, cells were incubated with formaldehyde by directly adding it into the medium (final concentration, 1%) at room temperature for 10 min. Then the cell pellet was lysed on ice for 10 min and sonicated under conditions that cause DNA to be broken into 200-to 800-bp fragments. Sonicated cell lysate was precleared with 75 µl of salmon sperm DNA/agarose. One hundred micrograms of the supernatant was used to isolate DNA as the internal control, and 3 mg was used for immunoprecipitation with antibodies to specific transcription factors overnight at 4°C. The protein/DNA complex was eluted from agarose in elution buffer and the DNA/protein complex was reversed by adding 5 M NaCl and incubating the mixture at 65°C for 4 h. The DNA was extracted with phenol:chloroform:isoamyl alcohol (25:24:1). Primers used for PCR in the ChIP assay were forward, 5′-CAGTACGTGGAAATCCTTATCA-3′; and reverse, 5′-GTGGA ATAGAGTGGGAGCAT-3′.
Statistical analysis
SigmaStat software was used for statistical analysis, and statistical significance was accepted when P < 0.05. Comparison of variants between experimental groups was performed with ANOVA and the Tukey test.
Results
Expression of GGT mRNA V in rat tissues
Transcription of the GGT gene from its distal promoter (GP5) was first detected in rat HTC and H5 hepatoma cells [30]. Promoter 5 generates an mRNA V primary transcript that, by alternative splicing of exon 2, giving rise to the V-1 and V-2 mature transcripts. mRNA V-1 contains exon 2 and is 252 nt longer than mRNAV-2 (Fig. 1A). To determine the distribution of mRNA V in normal rat tissues, specific PCR primers were designed both to target GGT mRNA V and to produce amplicons of 440 and 188 nt for V-1 and V-2, respectively. As shown in Fig. 1B, after 40 cycles of PCR, mRNA V-2 was detected in rat lung, spleen, ovary, testicle, and embryo; its expression was relatively lower in liver, kidney, and brain; and it was undetectable in heart and thymus. Among all the tissues examined, lung expresses the highest level of GGT mRNAV-2. The amplification of GGT mRNA V-1, which was expected to produce a 440-nucleotide amplicon, was not detected in any of the tissues examined, revealing that GGT mRNA V-2 is the predominant type V mRNA in these tissues.
Fig. 1.
Tissue distribution of rat GGT mRNA V. (A) Schematic representation of the two GGT V cDNA sequences. Black boxes represent the reading frame, gray boxes correspond to the 5′-UTR region common to all rat GGT mRNAs, the open box corresponds to the sequence found in the mRNA IV-1, and the dark gray boxes correspond to the sequence specific for cDNAs V-1 and V-2. (B) Specific primer pair against both rat GGT cDNAV-1 and V-2 was designed (as mapped in panel A), and PCR was performed by using total RNA from assorted rat tissues as templates after reverse transcription; β-actin was used as an internal control of PCR.
Increase of GGT mRNA V-2 by HNE in L2 cells
Previously, we have demonstrated that GGT gene expression was increased by HNE in L2 cells, and GGT mRNA V-2 was the major type of GGT mRNA being induced [31]. To further examine the mechanism of GGT mRNA V-2 induction by HNE, we first determined its expression pattern in response to HNE. The physiological concentration of HNE in the plasma is reported to be less than 1 µM [23,39], but its concentration can reach as high as 10 µM to 5 mM in plasma membrane under conditions of oxidative stress [40,41]. HNE concentrations used in this study were between 5 and 20 µM. At this level, HNE did not cause any morphological changes or affect the viability of L2 cells as determined by the trypan blue exclusion method (data not shown).
Exposure of L2 cells to increasing concentrations of HNE resulted in a dose-dependent induction in a steady-state mRNA V-2 level up to 4.5-fold over basal level in cells treated with 20 µM HNE for 12 h (Fig. 2). The transcription was significantly increased by 5 µM HNE, and the induction was maximal in cells treated with 20 µM HNE. The response was also time dependent, and the maximal induction was obtained after a 12-h HNE exposure. To achieve a degree of induction sufficient for mechanism studies, 15 µM HNE was used in the subsequent experiments.
Fig. 2.
Increase of GGT mRNA V-2 by HNE in L2 cells. RNA from L2 cells treated with/without HNE for 6 to 24 h as indicated was reverse-transcribed and real-time PCR was performed. n = 3, * p < 0.05, ** p < 0.01 compared with vehicle control.
Functional analysis of GP5
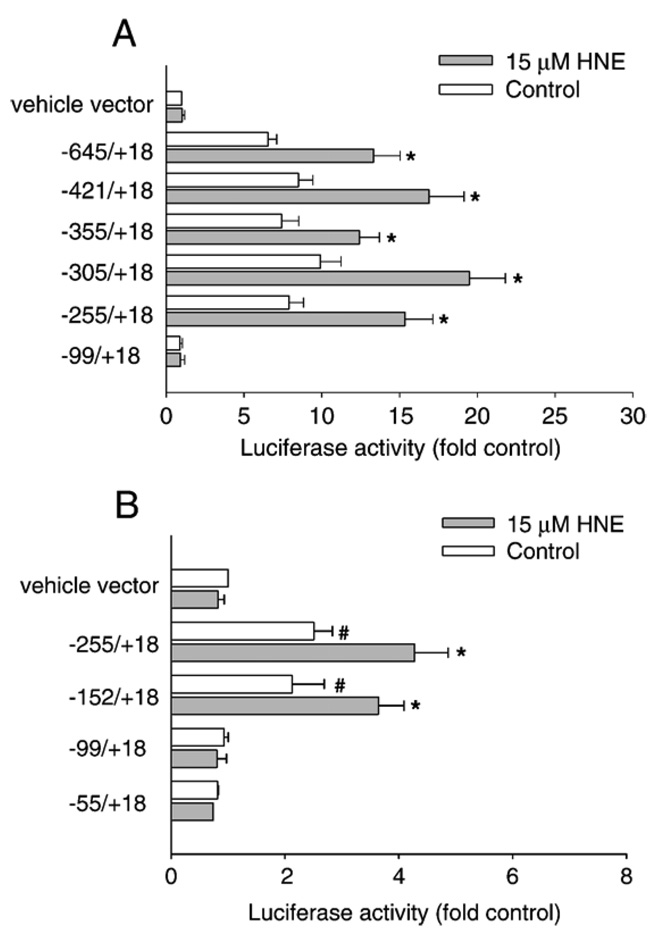
The transcription of GGT mRNA V-2 occurs from promoter 5, which contains several potential cis-acting elements in its proximal region, including several TRE sites and a consensus EpRE sequence (Fig. 3). To localize the regions or cis elements within GGT promoter 5 (GP5) that control the basal and HNE-inducible expression of GGT mRNA V-2, a series of GGT promoter 5/luciferase reporters were generated by inserting various lengths of DNA fragments derived from GGT promoter 5 into promoterless pGL-3 basic luciferase vector. L2 cells were transiently transfected with these constructs, and luciferase activity was determined in the absence and presence of 15 µM HNE. Luciferase activity of cells transfected with GP5 (−99/+18)-Luc was the same as that detected in cells transfected with pGL3-basic vector, and this construct did not respond to HNE. The extension of the sequence up to the nucleotide −255 in the GP5 (−255/+18)-Luc construct induced an 8-fold increase in the basal luciferase activity, which was further increased by about 2-fold in response to HNE treatment. Further extension of the GGT promoter sequence from −255 to −645 did not significantly increase the basal and HNE-inducible luciferase activity (Fig. 4A). These data suggest that the DNA fragment from −99 to −255 contains regulatory elements controlling both basal- and HNE-induced expression of GP5 in L2 cells.
Fig. 3.
Sequence of the proximal region of GP5. Consensus sequences for putative regulatory elements are indicated. The transcription start site is assigned the +1 position, and all other sequences have been numbered relative to this site. STRE, stress-response element; C/EBP, CCAAT-enhancer binding protein; SP-1, specificity protein 1; AP-2 activating protein 2.
Fig. 4.
Identification of regions in rat GGT promoter that is required for HNE responsiveness. A series of constructs were generated by cloning different lengths of DNA fragments derived from the proximal region of GP5 into promoterless pGL-3 basic luciferase vector (A) or pGL-3 promoter luciferase vector (B). Cells were transiently transfected with reporter vectors and then treated with or without HNE. Luciferase activity was determined 24 h after HNE treatment. Transfection efficiency was controlled by normalization with cotransfected β-galactosidase. n = 3, *i < 0.01 compared with vehicle control, #p < 0.01 compared with vehicle vector.
To further delineate the regulatory elements from −99 to −255, we deleted the promoter region downstream of −255 and cloned the deleted DNA fragments into a pGL-3 promoter vector. This luciferase vector is driven by an SV-40 promoter and is usually used to study enhancer effects. Consistent with results with pGL-3 basic vector constructs, luciferase activity was induced by HNE in cells transfected with GP5 (−155/+18)-pro-Luc but not in cells transfected with GP5 (−99/+18)-pro-Luc. This further reveals that the HNE-responsive elements were located from −99 to −155. When comparing luciferase expression with that in cells transfected with vehicle control vector (pGL-3 promoter vector), we found that this region also had the basal promoter activity (Fig. 4B).
Analysis of nucleotides containing the GP5 EpRE
Analysis of the nucleotide sequence between −99 and −155 of GP5 revealed the presence of a consensus EpRE core sequence (TGACACAGC) between −109 and −117. Because EpRE has been demonstrated to be responsible for the induction of phase II genes by electrophiles, including HNE, we considered this element a candidate enhancer of GP5. To test this hypothesis, constructs were made by cloning the GP5 sequence from −100 to −127 containing the consensus EpRE (TGACACAGC), as well as two mutated forms, M1 (GGACACAGC) and M2 (TGACACAAT), into pGL-3 promoter vector. When transfected into L2 cells, the construct containing the wild-type EpRE caused a significant increase in luciferase activity compared to that of cells transfected with vehicle vector, whereas vectors with the mutated EpRE failed to increase expression (M1 and M2; Fig. 5A). The EpRE sequence caused an increase in promoter activity even in the absence of added HNE, which we call basal expression. HNE treatment of L2 cells resulted in a 3-fold induction of luciferase activity only in cells transfected with GP5-EpRE-Luc, which contained the wild-type EpRE sequence, but not in cells transfected with the PGL3 luciferase vector alone or vectors containing the mutated form of EpRE (Fig. 5B).
Fig. 5.
Mutation analysis of EpRE in rat GP5. (A) Nucleotide sequence of oligonucleotides used to examine EpRE activity; mutated nucleotides are underlined. (B) Mutation in GP5 EpRE core sequence abrogated its HNE responsiveness. L2 cells were transfected with luciferase reporters generated by cloning oligonucleotides containing wild-type and mutated EpREs into pGL-3 promoter luciferase vector. (C) Mutation assay at whole gene level. Site-directed mutagenesis was performed with GP5 (−645/+18)-Luc as the template, and constructs were transient-transfected into L2 cells. n = 3, *p < 0.01 compared with vehicle control, #p < 0.01 compared with vehicle vector.
To further confirm the regulatory role of GP5 EpRE in vivo, site directed mutagenesis of the core EpRE sequence in GP5 (−645/+18)-Luc was introduced, i.e., from wild TGA XXXXGC to M1 GP5 (−645/+18)-Luc, GGAXXXXGC, and M2 GP5 (−645/+18)-Luc, TGAXXXXTA. As shown in Fig. 5C, the mutations completely abrogated the basal promoter activity of GP5 (−645/+18)-Luc and its response to HNE, suggesting that EpRE in GGT promoter 5 between −109 and −117 was responsible for both basal- and HNE-induced promoter activity.
Interaction of GP5 EpRE with DNA-binding proteins in vitro
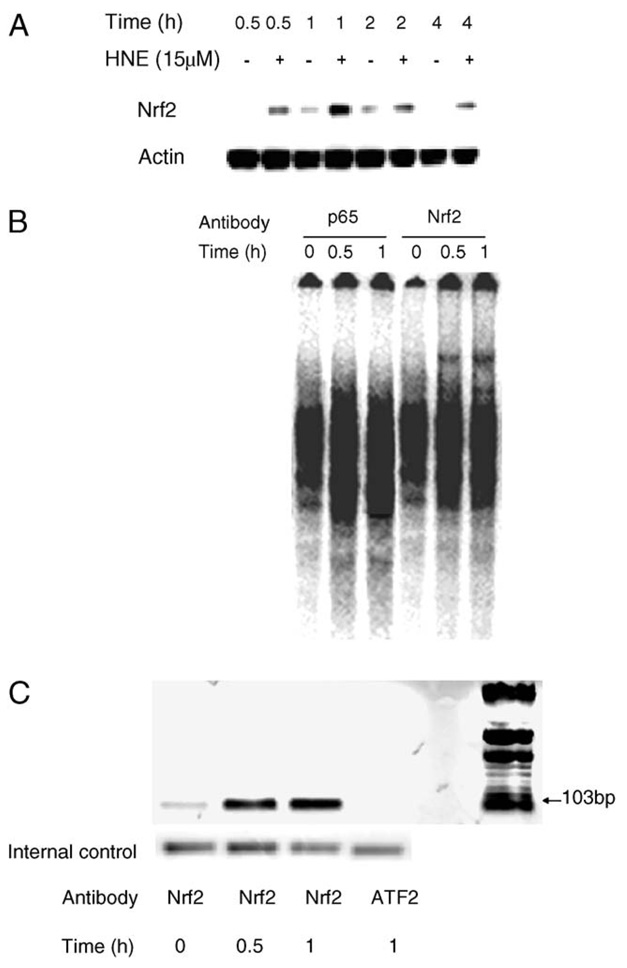
We next investigated the protein/DNA-binding activity of GP5 EpRE in gel-shift assay experiments. As shown in Fig. 6, a DNA/protein-binding complex was detected in nuclear extracts prepared from nontreated L2 cells, and this complex was significantly increased in intensity upon HNE treatment. The binding of this complex appeared to be specific because binding was abrogated by the addition of an excess amount of the wild-type oligonucleotide but not by the addition of nonspecific competitors (nuclear factor κB oligonucleotides) or a mutated form of GP5 EpRE (M1). It was noteworthy that the mutated EpRE M2 was able to outcompete the labeled wild-type EpRE, although not as efficiently as the cold wild-type EpRE did, suggesting that binding was diminished but not eliminated by this point mutation. Nonetheless, luciferase reporter containing this mutated EpRE could not drive promoter activity (Figs. 5B and 5C). The gel-shift data reveal a specific protein binding on the EpRE element of GP5 in L2 cells that is increased in response to HNE.
Fig. 6.
EMSA of GP5 EpRE probe with nuclear extracts from L2 cells. Nuclear extracts were prepared from L2 cells treated with/without 15 µM HNE for the indicated times. Synthetic oligonucleotides containing GP5 EpRE were annealed, end-labeled, and then incubated with nuclear extract. The DNA/protein complex was analyzed with EMSA. Representative image was generated by the Cyclone Storage Phosphor System from one typical experiment (top) and the semiquantitative graphical summary (bottom) was based on radioactivity counts generated by OptiQuant image analysis software. n = 3, *p < 0.01 compared with vehicle control; #p < 0.01 compared with the intensity of EpRE complex of 1 h after HNE treatment. N, nuclear factor κB oligonucleotides; C, cold GP5 EpRE; M1 and M2, mutation forms of GP5 EpRE.
Recruitment of Nrf2 to GP5 EpRE upon HNE exposure
Knowing EpRE is responsible for the HNE-induced GGT mRNA V-2 expression, we next tested whether Nrf2, the most frequent transcription factor that is involved in EpRE activation, is also involved in HNE-mediated GP5 EpRE complex formation. According to the dominant current thought, most Nrf2 is sequestered in the cytosol by Keap1 under unstimulated conditions. Upon stimulation with oxidants or electrophiles, Nrf2 dissociates from Keap1 and is translocated to the nucleus [42,43]. Thus, activation of Nrf2 would result in its increased nuclear content and increased binding to EpRE. We first determined the nuclear content of Nrf2. As shown in Fig. 7A, Nrf2 in the nucleus was significantly increased after HNE treatment. The maximal Nrf2 content was attained at 1 h after stimulation, and then it was decreased. Using supershift EMSA, we then investigated the possible binding of Nrf2 to GP5 EpRE. Nrf2 antibody picked up a significant shift of EpRE/protein complex. In a control, using antibody to the p65 subunit of NF-κB, no shift band was observed (Fig. 7B), suggesting that the Nrf2 shift band is not due to a nonspecific reaction.
Fig. 7.
HNE increased Nrf2 nuclear content and its recruitment to GP5 EpRE. Three experiments were performed and one typical result is shown here. (A) Nuclear content of Nrf2 was increased after HNE treatment. L2 cells were treated with/without 15 µM HNE for indicated times, nuclear extracts were prepared, and then Western blotting was performed. (B) Supershift assays of Nrf2. Eight micrograms of nuclear extract and 4 µg of Nrf2 antibody were used in the supershift EMSA assay. The DNA/protein complex was analyzed with EMSA. Representative image was generated by the Cyclone Storage Phosphor System. P65: antibody to the p65 subunit of NF-κB protein. (C) Increased recruitment of Nrf2 to GP5 EpRE in vivo after HNE exposure. L2 cells were stimulated with 15 µM HNE for indicated times, and then the cells were fixed with 1% formaldehyde. After being lysed, the cell lysate was sonicated to shear the chromatin into 0.2- to 0.8-kb fragments, which were then immunoprecipitated with 4 µg of anti-Nrf2 antibody. Nrf2-coprecipitating DNA was analyzed by PCR with specific primers amplifying EpRE-containing fragment of GGT promoter 5. Content of the PCR products amplified from the total DNA sample from the samples before immunoprecipitation was used as the internal control.
To confirm the recruitment of Nrf2 to GP5 EpRE in vivo, we performed ChIP assays. Fig. 7C shows that there was a slight binding of Nrf2 to GP5 EpRE under unstimulated conditions and that HNE treatment significantly increased the binding of Nrf2 to GP5 EpRE. Overall, our data suggest that Nrf2 is activated and binds to GP5 EpRE upon HNE exposure.
Discussion
Rat GGT has seven potential types of transcripts that are expressed in a cell-/tissue-specific pattern [27]; for example, mRNAs I and II are mainly expressed in kidney, whereas IV is mainly expressed in small intestine and epididymis. mRNA V was first identified in rat undifferentiated hepatoma H5 and HTC cells [30]. Our results here show that GGT mRNA V (V-2) was expressed predominantly in rat lung, spleen, ovary, testicle, and embryo and to a lesser extent in liver, kidney, and brain but not in heart and thymus (Fig. 1B). The highest level of GGT mRNA V-2 expression was found in the adult lung, where the GGT mRNA III expression has been reported previously [29]. Considering the critical roles of GGT in GSH homeostasis and metabolism of GSH S-conjugates, the wide tissue distribution of mRNA V suggests that it may play an important role in protecting cells from oxidative injury.
We have shown that the EpRE localized between −109 and −117 upstream of the transcription start site in the proximal regulatory region of rat GGT promoter 5 (GP5 EpRE) was responsible for the basal promoter activity of GP5 in L2 cells. In this cell line, we observed that the AP-1/hepatocyte nuclear factor (HNF) 3 overlapping site located between −27 and −48, which was reported to be essential for the basal expression of GGT mRNA in undifferentiated hepatoma H5 cells [30], is not involved in the basal activity of GP5. HNF3 is a member of the forkhead winged helix family, and its function is often controlled by regulatory factors which have binding sites overlapping with HNF3 motif [44]. In the aldose B gene promoter, for example, HNF3 restrained the binding of HNF1 to the overlapping binding site and decreased the gene expression [45]; in the human glucose transporter type 2 gene, interaction of HNF1 and HNF3 at the overlapping site increased promoter activity [46]. Interaction between a specific member of the AP-1 family and HNF3 β factor at a composite site activated the expression of the CC-10 gene in the NCIH441 adenocarcinoma cells; however, in another lung adenocarcinoma cell line that expressed HNF3 α instead of HNF3 β, CC-10 was silent [47]. A potential reason that the AP-1/HNF3-binding site between −27 and −48 is not involved in basal promoter activity in L2 cells is that the expression or posttranslational modification of HNF3-binding proteins is different between rat hepatoma H5 cells and lung epithelial L2 cells. It may also be possible that HNF3 functions as a potentiator that modulates the effect of other transcription factors [48]. In other words, HNF3 may occupy the binding site but not be sufficient to activate gene expression without the binding of other transcription factors to their respective regulatory elements. A precise description of the proteins binding to the AP-1/HNF3 overlapping site, as well as their cellular distribution in L2 cells and hepatoma H5 cells, would help to elucidate the mechanism underlying GGT mRNA V basal expression in different cell lines; however, this is beyond the scope of the current investigation.
Our results demonstrated that GP5 promoter activity was induced by the electrophile HNE in L2 cells in time- and dose-dependent manner and that the region between −99 and −155 in the promoter/enhancer sequence was responsive to HNE. Mutations of the candidate EpRE (M1 and M2) abrogated HNE-induced promoter activity. Both results reveal that EpRE is the cis-acting element responsible for HNE-mediated mRNA V-2 induction. Several TRE (AP-1 binding) sequences are also found in the proximal region of promoter 5 (Fig. 3). Although a TRE adjacent to EpRE was reported to be involved in regulation of catalytic subunit of glutamate cysteine ligase (GCLc) [49–51], it appears that adjacent TRE sites were not involved in basal or HNE-induced GP5 expression. In the gel-shift experiment, HNE treatment resulted in increased intensity of GP5 EpRE/ protein-binding complex, and this increase was concordant with the increased HNE responsiveness of the EpRE-driven constructs in the luciferase reporter assay. Thus, a correlation between the binding of specific proteins to the EpRE sequence and the HNE-induced gene expression was observed. We also noted a binding to this element in the absence of HNE treatment, and this binding can be associated with an effect of the EpRE observed under basal conditions. The promoter region from −98 to −150, which contains this EpRE, also contributes to the basal level of GP5 in hepatoma cells [30]; however, the specific contributions of the EpRE to this activity have not been demonstrated.
Being similar to typical EpRE sequences from other genes, GP5 EpRE (TGACACAGC) is composed of embedded TRE and GC boxes. Mutation of the GC box (M2) resulted in the loss of HNE responsiveness of the reporter gene, although the ability of this sequence to compete with wild-type EpRE in the gel-shift assay remained. This suggests that the GC box in GP5 EpRE is essential for the responsiveness of this EpRE to HNE. Because the GC box was not required for AP-1 binding but was critical for the induction of gene expression via EpRE [52,53], the motif responsible for both basal and HNE-inducible promoter activity appeared to be EpRE rather than the embedded AP-1-binding sequence. This is consistent with a previous report on the roles of the GC box in the EpRE of modulatory subunit of glutamate cysteine ligase (GCLm) [54], in which a GC box mutation abrogated β-naphthoflavone-induced EpRE function and had reduced binding efficiency. Studies on the role of the GC box in constitutive gene expression, however, are controversial. Similarly to what was observed here with rat GGT promoter 5, deletion of the GC box abrogated EpRE-mediated basal expression of the rat GSH S-transferase Ya [55] and cystine/glutamate exchange transporter [56]; however, deletion of the GC box had no effect on the constitutive expression of hGCLm and mice heme oxygenase-1 (HO-1) [54,57].
In this study, we also found an increased nuclear content and EpRE binding of Nrf2 after HNE stimulation (Fig. 7). Nrf2 is a basic leucine zipper protein. Its role in oxidant defense has been well demonstrated in Nrf2-deficient mice. These mice express decreased levels of various phase II enzymes and antioxidant genes and are extremely susceptible to oxidant-induced injuries [58–62]. It is now clear that the Nrf2 activity is precisely regulated. According to current thinking, in the resting state, most Nrf2 is sequestered in the cytoplasm through its association with Keap1, which anchors to the cytoskeleton [63,64]. It was found that Keap1 could function as ubiquitin-E3 ligase and mediate the rapid turnover of Nrf2 by proteosomal degradation under normal physiological conditions [42,43]. Upon exposure to stress, Nrf2 dissociates from Keap1 and thereby escapes from degradation. It is then translocated to the nucleus to form heterodimers with other leucine zipper proteins such as c-Jun and small Mafs. These complexes transactivate gene expression by binding to EpRE [65–67]. Two mechanisms have been proposed for Nrf2-Keap1 dissociation, i.e., Nrf2 phosphorylation and Keap1 modification. The former mechanism considers that phosphorylation of Nrf2 is required for its dissociation. This hypothesis is supported by the finding that oxidant-mediated Nrf2-Keap1 dissociation could be blocked through inhibiting Nrf2 phosphorylation, which occurs through activation of signaling pathways including those involving protein kinase C [68,69]. An alternative hypothesis of Nrf2 liberation, modification of Keap1, proposes that some reactive –SH groups on Keap1 act as oxidative stress sensors and that modification of them by ROS or electrophiles disrupts the Nrf2-Keap1 complex leading to Nrf2 dissociation. This hypothesis is supported by evidence of continuous Nrf2 activation by mutation of the active cysteine residues of Keap1 [67,70,71] and by the finding of conjugate formation between the electrophiles and the thiol group in Keap1 [72]. It seems that in the case of Nrf2 activation by HNE, Keap1 modification may be involved. Levonen et al. found that HNE could conjugate with Keap1 in vivo, and the author proposed that this might be the underlying mechanism of HNE-mediated Nrf2 nuclear translocation [72]. Nonetheless, since HNE has been found to activate various signaling pathways including PKC [73–79], the possibility of Nrf2 phosphorylation by HNE-mediated signaling pathways cannot be excluded.
EpRE/Nrf2 signaling has been found to be responsible for the induction of many phase II and antioxidant genes in response to ROS or electrophiles, including NADPH:quinine oxidoreductase-1 (NQO1) [80], HO-1 [81,82], glutamate cysteine ligase [35,49–51], GSH S-transferase [83], and multidrug resistance protein [84]. Here we demonstrated that the induction of one major type of mRNA of GGT, a key enzyme involved in GSH homeostasis and GSH S-conjugates metabolism, is also mediated through EpRE/Nrf2 signaling in response to electrophile (HNE). Nonetheless, the EpRE-binding complexes are composed of variable mixtures of transcription factors. In addition to Nrf2, many other transcription factors, which may or may not be the dimerization partner of Nrf2, could also be activated and bind to EpRE, and thereby induce expression of EpRE-driven genes upon exposure to oxidants or electrophiles. These redox-sensitive EpRE-binding proteins include other members of erythroid 2-related factor (Nrf) family Nrf1 and Nrf3, small Maf protein family members (MafG/K/F), and Jun (c-Jun, JunB, JunD) and Fos family members (c-Fos, FosB, Fra1, Fra2, etc.) (for reviews, see [36,37]). The transcription factors bound to the EpRE in response to stimuli may be cell, inducer, or gene specific. For example, under stimulated conditions, the transcription factors binding to a consensus human glutamate cysteine ligase (GCL) EpRE complex included Nrf2, c-Jun, JunB, and JunD in HBE1 cells [35]; in HepG2 cells, the proteins binding to a consensus GCL EpRE complex were composed of Nrf1/2, JunD, and small Maf proteins [85,86]. There are reports suggesting that the signaling transduction pathways involved in EpRE activation are also in a gene-, cell-, or stimulator-specific manner. An example of this is the signaling pathways involved in the EpRE-mediated NQO-1 and GCL induction in HepG2 cells; protein kinase C (PKC) and ERK/p38MAPK were involved, respectively in their EpRE activation [66,69]. As to the signaling pathways responsible for GGT induction by HNE, recent studies from our laboratory suggest that ERK1/2 and P38MAPK mediate the induction of GGT mRNA V-2 through regulation of the activation of Nrf2 [31,87]. The present study, however, focused on the identification of the cis element regulating GGT V2 expression, verifying whether it was the element to which Nrf2 bound and the tissue distribution of GGT V2 expression.
In summary, we find that GGT mRNA V is widely distributed in rat tissues and is most abundant in the lung; we also find that, in the rat lung L2 cell line, its expression is regulated at the transcriptional level through an EpRE sequence in the GGT promoter 5 proximal region. HNE treatment increased the recruitment of Nrf2 to GP5 EpRE. The data also suggest that HNE could induce GGT mRNA V expression via this EpRE. Based on the evidence that GGT mRNA V-2 is abundant in the lung and that the induction of mRNA V-2 is through EpRE, it can be inferred that EpRE/Nrf2 signaling is at least partially involved in GGT induction by other oxidative stress generators, including the redox-cycling quinones [11–14], NO2 [10], and hyperoxia [15]. Because every investigated type of oxidative stress results in increased HNE production [41,88], the underlying mechanism of GGT induction by these agents likely involves the production of the common inducer HNE.
Abbreviations
- GGT
γ-glutamyl transpeptidase
- EpRE
electrophile-response element
- Nrf2
NF-E2-related factor 2
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- HNE
4-hydroxynonenal
- 5′-UTR
5′-untranslated region
- FBS
fetal bovine serum
- PCR
polymerase chain reaction
- DTT
dithiothreitol
- TBS
Tris-buffered saline
- EMSA
electrophoretic mobility-shift assay
- HNF
hepatocyte nuclear factor
References
- 1.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express γ-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- 2.Griffith OW, Meister A. Excretion of cysteine and γ-glutamylcysteine moieties in human and experimental animal γ-glutamyl transpeptidase deficiency. Proc. Natl. Acad. Sci. USA. 1980;77:3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in γ-glutamyl transpeptidase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas E, Valverde M, Kala SV, Kala G, Lieberman MW. Accumulation of DNA damage in the organs of mice deficient in γ-glutamyltranspeptidase. Mutat. Res. 2000;447:305–316. doi: 10.1016/s0027-5107(99)00191-8. [DOI] [PubMed] [Google Scholar]
- 5.Barrios R, Shi ZZ, Kala SV, Wiseman AL, Welty SE, Kala G, Bahler AA, Ou CN, Lieberman MW. Oxygen-induced pulmonary injury in γ-glutamyl transpeptidase-deficient mice. Lung. 2001;179:319–330. doi: 10.1007/s004080000071. [DOI] [PubMed] [Google Scholar]
- 6.Meister A. The γ-glutamyl cycle. Diseases associated with specific enzyme deficiencies. Ann. Intern. Med. 1974;81:247–253. doi: 10.7326/0003-4819-81-2-247. [DOI] [PubMed] [Google Scholar]
- 7.Stark AA, Porat N, Volohonsky G, Komlosh A, Bluvshtein E, Tubi C, Steinberg P. The role of γ-glutamyl transpeptidase in the biosynthesis of glutathione. Biofactors. 2003;17:139–149. doi: 10.1002/biof.5520170114. [DOI] [PubMed] [Google Scholar]
- 8.Paolicchi A, Sotiropuolou M, Perego P, Daubeuf S, Visvikis A, Lorenzini E, Franzini M, Romiti N, Chieli E, Leone R, Apostoli P, Colangelo D, Zunino F, Pompella A. γ-Glutamyl transpeptidase catalyses the extracellular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney. Eur. J. Cancer. 2003;39:996–1003. doi: 10.1016/s0959-8049(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths SA, Good VM, Gordon LA, Hudson EA, Barrett MC, Munks RJ, Manson MM. Characterization of a promoter for γ-glutamyl transpeptidase activated in rat liver in response to aflatoxin B1 and ethoxyquin. Mol. Carcinog. 1995;14:251–262. doi: 10.1002/mc.2940140405. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Oakes SM, Williams MC, Takahashi S, Miura T, Joyce-Brady M. Nitrogen dioxide exposure activates γ-glutamyl transferase gene expression in rat lung. Toxicol. Appl. Pharmacol. 1997;143:388–396. doi: 10.1006/taap.1996.8087. [DOI] [PubMed] [Google Scholar]
- 11.Kugelman A, Choy HA, Liu R, Shi MM, Gozal E, Forman HJ. γ-Glutamyl transpeptidase is increased by oxidative stress in rat alveolar L2 epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994;11:586–592. doi: 10.1165/ajrcmb.11.5.7946387. [DOI] [PubMed] [Google Scholar]
- 12.Liu RM, Shi MM, Giulivi C, Forman HJ. Quinones increase γ-glutamyl transpeptidase expression by multiple mechanisms in rat lung epithelial cells. Am. J. Physiol. 1998;274:L330–L336. doi: 10.1152/ajplung.1998.274.3.L330. [DOI] [PubMed] [Google Scholar]
- 13.Liu RM, Hu H, Robison TW, Forman HJ. Differential enhancement of γ-glutamyl transpeptidase and γ-glutamylcysteine synthetase by tert-butylhydroquinone in rat lung epithelial L2 cells. Am. J. Respir. Cell Mol. Biol. 1996;14:186–191. doi: 10.1165/ajrcmb.14.2.8630269. [DOI] [PubMed] [Google Scholar]
- 14.Liu RM, Hu H, Robison TW, Forman HJ. Increased γ-glutamylcysteine synthetase and γ-glutamyl transpeptidase activities enhance resistance of rat lung epithelial L2 cells to quinone toxicity. Am. J. Respir. Cell Mol. Biol. 1996;14:192–197. doi: 10.1165/ajrcmb.14.2.8630270. [DOI] [PubMed] [Google Scholar]
- 15.Knickelbein RG, Ingbar DH, Seres T, Snow K, Johnston RB, Jr, Fayemi O, Gumkowski F, Jamieson JD, Warshaw JB. Hyperoxia enhances expression of γ-glutamyl transpeptidase and increases protein S-glutathiolation in rat lung. Am. J. Physiol. 1996;270:L115–L122. doi: 10.1152/ajplung.1996.270.1.L115. [DOI] [PubMed] [Google Scholar]
- 16.Pross M, Schulz HU, Flechsig A, Manger T, Halangk W, Augustin W, Lippert H, Reinheckel T. Oxidative stress in lung tissue induced by CO(2) pneumoperitoneum in the rat. Surg. Endosc. 2000;14:1180–1184. doi: 10.1007/s004640000189. [DOI] [PubMed] [Google Scholar]
- 17.Ogihara T, Hirano K, Morinobu T, Kim HS, Hiroi M, Ogihara H, Tamai H. Raised concentrations of aldehyde lipid peroxidation products in premature infants with chronic lung disease. Arch. Dis. Child Fetal Neonatal Ed. 1999;80:F21–F25. doi: 10.1136/fn.80.1.f21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton RF, Jr, Li L, Eschenbacher WL, Szweda L, Holian A. Potential involvement of 4-hydroxynonenal in the response of human lung cells to ozone. Am. J. Physiol. 1998;274:L8–L16. doi: 10.1152/ajplung.1998.274.1.L8. [DOI] [PubMed] [Google Scholar]
- 19.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, DeBoer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 20.Kirichenko A, Li L, Morandi MT, Holian A. 4-Hydroxy-2-nonenal-protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol. Appl. Pharmacol. 1996;141:416–424. doi: 10.1006/taap.1996.0307. [DOI] [PubMed] [Google Scholar]
- 21.Bouhafs RK, Samuelson A, Jarstrand C. Lipid peroxidation of lung surfactant due to reactive oxygen species released from phagocytes stimulated by bacteria from children with cystic fibrosis. Free Radic. Res. 2003;37:909–917. doi: 10.1080/1071576031000124525. [DOI] [PubMed] [Google Scholar]
- 22.Aoshiba K, Koinuma M, Yokohori N, Nagai A. Immunohistochemical evaluation of oxidative stress in murine lungs after cigarette smoke exposure. Inhal. Toxicol. 2003;15:1029–1038. doi: 10.1080/08958370390226431. [DOI] [PubMed] [Google Scholar]
- 23.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 24.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993;57:779S–785S. doi: 10.1093/ajcn/57.5.779S. discussion 785S–786S. [DOI] [PubMed] [Google Scholar]
- 25.Zarkovic N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Aspects Med. 2003;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 26.Dianzani MU. 4-Hydroxynonenal from pathology to physiology. Mol. Aspects Med. 2003;24:263–272. doi: 10.1016/s0098-2997(03)00021-9. [DOI] [PubMed] [Google Scholar]
- 27.Chikhi N, Holic N, Guellaen G, Laperche Y. γ-Glutamyl transpeptidase gene organization and expression: a comparative analysis in rat, mouse, pig and human species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999;122:367–380. doi: 10.1016/s0305-0491(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 28.Markey CM, Rudolph DB, Labus JC, Hinton BT. Oxidative stress differentially regulates the expression of γ-glutamyl transpeptidase mRNAs in the initial segment of the rat epididymis. J. Androl. 1998;19:92–99. [PubMed] [Google Scholar]
- 29.Joyce-Brady M, Oakes SM, Wuthrich D, Laperche Y. Three alternative promoters of the rat γ-glutamyl transferase gene are active in developing lung and are differentially regulated by oxygen after birth. J. Clin. Invest. 1996;97:1774–1779. doi: 10.1172/JCI118605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura S, Lahuna O, Suzuki T, Brouillet A, Chobert MN, Laperche Y. A specific distal promoter controls γ-glutamyl transpeptidase gene expression in undifferentiated rat transformed liver cells. Biochem. J. 1997;326:311–320. doi: 10.1042/bj3260311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Dickinson DA, Liu RM, Forman HJ. 4-Hydroxynonenal increases γ-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic. Biol. Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc. Natl. Acad. Sci. USA. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 34.Favreau LV, Pickett CB. The rat quinone reductase antioxidant response element. Identification of the nucleotide sequence required for basal and inducible activity and detection of antioxidant response element-binding proteins in hepatoma and non-hepatoma cell lines. J. Biol. Chem. 1995;270:24468–24474. doi: 10.1074/jbc.270.41.24468. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 36.Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 37.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-Hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 39.Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J. Lipid Mediat. Cell Signal. 1995;11:51–61. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzer E, Muller O, Arese P, Siems WG, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996;388:119–122. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- 41.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol. Metab. 2000;11:281–285. doi: 10.1016/s1043-2760(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 45.Gregori C, Kahn A, Pichard AL. Activity of the rat liver-specific aldolase B promoter is restrained by HNF3. Nucleic Acids Res. 1994;22:1242–1246. doi: 10.1093/nar/22.7.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha JY, Kim H, Kim KS, Hur MW, Ahn Y. Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene. Cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J. Biol. Chem. 2000;275:18358–18365. doi: 10.1074/jbc.M909536199. [DOI] [PubMed] [Google Scholar]
- 47.Sawaya PL, Stripp BR, Whitsett JA, Luse DS. The lung-specific CC10 gene is regulated by transcription factors from the AP-1, octamer, and hepatocyte nuclear factor 3 families. Mol. Cell. Biol. 1993;13:3860–3871. doi: 10.1128/mcb.13.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaret K. Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev. Biol. 1999;209:1–10. doi: 10.1006/dbio.1999.9228. [DOI] [PubMed] [Google Scholar]
- 49.Mulcahy RT, Gipp JJ. Identification of a putative antioxidant response element in the 5′-flanking region of the human γ-glutamylcysteine synthetase heavy subunit gene. Biochem. Biophys. Res. Commun. 1995;209:227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- 50.Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and beta-naphthoflavone-induced expression of the human γ-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J. Biol. Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 51.Moinova HR, Mulcahy RT. An electrophile responsive element (EpRE) regulates beta-naphthoflavone induction of the human γ-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J. Biol. Chem. 1998;273:14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen T, Rushmore TH, Pickett CB. Transcriptional regulation of a rat liver glutathione S-transferase Ya subunit gene. Analysis of the antioxidant response element and its activation by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. J. Biol. Chem. 1994;269:13656–13662. [PubMed] [Google Scholar]
- 53.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 54.Wild AC, Gipp JJ, Mulcahy T. Overlapping antioxidant response element and PMA response element sequences mediate basal and beta-naphthoflavone-induced expression of the human γ-glutamylcysteine synthetase catalytic subunit gene. Biochem. J. 1998;332(Pt 2):373–381. doi: 10.1042/bj3320373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 57.Inamdar NM, Ahn YI, Alam J. The heme-responsive element of the mouse heme oxygenase-1 gene is an extended AP-1 binding site that resembles the recognition sequences for MAF and NF-E2 transcription factors. Biochem. Biophys. Res. Commun. 1996;221:570–576. doi: 10.1006/bbrc.1996.0637. [DOI] [PubMed] [Google Scholar]
- 58.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 59.Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J. Biol. Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 60.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 61.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 62.Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 63.Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox. Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 65.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem. Biophys. Res. Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]
- 67.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 70.Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kakishita H, Hattori Y. Vascular smooth muscle cell activation and growth by 4-hydroxynonenal. Life Sci. 2001;69:689–697. doi: 10.1016/s0024-3205(01)01166-3. [DOI] [PubMed] [Google Scholar]
- 74.Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol. Pharmacol. 2000;58:535–554. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- 75.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1- binding activity through caspase activation in neurons. J. Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 76.Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O. H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp. Neurol. 2003;180:144–155. doi: 10.1016/s0014-4886(02)00059-6. [DOI] [PubMed] [Google Scholar]
- 77.Bruckner SR, Estus S. JNK3 contributes to c-jun induction and apoptosis in 4-hydroxynonenal-treated sympathetic neurons. J. Neurosci. Res. 2002;70:665–670. doi: 10.1002/jnr.10437. [DOI] [PubMed] [Google Scholar]
- 78.Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem. -Biol. Interact. 2001;130–132:943–954. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- 79.Kumagai T, Nakamura Y, Osawa T, Uchida K. Role of p38 mitogen-activated protein kinase in the 4-hydroxy-2-nonenal-induced cyclooxygenase-2 expression. Arch. Biochem. Biophys. 2002;397:240–245. doi: 10.1006/abbi.2001.2601. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Jaiswal AK. Human antioxidant-response-element-mediated regulation of type 1 NAD(P)H:quinone oxidoreductase gene expression. Effect of sulfhydryl modifying agents. Eur. J. Biochem. 1994;226:31–39. doi: 10.1111/j.1432-1033.1994.tb20023.x. [DOI] [PubMed] [Google Scholar]
- 81.Gong P, Stewart D, Hu B, Li N, Cook J, Nel A, Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J(2) is mediated by the stress response elements and transcription factor Nrf2. Antioxid. Redox. Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- 82.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 83.Liu S, Pickett CB. The rat liver glutathione S-transferase Ya subunit gene: characterization of the binding properties of a nuclear protein from HepG2 cells that has high affinity for the antioxidant response element. Biochemistry. 1996;35:11517–11521. doi: 10.1021/bi960572p. [DOI] [PubMed] [Google Scholar]
- 84.Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 85.Moinova HR, Mulcahy RT. Up-regulation of the human γ-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem. Biophys. Res. Commun. 1999;261:661–668. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- 86.Wild AC, Moinova HR, Mulcahy RT. Regulation of γ-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 87.Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. 4-Hydroxynonenal induces rat γ-glutamyl transpeptidase through MAPK mediated EpRE/Nrf2 signaling. Am. J. Respir. Cell Mol. Biol. 2005 doi: 10.1165/rcmb.2005-0280OC. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid. Redox. Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]