Abstract
Magnetic nanoparticles (MNPs) possess unique magnetic properties and the ability to function at the cellular and molecular level of biological interactions making them an attractive platform as contrast agents for magnetic resonance imaging (MRI) and as carriers for drug delivery. Recent advances in nanotechnology have improved the ability to specifically tailor the features and properties of MNPs for these biomedical applications. To better address specific clinical needs, MNPs with higher magnetic moments, non-fouling surfaces, and increased functionalities are now being developed for applications in the detection, diagnosis, and treatment of malignant tumors, cardiovascular disease, and neurological disease. Through the incorporation of highly specific targeting agents and other functional ligands, such as fluorophores and permeation enhancers, the applicability and efficacy of these MNPs have greatly increased. This review provides a background on applications of MNPs as MR imaging contrast agents and as carriers for drug delivery and an overview of the recent developments in this area of research.
Keywords: Magnetic nanoparticle, MRI, Contrast agent, Drug delivery, Targeting, DNA, siRNA, Peptide, Ligand, Cancer, Biodistribution
1. Introduction
Magnetic nanoparticles (MNPs) are a major class of nanoscale materials with the potential to revolutionize current clinical diagnostic and therapeutic techniques. Due to their unique physical properties and ability to function at the cellular and molecular level of biological interactions, MNPs are being actively investigated as the next generation of magnetic resonance imaging (MRI) contrast agents [1] and as carriers for targeted drug delivery [2, 3]. Although the early research in the field can be dated back several decades, the recent surge of interest in nanotechnology has significantly expanded the breadth and depth of the MNP research. With a wide range of applications in the detection, diagnosis, and treatment of illnesses, such as cancer [4], cardiovascular disease [5], and neurological disease [6], MNPs may soon play a significant role in meeting the healthcare needs of tomorrow.
Numerous forms of MNP with various chemical compositions have been proposed and evaluated for biomedical applications to exploit nanoscale magnetic phenomena, such as enhanced magnetic moments and superparamagnetism. Like other nanomaterial-based systems, advances in nanotechnology now allow for precise engineering of the critical features of these fine particles. Composition, size, morphology and surface chemistry can now be tailored by various processes to not only improve magnetic properties but also affect the behavior of nanoparticles in vivo [7, 8]. In its simplest form, a biomedical MNP platform is comprised of an inorganic nanoparticle core and a biocompatible surface coating that provides stabilization under physiological conditions. Additionally, the application of suitable surface chemistry allows for the integration of functional ligands [9]. This modular design enables MNPs to perform multiple functions simultaneously, such as in multi-modal imaging [10], drug delivery and real-time monitoring, as well as combined therapeutic approaches.
The ability of MNPs to enhance proton relaxation of specific tissues and serve as MR imaging contrast agents is one of the most promising applications of nanomedicine. MNPs in the form of superparamagnetic iron oxides (SPIO) have been actively investigated as MR imaging contrast agents for over two decades [11]. With applications, such as bowel contrast agents (i.e., Lumiren® and Gastromark®) and liver/spleen imaging (i.e., Endorem® and Feridex IV®) [12, 13], already on the market, SPIOs have led the way for MNPs into the clinic. Several forms of ultrasmall superparamagnetic iron oxides (USPIO) have undergone clinical trials with one of the most notable being Combidex® which is in late stage clinical trials for use in the detection of lymph node metastases [14].
As therapeutic tools, MNPs have been evaluated extensively for targeted delivery of pharmaceuticals through magnetic drug targeting (MDT) [15, 16] and by active targeting through the attachment of high affinity ligands [17, 18, 19]. In the spirit of Ehrlich’s “Magic Bullet” [20], MNPs have the potential to overcome limitations associated with systemic distribution of conventional chemotherapies. With the ability to utilize magnetic attraction and/or specific targeting of disease biomarkers, MNPs offer an attractive means of remotely directing therapeutic agents specifically to a disease site, while simultaneously reducing dosage and the deleterious side-effects associated with non-specific uptake of cytotoxic drugs by healthy tissue. Also referred to as magnetic targeted carriers (MTC), colloidal iron oxide particles in early clinical trials have demonstrated some degree of success with the technique and shown satisfactory toleration by patients [21, 22]. Although not yet capable of reaching levels of safety and efficacy for regulatory approval, pre-clinical studies indicated that some of the shortcomings of MDT technology, such as poor penetration depth and diffusion of the released drug from the disease site, can be overcome by improvements in MTC design [23, 24]. Furthermore, the use of MNP as carriers in multifunctional nanoplatforms as a means of real-time monitoring of drug delivery is an area of intense interest [25, 26].
A significant challenge associated with the application of these MNP systems is their behavior in vivo. The efficacy of many of these systems is often compromised due to recognition and clearance by the reticuloendothelial system (RES) prior to reaching target tissue, as well as by an inability of to overcome biological barriers, such as the vascular endothelium or the blood brain barrier. The fate of these MNP upon intravenous administration is highly dependent on their size, morphology, charge, and surface chemistry. These physicochemical properties of nanoparticles directly affect their subsequent pharmacokinetics and biodistribution [27]. To increase the effectiveness of MNPs, several techniques, including reducing size and grafting non-fouling polymers, have been employed to improve their “stealthiness” and increase their blood circulation time to maximize the likelihood of reaching targeted tissues [28, 29].
Next-generation MNP-based MR imaging contrast agents and carriers for drug delivery incorporate novel nanocrystalline cores, coating materials, and functional ligands to improve the detection and specific delivery of these nanoparticles. New formulations of MNP cores, such as doped iron oxide nanocrystals, metallic/alloy nanoparticles, and nanocomposites, offer high magnetic moments increasing their signal-to-background ratios under MRI. Concurrently, the use of new surface coatings, such as stable gold or silica shell structures, allows for the application of otherwise toxic core materials, as well as more thorough coating through the formation of self-assembled monolayers (SAMS) on the nanoparticle surface. In addition, recent studies and reviews indicate an increasing role of cellular mechanics in diseases such as malaria [30, 31] and cancer metastasis [32, 33, 34]. As such, there is potential for next-generation platforms to incorporate surface qualities that would enable probing and/or monitoring of local physical mechanistic changes at a length scale that would greatly assist in improving disease detection, monitoring, and treatment.
Although many are still early in pre-clinical evaluation, with work still necessary to address the metabolism and potential long-term toxicity of these MNPs, efforts such as that of the National Cancer Institute (NCI) Nanotechnology Characterization Laboratory (NCL) are accelerating the evaluation of these new nanomaterials allowing for even quicker development in this already rapidly growing field. This review examines some of the recent developments in MNP technology and provides a brief background of their applications as MR imaging contrast agents and as carriers for drug delivery.
2. Magnetic nanoparticles
2.1. Magnetic properties
The penetration of magnetic fields through human tissue and the ability to remotely detect or manipulate magnetic materials has been investigated for use in medicine for centuries [35]. One of the more recent and significant applications of these properties has been in MRI as a non-invasive imaging modality capable of providing high resolution anatomical images. However, the potential of current clinical medical imaging can be greatly expanded through the use of MNPs to improve differentiation of malignant and healthy tissue. In addition, upon location of a malignancy or lesion, external magnetic fields can then be controlled to direct particle accumulations to deliver therapeutics. To better understand the advantages of MNPs as MRI contrast agents, we briefly review some of the fundamental concepts of magnetism and the properties of MNPs. More thorough and detailed discussion of this topic can be found in the literature [2, 36].
The classification of a material’s magnetic properties is based on its magnetic susceptibility (χ), which is defined by the ratio of the induced magnetization (M) to the applied magnetic field (H). In diamagnetic materials, the magnetic moment is antiparallel to H resulting in very small and negative susceptibilities (−10−6 to −10−3). They do not retain magnetic properties when the external field is removed. Materials with magnetic moments aligned parallel to H and susceptibilities on the order of 10−6 to 10−1 are described as paramagnetic. While in ferri- and ferromagnetic materials, magnetic moments also align parallel to H, coupling interactions between the electrons of the material result in ordered magnetic states, i.e., magnetic domains, and large spontaneous magnetization. The susceptibilities of these materials depend on their atomic structures, temperature, and the external field H.
At small sizes (on the order of tens of nanometers), ferri- or ferro-magnetic materials, such as MNPs, become a single magnetic domain and therefore maintain one large magnetic moment. However, at sufficiently high temperatures (i.e., blocking temperature TB) thermal energy is sufficient to induce free rotation of the particle resulting in a loss of net magnetization in the absence of an external field. This superparamagnetic property, marked by the lack of remnant magnetization after removal of external fields, enables the particles to maintain their colloidal stability and avoid aggregation making it feasible for their use in biomedical applications. Furthermore, the coupling interactions within these single magnetic domains result in much higher magnetic susceptibilities than paramagnetic materials.
Although superparamagnetism is a favorable property of small particles, the reduction of particle size is not without some consequences. As particle sizes decrease, surface-to-volume ratios increase resulting in pronounced surface effects, such as noncollinear spins, spin canting, and spin-glass-like behavior, which can significantly impact the magnetic properties of the material [37]. Typically, the saturation magnetization (Ms) values of nanoparticles, corresponding to the complete alignment of all individual moments in a sample, are smaller than their corresponding bulk phases due to disordered crystal structure resulting from high surface curvature, which increases with particle size reduction. Furthermore, significant differences in magnetic properties are observed with MNPs obtained through different chemical processes. More detailed explanations of the physical properties of MNP and nanoscale magnetic phenomena can be found in recent reviews in this area [37, 38].
2.2. Iron oxide nanoparticles
Colloidal iron oxide nanoparticles, such as SPIO and USPIO, have been the most extensively investigated MNPs for biomedical applications due to their excellent biocompatibility and ease of synthesis. Typically composed of nanocrystalline magnetite (Fe3O4) or maghemite (γFe2O3) protected with a polymeric coating, these ferrite nanoparticles possess a spinel crystal structure with oxygen ions forming a close-packed cubic lattice and iron ions located at interstices. In the case of Fe3O4, magnetization arises from electron hopping between the Fe2+ and Fe3+ ions that coexist at the octahedral sites. In addition to magnetic properties, the favorable biocompatibility and biodegradability of these MNPs have contributed greatly to their widespread use in biomedical applications. Upon metabolism, iron ions are added to the body’s iron stores and eventually incorporated by erythrocytes as hemoglobin allowing for their safe use in vivo [39].
Iron oxide nanoparticles have been produced by a variety of synthesis processes ranging from traditional wet chemistry solution-based methods to more exotic techniques such as laser pyrolysis or chemical vapor deposition [7, 8, 40]. Currently, SPIO and USPIO utilized or under investigation for clinical application as MRI contrast agents are predominately synthesized by an aqueous co-precipitation process in the presence of the coating material [41, 42]. In these hydrolytic processes, the control of the solution pH value and the presence of the coating material serving as a surfactant are critical to particle formation and properties. Unfortunately, magnetization can vary vastly among synthesis methods even within particles of similar size due to incorporation of impurities disrupting the crystal structure, as well as the surface effects described previously. Typically, Ms values of magnetite nanoparticles obtained by these methods are in the range of 30–50 emu/g, which is lower than the 90 emu/g reported for their bulk form [8].
Recently, the use of high-temperature decomposition of organometallic precursors has been examined to produce iron oxide nanoparticles with marked improvements in size control, size distributions, and crystallinity [43, 44]. In this process, the size of the nanoparticle is controlled by varying the reaction temperature or changing the metal precursor. Sizes could be further tuned by a seed-mediated growth process to obtain larger particles. Utilizing this process, Sun et al. demonstrated the ability to synthesize highly uniform spherical Fe3O4 particles with size variation within 2 nm and mean diameters from 4 to 20 nm [43]. One drawback of this approach is the use of hydrophobic oleic acid and oleylamine surfactants in the process which results in a hydrophobic coating on the particle surface necessitating additional modification to achieve nanoparticle solubility in aqueous media. Approaches such as the addition of an amphiphilic polymer or surface surfactant exchange have been utilized to overcome this problem [45].
The need to improve magnetic properties for applications, such as molecular imaging, has generated interest in the development of metal doped iron oxides due to their enhanced magnetic properties. These spinel metal ferrites with a composition of MFe2O4, where M is +2 cation of Mn, Fe, Co or Ni, have been fabricated by various methods to tune specific magnetic properties [40]. Recently, Lee et al. reported the synthesis and characterization of MnFe2O4, FeFe2O4, CoFe2O4, and NiFe2O4 by high-temperature reaction between divalent metal chloride and iron tris-2,4-pentadioate [44]. Through comparison of various metal-doped ferrite nanoparticles, this group has demonstrated that MnFe2O4 nanoparticles are nontoxic in vitro and possess higher magnetic susceptibility than magnetite nanoparticles, suggesting that they may be used as an ultrasensitive MR imaging probe. Cobalt and nickel ferrites have also been investigated recently for in vivo biomedical applications despite known toxicities of these elements. Baldi et al. has examined the synthesis and coating of CoFe2O4 MNPs for use as magnetic nanocarriers [46]. Utilizing a polyol-based synthesis method this group produced 5.4 nm particles coated with mono- and difunctional phosphonic and hydroxamic acids. Cobalt leakage was monitored through inductively coupled plasma atomic emission spectroscopy (ICP-AES) and found to correspond with quality of surface coverage by the attached ligand. Similarly, Rana et al. recently investigated the use of nanocrystalline NiFe2O4 as drug carriers [47].
2.3. Metallic nanoparticles
Metallic MNPs, made of iron, cobalt, or nickel, are often overlooked for biological applications due to their chemical instability. Readily forming oxides in the presence of water and oxygen, these metallic MNPs are typically protected by coatings, such as gold or silica, to form a core-shell structure. Despite complex synthesis processes, research continues on these metallic nanoparticles due to the unique advantages some of these MNPs can offer. For example, iron nanoparticles possess relatively high magnetization and are able to maintain superparamagnetism at larger particle sizes compared to their oxide counterparts [48].
For iron nanoparticles, Peng et al. demonstrated that crystalline Fe3O4 shells were capable of providing a robust protective coating, while amorphous coatings could not protect the metallic core from deep oxidation [49]. In this study, a thermal degradation process was used initially to create iron nanoparticles, while the oxide coating was formed and its thickness was tuned by controlled oxidation utilizing an oxygen transferring agent. Fe/Fe3O4 nanoparticles were produced with a core radius of 4 nm and oxide thickness of 2.5 nm. Magnetic characterization of these MNPs confirmed that the particles were superparamagnetic and possessed a Ms of 102.6 emu/g Fe. Alternatively, Qiang et al. recently reported a method of producing stable Fe/Fe3O4 nanoparticles using a nanocluster deposition system. The group demonstrated the ability to vary core sizes from 2 to 100 nm and shell thicknesses from 2.5 to 5 nm by controlling growth parameters [50]. Nanoparticles generated by this process with a size less than 10 nm exhibited a Ms of approximately 80 emu/g Fe.
2.4. Bi-metallic nanoparticles
Bimetallic or metal alloy nanoparticles can also exhibit superparamagnetic properties making them attractive candidates as MRI contrast agents or magnetic carriers for drug delivery. Recent advances in the synthesis and surface modification of FePt nanoparticles have made these MNPs a viable option for biomedical applications [51]. Typically obtained from a variety of processes, such as vacuum-deposition or solution phase synthesis, FePt nanoparticles are known to possess a chemically disordered face-centered cubic (fcc) or chemically ordered face-centered tetragonal (fct) structure, both of which result in near-equal atomic percentages of Fe and Pt [51]. Interactions between the two chemical species lead to greater chemical stability in comparison to other high moment metallic nanoparticles. Furthermore, the surface chemistry of these MNPs allows for binding of carboxylate- and amine-based surfactants which may be utilized to improve the water solubility of these nanoparticles.
Hong et al. reported the modification of FePt nanoparticles with thiol terminated poly(ethylene glycol) (PEG) and dopamine ligands to form a mixed-monolayer-functionalized MNPs [52]. Utilizing FePt nanoparticles prepared by the method introduced by Sun et al. [53], this group demonstrated that these coated MNPs were stable in biologically relevant media such as PBS and cell culture medium. Furthermore, the ability to bind DNA and protein to the surface of these MNPs was also demonstrated through the incorporation of charge functionality. Recently, Gao et al. developed a process to create FePt nanoparticles encapsulated with a shell composed of CoS2 or CdO to serve as multifunctional nanostructures with cytotocity toward cancer cells or to provide fluorescence detectability [54, 55]. Although the toxicity of FePt nanoparticles themselves has not been thoroughly evaluated with only limited in vitro cytotoxicity assays reported throughout the literature [56], inert coatings such as gold shells are actively being investigated to improve the biocompatibility of these MNPs [57].
Another form of binary metallic MNPs receiving increased attention are those composed of FeCo [58]. With extremely high Ms values, these nanoalloys require protective coatings to prevent them from oxidation and corrosion. Bai and Wang have reported a method of synthesizing high magnetic moment nanoparticles with 10–20 nm Fe60Co40 cores and 1–3 nm gold or silver shells through a physical deposition process [59]. Cubic nanoparticles synthesized by this method were found to be superparamagnetic and have a Ms three times as high as that of comparable iron oxide nanoparticles.
Recently, Seo et al. reported the development of FeCo nanocrystals coated with a single-graphitic shell that were soluble and stable in aqueous solutions [60]. Synthesized through chemical vapor deposition (CVD), 7 nm and 4 nm FeCo cores were produced with compositions of Fe40Co60 and Fe12Co88, respectively. The graphitic shell was then applied by heating in H2 and subsequent methane CVD. The Ms of the 7 nm and 4 nm nanocrystals were 215 emu/g and 162 emu/g, respectively. In addition to providing protection from oxidation and potential toxicity, the graphitic coating also provides near-infrared optical absorbance allowing for potential use of photothermal ablation as a therapeutic application.
3. Surface coatings and functionalization
3.1. Polymeric coatings
Surface coatings are an integral component of all MNP platforms for biomedical applications. Although not attracted magnetically, due to their superparamagnetic properties, nanoparticles still have a significant tendency to agglomerate as a result of their high surface energy. Colloidal electrostatic stabilization arising from repulsion of surface charges on the nanoparticles is typically not adequate to prevent aggregation in biological solutions due to the presence of salts or other electrolytes that may neutralize this charge. Furthermore, upon intravenous injection the surfaces of MNPs are subjected to adsorption of plasma protein, or opsonization, as the first step in their clearance by the RES. Evading uptake by the RES and maintaining a long plasma half-life is a major challenge for many MNP applications in medicine [61].
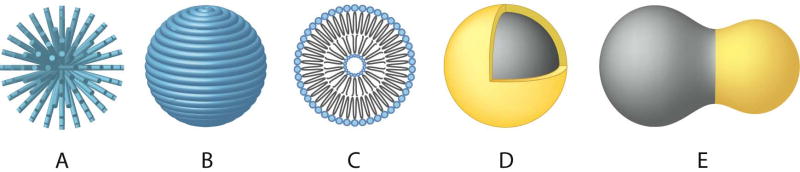
Polymeric coatings provide a steric barrier to prevent nanoparticle agglomeration and avoid opsonization. In addition, these coatings provide a means to tailor the surface properties of MNPs such as surface charge and chemical functionality. Some critical aspects with regard to polymeric coatings that may affect the performance of a MNP system include the nature of the chemical structure of the polymer (e.g. hydrophilicity/hydrophobicity, biodegradation characteristics, etc.), the length or molecular weight of the polymer, the manner in which the polymer is anchored or attached (e.g. electrostatic or covalent bonding), the conformation of the polymer, and the degree of particle surface coverage. Various monomeric species, such as bisphosphonates [62], dimercaptosuccinic acid (DMSA) [63] and alkoxysilanes [64, 65], have been evaluated as anchors to facilitate attachment of polymer coatings on MNPs. The molecular weight and geometric orientation of the polymer on the surface of the particles in the form of loops, trains, and tails [66] or as end-grafted brushes (Fig. 1A) or as fully encapsulated polymer shells (Fig. 1B) not only affect the antifouling characteristics of the nanoparticle, but also contribute to their effective hydrodynamic size, which is another key factor in avoiding recognition by the RES.
Figure 1.
MNP structures and coating schemes. (A) End-grafted polymer coated MNP. (B) MNP fully encapsulated in polymer coating. (C) Liposome encapsulated MNP. (D) Core-shell MNP. (E) Heterodimer MNP.
A variety of natural and synthetic polymers have been evaluated for use as coatings on MNPs. Readers are directed to several reviews on the topic for a comprehensive analysis of these materials [8, 67]. One of the most widely utilized and successful polymer coatings, in terms of in vivo applications, has been the polysaccharide dextran [1, 11]. Weissleder and co-workers have developed various formulations of dextran-coated iron oxide nanoparticles also referred to as monocrystalline iron oxide nanoparticles (MION) [42] and cross-linked iron oxide nanoparticles (CLIO) [68], which have been evaluated extensively for a variety of MR imaging applications. Chemical functionality was established by treating CLIO with ammonia to provide primary amino groups for the attachment of biomolecules such as proteins or peptides [69, 70].
Poly(ethylene glycol) (PEG) is another widely used polymer for nanoparticle coating in biomedical applications [28, 71]. The antifouling nature of PEG has been shown to reduce nanoparticle uptake by macrophages [18] and extend blood circulation time in vivo [72]. Various methods have been utilized to attach PEG to MNPs including silane grafting to oxide surfaces [73], polymerization at the surface of MNPs [74], and modification through sol-gel approaches [75].
To control polymer conformation and provide stable covalent linkages to the surface of iron oxide nanoparticles, Kohler et al. developed bifunctional PEG silanes capable of forming self-assembled monolayers (SAMs) and increasing the packing density of the polymer chains onto the nanoparticles surface [18, 19, 76, 77]. In addition, terminal amine or carboxyl groups extending out from the nanoparticle surface provide sites for conjugation of functional ligands, as demonstrated by the attachment of folic acid in this study. Similarly, Lee et al. reported the development of a protein resistant poly(TMSMA-r-PEGMA) copolymer comprised of silane anchoring groups and PEG branches [78]. Utilizing this polymer to coat magnetite nanoparticles, this group demonstrated the accumulation of the MNPs in xenograft tumors in mice as identified by MRI contrast enhancement.
Recently, there has been an increased interest on the modification of polymers or development of copolymers to allow for in situ coating of MNPs during nanoparticle synthesis [78, 79]. These processes, often termed “one-pot” synthesis methods, have several advantages over stepwise surface modification, including reduced agglomeration due to immediate coating of the particles and less processing procedures [80, 81, 82]. However, the presence of polymers during nanocrystal nucleation and growth can have a significant impact on the crystal structure and morphology of the MNPs obtained through these processes. For example, Lee et al found that crystallinity decreased with increasing concentration of poly(vinyl alcohol) (PVA) present during the synthesis of iron oxide particles through a precipitation reaction [83]. As noted, the imperfections in the crystal structure of these MNP can be detrimental to their magnetic properties. Another consideration to take into account while utilizing polymer coatings is their effects on the nanoparticle magnetic properties [84, 85].
3.2. Liposomes and micelles
The development of liposomes as drug delivery vehicles can be considered one of the earliest forms of nanomedicine. These phospholipid bilayered membrane vesicles (Fig. 1C) can range from 100 nm up to 5 μm in size and have been utilized for the delivery of small molecules, proteins and peptides, DNA, and MR imaging contrast agents [86]. An advantage of liposome encapsulation is that their in vivo behavior has been well established with processes such as PEGylation resulting in long circulation times. Another favorable feature of liposomes is the ability to encapsulate a large number of MNP cores and deliver them together, avoiding dilution, to a target site. Combining a therapeutic agent in the payload further enhances the multifunctionality of these delivery vehicles. Similarly, multifunctional micelles formed with amphiphilic block copolymers have also been used to entrap MNPs for these applications [87, 88].
Martina et al. developed magnetic-fluid-loaded liposomes (MFLs) by encapsulating maghemite nanocrystals within unilamellar vesicles of egg phosphatidylcholine and DSPE-PEG2000 [89]. MFLs with hydrodynamic size of 195 ± 33 nm were formed by film hydration coupled with sequential extrusion and were capable of encapsulating up to 1.67 mol of iron per mol of lipid. In vivo evaluation in mice using MR angiography demonstrated that these MFLs were still present in the blood 24 hours after intravenous injection confirming their long-circulating behavior.
3.3. Core-shell structures
In addition to organic coatings, core-shell structures (Fig. 1D) utilizing biocompatible silica or gold to encapsulate the MNPs have become another attractive approach for developing MRI contrast agents or MTCs for drug delivery. As mentioned in the previous sections, these inert coatings, or shells, provide both protection against chemical degradation of magnetic cores and prevent the release of potentially toxic components. Furthermore, functionalization chemistries are generally better established with these materials than those that comprise MNPs.
Silica shells are attractive options to serve as protective coatings on MNPs due to their stability under aqueous conditions and ease of synthesis. Sol-gel processes using tetraethoxysilane (TEOS) are generally utilized throughout the literature to produce coatings of controlled thickness [75, 90]. The use of functional alkoxysilanes, such as 3-aminopropyltriethyoxysilane (APS), allows for surface reactive groups to be easily added to these core-shell structures. In addition, the ability to encapsulate functional molecules, such as alternative imaging or therapeutic agents, within this protective matrix is a unique feature to these nanostructures [91, 92]. Recently, Ma et al. described one such multifunctional core-shell MNP composed of iron oxide cores of approximately 10 nm surrounded by a shell of SiO2 10–15 nm thick [93]. In this study, an organic dye, Tris(2,2′-bipyridine) ruthenium, was doped inside a second silica shell to provide luminescence and prevent quenching by interaction with the magnetic core. With this core-shell structure exhibiting superparamagnetic and luminescent properties, the authors of this work proposed this nanostructure for use in biomedical imaging applications.
Gold offers several advantages as a coating material for MNPs due to its low chemical reactivity and unique ability to form SAMs on their surface using alkanethiols [94, 95]. Unfortunately, this chemical inertness may also lead the difficulty in forming gold shells over MNPs. Recent advances in synthesizing gold-coated iron nanoparticles through a variety of methods ranging from reversed microemulsion, combined wet chemical, to laser irradiation have been reviewed by Lu et al [37]. Alternatively, heterodimer MNPs (Fig. 1E) can be produced by similar processes as gold core-shell structures representing another unique class of MNP [96].
3.4. Functional ligands
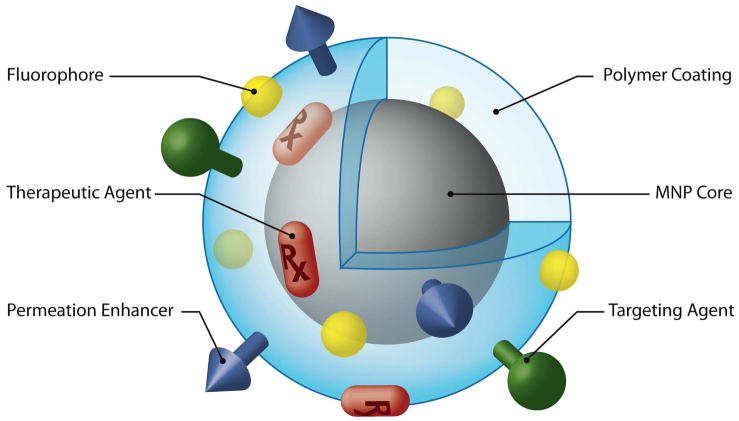
As discussed throughout this review, the ability to add components to MNPs in a modular fashion allows for specific features and functional moieties to be interchanged or combined. Ligands such as targeting agents, permeation enhancers, optical dyes, and therapeutic agents can all be conjugated on the surface or incorporated within these nanostructures (Fig. 2). To perform such nanoscale engineering, bioconjugation chemistries and techniques utilized for protein coupling have been studied [97, 98]. Techniques such as avidin-biotin binding, use of heterobifunctional linkers to form amide, ester, or disulfide bonds, and more recently “click” chemistries [99, 100], have all been shown to be useful in attaching functional ligands to MNPs. In addition to understanding the mechanisms of these reactions, those utilizing these techniques on MNPs may also find it useful to review basic concepts of colloidal science to avoid unwanted flocculation or aggregation during these processes [101].
Figure 2.
MNP possessing various ligands to enable multifunctionality from a single nanoparticle platform.
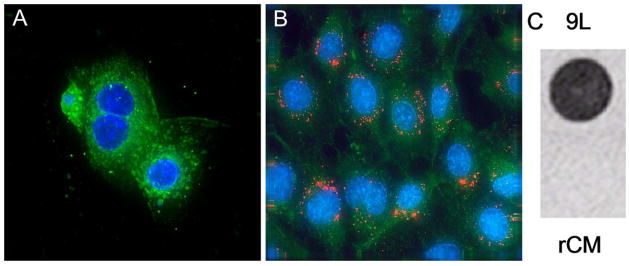
One example of adding functionality to MNPs has been the combination of organic dyes or fluorophores as optical imaging agents to allow for detection by multiple imaging modalities. Several groups have demonstrated the fluorescent imaging of cells in vitro after internalization of FITC [18] or rhodamine [102, 103] labeled MNPs. Recently, the conjugation of near-infrared fluorescent (NIRF) dyes to MNPs has received significant attention due to the deep penetration of NIRF light through tissues [104]. The integration of NIRF detectability may allow for these nanoparticles to be used for both presurgical planning by MRI and intraoperative resection of malignant tissues by optical imaging. Since both MRI and optical signals come from the same nanoparticles, the MR image can serve as a roadmap to the fluorescently labeled tumor cells. Josephson and co-workers have attached NIRF Cy5.5 dyes to CLIO MNPs and demonstrated in vivo accumulation of nanoparticles at tumor margins through macrophage uptake to improve brain tumor delineation [105, 106, 107]. Veiseh et al. constructed a multimodal agent composed of PEG-coated iron oxide nanoparticles conjugated to both Cy5.5 and a targeting agent, chlorotoxin, to improve specificity and internalization of nanoparticles into 9L glioma cells (Fig. 3) [19].
Figure 3.
Confocal fluorescent images of cells incubated with chlorotoxin-targeted iron oxide nanoparticles conjugated to Cy5.5. A: rat cardiomyocytes (rCM) representing normal cells. B: 9L glioma cells. C: MR phantom image of 9L (top) and rCM (bottom) cells cultured with the chlorotoxin-targeted nanoparticles (4.7 T, spin echo pulse sequence, TR 3000 ms, TE 30 ms) [19]. Reproduced with permission of the American Chemical Society.
4. Pharmacokinetics and biodistribution
4.1. Blood half-life
The need to extend nanoparticles’ blood circulation time to allow for their accumulation in target tissues has long been recognized as one of the primary challenges in the development of MNPs [29]. The ability to evade uptake by the RES are critical to achieving a long blood half-life. Like other colloidal carriers, the physicochemical properties of these MNP platforms, such as size, morphology, charge, and surface chemistry, dictate their fate in vivo.
The overall size of MNPs must be sufficiently small to evade rapid splenic filtration but large enough to avoid renal clearance. Nanoparticles larger than 200 nm are sequestered by phagocytotic cells of the spleen [108], while particles smaller than 5.5 nm are rapidly removed through renal clearance [109]. In addition to size, the shape and flexibility of MNPs have been suggested as physical characteristics that require more investigation to improve their performance in vivo [110]. Particles that escape filtration are then subject to opsonization resulting in recognition and clearance by Kupffer cells and other tissue macrophages. As described in the previous sections, various coatings including hydrophilic polymers, such as PEG, have been utilized to create a non-fouling coating on the particle surface [111].
In addition to the bio-fouling nature of MNPs, surface charge plays a critical role in blood half-lives of colloids and polymers. Positively charged polymers and particles tend to nonspecifically stick to cells [112]. This nonspecific adsorption can have a significant impact on blood-half life as demonstrated in a study by Papisov et al., where the circulation time of cationic poly-L-lysine coated MION was found to be only 1–2 min in comparison to 2–3 hrs for their uncharged variant [113]. Strong negative charges on the particle surface are also detrimental in that they result in increased liver uptake [27]. Therefore, it is generally agreed that nanoparticles with a neutral surface experience extended blood circulation times.
4.2. Passive targeting
The development of long-circulating nanoparticles has allowed for many MNP platforms to exploit structural abnormalities in the vasculature of particular pathologies, such as tumors, inflammatory, and infectious sites. This phenomenon, known as the enhance permeability and retention (EPR) effect [114, 115], is based on the mechanism that these tissues possess “leaky” vasculature which allows macromolecules and nanoparticles to extravasate and accumulate more readily. In the case of tumors, poorly organized vascular beds also result in impaired lymphatic drainage from these tissues. This non-specific accumulation, or passive targeting, has been demonstrated with nanoparticles ranging from 10–500 nm in diameter [115].
Passive targeting can also occur through the inherent clearance by the RES. Comprised of bone marrow progenitors, blood monocytes, and tissue macrophages, the uptake of MNPs by these phagocytic cells provides a means of delivering contrast agents and drug carriers to related organs. This RES-mediated targeting is the basis for the first clinical application of MNPs in the form of Ferumoxides AMI-25 (Endorem® and Feridex IV®) for liver imaging [116]. The rapid uptake of these MNPs by Kupffer cells of healthy hepatic parenchyma allows for their differentiation from diseased tissue by the contrast enhancement observed under MRI [117].
4.3. Active targeting
One promising approach toward increasing the local accumulation of MNPs in diseased tissue, known as active targeting or specific targeting, is by the conjugation of targeting molecules that possess high affinity toward unique molecular signatures found on malignant cells [118]. Often augmented by the EPR effect, these receptor-ligand or antigen-antibody interactions provide an effective strategy to improve the residence time in malignant tissues, such as tumors (Fig. 4). Targeting ligands, such as proteins [119, 120], peptides [69], aptamers [121, 122, 123] and small molecules [124], have been investigated to increase the site specific accumulation of MNPs. In some cases, specific binding can also facilitate internalization of the nanoparticle by receptor-mediated endocytosis.
Figure 4.
Illustration of tissue specific delivery of MNPs through active targeting facilitated by “leaky” vasculature. (A) Internalization of nanoparticles by (A) receptor-mediated endocytosis and formation of an endosome. (B) Endosomal acidification by proton pumps results in elevated osmotic pressure, swelling, and (C) rupture of the endosome allowing for release of the nanoparticle and conjugated therapeutic agents.
Monoclonal antibodies (mAbs) were the first targeting agents to exploit molecular recognition to deliver MNPs [125, 126, 127, 128] and continue to be widely used due to their high specificity. Recently, the development of Herceptin™, an FDA-approved mAb to the HER2/neu (erbB2) receptor, has made it a popular targeting agent for nanoparticles [129, 130]. Huh et al. demonstrated specific delivery of Herceptin™ targeted DMSA-coated magnetite nanoparticles to NIH3T6.7 cells expressing the HER2/neu cancer marker in vivo [131]. MR imaging of mice bearing xenograft tumors showed a T2 decrease of ~20% as a result of accumulation of this nanoprobe. One drawback of mAbs is their large size and inherent immunogenicity which can cause conjugated nanoparticles to diffuse poorly through biological barriers [132, 133]. Another area of extensive investigation has been the targeting of MNPs to receptors overexpressed on tumor neovasculature. The formation of new blood vessels, or angiogenesis, is an essential component of tumor growth and has been shown to be highly specific for neoplasia [134]. A relatively large number of angiogenesis markers, which include the αvβ3 integrin, vascular endothelial growth factor (VEGF), cell surface nucleolin, and heparin sulfates, have been identified as potential targets for the delivery diagnostic and therapeutic agents [135, 136]. Targeting agents, such as the Arg-Gly-Asp (RGD) peptide demonstrating high affinity for the αvβ3 integrin, have been evaluated for the delivery of MNPs to a variety of neoplastic tissues including breast tumors, malignant melanomas, and squamous cell carcinomas [137, 138, 139]. In a recent study by Reddy et al., the F3 peptide, which binds to nucleolin expressed on tumor endothelium and cancer cells, was utilized to deliver a multifunctional MNP to brain tumors [140]. Through combination with photodynamic therapy (PDT), this group was able to monitor the treatment efficacy of 9L gliomas in rats using the MNP component as a contrast agent for MRI.
Chlorotoxin (CTX), a peptide originally purified from the venom of the Leiurus quinquestriatus scorpion, has also been shown to be an effect targeting agent for tumors of neuroectodermal origin [141, 142, 143]. Studies suggest the target of CTX is associated with the membrane-bound matrix metalloproteinase-2 (MMP-2) protein complex, which is up-regulated on gliomas, as well as a variety of other tumors [144]. CTX has been shown to be an effective targeting agent to deliver MNPs to brain tumor cells [19].
The use of short peptides and small molecules as targeting agents also offers the advantage of increased binding affinity through multivalent attachment [124, 145]. This targeting phenomenon has been examined with folic acid, a vitamin whose receptor is overexpressed on the surface of many human tumor cells, including ovarian, lung, breast, endometrial, renal, and colon cancers [146, 147]. In our previous work, we demonstrated the highly selective binding of folic acid conjugated MNPs to a variety of tumor cells to improve their detectability by MRI [76, 148]. Another advantage of utilizing small molecules as targeting agents is that they are generally more robust than proteins or peptides thereby reducing possibility of loss of functionality through the synthesis of such MNPs.
4.4. Intracellular delivery and controlled release
An essential step in the use of MNPs for drug delivery is the internalization of the MNP and/or its therapeutic payload, as well as the subsequent release of these therapeutic agents to cell cytoplasm for desired actions to take place. Several mechanisms have been proposed to describe the uptake of nanoparticles into cells, including receptor-mediated endocytosis [61] and internalization by caveolae structures [149]. Nanoparticle size and surface properties play a critical role in moving across the plasma membrane. Nanoparticles smaller than 50 nm or those coated with lipophilic polymers, such as PEG, have been shown to efficiently diffuse through cell membranes [18, 150]. In addition, permeation enhancers, such as the Tat peptide, can also be attached to MNPs to facilitate delivery to cytoplasm [69, 151, 152]. Using Tat-labeled CLIO MNPs, Koch et al. demonstrated the effective internalization and slow excretion of the nanoparticles for cell tracking and drug delivery applications [153].
Upon particle internalization by target cells, another significant challenge for MNPs to serve as drug carriers is the release of the therapeutic agent to targeted subcellular organelles, such as the nucleus or mitochondria, prior to being trafficked to lysosomes where their biological activity may be destroyed. Although cleavage from MNP carriers under the hostile environment of the lysosomes may be suitable for some stable therapeutics [154], the effectiveness of other compounds, such as protein/peptides and oligonucleotides, may be severely compromised. Strategies to achieve endosomal release after cellular internalization include tailoring of cleavable linkers responsive to pH, osmolarity, or enzymatic activity [155, 156]. In addition, integration of cationic polymers to induce osmotic swelling, or “proton sponge” effect (Fig. 3), has also been examined to facilitate escape from endosomes [157].
4.5. Biodistribution and clearance
The long-term fate of MNPs in vivo is a major concern in the development of these nanoparticle platforms. Although general guidelines, such as those discussed in regard to the physicochemical properties of MNPs, may provide some insight on their behavior in the body, no universal set of criteria has been elucidated to predict this critical aspect of nanomedicine [158]. Mechanisms of clearance can vary significantly depending on the wide range of structures that are employed in the development of MNPs. One can make the obvious distinction that the metabolism, clearance, and toxicity profiles associated with a gold-coated FePt core-shell nanoparticle will be drastically different from that of an iron oxide filled liposome. These unique structures therefore necessitate their individual evaluation. Recently, increased emphasis has been placed on standardizing preclinical characterization of biomedical nanoparticles to better elucidate structure-activity relationships (SARs) [159].
5. MR imaging
5.1. Magnetic properties and MRI contrast enhancement
MR imaging is one of the most powerful noninvasive imaging modalities utilized in clinical medicine today [160, 161]. MR imaging is based on the property that hydrogen protons will align and process around an applied magnetic field, B0. Upon application of a transverse radiofrequency (rf) pulse, these protons are perturbed from B0. The subsequent process through which these protons return to their original state is referred to as the relaxation phenomenon. Two independent processes, longitudinal relaxation (T1-recovery) and transverse relaxation (T2-decay), can be monitored to generate an MR image. Local variation in relaxation, corresponding to image contrast, arises from proton density as well as the chemical and physical nature of the tissues within the specimen.
Upon accumulation in tissues, MNPs provide MR contrast enhancement (i.e., changes in signal intensity) by shortening both the longitudinal and transverse relaxation of surrounding protons. However, T1 shortening processes require a close interaction between protons and T1-agents which can be hindered by the thickness of the coating on the MNP. The effect of MNP on T2 shortening is caused by the large susceptibility difference between the particles and surrounding medium resulting in microscopic magnetic field gradients. Diffusion of protons through these field gradients leads to dephasing of the proton magnetic moments (i.e., irreversible loss of phase coherence) and thus decreased transverse relaxation times of protons [1, 11]. As a result of the more pronounced T2 effect, superparamagnetic nanoparticles are typically used to provide negative (hypointense) contrast enhancement using T2-weighted pulse sequences. The effects of MNP composition and size on proton relaxivity have been evaluated empirically with iron oxides [162, 163], however, aspects such as aggregation within cells and the effect of more complicated multilayered coatings must still be investigated.
The effectiveness of a contrast agent can be described by its relaxivity, which is the proportionality constant of the measured rate of relaxation, or R1 (1/T1) and R2 (1/T2), over a range of contrast agent concentrations. The relaxivity of a sample varies with not only the magnetic properties of the contrast agent, but also experimental variables such as field strength, temperature, and the medium in which the measurements are made. Therefore, care should be taken to note these parameters when making comparison of contrast agents found throughout the literature.
5.2. Applications
5.2.1. Cancer imaging
MNPs have been examined extensively as MRI contrast agents to improve the detection, diagnosis, and therapeutic management of solid tumors. Currently, clinical imaging of liver tumors and metastases through RES-mediated uptake of SPIOs has been capable of distinguishing lesions as small as 2–3 mm [1, 164]. In addition, USPIOs have been shown to be effective in identification of lymph-node metastases with a diameter of 5–10 mm under MRI [14]. This non-invasive approach has broad implications as identification of lymphatic dissemination is an essential component of staging and determining the approaches to treatment of diseases such as prostate, breast, and colon cancers [165].
Another clinical application of USPIO MNPs under evaluation is their use in improving the delineation of brain tumor boundaries and quantify tumor volumes [166, 167]. Current approaches utilizing gadolinium chelate-based contrast agents are typically limited by edema surrounding tumor and diffusion of these small molecules from the tumor vasculature. In comparison, MNP-based contrast agents offer prolonged delineation of tumor margins due to enhanced cellular internalization and slower clearance from the tumor site [166, 168]. Although it has been shown that these USPIOs will not replace gadolinium chelates, they have been demonstrated to be helpful in distinguishing neoplastic tissue from areas of radiation necrosis [169].
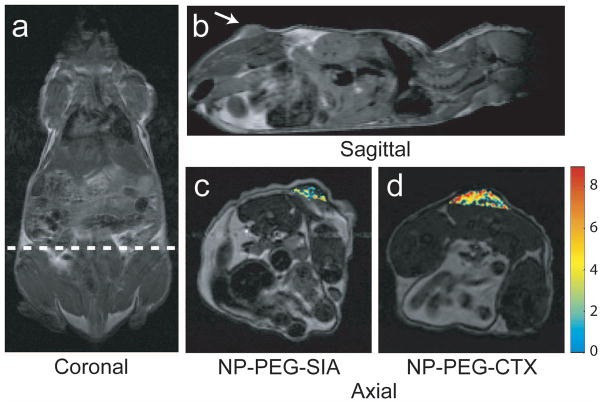
The next generation of active targeting MNPs currently being investigated have the potential to offer significantly improved tumor detection and localization by exploiting the unique molecular signatures of these diseases [170]. For example, Sun et al. recently demonstrated the specific accumulation of CTX-targeted iron oxide nanoparticles in 9L glioma flank xenografts resulting in more thorough contrast enhancement of tumors in comparison to non-targeted control nanoparticles (Fig. 5) [171].
Figure 5.
MRI anatomical image of a mouse in the (a) coronal plane with the dotted line displaying the approximate location of the axial cross sections displayed in (c) and (d). Anatomical image in the (b) sagittal plane displaying the location of the 9L xenograft tumor. Change in R2 relaxation values for the tumor regions (superimposed over anatomical MR images) for mouse receiving (c) non-targeting PEG coated iron oxide nanoparticles and (d) CTX-targeted PEG coated iron oxide nanoparticles 3 hrs post nanoparticle injection. Reproduced with permission of the Wiley-VCH Verlag GmbH & Co.
5.2.2. Cardiovascular disease imaging
MNPs have been proposed as MRI contrast agents for several clinical applications in cardiovascular medicine including myocardial injury, atherosclerosis, and other vascular disease [172, 173]. The uptake of MNPs by macrophages, which have been shown to be a marker of unstable atheromatous plaques [1, 6], has also been exploited to visualize these lesion prone arterial sites. Clinical studies have demonstrated that MR imaging using USPIOs may be useful in evaluating the risk of acute ischaemic events [174, 175]. Recently, Kelly et al. have identified 30 families of new peptides that bind to atherosclerotic lesions, through in vivo phage display [176]. From this study the adhesion molecule VCAM-1 was identified as a target for endothelial and macrophage cells responsible for atherosclerosis. Utilizing a VCAM-1 targeted peptide sequence, the group demonstrated specific binding of MNPs and MRI contrast enhancement of early lesions in juvenile mice as well as resected human carotid artery plaques [177].
5.2.3. Molecular imaging
Molecular imaging has been defined as the non-invasive in vivo visual representation, characterization, and quantification of biological processes at the cellular and molecular levels [178]. For instance, molecular imaging allows sensitive and specific monitoring of key molecular targets and host responses associated with early events in carcinogenesis [178, 179]. By coupling advances in medical imaging technology with those in molecular and cell biology, this growing research discipline offers the potential to have a major impact on early disease detection, individualized treatment, and drug development [178, 179]. Due to their ability to serve as molecularly targeted imaging agents, MNPs are now and will continue to play an integral role in this developing field. In addition to the targeted imaging applications reviewed in the previous sections, exciting novel molecular imaging applications of MNPs, such as in the imaging of cell migration/trafficking [180], apoptosis detection [181], and imaging of enzyme activities [155, 156, 182], are currently being investigated. Using MNPs targeted to macrophages in lymph nodes, MRI was able to reveal millimeter-sized metastases in nonenlarged lymph nodes [14], a size dimension beyond the detection threshold of many other imaging techniques. For monitoring of treatment-induced cell death, the MNPs were surface-modified with annexin V for apoptosis detection [181]. Annexin V is a protein with high binding affinity to membrane phosphatidylserine, externalized during the early execution phase of apoptosis. MNPs conjugated with annexin V would allow non-invasive quantification of apoptotic response in vivo and enable efficient optimization of therapy.
6. Drug Delivery
6.1. Magnetic drug targeting (MDT)
The primary shortcoming of most chemotherapeutic agents is their relative non-specificity and thus potential side effects to healthy tissues. To overcome this problem, MDT utilizes the attraction of MNP carriers to an external magnetic field to increase site specific delivery of therapeutic agents [2, 3]. In general, this process involves the attachment of a cytotoxic drug to a biocompatible MNP carrier (a.k.a. magnetic targeted carrier or MTC), intravenous injection of these MTCs in the form of a colloidal suspension, application of a magnetic field gradient to direct the MTC to the pathological site, and release of the therapeutic agent from the MTC. Although seemingly straightforward, there are many variables that complicate the execution of this technique. Parameters such as the physicochemical properties of the drug-loaded MNP, field strength and geometry, depth of the target tissue, rate of blood flow, and vascular supply, all play a role in determining the effectiveness of this method of drug delivery [3, 16].
Early clinical trials of colloidal iron oxide MTCs loaded with epirubicin and directed toward solid tumors have demonstrated successful accumulation in the target site in about half the patients in this study [21, 22]. These MTCs were also shown to be well tolerated by patients. Unfortunately, several problems have been identified with this technique including the possibility of embolization of the blood vessels, difficulty in scaling up from animal models due to limited field penetration of commercial magnets, control of drug diffusion after release from the MTC, and toxic responses to the MTCs. To address some of these issues and develop a theoretical basis for this technique, Grief and Richardson created a mathematical model incorporating the effects of hydrodynamics within blood vessels, particle volumes, magnetic field strength, and even the effects of cells within the plasma [183]. In this study the authors concluded that MDT could only be used effectively for targets close to the surface of the body.
Given this limitation, Alexiou et al. recently demonstrated the successful in vivo delivery of MCT composed of starch coated USPIO loaded with mitoxantrone into VX2-squamous cell carcinomas on the hind limbs of New Zealand White Rabbits [184, 185]. The group demonstrated the effectiveness of these MCTs to completely eliminate tumors after approximately 35 days of treatment.
6.2. Targeting multifunctional carriers
As described in the previous sections, the attachment of targeting agents to MNPs can be used to increase the specific accumulation of nanoparticles within diseased tissue. By integrating therapeutic agents, these multifunctional MNPs can serve strictly as a vehicle for drug delivery. One advantage of these MNPs, as well as other nanoparticle carriers, is their high surface area-to-volume ratios allowing for a large number of therapeutic molecules to be attached to individual nanoparticles. Additionally, while utilizing an active targeting strategy for specific delivery, the magnetic properties of the nanoparticle may be used to provide imaging modality for monitoring of drug delivery through MRI [26], or an alternative source of treatment through magnetic fluid hyperthermia (MFH) therapy [186].
6.3. Therapeutic agents
6.3.1. Conventional chemotherapeutic agents
MNPs have been evaluated as drug carries for a variety of chemotherapeutic agents. Traditional drugs such as etoposide, doxorubicin, and methotrexate have been attached or encapsulated in MNPs for potential treatment of diseases ranging from rheumatoid-arthritis to highly malignant prostate and breast tumors [25, 26, 187, 188]. With the wide variety of nanostructures described in the previous sections, carriers can be designed with specific characteristics to enhance the efficacy of these therapeutic agents over that achieved by typical systemic delivery. Characteristics such as loading capacities and drug release profiles can now be tailored by controlling structural features and chemical bonding within the MNP conjugate.
Yang et al. investigated the synthesis and release characteristics of poly(ethyl-2-cyanoacrylate) (PECA) coated magnetite nanoparticles containing anti-cancer agents cisplatin and gemcitabine [189]. In this study, cisplatin was shown to exhibit a sustained release behavior due to its hydrophobicity in comparison to the more rapid release of the hydrophilic gemcitabine. Kohler et al. demonstrated a sustained release of methotrexate (MTX) in breast and brain tumor cells delivered by iron oxide nanoparticles [25, 26]. In this study, the authors covalently attached MTX to amine functionalized nanoparticles through amide bonds to ensure stability of the drug conjugate under intravenous conditions. Cleavage of the MTX from the MNPs was evaluated over a range of pH values and in the presence of lysozymes to mimic conditions present in the lysosomal compartments. Through the use the covalent linkage the group demonstrated the controlled release of MTX to the cellular cytosol and the subsequent cytotoxicity to these cancer cells.
6.3.2. Proteins and peptides
In addition to drug molecules, MNPs have been investigated as carriers of therapeutic proteins and peptides. As described in the previous section, Herceptin™, also known as trastuzumab, has been conjugated to MNPs as a mAb targeting agent [131]. However, it also exhibits a therapeutic effect causing cells to undergo arrest during the G1 phase of the cell cycle and thereby reduces cell proliferation [190]. By incorporating Herceptin™ into magnetite nanoparticle loaded liposomes, Ito et al. demonstrated an antiproliferative effect on breast tumor cells [191]. In this in vitro study, similar therapeutic effects were observed for the nanoparticle conjugates as that of the free mAb at equal concentrations. Furthermore, the group exploited the magnetic properties of the magnetite nanoparticles to induce hyperthermia resulting in a combined therapeutic approach with an increased cytotoxic effect.
As described earlier in this review, CTX, a peptide with high affinity for a variety of tumors, is currently being evaluated for applications in cancer imaging and therapy. In addition to serving as a targeting agent, CTX also exhibits the ability to inhibit tumor invasion, which is particularly useful in the treatment of highly invasive brain tumors such as gliomas [144]. Although the mechanism of this therapeutic effect continues to be investigated, it is believed that CTX’s role as a Cl−-channel inhibitor affects tumor cells’ ability to regulate volume changes which allows for their migration into narrow extracellular spaces [192]. The attachment of CTX peptides to MNPs and enhanced internalization by target cells [19] are expected to further improve therapeutic effect over that of the free peptide alone.
6.3.3. DNA and siRNA
Antisense and gene therapy have been areas of intense research in recent years due to their potential to generate a significant impact on medicine. However, the delivery of genes and their resulting transfection efficiencies are often limited by their short half-life in vivo, lack of specificity, and poor diffusion across cell membranes [193, 194]. The use of MNPs as carriers for antisense oligodioxynucleotides (ODNs) or gene vectors overcomes many of the problems associated with the delivery of these therapeutic agents [195, 196]. Also referred to as magnetofection, this technique has been successfully applied for in vitro transfection and is currently being optimized for in vivo applications [197]. Recently, Pan et al. developed a dendrimer-modified MNP to deliver antisense survivin ODNs to breast and liver cancer cells [198]. By complexing ODNs to the positively charged polyamidoamine (PAMAM) coated MNPs, the authors demonstrated down regulation of the survivin gene and protein within 15 min as well as inhibited cell growth in a concentration and time dependent manner.
MNPs have also been investigated as carriers for the delivery of small interfering RNA (siRNA) [199]. In recent years, RNA interference (RNAi) has emerged as a highly promising therapeutic platform [149, 200]. In vitro magnetofection kits utilizing cationic polymer coated MNPs are now available commercially and used routinely in laboratories. The translation of these in vitro applications of siRNA delivery for in vivo use is currently being investigated with MNPs, such as polyethylenimine (PEI) coated iron oxides [199, 201]. Medarova et al. recently reported on the development of a MNP-based probe for siRNA delivery and imaging in vivo [202]. In this work, MNPs labeled with a NIFR dye and covalently bound with siRNA were shown to silence green fluorescent protein (GFP) production in a GFP expressing xenograft tumor mouse model. Exploiting the EPR effect, the group demonstrated the feasibility of in vivo tracking of MNP up-taken by tumor with MRI and optical imaging. However, similar to DNA-based therapy, the primary challenge to this therapeutic strategy continues to be the targeted delivery of siRNA to specific tissues. Further enhancement of therapeutic efficacy of these MNP-based siRNA carriers through active targeting is currently being investigated.
7. Conclusions
The development of MNPs has been greatly accelerated in the past decade by advances in nanotechnology, molecular cell biology, and small-animal imaging instrumentation. MNPs of various formulations have been developed to diagnose and treat diseases for which conventional therapy has shown limited efficacy. In particular, the use of MNPs as MRI contrast agents and drug carriers have drawn enormous attention, as it holds great potential of providing new opportunities for early cancer detection and targeted therapies. The approach will not only minimize the invasive procedure, but also reduce side effects to healthy tissues, which are two primary concerns in conventional cancer therapies.
Improving imaging contrast, biocompatibility, and specific targeting capability remains the mainstay of MNP development for medicine. To improve MRI signal-to-background ratios, MNP cores with high magnetic moments, such as doped iron oxide nanocrystals, metallic/alloy nanoparticles, and nanocomposites, have been developed. To improve biocompatibility, surface coatings, such as gold, silica and a number of biocompatible polymers have been investigated. The use of gold or silica as shell materials allows the application of toxic materials as nanoparticle cores with strong magnetic properties. The conjugation of biocompatible polymers, such as dextran, PEG, or other protein resistant polymers, as surface coating for MNPs, prevents nanoparticles from aggregation and opsonization, evades nanoparticle uptake by the RES, and increase colloidal stability in physiological solutions and blood circulation time. Specific targeting capability is commonly achieved by conjugation of peptides, aptamers, and small biomolecules with high affinity to target cells, on the surface of MNPs, aimed to increase the local accumulation and retention of the MNPs in pathological sites while reducing side effects. Interestingly, some targeting agents, such as MTX and CTX, also exhibit therapeutic effects for target cells, which allows the MNPs to serve multiple functions including diagnosis, treatment, and even treatment monitoring. It is worthwhile mentioning that such targeting agents are not common, and the multifunction of MNPs is usually achieved by conjugation of multiple agents. MNPs serving as multimodal imaging agents or multifunctional carriers are actively pursued. With continued advances in nanomaterials synthesis technology, surface chemistry, and knowledge in interactions of materials with biological systems, such a strategic approach is becoming a commonplace.
Improvements to MNP technology, such as enhanced magnetic properties, non-biofouling surface coatings, and the integration of multifunctional ligands, continue to be evaluated in an effort to bring these nanostructures from the bench-top to the clinic. A critical component of this translation is the continued investigation into the relationships between the physicochemical properties of these nanostructures and their behavior in vivo, which is currently poorly understood. Although advances have been made in some aspects, such as avoiding RES-uptake and enhanced site-specific accumulation of MNPs, greater insight into the mechanisms dictating the fate of nanoparticles in vivo is needed. By incorporating advances in nanoscale engineering, molecular imaging, and novel therapeutics, MNP platforms have the potential to enable physicians to diagnose and treat diseases, such as cancer and cardiovascular disease, with greater effectiveness than ever before.
Acknowledgments
This work was supported in part by NIH/NCI Nanoplatform grant (R01CA119408), NIH/NCI grant (R01CA134213), and NIH/NIBIB grant (R01EB006043).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Advanced Drug Delivery Reviews. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. Journal of Physics D-Applied Physics. 2003;36:R167–R181. [Google Scholar]
- 3.Dobson J. Magnetic nanoparticles for drug delivery. Drug Development Research. 2006;67:55–60. [Google Scholar]
- 4.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nature Reviews Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 5.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. Journal of Magnetic Resonance Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 6.Corot C, Petry KG, Trivedi R, Saleh A, Jonkmanns C, Le Bas JF, Blezer E, Rausch M, Brochet B, Foster-Gareau P, Baleriaux D, Gaillard S, Dousset V. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol. 2004;39:619–25. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- 7.Tartaj P, Morales MD, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D-Applied Physics. 2003;36:R182–R197. [Google Scholar]
- 8.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 9.McNeil SE. Nanotechnology for the biologist. J Leukoc Biol. 2005;78:585–94. doi: 10.1189/jlb.0205074. [DOI] [PubMed] [Google Scholar]
- 10.Frullano L, Meade TJ. Multimodal MRI contrast agents. Journal of Biological Inorganic Chemistry. 2007;12:939–949. doi: 10.1007/s00775-007-0265-3. [DOI] [PubMed] [Google Scholar]
- 11.Weissleder R, Bogdanov A, Neuwelt EA, Papisov M. Long-Circulating Iron-Oxides for Mr-Imaging. Advanced Drug Delivery Reviews. 1995;16:321–334. [Google Scholar]
- 12.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–31. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 13.Bonnemain B. Superparamagnetic agents in magnetic resonance imaging: Physicochemical characteristics and clinical applications - A review. Journal of Drug Targeting. 1998;6:167–174. doi: 10.3109/10611869808997890. [DOI] [PubMed] [Google Scholar]
- 14.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New England Journal of Medicine. 2003;348:2491–U5. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 15.Senyei A, Widder K, Czerlinski G. Magnetic Guidance Of Drug-Carrying Microspheres. Journal Of Applied Physics. 1978;49:3578–3583. [Google Scholar]
- 16.Neuberger T, Schopf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. Journal Of Magnetism And Magnetic Materials. 2005;293:483–496. [Google Scholar]
- 17.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–55. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Kohler N, Zhang MQ. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 19.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang MQ. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Letters. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 20.Winau F, Westphal O, Winau R. Paul Ehrlich--in search of the magic bullet. Microbes Infect. 2004;6:786–9. doi: 10.1016/j.micinf.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Lubbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dorken B, Herrmann F, Gurtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke AJ, Ohlendorf D, Huhnt W, Huhn D. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996;56:4686–93. [PubMed] [Google Scholar]
- 22.Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95:200–6. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- 23.Lubbe AS, Bergemann C, Brock J, McClure DG. Physiological aspects in magnetic drug-targeting. Journal Of Magnetism And Magnetic Materials. 1999;194:149–155. [Google Scholar]
- 24.Gallo JM, Hafeli U. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy and clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Research. 1997;57:3063–3064. [PubMed] [Google Scholar]
- 25.Kohler N, Sun C, Wang J, Zhang MQ. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir. 2005;21:8858–8864. doi: 10.1021/la0503451. [DOI] [PubMed] [Google Scholar]
- 26.Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang MQ. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006;2:785–792. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]
- 27.Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. Journal of Microencapsulation. 1996;13:245–255. doi: 10.3109/02652049609026013. [DOI] [PubMed] [Google Scholar]
- 28.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 29.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 30.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–38. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puig-de-Morales-Marinkovic M, Turner KT, Butler JP, Fredberg JJ, Suresh S. Viscoelasticity of the human red blood cell. Am J Physiol Cell Physiol. 2007;293:C597–605. doi: 10.1152/ajpcell.00562.2006. [DOI] [PubMed] [Google Scholar]
- 32.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–49. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 33.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 34.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Mourino MR. From Thales to Lauterbur, or from the lodestone to MR imaging: magnetism and medicine. Radiology. 1991;180:593–612. doi: 10.1148/radiology.180.3.1871268. [DOI] [PubMed] [Google Scholar]
- 36.Morrish A. The Physical Principles of Magnetism. IEEE Press; New York: 2001. [Google Scholar]
- 37.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angewandte Chemie-International Edition. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 38.Lin XM, Samia ACS. Synthesis, assembly and physical properties of magnetic nanoparticles. Journal Of Magnetism And Magnetic Materials. 2006;305:100–109. [Google Scholar]
- 39.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, Jacobs P, Lewis J. Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR Am J Roentgenol. 1989;152:167–73. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 40.Willard MA, Kurihara LK, Carpenter EE, Calvin S, Harris VG. Chemically prepared magnetic nanoparticles. International Materials Reviews. 2004;49:125–170. [Google Scholar]
- 41.Molday RS, Mackenzie D. Immunospecific Ferromagnetic Iron-Dextran Reagents for the Labeling and Magnetic Separation of Cells. Journal of Immunological Methods. 1982;52:353–367. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- 42.Shen T, Weissleder R, Papisov M, Bogdanov A, Jr, Brady TJ. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 43.Sun SH, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li GX. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. Journal of the American Chemical Society. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13:95–9. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 45.Xu CJ, Sun SH. Monodisperse magnetic nanoparticles for biomedical applications. Polymer International. 2007;56:821–826. [Google Scholar]
- 46.Baldi G, Bonacchi D, Franchini MC, Gentili D, Lorenzi G, Ricci A, Ravagli C. Synthesis and coating of cobalt ferrite nanoparticles: a first step toward the obtainment of new magnetic nanocarriers. Langmuir. 2007;23:4026–8. doi: 10.1021/la063255k. [DOI] [PubMed] [Google Scholar]
- 47.Rana S, Gallo A, Srivastava RS, Misra RDK. On the suitability of nanocrystalline ferrites as a magnetic carrier for drug delivery: Functionalization, conjugation and drug release kinetics. Acta Biomaterialia. 2007;3:233–242. doi: 10.1016/j.actbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Huber DL. Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1:482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- 49.Peng S, Wang C, Xie J, Sun S. Synthesis and stabilization of monodisperse Fe nanoparticles. J Am Chem Soc. 2006;128:10676–7. doi: 10.1021/ja063969h. [DOI] [PubMed] [Google Scholar]
- 50.Qiang Y, Antony J, Sharma A, Nutting J, Sikes D, Meyer D. Iron/iron oxide core-shell nanoclusters for biomedical applications. Journal of Nanoparticle Research. 2006;8:489–496. [Google Scholar]
- 51.Sun SH. Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Advanced Materials. 2006;18:393–403. [Google Scholar]
- 52.Hong R, Fischer NO, Emrick T, Rotello VM. Surface PEGylation and ligand exchange chemistry of FePt nanoparticles for biological applications. Chemistry of Materials. 2005;17:4617–4621. [Google Scholar]
- 53.Sun SH, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 2000;287:1989–1992. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 54.Gao J, Liang G, Zhang B, Kuang Y, Zhang X, Xu B. FePt@CoS(2) yolk-shell nanocrystals as a potent agent to kill HeLa cells. J Am Chem Soc. 2007;129:1428–33. doi: 10.1021/ja067785e. [DOI] [PubMed] [Google Scholar]
- 55.Gao J, Zhang B, Gao Y, Pan Y, Zhang X, Xu B. Fluorescent magnetic nanocrystals by sequential addition of reagents in a one-pot reaction: a simple preparation for multifunctional nanostructures. J Am Chem Soc. 2007;129:11928–35. doi: 10.1021/ja0731017. [DOI] [PubMed] [Google Scholar]
- 56.Kim DK, Kan D, Veres T, Normadin F, Liao JK, Kim HH, Lee SH, Zahn M, Muhammed M. Monodispersed Fe-Pt nanoparticles for biomedical applications. Journal Of Applied Physics. 2005;97 [Google Scholar]
- 57.de la Presa P, Multigner M, Morales MP, Rueda T, Fernandez-Pinel E, Hernando A. Synthesis and characterization of FePt/Au core-shell nanoparticles. Journal of Magnetism and Magnetic Materials. 2007;316:E753–E755. [Google Scholar]
- 58.Reiss G, Hutten A. Magnetic nanoparticles - Applications beyond data storage. Nature Materials. 2005;4:725–726. doi: 10.1038/nmat1494. [DOI] [PubMed] [Google Scholar]
- 59.Bai JM, Wang JP. High-magnetic-moment core-shell-type FeCo-Au/Ag nanoparticles. Applied Physics Letters. 2005;87 [Google Scholar]
- 60.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, Dai H. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat Mater. 2006;5:971–6. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 61.Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D-Applied Physics. 2003;36:R198–R206. [Google Scholar]
- 62.Portet D, Denizot B, Rump E, Lejeune JJ, Jallet P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. Journal Of Colloid And Interface Science. 2001;238:37–42. doi: 10.1006/jcis.2001.7500. [DOI] [PubMed] [Google Scholar]
- 63.Fauconnier N, Pons JN, Roger J, Bee A. Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. Journal Of Colloid And Interface Science. 1997;194:427–433. doi: 10.1006/jcis.1997.5125. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C, Wangler B, Morgenstern B, Zentgraf H, Eisenhut M, Untenecker H, Kruger R, Huss R, Seliger C, Semmler W, Kiessling F. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: A promising tool to label cells for magnetic resonance imaging. Langmuir. 2007;23:1427–1434. doi: 10.1021/la061879k. [DOI] [PubMed] [Google Scholar]
- 65.Mornet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues A, Portier J, Pollert E, Duguet E. Magnetic nanoparticle design for medical applications. Progress In Solid State Chemistry. 2006;34:237–247. [Google Scholar]
- 66.Sato T. Stabilization of colloidal dispersions by polymer adsoption. Dekker; New York: 1980. [Google Scholar]
- 67.Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 68.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999;10:186–91. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 69.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 2002;13:264–8. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 70.Schellenberger EA, Bogdanov A, Jr, Hogemann D, Tait J, Weissleder R, Josephson L. Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Mol Imaging. 2002;1:102–7. doi: 10.1162/15353500200202103. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M, Ferrari M. Hemocompatible polyethylene glycol films on silicon. Journal of Biomedical Microdevices. 1998;1:81–89. [Google Scholar]
- 72.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable Long-Circulating Polymeric Nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 73.Butterworth MD, Illum L, Davis SS. Preparation of ultrafine silica- and PEG-coated magnetite particles. Colloids And Surfaces A-Physicochemical And Engineering Aspects. 2001;179:93–102. [Google Scholar]
- 74.Flesch C, Unterfinger Y, Bourgeat-Lami E, Duguet E, Delaite C, Dumas P. Poly(ethylene glycol) surface coated magnetic particles. Macromolecular Rapid Communications. 2005;26:1494–1498. [Google Scholar]
- 75.Lu Y, Yin YD, Mayers BT, Xia YN. Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Letters. 2002;2:183–186. [Google Scholar]
- 76.Zhang Y, Sun C, Kohler N, Zhang MQ. Self-assembled coatings on individual monodisperse magnetite nanoparticles for efficient intracellular uptake. Biomedical Microdevices. 2004;6:33–40. doi: 10.1023/b:bmmd.0000013363.77466.63. [DOI] [PubMed] [Google Scholar]
- 77.Kohler N, Fryxell GE, Zhang MQ. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. Journal of the American Chemical Society. 2004;126:7206–7211. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- 78.Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. Journal of the American Chemical Society. 2006;128:7383–7389. doi: 10.1021/ja061529k. [DOI] [PubMed] [Google Scholar]
- 79.Yuan JJ, Armes SP, Takabayashi Y, Prassides K, Leite CAP, Galembeck F, Lewis AL. Synthesis of biocompatible poly[2-(methacryloyloxy)ethyl phosphorylcholine]-coated magnetite nanoparticles. Langmuir. 2006;22:10989–10993. doi: 10.1021/la061834j. [DOI] [PubMed] [Google Scholar]
- 80.Lutz JF, Stiller S, Hoth A, Kaufner L, Pison U, Cartier R. One-pot synthesis of PEGylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules. 2006;7:3132–3138. doi: 10.1021/bm0607527. [DOI] [PubMed] [Google Scholar]
- 81.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Advanced Materials. 2006;18:2553. [Google Scholar]
- 82.Wang LY, Bao J, Wang L, Zhang F, Li YD. One-pot synthesis and bioapplication of amine-functionalized magnetite nanoparticles and hollow nanospheres. Chemistry-A European Journal. 2006;12:6341–6347. doi: 10.1002/chem.200501334. [DOI] [PubMed] [Google Scholar]
- 83.Lee J, Isobe T, Senna M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. Journal Of Colloid And Interface Science. 1996;177:490–494. [Google Scholar]
- 84.Mikhaylova M, Jo YS, Kim DK, Bobrysheva N, Andersson Y, Eriksson T, Osmolowsky M, Semenov V, Muhammed M. The effect of biocompatible coating layers on magnetic properties of superparamagnetic iron oxide nanoparticles. Hyperfine Interactions. 2004;156:257–263. [Google Scholar]
- 85.Mikhaylova M, Kim DK, Bobrysheva N, Osmolowsky M, Semenov V, Tsakalakos T, Muhammed M. Superparamagnetism of magnetite nanoparticles: Dependence on surface modification. Langmuir. 2004;20:2472–2477. doi: 10.1021/la035648e. [DOI] [PubMed] [Google Scholar]
- 86.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 87.Nasongkla N, Bey E, Ren JM, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao JM. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Letters. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 88.Lecommandoux S, Sandre O, Checot F, Perzynski R. Smart hybrid magnetic self-assembled micelles and hollow capsules. Progress in Solid State Chemistry. 2006;34:171–179. [Google Scholar]
- 89.Martina MS, Fortin JP, Menager C, Clement O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. Journal of the American Chemical Society. 2005;127:10676–10685. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 90.Stober W, Fink A, Bohn E. Controlled Growth Of Monodisperse Silica Spheres In Micron Size Range. Journal Of Colloid And Interface Science. 1968;26:62. [Google Scholar]
- 91.Tada DB, Vono LLR, Duarte EL, Itri R, Kiyohara PK, Baptista MS, Rossi LM. Methylene blue-containing silica-coated magnetic particles: A potential magnetic carrier for photodynamic therapy. Langmuir. 2007;23:8194–8199. doi: 10.1021/la700883y. [DOI] [PubMed] [Google Scholar]
- 92.Tan WH, Wang KM, He XX, Zhao XJ, Drake T, Wang L, Bagwe RP. Bionanotechnology based on silica nanoparticles. Medicinal Research Reviews. 2004;24:621–638. doi: 10.1002/med.20003. [DOI] [PubMed] [Google Scholar]
- 93.Ma DL, Guan JW, Normandin F, Denommee S, Enright G, Veres T, Simard B. Multifunctional nano-architecture for biomedical applications. Chemistry of Materials. 2006;18:1920–1927. [Google Scholar]
- 94.Prime KL, Whitesides GM. Self-Assembled Organic Monolayers - Model Systems For Studying Adsorption Of Proteins At Surfaces. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 95.Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation Of Monolayer Films By The Spontaneous Assembly Of Organic Thiols From Solution Onto Gold. Journal Of The American Chemical Society. 1989;111:321–335. [Google Scholar]
- 96.Choi JS, Jun YW, Yeon SI, Kim HC, Shin JS, Cheon J. Biocompatible heterostructured nanoparticles for multimodal biological detection. J Am Chem Soc. 2006;128:15982–3. doi: 10.1021/ja066547g. [DOI] [PubMed] [Google Scholar]
- 97.Aslam M, Dent A. Bioconjugation. Macmillan Reference Ltd; London, UK: 1998. [Google Scholar]
- 98.Hermanson G. Bioconjugate techniques. Academic Press; San Diego: 1996. [Google Scholar]
- 99.Sun EY, Josephson L, Weissleder R. “Clickable” nanoparticles for targeted imaging. Molecular Imaging. 2006;5:122–128. [PubMed] [Google Scholar]
- 100.Nandivada H, Jiang XW, Lahann J. Click chemistry: Versatility and control in the hands of materials scientists. Advanced Materials. 2007;19:2197–2208. [Google Scholar]
- 101.Myers D. Surfaces, interfaces, and colloids: principles and applications. VCH Publishers; New York: 1991. [Google Scholar]
- 102.Bertorelle F, Wilhelm C, Roger J, Gazeau F, Menager C, Cabuil V. Fluorescence-modified superparamagnetic nanoparticles: Intracellular uptake and use in cellular imaging. Langmuir. 2006;22:5385–5391. doi: 10.1021/la052710u. [DOI] [PubMed] [Google Scholar]
- 103.Lee JH, Jun YW, Yeon SI, Shin JS, Cheon J. Dual-mode nanoparticle probes for high-performance magnetic resonance and fluorescence imaging of neuroblastoma. Angewandte Chemie-International Edition. 2006;45:8160–8162. doi: 10.1002/anie.200603052. [DOI] [PubMed] [Google Scholar]
- 104.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 105.Josephson L, Kircher MF, Mahmood U, Tang Y, Weissleder R. Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes. Bioconjugate Chemistry. 2002;13:554–560. doi: 10.1021/bc015555d. [DOI] [PubMed] [Google Scholar]
- 106.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63:8122–5. [PubMed] [Google Scholar]
- 107.Trehin R, Figueiredo JL, Pittet MJ, Weissleder R, Josephson L, Mahmood U. Fluorescent nanoparticle uptake for brain tumor visualization. Neoplasia. 2006;8:302–11. doi: 10.1593/neo.05751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen LT, Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973;41:529–37. [PubMed] [Google Scholar]
- 109.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 111.Storm G, Belliot SO, Daemen T, Lasic DD. Surface Modification Of Nanoparticles To Oppose Uptake By The Mononuclear Phagocyte System. Advanced Drug Delivery Reviews. 1995;17:31–48. [Google Scholar]
- 112.Fujita T, Nishikawa M, Ohtsubo Y, Ohno J, Takakura Y, Sezaki H, Hashida M. Control of in vivo fate of albumin derivatives utilizing combined chemical modification. J Drug Target. 1994;2:157–65. doi: 10.3109/10611869409015905. [DOI] [PubMed] [Google Scholar]
- 113.Papisov MI, Bogdanov A, Schaffer B, Nossiff N, Shen T, Weissleder R, Brady TJ. Colloidal Magnetic-Resonance Contrast Agents - Effect Of Particle Surface On Biodistribution. Journal Of Magnetism And Magnetic Materials. 1993;122:383–386. [Google Scholar]
- 114.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 115.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 116.Clement O, Siauve N, Lewin M, de Kerviler E, Cuenod CA, Frija G. Contrast agents in magnetic resonance imaging of the liver: present and future. Biomedicine & Pharmacotherapy. 1998;52:51–58. doi: 10.1016/S0753-3322(98)80003-6. [DOI] [PubMed] [Google Scholar]
- 117.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, Wittenberg J, Ferrucci JT. Superparamagnetic Iron-Oxide - Clinical-Application as a Contrast Agent for Mr Imaging of the Liver. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 118.Weissleder R, Bogdanov A, Papisov M. Drug targeting in magnetic resonance imaging. Magn Reson Q. 1992;8:55–63. [PubMed] [Google Scholar]
- 119.Kresse M, Wagner S, Pfefferer D, Lawaczeck R, Elste V, Semmler W. Targeting of ultrasmall superparamagnetic iron oxide (USPIO) particles to tumor cells in vivo by using transferrin receptor pathways. Magn Reson Med. 1998;40:236–42. doi: 10.1002/mrm.1910400209. [DOI] [PubMed] [Google Scholar]
- 120.Kresse M, Wagner S, Pfefferer D, Lawaczeck R, Elste V, Semmler W. Targeting of ultrasmall superparamagnetic iron oxide (USPIO) particles to tumor cells in vivo by using transferrin receptor pathways. Magnetic Resonance in Medicine. 1998;40:236–242. doi: 10.1002/mrm.1910400209. [DOI] [PubMed] [Google Scholar]
- 121.Farokhzad OC, Jon S, Khademhosseini A, Tran TN, Lavan DA, Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64:7668–72. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 122.Herr JK, Smith JE, Medley CD, Shangguan D, Tan W. Aptamer-conjugated nanoparticles for selective collection and detection of cancer cells. Anal Chem. 2006;78:2918–24. doi: 10.1021/ac052015r. [DOI] [PubMed] [Google Scholar]
- 123.Yigit MV, Mazumdar D, Kim HK, Lee JH, Odintsov B, Lu Y. Smart “Turn-on” Magnetic Resonance Contrast Agents Based on Aptamer-Functionalized Superparamagnetic Iron Oxide Nanoparticles. Chembiochem. 2007;8:1675–1678. doi: 10.1002/cbic.200700323. [DOI] [PubMed] [Google Scholar]
- 124.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nature Biotechnology. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 125.Cerdan S, Lotscher HR, Kunnecke B, Seelig J. Monoclonal antibody-coated magnetite particles as contrast agents in magnetic resonance imaging of tumors. Magn Reson Med. 1989;12:151–63. doi: 10.1002/mrm.1910120202. [DOI] [PubMed] [Google Scholar]
- 126.Bulte JW, Hoekstra Y, Kamman RL, Magin RL, Webb AG, Briggs RW, Go KG, Hulstaert CE, Miltenyi S, The TH, et al. Specific MR imaging of human lymphocytes by monoclonal antibody-guided dextran-magnetite particles. Magn Reson Med. 1992;25:148–57. doi: 10.1002/mrm.1910250115. [DOI] [PubMed] [Google Scholar]
- 127.Weissleder R, Lee AS, Fischman AJ, Reimer P, Shen T, Wilkinson R, Callahan RJ, Brady TJ. Polyclonal human immunoglobulin G labeled with polymeric iron oxide: antibody MR imaging. Radiology. 1991;181:245–9. doi: 10.1148/radiology.181.1.1887040. [DOI] [PubMed] [Google Scholar]
- 128.Tiefenauer LX, Kuhne G, Andres RY. Antibody-magnetite nanoparticles: in vitro characterization of a potential tumor-specific contrast agent for magnetic resonance imaging. Bioconjug Chem. 1993;4:347–52. doi: 10.1021/bc00023a007. [DOI] [PubMed] [Google Scholar]
- 129.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Research. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 130.Artemov D, Mori N, Okollie B, Bhujwalla ZM. MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magnetic Resonance in Medicine. 2003;49:403–408. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 131.Huh YM, Jun YW, Song HT, Kim S, Choi JS, Lee JH, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J Am Chem Soc. 2005;127:12387–91. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- 132.Foon KA. Biological Response Modifiers - the New Immunotherapy. Cancer Research. 1989;49:1621–1639. [PubMed] [Google Scholar]
- 133.Jain RK. Transport of Molecules in the Tumor Interstitium - a Review. Cancer Research. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 134.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 135.Ruoslahti E. Specialization of tumour vasculature. Nature Reviews Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 136.Neri D, Bicknell R. Tumour vascular targeting. Nature Reviews Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 137.Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sunderland CJ, Steiert M, Talmadge JE, Derfus AM, Barry SE. Targeted nanoparticles for detecting and treating cancer. Drug Development Research. 2006;67:70–93. [Google Scholar]
- 139.Zhang CF, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Research. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 140.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YEL, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clinical Cancer Research. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 141.Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM. Tumor paint: A Chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Research. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 142.Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–73. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- 143.Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, Del Maestro R, Beliveau R. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis. 1999;17:555–66. doi: 10.1023/a:1006760632766. [DOI] [PubMed] [Google Scholar]
- 144.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–44. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 145.Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros I, Patri AK, Thomas T, Mule J, Baker JR. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharmaceutical Research. 2002;19:1310–1316. doi: 10.1023/a:1020398624602. [DOI] [PubMed] [Google Scholar]
- 146.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401. [PubMed] [Google Scholar]
- 147.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73:2432–43. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 148.Sun C, Sze R, Zhang MQ. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. Journal of Biomedical Materials Research Part A. 2006;78A:550–557. doi: 10.1002/jbm.a.30781. [DOI] [PubMed] [Google Scholar]
- 149.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 150.Mrsny R. Active Targeting Strategies in Cancer with a Focus on Potential Nanotechnology Applications. In: Amiji MM, editor. Nanotechnology for Cancer Therapy. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 151.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–4. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 152.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–96. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 153.Koch AM, Reynolds F, Kircher MF, Merkle HP, Weissleder R, Josephson L. Uptake and metabolism of a dual fluorochrome tat-nanoparticle in HeLa cells. Bioconjugate Chemistry. 2003;14:1115–1121. doi: 10.1021/bc034123v. [DOI] [PubMed] [Google Scholar]
- 154.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–58. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tung CH, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60:4953–8. [PubMed] [Google Scholar]
- 156.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–8. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 157.Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A Versatile Vector For Gene And Oligonucleotide Transfer Into Cells In Culture And In-Vivo - Polyethylenimine. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occupational Medicine-Oxford. 2006;56:307–311. doi: 10.1093/occmed/kql052. [DOI] [PubMed] [Google Scholar]
- 159.Patri AK, Dobrovolskaia MA, Stern ST, McNeil SE. Preclinical Characterization of Engineered Nanoparticles Intended for Cancer Therapeutics. In: Amiji MM, editor. Nanotechnology for Cancer Therapy. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 160.Mitchell D, Cohen M. MRI principles. Saunders; Philadelphia, PA: 2004. [Google Scholar]
- 161.Brown M, Semelka R. MRI: basic principles and applications. Wiley-Liss; Hoboken, NJ: 2003. [Google Scholar]
- 162.Josephson L, Lewis J, Jacobs P, Hahn PF, Stark DD. The Effects of Iron-Oxides on Proton Relaxivity. Magnetic Resonance Imaging. 1988;6:647–653. doi: 10.1016/0730-725x(88)90088-4. [DOI] [PubMed] [Google Scholar]
- 163.Jun YW, Huh YM, Choi JS, Lee JH, Song HT, Kim S, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J Am Chem Soc. 2005;127:5732–3. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 164.Semelka RC, Helmberger TK. Contrast agents for MR imaging of the liver. Radiology. 2001;218:27–38. doi: 10.1148/radiology.218.1.r01ja2427. [DOI] [PubMed] [Google Scholar]
- 165.Harisinghani MG, Weissleder R. Sensitive, noninvasive detection of lymph node metastases. PLoS Med. 2004;1:e66. doi: 10.1371/journal.pmed.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Enochs WS, Harsh G, Hochberg F, Weissleder R. Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent. J Magn Reson Imaging. 1999;9:228–32. doi: 10.1002/(sici)1522-2586(199902)9:2<228::aid-jmri12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 167.Neuwelt EA, Varallyay P, Bago AG, Muldoon LL, Nesbit G, Nixon R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol Appl Neurobiol. 2004;30:456–71. doi: 10.1111/j.1365-2990.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 168.Varallyay P, Nesbit G, Muldoon LL, Nixon RR, Delashaw J, Cohen JI, Petrillo A, Rink D, Neuwelt EA. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am J Neuroradiol. 2002;23:510–9. [PMC free article] [PubMed] [Google Scholar]
- 169.Taschner CA, Wetzel SG, Tolnay M, Froehlich J, Merlo A, Radue EW. Characteristics of ultrasmall superparamagnetic iron oxides in patients with brain tumors. AJR Am J Roentgenol. 2005;185:1477–86. doi: 10.2214/AJR.04.1286. [DOI] [PubMed] [Google Scholar]
- 170.Koo YE, Reddy GR, Bhojani M, Schneider R, Philbert MA, Rehemtulla A, Ross BD, Kopelman R. Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev. 2006;58:1556–77. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 171.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. doi: 10.1002/smll.200700784. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115:2076–86. doi: 10.1161/CIRCULATIONAHA.106.658930. [DOI] [PubMed] [Google Scholar]
- 173.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–80. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 174.Kooi ME, Cappendijk VC, Cleutjens K, Kessels AGH, Kitslaar P, Borgers M, Frederik PM, Daemen M, van Engelshoven JMA. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 175.Trivedi RA, U-King-Im JM, Graves MJ, Cross JJ, Horsley J, Goddard MJ, Skepper JN, Quartey G, Warburton E, Joubert I, Wang LQ, Kirkpatrick PJ, Brown J, Gillard JH. In vivo detection of macrophages in human carotid atheroma - Temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 176.Kelly KA, Nahrendorf M, Yu AM, Reynolds F, Weissleder R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Molecular Imaging And Biology. 2006;8:201–207. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- 177.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 178.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–33. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 179.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–71. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 180.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–99. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 181.Schellenberger EA, Sosnovik D, Weissleder R, Josephson L. Magneto/optical annexin V, a multimodal protein. Bioconjugate Chemistry. 2004;15:1062–1067. doi: 10.1021/bc049905i. [DOI] [PubMed] [Google Scholar]
- 182.Sosnovik DE, Weissleder R. Emerging concepts in molecular MRI. Curr Opin Biotechnol. 2007;18:4–10. doi: 10.1016/j.copbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 183.Grief AD, Richardson G. Mathematical modelling of magnetically targeted drug delivery. Journal Of Magnetism And Magnetic Materials. 2005;293:455–463. [Google Scholar]
- 184.Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lubbe AS. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60:6641–8. [PubMed] [Google Scholar]
- 185.Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C, Huenges E, Nawroth T, Arnold W, Parak FG. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J. 2006;35:446–50. doi: 10.1007/s00249-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 186.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. Journal Of Materials Chemistry. 2004;14:2161–2175. [Google Scholar]
- 187.Schulze K, Koch A, Schopf B, Petri A, Steitz B, Chastellain M, Hofmann M, Hofmann H, von Rechenberg B. Intraarticular application of superparamagnetic nanoparticles and their uptake by synovial membrane - an experimental study in sheep. Journal Of Magnetism And Magnetic Materials. 2005;293:419–432. [Google Scholar]
- 188.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 189.Yang J, Lee H, Hyung W, Park SB, Haam S. Magnetic PECA nanoparticles as drug carriers for targeted delivery: Synthesis and release characteristics. Journal Of Microencapsulation. 2006;23:203–212. doi: 10.1080/02652040500435444. [DOI] [PubMed] [Google Scholar]
- 190.Kute T, Lack CM, Willingham M, Bishwokama B, Williams H, Barrett K, Mitchell T, Vaughn JP. Development of Herceptin resistance in breast cancer cells. Cytometry A. 2004;57:86–93. doi: 10.1002/cyto.a.10095. [DOI] [PubMed] [Google Scholar]
- 191.Ito A, Kuga Y, Honda H, Kikkawa H, Horiuchi A, Watanabe Y, Kobayashi T. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 2004;212:167–75. doi: 10.1016/j.canlet.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 192.McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 194.Juliano RL, Alahari S, Yoo H, Kole R, Cho M. Antisense pharmacodynamics: Critical issues in the transport and delivery of antisense oligonucleotides. Pharmaceutical Research. 1999;16:494–502. doi: 10.1023/a:1011958726518. [DOI] [PubMed] [Google Scholar]
- 195.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–9. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 196.Krotz F, de Wit C, Sohn HY, Zahler S, Gloe T, Pohl U, Plank C. Magnetofection - A highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Molecular Therapy. 2003;7:700–710. doi: 10.1016/s1525-0016(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 197.Dobson J. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther. 2006;13:283–7. doi: 10.1038/sj.gt.3302720. [DOI] [PubMed] [Google Scholar]
- 198.Pan BF, Cui DX, Sheng Y, Ozkan CG, Gao F, He R, Li Q, Xu P, Huang T. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Research. 2007;67:8156–8163. doi: 10.1158/0008-5472.CAN-06-4762. [DOI] [PubMed] [Google Scholar]
- 199.Schillinger U, Brill T, Rudolph C, Huth S, Gersting S, Krotz F, Hirschberger J, Bergemann C, Plank C. Advances in magnetofection - magnetically guided nucleic acid delivery. Journal Of Magnetism And Magnetic Materials. 2005;293:501–508. [Google Scholar]
- 200.Ryther RCC, Flynt AS, Phillips JA, Patton JG. siRNA therapeutics: Big potential from small RNAs. Gene Therapy. 2005;12:5–11. doi: 10.1038/sj.gt.3302356. [DOI] [PubMed] [Google Scholar]
- 201.Mykhaylyk O, Vlaskou D, Tresilwised N, Pithayanukul P, Moller W, Plank C. Magnetic nanoparticle formulations for DNA and siRNA delivery. Journal Of Magnetism And Magnetic Materials. 2007;311:275–281. [Google Scholar]
- 202.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–7. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]