Abstract
Background
Functional variants in the catechol-O-methyltransferase (COMT) gene have been shown to impact cognitive function, cortical physiology and risk for schizophrenia. A recent study showed that previously reported effects of the functional val158met SNP (rs4680) on brain function are modified by other functional SNPs and haplotypes in the gene, though it was unknown if these effects are also seen in brain structure.
Methods
We used voxel-based morphometry to investigate the impact of multiple functional variants in COMT on gray matter volume in a large group of 151 healthy volunteers from the CBDB/NIMH Genetic Study of Schizophrenia.
Results
We found that the previously described rs4680 val risk variant affects hippocampal and dorsolateral prefrontal (DLPFC) gray matter volume. In addition, we found that this SNP interacts with a variant in the P2 promoter region (rs2097603) in predicting changes in hippocampal gray matter volume consistent with a nonlinear effect of extracellular dopamine.
Conclusions
We report evidence that interacting functional variants in COMT affect gray matter regional volume in hippocampus and DLPFC, providing further in vivo validation of the biological impact of complex genetic variation in COMT on neural systems relevant for the pathophysiology of schizophrenia and extending observations of nonlinear dependence of prefrontal neurons on extracellular dopamine to the domain of human brain structure.
Introduction
Catechol-O-methyltransferase (COMT) is a major enzyme degrading catecholamines, especially dopamine (DA) (Bertocci et al., 1991; Grossman et al., 1992). The COMT gene is located on chromosome 22q11.22-23 and consists of two promoters and six exons which encode both the membrane-bound (MB-COMT) and soluble (S-COMT) forms of COMT. Of the two confirmed isoforms, MB-COMT is predominantly expressed in the central nervous system at neuronal dendritic processes throughout the cortex, cerebellum, amygdala, putamen, thalamus, spinal cord, and hippocampus (Hong et al., 1998; Masuda et al., 2003). COMT appears to be concentrated in the extrasynaptic spaces of the prefrontal cortex and hippocampus (Deutch and Roth, 1990; Matsumoto et al., 2003a; Matsumoto et al., 2003b). Since prefrontal dopamine transporters are scarce, COMT is thought to play a key role in clearing dopamine in the prefrontal cortex, which has been demonstrated directly by a two- to threefold increase in baseline frontal dopamine in male COMT knockout mice (Gogos et al., 1998). An evolutionarily recent functional single nucleotide polymorphism (SNP) in COMT results in the amino acid substitution of valine (val) with methionine (met) at codon 158 of MB-COMT (rs4680, (Savitz et al., 2006)). This substitution leads to a significant (approximately 40%) decrease in enzymatic activity in the brain and lymphocytes (Chen et al., 2004a) of the met-allele compared to the val-containing polypeptide. Consequently, met-carriers are presumably associated with a higher level of prefrontal extra-cellular dopamine (Chen et al., 2004b; Lachman et al., 1996). A large body of work has demonstrated an impact of this genetic variant on neural function related to cognitive and affective processing. Several studies have shown that met/met homozygous individuals have increased PFC signal to noise (Egan et al., 2001; Mattay et al., 2003; Meyer-Lindenberg et al., 2005) and improved performance on prefrontally dependent cognitive tasks like working memory, while those with the high activity val-allele have relatively poorer performance and “inefficient” dorsolateral prefrontal function (Bilder et al., 2002; Bilder et al., 2004; Egan et al., 2001; Malhotra et al., 2002; Mattay et al., 2003; Meyer-Lindenberg et al., 2005; Savitz et al., 2006). Sub-optimal DA activity in the prefrontal cortex could contribute to some of the cognitive abnormalities found in schizophrenia (Meyer-Lindenberg et al., 2002; Meyer-Lindenberg and Weinberger, 2006; Weinberger, 1999b), namely working memory, perserverative errors on the Wisconsin Card Sorting Test (WCST), verbal fluency, and IQ (Bearden et al., 2004; Egan et al., 2001; Rosa et al., 2004). The COMT val/met polymorphism is also associated with functional variation in hippocampal processing of emotional stimuli (Drabant et al., 2006; Heinz and Smolka, 2006) and of episodic memory (Bertolino et al., 2004).
COMT is located in a region implicated in schizophrenia by linkage studies (Owen et al., 2004) as well as the 22q11.2 deletion syndrome, which is associated with strongly increased risk for psychosis (Sanders et al., 2005; Yan et al., 1998; Zinkstok and van Amelsvoort, 2005). However, despite the large body of convergent evidence implicating COMT val158met in brain function, the strength of association with manifest psychiatric illness has been variable, and the attributable risk low (Fan et al., 2005; Munafo et al., 2005). One reason for this discrepancy could be that additional loci within COMT have an effect on gene function, ultimately affecting enzyme activity and adding complexity to the functional and clinical implications of COMT variation. Independent functional effects of three genetic variants in COMT have indeed been demonstrated. A cis functional variant (rs2097603) linked upstream in the P2 promoter, driving transcription of the predominant form of COMT in the brain (MB-COMT), affects COMT activity in lymphocytes and post-mortem brain tissue (Chen et al., 2004a). Another variant in the 3′ untranslated region (rs165599), highly associated with schizophrenia in a large sample of Israelis of Ashkenazi descent (Shifman et al., 2002), was found to differentially affect expression of rs4680 alleles in human brain tissue (Bray et al., 2003), possibly through altering an miRNA binding site (Barenboim, Lipska and Weinberger, unpublished results). A further synonymous variant has been identified only 60 nucleotides from val/met and impacts COMT translation through an alteration of mRNA secondary structure (Nackley et al., 2006). Furthermore, recent studies show that genetic variants in COMT may functionally interact with regard to brain function in healthy humans (Meyer-Lindenberg et al., 2006), suggesting a possible impact on other neural parameters such as brain structure.
The relationship between brain structure and the COMT val158met variant, studied in isolation, is unclear. Two studies reported no associations between genotype and brain volume in healthy controls (Ho et al., 2005; Zinkstok et al., 2006), and two reported genotype effects in patients with schizophrenia (Ohnishi et al., 2006) or subjects at risk for psychosis (McIntosh et al., 2006a). All of these earlier studies have involved small samples and none have investigated structural correlates of other functional SNPs or haplotypes in COMT.
The current study sought to investigate the impact of several COMT polymorphisms on gray matter volume in healthy subjects using optimized voxel-based morphometry (VBM). VBM has been useful in identifying alterations in gray matter (GM) related to genetic variation brain-derived neurotrophic factor (BDNF), 5-HTTLPR, and DISC1 (Callicott et al., 2005; Pezawas et al., 2005; Pezawas et al., 2004). We utilized a previously described regression-based approach to probe the influence of probabilistic haplotype variation in COMT on brain structure (Meyer-Lindenberg et al., 2006). We investigated a 2-SNP haplotype composed of rs4680 and a P2 promoter region SNP (rs2097603) and a 3-SNP haplotype of rs2097603 – rs4680 – rs165599, previously found to have a statistically strong impact on prefrontal function (Meyer-Lindenberg et al., 2006) and risk for schizophrenia (Nicodemus et al., 2007). Our large sample also allowed us to control for occult genetic stratification in genes such as BDNF, accounting for a potential confound in interpreting dopaminergic affects on the brain (Meyer-Lindenberg and Weinberger, 2006; Savitz et al., 2006).
We report evidence that interacting functional variants in COMT affect gray matter regional volume in hippocampus and DLPFC, providing further in vivo validation of the biological impact of complex genetic variations within COMT on brain structure.
Methods and Materials
Subjects
Subjects were selected from a larger population after careful screening (Egan et al., 2001), ensuring they were free of any current or lifetime history of psychiatric or neurological illness, psychiatric treatment, and drug or alcohol abuse. Only Caucasians of European ancestry were studied to avoid stratification artifacts. Subjects gave written informed consent and participated in the study according to the guidelines of the National Institute of Mental Health Institutional Review Board. For customized template creation, a sample of 171 subjects meeting these criteria was used (aged 19-61; mean, 32.36 ± 9.43); from these, all scans from subjects with available COMT genotype data were used for the VBM analysis (72 male; 79 females; age range 19-61) (Table 1).
Table 1.
Demographics by Genotype, separated by SNP
| val158met SNP (rs4680) | Val/Val | Val/Met | Met/Met | All | ANOVA |
|---|---|---|---|---|---|
| Variable | (n = 38) | (n = 78) | (n = 35) | (n = 151) | p-value |
| Age | 31.1 (8.9) | 34.1 (10.4) | 32.4 (8.6) | 32.9 (9.7) | p = 0.281* |
| Gender (M/F) | 19/19 | 35/43 | 18/17 | 72/79 | p = 0.773* |
| Education years | 16.4 (3.9) | 16.8 (2.7) | 17.3 (3.2) | 16.8 (3.1) | p = 0.505* |
| WAIS full scale IQ | 108.9 (8.9) | 107.6 (8.8) | 106.1 (7.8) | 107.6 (8.6) | p = 0.373* |
| Handedness | 84.6 (44.3) | 76.4 (53.2) | 85.4 (38.6) | 80.5 (47.9) | p = 0.539* |
| P2 promoter SNP (rs2097603) | A/A | A/G | G/G | All | ANOVA |
| Variable | (n = 42) | (n = 81) | (n = 23) | (n = 146) | p-value |
| Age | 34.4 (10.6) | 31.6 (9.0) | 35.9 (10.2) | 33.1 (9.8) | p = 0.108* |
| Gender (M/F) | 20/22 | 40/41 | 9/14 | 69/77 | p = 0.689* |
| Education years | 17.0 (3.8) | 16.6 (3.0) | 17.0 (2.6) | 16.8 (3.2) | p = 0.763* |
| WAIS full scale IQ | 109.5 (9.0) | 107.0 (8.6) | 106.0 (7.9) | 107.5 (8.7) | p = 0.156* |
| Handedness | 83.6 (45.3) | 79.6 (48.2) | 84.3 (41.3) | 81.5 (46.1) | p = 0.859* |
| 3″ flanking SNP (rs737865) | A/A | A/G | G/G | All | ANOVA |
| Variable | (n = 65) | (n = 64) | (n = 13) | (n = 142) | p-value |
| Age | 33.0 (10.0) | 32.8 (9.8) | 31.6(9.3) | 32.8 (9.6) | p = 0.793* |
| Gender (M/F) | 29/36 | 34/30 | 5/8 | 68/74 | p = 0.491* |
| Education years | 16.5 (3.7) | 16.6 (3.4) | 17.8 (2.3) | 16.8 (3.2) | p = 0.400* |
| WAIS full scale IQ | 105.3 (9.1) | 106.0 (12.0) | 113.3 (6.4) | 107.4 (8.6) | p = 0.004 |
| Handedness | 86.5 (35.0) | 77.9 (50.9) | 97.7 (6.0) | 79.9 (49.1) | p = 0.059* |
Means (± SD).
There is no significant difference between genotype for any variable
Genotyping and haplotype construction
COMT genotype frequencies were in Hardy-Weinberg equilibrium. Genotyping for the three SNPs studied here, (1) P2 promoter SNP (rs2097603), (2) val158met SNP (rs4680) and (3) 3′ flanking SNP (rs165599) were performed as described previously using the Taqman 5′ endonuclease assay (Chen et al., 2004a). There were no significant differences in age, gender, IQ, handedness, or years of education between genotype groups for the val158met SNP or the P2 promotor SNP. There was a significant difference in IQ for the 3′ flanking SNP genotype groups A/A and G/G in IQ (p<.024), thus IQ was included as a nuisance covariate in all imaging analyses. Linkage disequilibrium between markers was determined by GOLD (Abecasis and Cookson, 2000). We constructed likely 2- and 3-SNP haplotypes in our samples by use of PHASE 2.1 (Stephens and Donnelly, 2003). Since we had 151 subjects, only haplotypes with estimated frequency > 3% were used in subsequent analysis to ensure we had sufficient numbers of scans per haplotype. Frequencies are shown in Table 2.
Table 2.
Genotype (coding strand)/estimated haplotype frequencies (%)
| Genotype/haplotype | Genotype/estimated haplotype frequency |
|---|---|
| rs2097603(A → G) | 42 (AA) 81 (AG) 23 (GG) |
| rs4680 (G-val → A-met) | 38 (val/val) 78 (val/met) 35 (met/met) |
| rs165599 (A → G) | 65 (AA) 64 (AG) 13 (GG) |
| 2-SNP haplotype (rs2097603–rs4680) | |
| A-val | 39 ± .3 |
| A-met | 17 ± .8 |
| G-val | 11 ± .1 |
| G-met | 31 ± .7 |
Structural image processing and analyses
Three-dimensional volumes of T1-weighted SPGR images were acquired on a 1.5T GE scanner with a voxel resolution of 0.975 × 0.975 × 1.5 mm3 (TR/TE/NEX 24/5/1; flip angle 45°; matrix size 256 × 256; FOV 24 × 24 cm; 124 sagittal slices). Data were analyzed on a Linux workstation (Red Hat 8.0) using MATLAB 6.51SP1 (Mathworks, Natick, MA) using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Imaging Neuroscience, London, UK). Additional imaging software packages used were Analysis of Functional NeuroImages (AFNI) (Cox, 1996), and Surface Mapping with AFNI (SUMA) (Argall et al., 2005).
Template creation was based on a sample of 171 healthy control subjects recruited as described above. An iterative procedure was used to make the customized template optimally suited for our sample, as described previously (Pezawas et al., 2004).
Structural imaging data were preprocessed using AFNI to set the origins followed by optimized VBM, performed in SPM2, using the non-uniformity correction option to correct for image inhomogeneities (Ashburner and Friston, 2000; Good et al., 2001). Relative volume was assessed by correcting for volume changes made during the normalization transformation and images were modulated by multiplication with the determinant of the Jacobian matrix from the spatial normalization function. An integration of modulated gray matter voxel values resulted in estimates for total gray matter volume. The normalized segmented images were smoothed using a 10mm FWHM isotropic Gaussian kernel.
The normalized and smoothed gray matter images were analyzed using the General Linear Model as implemented in SPM2. For the three single SNP analyses we used a multiple regression model that included age, second-order polynomial age expansions, gender, and IQ as nuisance covariates of no interest to assess genetic affects on absolute volumes. In addition we ran an identical analysis, also adding total gray matter volume in the design matrix to assess genetic affects on relative volumes. Voxel-by-voxel t-tests were used at the level of the whole brain to determine global differences in gray matter volumes based on COMT val158met, grouping alleles as val/val homozygotes, val/met heterozygotes, and met/met homozygotes. In addition, for the P2 Promotor SNP (rs2097603) and the 3′ flanking SNP (rs165599) global differences in gray matter volume were tested between the AA, AG, and GG allele carriers.
SPM2 was also used for an adapted haplotype trend regression model. We tested for the association between both absolute and relative gray matter volumes and the estimated haplotype frequencies with the same nuisance covariates as before (42). Each of the haplotype combinations (for both the 2SNP and 3SNP haplotypes) were contrasted within the analysis for significant global gray matter variation.
Previous data has demonstrated the influence of genetic variation in COMT on the function of DLPFC (i.e. Brodmann's areas (BA) 9, 10, 45, and 46) and hippocampus (Bertolino et al., 2006; Drabant et al., 2006; Harrison and Weinberger, 2005; Smolka et al., 2005), so we used a region of interest (ROI) approach to specifically probe effects in these areas. The choice of the a-priori DLPFC ROI was based on the maximum effect in several studies and consisted of BA 9, 10, 45 and 46 (Callicott et al., 2003; Egan et al., 2001). ROIs were made based on the WFU PickAtlas (http://www.fmri.wfubmc.edu; Advanced Neuroscience Imaging Research Core, Wake Forest University, Winston-Salem, NC, USA), selecting the above mentioned areas with a dilation of 1 mm. Gray matter volume changes were assessed statistically using t-contrasts after small-volume correction for these ROIs in each of the 3 single SNP analyses, as well as the two haplotype analyses. Family-wise error rate estimations were used to correct for multiple comparisons across voxels, where a corrected probability of p < 0.05 was considered significant.
Results
Single SNP analyses
We found gray matter volume differences in both medial temporal and frontal structures based on the val158met SNP. Specifically, at the whole-brain level, we found significant decreases in volume in a cluster of voxels including the left hippocampus and parahippocampal gyrus in val-allele carriers and val homozygotes relative to met homozygotes (p<.05 FWE corrected, Figure 1a). Within our hippocampal ROI, we identified significant bilateral hippocampal reductions in val-carriers (p<.01 FWE corrected MNI coordinates (-24, -12, -27) and (24, -13, -26)). In the reverse contrast (val/val > val/met > met/met), our a-priori ROI in DLPFC (including BA 9, 10, 45, and 46) showed a trend for volume reductions in the met-carriers (p<.068 FWE corrected, Figure 1b). No additional voxels showed significant association at the whole-brain level.
Figure 1.
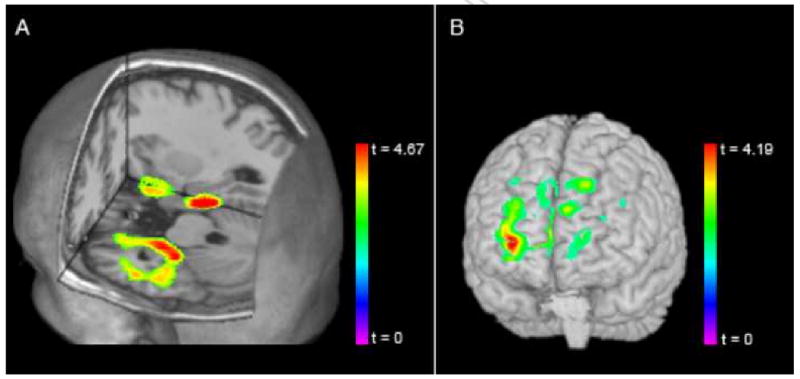
A. Whole brain VBM results overlaid on MRICRON 3-D mean 154 brain showing significant decreases in volume in a cluster of voxels including the left hippocampus and parahippocampal gyrus in val-allele carriers and val homozygotes relative to met homozygotes. B. Overlay demonstrating a trend for volume reductions in met-carriers in (val/val > val/met > met/met) an ROI in the DLPFC (including BA 9, 10, 45, and 46).
Independent analysis of the P2 promoter and 3′ flanking SNPs revealed no significant effect of genetic variation for either SNP alone on brain structure. This included investigation of absolute and relative volumes at the whole brain level as well as in our hippocampal and DLPFC regions of interest.
2-SNP & 3-SNP Haplotype analyses
Analysis of the 2-SNP haplotype, composed of the P2 promoter SNP and val158met SNP, revealed a significant effect (in ROI) for variation of val158met when contrasted on the P2 promoter-A background, that is, the P2-A allele & val/val (A-val) > P2-A allele & met-carriers (A-met) (p < 0.06, whole-brain corrected, p < 0.01, corrected in hippocampal ROI, (-34, -14, -13), Figure 2). There was also a trend for an effect of variation in the hippocampal ROI in the contrast of the P2-G allele & met-carriers (G-met) > P2-G allele & val-carriers (G-val). When ordered by putative genetic variation of enzymatic activity (Meyer-Lindenberg et al., 2006), such that the highest activity was for A-val, then G-val (effect of reduced expression at promoter SNP in val-carriers), then A-met and finally G-met (corresponding to the met-carriers on each of the two P2 promoter backgrounds), a nonlinear U-shape relationship of haplotype effects was found in absolute volumes, where both A-met and G-val were associated with lower hippocampal volumes than A-val and G-met haplotypes (Figure 3).
Figure 2.
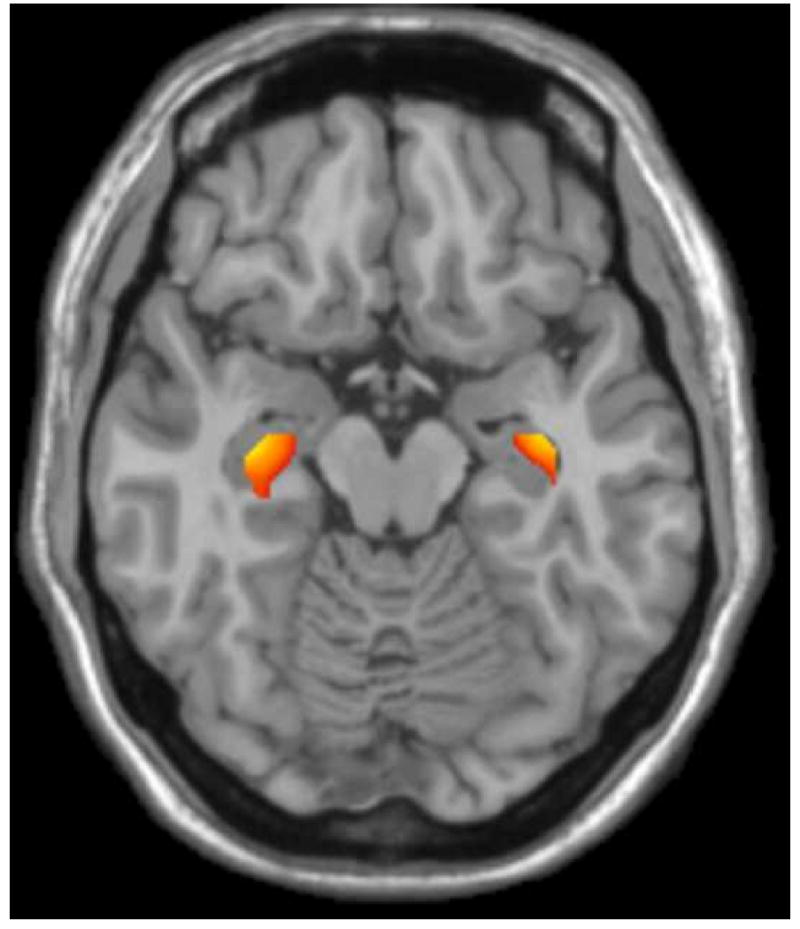
Results overlaid on MNI T1 axial slice from the analysis of the 2-SNP haplotype, composed of the P2 promoter SNP and val158met SNP. Displayed is the significant effect of val158met variation on gray matter volume when contrasted on the P2 promoter-A background (P2-A allele & val/val (A-val) > P2-A allele & met-carriers (A-met)), (p < 0.06, whole-brain corrected, p < 0.01, corrected in hippocampal ROI, (-34, -14, -13)).
Figure 3.
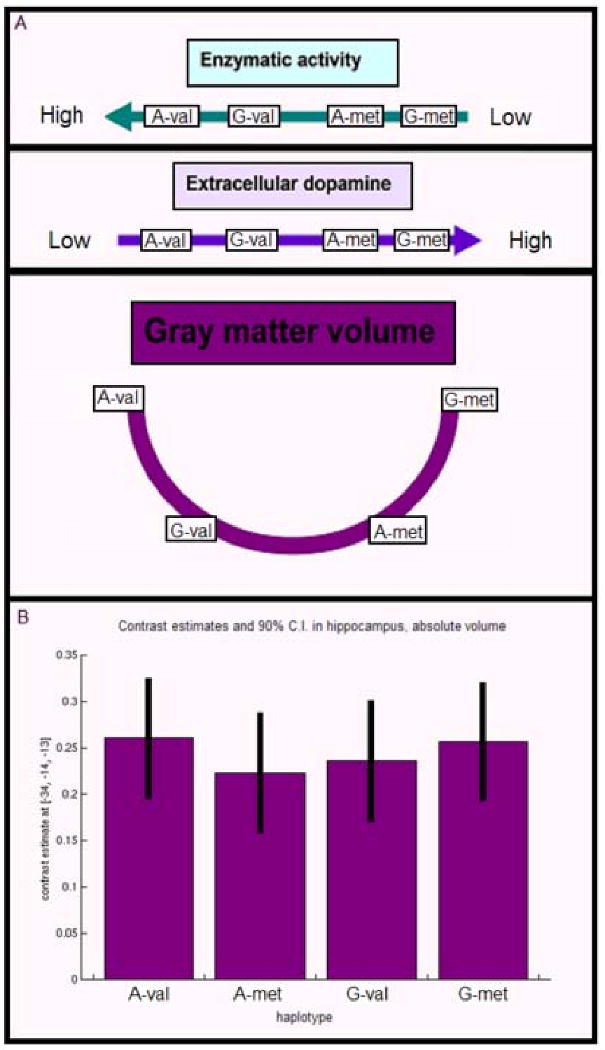
Figure 3 (B) demonstrates the trend for an effect of variation in the hippocampal ROI in the contrast of the P2-G allele & met-carriers (G-met) > P2-G allele & val-carriers (G-val). (A) When ordered by putative genetic variation of enzymatic activity, such that the highest activity was for A-val, then G-val (effect of reduced expression at promoter SNP in val-carriers), then A-met and finally G-met (corresponding to the met-carriers on each of the two P2 promoter backgrounds), a nonlinear U-shape relationship of haplotype effects was found in absolute hippocampal volumes, where both A-met and G-val were associated with lower volumes than A-val and G-met haplotypes
The 3-SNP haplotype analysis, consisting of the P2 promoter, val158met, and the 3′ flanking SNP (rs2097603-rs4680-rs165599), did not uncover significant effects in either hippocampus or DLPFC. However, based on previous findings with this haplotype in a functional MRI dataset, we investigated effects in BA 45, corresponding to the locale of our previous observation of maximal functional effects of this gene (Meyer-Lindenberg et al., 2006); this yielded a non-significant trend that was congruent with this earlier study (A-val-A had less gray matter volume than A-val-G, p < 0.15, corrected in BA 45 ROI).
Discussion
Our results show an impact of COMT genetic variation on brain structure that is characterized by allelic variation in both the val158met SNP and a 2-SNP haplotype consisting of val158met and the P2 promoter SNP. In our study, the functional COMT val158met polymorphism significantly influenced hippocampal cortical volume. While the individual analysis of the P2 promoter and 3′ SNPs did not reveal a significant effect on brain structure, the 2-SNP haplotype revealed the complexity of the effects of multiple variants on gray matter volume, similar to previous results on brain function (Meyer-Lindenberg et al., 2006). Our data implies that complex genetic variation in COMT may impact gray matter volume, possibly through neurotrophic or neurotoxic effects of varying levels of DA.
Previous work involving val158met and brain structure has resulted in conflicting results. Interestingly, Kates et al. found a COMT by gender interaction on the DLPFC in children with velo-cardio-facial syndrome (Kates et al., 2006). A recent paper studying young healthy adults found no group differences in regional gray matter density as a function of genotype (Zinkstok et al., 2006), indicating that regional volume measures, as opposed to density measures, may be more sensitive indicators of genotype-related alterations. Issues related to reduced sensitivity may also underlie a negative result by Ho and colleagues, who found no effect of this genotype on total frontal gray matter in patients and controls, but did not study regional volume change (Ho et al., 2005). In a group of subjects at high risk for psychosis, McIntosh and coworkers (McIntosh et al., 2006a) saw increased volume in met homozygotes relative to val homozygotes in the anterior cingulate. No relevant effect was observed in this location in our study, nor in the studies by Zinstock et al and Ho et al. It appears possible that the at-risk group showed different genotype effects because they are expected have more interacting genetic variants predisposing them to psychosis, which might impact on expression of the neural phenotype (Harrison and Weinberger, 2005). This interpretation could also apply to the results of Ohnishi and coworkers, who observed no genotype effects in controls, but did find volume effects in the cingulate and left medial temporal gyrus in patients, at very lenient statistical thresholds, who carried val, with a gene by diagnosis interaction in left parahippocampus-amygdala (Ohnishi et al., 2006). These authors used yet another methodological approach, tensor-based morphometry, which does not account for local gray matter intensity.
While the discrepant findings could be accounted for by methodological, study population and statistical threshold differences, it also appears likely that the val158met variant, in isolation, may not identify a sufficiently homogeneous population of subjects. This parallels the inconsistent association of COMT with schizophrenia (Fan et al., 2005; Munafo et al., 2005), and suggests that additional loci within COMT have an effect on gene function, ultimately affecting enzyme activity and adding complexity to the neural and clinical implications of COMT variation. Regarding risk for schizophrenia, this idea receives strong support from several studies. A large Ashkenazi sample showed highly significant association for two other polymorphisms (in intron 1, rs737865, and 3′ UTR (rs165599), as well as for a core haplotype of three markers including val (P=9.5 × 10 -8) (Reenila and Mannisto, 2001). Handoko et al, in a recent analysis of these SNPs and this three-marker haplotype (rs737865-rs4680-rs165599), found that while none of the individual SNPs showed significant association (when corrected for multiple comparisons), the haplotype showed significant evidence of association (Handoko et al., 2005). Nicodemus et al (2007) (Nicodemus et al., 2007) recently reported that diplotypes comprised of haplotypes shown to be most deleterious in terms of prefrontal function in our earlier fMRI analysis (Meyer-Lindenberg et al., 2006) were significantly enriched in a case control sample of patients with schizophrenia that was negative for any of the individual SNPs in the haplotype.
Our data indeed demonstrate that interacting functional variants impact on regional brain volume. We found that while overall, val-allele carrying subjects had less hippocampal volume than those homozygote for the met allele, two subpopulations with relatively higher or lower volume could be distinguished for both val- and met-allele chromosomes based on the P2 promoter genotype. Specifically, we found a u-shaped relationship between hippocampal volume and presumed COMT activity, such that very low activity (COMT-met combined with the low activity G-allele of the P2 promoter) and very highly active COMT variants (val158 on the ancestral P2-A allele) had larger volumes than haplotypes with predicted intermediate COMT activity (P2-G allele and val158, and P2-A allele and 158met) (Figure 3). This nonlinearity in genotype relating to structure mirrors our previous findings on the effects of these variants on brain function (Meyer-Lindenberg et al., 2006). Preclinical data suggest that these functional effects are related to a lawful effect of D1-mediated extracellular dopamine stimulation on the firing rate and tuning of prefrontal neurons, which exhibit a middle range of dopamine at which ‘tuning,’ --or ratio of task-related to task-unrelated neural firing--(Vijayraghavan et al., 2007; Williams and Goldman-Rakic, 1995), is optimal. This may relate to risk for schizophrenia through reduced prefrontal signal to noise in the context of reduced prefrontal D1 stimulation (Meyer-Lindenberg and Weinberger, 2006; Vijayraghavan et al., 2007). Neuroimaging data further indicate that the position on this u-shaped curve is a trait-like enduring characteristic (Meyer-Lindenberg et al., 2005).
Our data suggest a similar impact of trait-like variation in dopaminergic neurotransmission (in particular: extracellular dopamine concentration) on neural growth and survival in humans. Several lines of preclinical evidence support this hypothesis. Establishment of dopaminergic connections coincides with neuronal growth and differentiation in striatum (Fishell and van der Kooy, 1987) and dopamine induces the neurotrophic factor, BDNF, in neuronal culture (Kuppers and Beyer, 2001). These effects have been linked to D1 receptor stimulation, but also to antioxidative activity (Ma and Zhou, 2006). Reductions in dopamine signaling in knockout models of the dopamine D1 receptor (Xu et al., 1994) and tyrosine hydroxylase (Zhou and Palmiter, 1995) severely impair neuronal differentiation. While these data show that dopamine stimulation is relevant for growth and differentiation of dopaminoceptive neurons, clinical and preclinical work also indicates that excess dopamine can impair neuronal integrity and survival. Excess extracellular dopamine is neurotoxic (Santiago et al., 2000). In mouse knockouts for the dopamine transporter, which exhibit chronically elevated extracellular dopamine levels, BDNF gene expression is reduced in frontal cortex (Fumagalli et al., 2003). In the brain of human methamphetamine abusers, pronounced reductions in cortical volume were found in medial prefrontal and limbic cortex, as well as in hippocampus (Thompson et al., 2004). Taken together, these data indicate that both over- and under-stimulation with dopamine may result in impaired neuronal survival and growth, implying that an optimum range for extracellular dopamine in cortex and hippocampus may exist for structural integrity.
To our knowledge, this is the first report of combined genetic variants in COMT impacting on cortical structure. This complements previous findings in function (Meyer-Lindenberg et al., 2006), enzymatic activity (Chen et al., 2004a), and mRNA expression (Matsumoto et al., 2003b), and may provide further understanding for the putative involvement of COMT variants in risk for schizophrenia (Nicodemus et al., 2007).
Although predominantly studied within the context of prefrontal function and molecular subtleties; we clearly identified structural effects in the hippocampus and parahippocampal gyrus, as well as a trend in the prefrontal cortex. This is in agreement with the abundance of COMT in these structures (Hong et al., 1998) and, again, may be involved in the risk for schizophrenia since both these regions have been prominently involved in the pathophysiology of the illness (Weinberger, 1999a). In addition VBM studies of subjects at high-risk for schizophrenia have shown gray matter volume reductions in the hippocampus and prefrontal cortex (Job et al., 2005; Job et al., 2003; McIntosh et al., 2004; McIntosh et al., 2006b). It is interesting to note that structural effects of variation of rs4680 were opposite in direction in these two structures (i.e. met-alleles predicted decreased volume in the hippocampus, but relative (non-significant) increases in PFC), although the mechanism of this observation, which could include differential effects of extracellular dopamine on allocortex and neocortex or compensatory PFC-hippocampal interactions, is unclear. However, we previously observed opposite effects of COMT variation on hippocampal activation and connectivity (Drabant et al., 2006) from those found in PFC (Egan et al., 2001; Mattay et al., 2003; Meyer-Lindenberg et al., 2006), indicating that a similar phenomenon is present in functional activation as well. Therefore, whatever the neurobiological proximate mechanisms, there is a multimodal convergence showing qualitative differences in the impact of COMT genetic variation in different brain regions.
We were careful to control for potential confounding factors such as occult stratification and genetic admixture using this genomic control analysis. However, replication of these results in independent samples is necessary. It should be noted that the genetics even of intermediate phenotypes is still likely to be complex, and other genetic variants, for example in BDNF, are also expected to have effects (Ahtila et al., 1995) that may interact with those studied here. A focused study of such epistatic interactions in adequately powered samples will be a fruitful further research avenue.
Finally, the scope of VBM, while it has proved very useful for the analysis of genetic variation not constrained by traditional anatomical landmarks, is limited to regional volume change in gray matter and should be supplemented by analyses looking at cortical thickness, shape, or density. These analyses would be particularly useful considering the location of the genotype effect in our sample, as more ventral regions surrounded by CSF are particularly susceptible to segmentation error and partial volume affects. In addition, MRI gray matter signal should not be naively assumed to reflect neuronal volume, since it is affected by hydration, medication, scanner sequence, and environmental exposures (Davatzikos, 2004; McClure et al., 2006). Spatial normalization in VBM can not perfectly register individuals (Nieto-Castanon et al., 2003), and significant differences, especially those not corrected for multiple comparisons, may be a function of misregistration (Bookstein, 2001). To date, data on hippocampal volume from MRI and post-mortem imaging literature do not always correspond (Weinberger, 1999a), and VBM may be reflecting other more complex changes going on at the neuronal level other than gross volume loss. However, this study implemented the same scanner sequence on a large group of healthy controls, used a custom template, and used an optimized VBM protocol, therefore reducing possible partial volume effects and reducing noise in this dataset (Good et al., 2001).
Overall this study provides evidence that interacting functional variants in COMT affect gray matter regional volume in human brain, providing further in vivo validation of the biological impact of complex genetic variation in COMT on neural systems relevant for the pathophysiology of schizophrenia, especially hippocampus and DLPFC, and extending observations of nonlinear dependence of prefrontal neurons on extracellular dopamine to the domain of brain structure.
Acknowledgments
This study was supported by the NIMH/IRP and by a bench-to-bedside award by NIMH, NIAAA and ORD to A.M.L. We thank Katherine Hobbs and Aaron Goldman for their assistance in data preprocessing and Joshua Buckholtz for his advice in the process of manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- Ahtila S, Kaakkola S, Gordin A, Korpela K, Heinavaara S, Karlsson M, Wikberg T, Tuomainen P, Mannisto PT. Effect of entacapone, a COMT inhibitor, on the pharmacokinetics and metabolism of levodopa after administration of controlled-release levodopa-carbidopa in volunteers. Clin Neuropharmacol. 1995;18:46–57. doi: 10.1097/00002826-199502000-00006. [DOI] [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp. 2005 doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM, Saitta SC, Harris SE, Moss E, Wang PP, Zackai E, Emanuel BS, Simon TJ. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry. 2004;161:1700–1702. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm HW, Malherbe P. Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc Natl Acad Sci U S A. 1991;88:1416–1420. doi: 10.1073/pnas.88.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, Altamura M, Nappi G, Papa S, Callicott JH, Mattay VS, Bellomo A, Scarabino T, Weinberger DR, Nardini M. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Kunz M, Chakos M, Cooper TB, Lieberman JA. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O'Donovan MC. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004a;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang X, O'Neill AF, Walsh D, Kendler KS. Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Mol Psychiatry. 2004b;9:962–967. doi: 10.1038/sj.mp.4001519. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. discussion 402-363. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, Feng GY, St Clair D, He L. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry. 2005;57:139–144. doi: 10.1016/j.biopsych.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Fishell G, van der Kooy D. Pattern formation in the striatum: developmental changes in the distribution of striatonigral neurons. J Neurosci. 1987;7:1969–1978. doi: 10.1523/JNEUROSCI.07-07-01969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol Psychiatry. 2003;8:898–899. doi: 10.1038/sj.mp.4001370. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grossman MH, Emanuel BS, Budarf ML. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1----q11.2. Genomics. 1992;12:822–825. doi: 10.1016/0888-7543(92)90316-k. [DOI] [PubMed] [Google Scholar]
- Handoko HY, Nyholt DR, Hayward NK, Nertney DA, Hannah DE, Windus LC, McCormack CM, Smith HJ, Filippich C, James MR, Mowry BJ. Separate and interacting effects within the catechol-O-methyltransferase (COMT) are associated with schizophrenia. Mol Psychiatry. 2005;10:589–597. doi: 10.1038/sj.mp.4001606. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17:359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10:229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Hong J, Shu-Leong H, Tao X, Lap-Ping Y. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport. 1998;9:2861–2864. doi: 10.1097/00001756-199808240-00033. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W, Higgins AM, Kucherlapati R, Shprintzen RJ. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome) Am J Med Genet B Neuropsychiatr Genet. 2006;141B:274–280. doi: 10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, Goldberg R, Kucherlapati R, Papolos DF. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67:468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhou J. Dopamine promotes the survival of embryonic striatal cells: involvement of superoxide and endogenous NADPH oxidase. Neurochem Res. 2006;31:463–471. doi: 10.1007/s11064-006-9038-6. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Masuda M, Tsunoda M, Imai K. High-performance liquid chromatography-fluorescent assay of catechol-O-methyltransferase activity in rat brain. Anal Bioanal Chem. 2003;376:1069–1073. doi: 10.1007/s00216-003-2025-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003a;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003b;28:1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure RK, Phillips I, Jazayerli R, Barnett A, Coppola R, Weinberger DR. Regional change in brain morphometry in schizophrenia associated with antipsychotic treatment. Psychiatry Res. 2006;148:121–132. doi: 10.1016/j.pscychresns.2006.04.008. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, Moorhead TW, Owens DG, Miller P, Porteous D, Lawrie SM, Johnstone EC. Relationship of Catechol-O-Methyltransferase Variants to Brain Structure and Function in a Population at High Risk of Psychosis. Biol Psychiatry. 2006a doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006b;141:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, Mattay VS, Egan M, Weinberger DR. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–877. 797. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry. 2005;10:765–770. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, Rujescu D, Weinberger DR. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. Neuroimage. 2003;19:1303–1316. doi: 10.1016/s1053-8119(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Hashimoto R, Mori T, Nemoto K, Moriguchi Y, Iida H, Noguchi H, Nakabayashi T, Hori H, Ohmori M, Tsukue R, Anami K, Hirabayashi N, Harada S, Arima K, Saitoh O, Kunugi H. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129:399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004;9:14–27. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenila I, Mannisto PT. Catecholamine metabolism in the brain by membrane-bound and soluble catechol-o-methyltransferase (COMT) estimated by enzyme kinetic values. Med Hypotheses. 2001;57:628–632. doi: 10.1054/mehy.2001.1430. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, Fananas L. New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry. 2004;161:1110–1112. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- Sanders AR, Rusu I, Duan J, Vander Molen JE, Hou C, Schwab SG, Wildenauer DB, Martinez M, Gejman PV. Haplotypic association spanning the 22q11.21 genes COMT and ARVCF with schizophrenia. Mol Psychiatry. 2005;10:353–365. doi: 10.1038/sj.mp.4001586. [DOI] [PubMed] [Google Scholar]
- Santiago M, Matarredona ER, Granero L, Cano J, Machado A. Neurotoxic relationship between dopamine and iron in the striatal dopaminergic nerve terminals. Brain Res. 2000;858:26–32. doi: 10.1016/s0006-8993(99)02485-3. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999a;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Schizophrenia: new phenes and new genes. Biol Psychiatry. 1999b;46:3–7. [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Yan W, Jacobsen LK, Krasnewich DM, Guan XY, Lenane MC, Paul SP, Dalwadi HN, Zhang H, Long RT, Kumra S, Martin BM, Scambler PJ, Trent JM, Sidransky E, Ginns EI, Rapoport JL. Chromosome 22q11.2 interstitial deletions among childhood-onset schizophrenics and “multidimensionally impaired”. Am J Med Genet. 1998;81:41–43. [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, Schmitz N, van Amelsvoort T, de Win M, van den Brink W, Baas F, Linszen D. The COMT val158met polymorphism and brain morphometry in healthy young adults. Neurosci Lett. 2006;405:34–39. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, van Amelsvoort T. Neuropsychological profile and neuroimaging in patients with 22Q11.2 Deletion Syndrome: a review. Child Neuropsychol. 2005;11:21–37. doi: 10.1080/09297040590911194. [DOI] [PubMed] [Google Scholar]


