Abstract
Schizophrenia is associated with subtle developmental compromise, but the degree to which this is also associated with heritability and genetic risk is uncertain. The goal of the current study was to investigate the childhood, adolescent, and early adulthood social and academic function of patients with schizophrenia, their healthy siblings, and normal controls, using the Premorbid Adjustment Scale (PAS). Generalized Estimating Equations were conducted to account for nesting of subjects within families. Patients (N=286) scored significantly worse than their healthy siblings (N=315) at every age period; siblings scored significantly worse than controls (N=261) at every age period. In probands, PAS scores got worse after early adolescence while control and proband scores improved after late adolescence. Furthermore, patient PAS scores significantly predicted the scores of their own discordant siblings in childhood and late adolescence. This effect approached significance in early adolescence and in the general scale. Thus, the most premorbidly impaired patients tended to have non-ill siblings with worse premorbid adjustment scores than the siblings of less impaired probands. The results suggest that both patients and many of their siblings share poor adjustment in childhood and adolescence, possibly due to shared genetic or environmental risk factors.
Keywords: Schizophrenia, Premorbid Adjustment Scale, Premorbid, Siblings
1. Introduction
1.1 Background
There is abundant evidence that at least some individuals who develop schizophrenia as adults demonstrate signs of subtle neurodevelopmental compromise long before the illness becomes evident. This evidence has emerged from the direct study of children born to mothers with schizophrenia (Fish et al., 1992), studies of home movies of those who later develop the illness (Walker & Lewine, 1994), and the study of developmental milestones and school achievement in birth cohorts (Jones et al., 1994; Isohanni, et al., 1994).
Abnormalities of premorbid social behavior and academic performance can be seen as reflections of some of these neurodevelopmental problems. Such abnormalities have previously been described in discordant monozygotic twins and in non-ill children of mothers with schizophrenia [including as measured with the Premorbid Adjustment Scale (Dworkin et al., 1991; Reichenberg et al., 2000)]. There is also a substantial literature demonstrating an association between various cognitive abilities in childhood and the development of illness in adulthood (e.g. Reichenberg et al., 2006; Smith et al., 2006; Sorensen et al., 2006). Moreover, there is evidence that high scores on self report measures of social anhedonia may be related to increased risk for schizophrenia or to other intermediate phenotypes linked to schizophrenia (Gooding, et al., 2005; Gooding et al., 2006; Blanchard et al., 2000). Retrospective rating scales designed to measure these differences in social and academic function have proven useful.
1.2 The Premorbid Adjustment Scale
One of the most widely used retrospective rating scales is the Premorbid Adjustment Scale [PAS] (Cannon-Spoor et al., 1982), which assesses the “degree of achievement of developmental goals” over the course of childhood, adolescence, and where applicable, adulthood. The scale is divided into a general scale and four distinct developmental age periods—childhood to age 11, early adolescence to age 15, late adolescence to age 18, and adulthood. Individual items in the childhood and adolescence categories assess premorbid adjustment by asking about sociability and social withdrawal, peer relationships, scholastic performance, adaptation to school, and ability to form socio-sexual relationships. Ratings in the adult period focus on social relationships while the General Scale ratings are broader, including educational achievement, social relationships, level of interest in and enjoyment of major life activities (work, family, etc). While retrospective reports from patients about their childhood and developmental adjustment may be subject to a variety of biases, there is an impressive body of data suggesting that premorbid adjustment and PAS ratings, specifically, are related to important clinical features of the illness. For example, subjects with poor premorbid adjustment have been shown to have more negative symptoms and a more unremitting course of illness (Bailer, Brauer, & Rey, 1996), longer duration of hospitalizations (Levitt et al., 1994), poor long-term outcome (Childers & Harding, 1990), poor adult social and vocational functioning (San et al., 2007), early and insidious onset of illness (Gupta et al., 1995; Vyas et al., 2007), poorer medication compliance (DeQuardo et al., 1994), and larger cerebral ventricles (Weinberger et al., 1980). Indeed, it has been suggested that poor premorbid adjustment may be indicative of a specific phenotype of increased genetic (Schmael et al., 2007) and/or environmental risk in schizophrenia.
1.3 Current Study
In this study, we examined PAS scores in a large sample of patients with schizophrenia, their unaffected siblings, and a control group free of any axis I disorders. We undertook this investigation to examine whether PAS ratings may also mark effects shared within a family (either genetic or shared environmental effects). While attempts to examine specific susceptibility genes or environmental risk factors for schizophrenia would be beyond the scope of this paper, the aforementioned relationships between PAS scores and more pronounced clinical features of the illness suggest that the PAS is sensitive to and taps these risk factors. Furthermore, the good discriminative (Cannon-Spoor et al., 1982) and predictive validity (Brill et al., 2007) of the PAS in this context adds additional support to its utility in the measurement of adaptive functioning.
1.4 Hypotheses
Patients with schizophrenia share, on average, more heritable and environmental risk factors with their siblings than with non-related controls. Because these factors are present before the illness becomes evident, we hypothesized that patients would show worse PAS scores than siblings or controls. Furthermore, we hypothesized that siblings would show more abnormal scores than those of control participants. As previously mentioned, it is suggested that more abnormal premorbid adjustment ratings reflect increased genetic and/or environment risk Thus, we also hypothesized that siblings of patients with the most abnormal childhood and adolescent PAS scores would show the highest (most abnormal) PAS scores in the sibling group. To our knowledge, no study has investigated the PAS in such a large sample of patients, discordant siblings, and controls.
2. Method
Participants were 286 probands with schizophrenia (male: 216 (75.5%), female: 70, (24.5%), 315 of their healthy biological siblings (male: 129 (41%), female: 186, (59%) and 261 healthy control participants (male: 117 (44.8%), female: 144, (55.2%). Overall, 53.6% of the participants were male and 46.4% were female.1 The sample was comprised of Caucasian, African-American, Hispanic, and Alaskan native or Native American individuals, as well as individuals who defined themselves as “mixed race” and individuals of Asian decent. Ages in the sample ranged from 16-64, but 95% of subjects were between the ages of 19 and 54. Demographic information parsed by diagnostic group is shown in Table 1. All individuals were recruited to participate in the Sibling Study in the Genes, Cognition, and Psychosis Program at the National Institute of Mental Health, a study of neurobiological phenotypes related to genetic risk for schizophrenia; details of recruitment for this study are described elsewhere (Egan et al., 2000). All participants gave written informed consent after receiving a full oral and written description of study procedures.
Table 1.
Demographics of the current sample, parsed by diagnostic status.
| Demographic Characteristics of Diagnostic Groups | |||
|---|---|---|---|
| Controls | Siblings | Probands | |
|
|
|
|
|
| Age* | 34.38 ± 10.05 | 36.94 ± 9.96 | 36.2 ± 9.44 |
| Years of Education* | 16.72 ± 2.85 | 15.87 ± 2.48 | 13.81 ± 2.14 |
| Family SES1 | 48.1 ± 13.28 | 52.86 ± 12.77 | 51.79 ± 12.54 |
| Gender | |||
| Male | 117 (44.8%) | 129 (41%) | 216 (75.5%) |
| Female | 144 (55.2%) | 186 (59%) | 70 (24.5%) |
| Race/Ethnicity | |||
| African American | 34 (13%) | 10 (3.2%) | 13 (4.5%) |
| Hispanic | 7 (2.7%) | 6 (1.9%) | 7 (2.4%) |
| Caucasian | 204 (78.2%) | 282 (89.5) | 249 (87.1%) |
| Native American or Alaskan Native | 1 (.4%) | 2 (.6%) | 2 (.7%) |
| Asian | 8 (3.1%) | 2 (.6%) | 3 (1%) |
| Mixed Race | 7 (2.7%) | 13 (4.1 %) | 12 (4.2 %) |
Value plus or minus one standard deviation
2.1 Diagnostic Status
In short, all probands fulfilled criteria for a DSM-IV diagnosis of schizophrenia or schizoaffective disorder in the absence of any other current axis I diagnosis. Diagnoses were made by either a clinical psychologist or psychiatrist using a revised version of the Standard Clinical Interview for DSM-IV (SCID). Siblings were all first degree full siblings of the probands included in the study. They were also free of any current axis I or II diagnosis and considered to be in full remission from any prior lifetime axis I or II diagnoses. We excluded any siblings who had a history of psychosis, even if it was in full remission at the time of the study. Healthy controls met the same criteria and had no family history of schizophrenia spectrum disorders. Additionally, all participants were free of any alcohol or drug abuse for at least six months before recruitment.
2.2 Data Collection
The original developer of the scale, Eleanor Spoor, completed all five subtests of the PAS with each subject, obtaining retrospective reports for all previous age periods. However, if a diagnosis of schizophrenia was met before adulthood, PAS subscales that covered the post-onset age periods were excluded; “premorbid” was defined as the period ending 6 months before evidence of psychosis (as evidenced by contact with a mental health professional, appearance of florid symptoms, etc.). Data for the General subscale was collected from all participants. The interviewer was theoretically blind to the diagnostic status of participants. However, due to frequently shared last names between patients and siblings, as well as issues with the scheduling of participants (family members were typically run through the protocol within days or weeks of each other), complete blindness to diagnostic status was not present in the current study.
2.3 Data Analysis
One of the three major assumptions of many parametric statistical tests (including Linear Regression and the ANOVA family) is independence of observations. However, because patients and siblings in this study are nested within family clusters, their PAS scores are theoretically intercorrelated and therefore not independent of each other. When using a regression framework, this within-cluster correlation typically leads to underestimated, or biased, standard errors and increased likelihood of Type I errors (particularly with small sample sizes). General Estimating Equations (GEEs) are increasingly becoming the standard for analyzing family data. They adjust the standard errors used in regression models to account for this within-cluster correlation of dependant variables, resulting in unbiased regression parameters. Further, they correct for the contribution of many data points by families with multiple patients and siblings, and allow for the inclusion of traditional covariates. GEE models are also robust to violations of normality and homogeneity of variance. Thus, we used GEE models with each of the PAS subtests, using an unstructured working correlation matrix. This allows for the actual and theoretical correlation (non-independence) of PAS scores within families. After finding significant differences between diagnostic groups in age, gender, and family SES, we included them as covariates in the models. Subsequently, we performed omnibus Wald Chi-Square analyses on the GEE adjusted marginal means to test whether there was a main effect of diagnostic group on PAS subtest scores. We also conducted pairwise comparisons on the resultant group means (analogous to those done in an ANCOVA) to investigate whether diagnostic groups differed from each other.
Visual inspection of these data suggested that proband PAS scores increased over time while scores in the other two groups did not. To investigate this observation, the adjusted marginal means and standard errors provided by each PAS subtest GEE analysis (see table 2) were used to conduct within-groups analytical comparisons (computed with the Welch's independent samples t statistic). Though these means are not technically independent, they benefited from the adjustments made by the generalized estimating equation models. Further, it was not possible to conduct dependant samples tests because the GEE analyses conducted did not provide adjusted scores at the individual participant level. Analogous contrasts were performed for the sibling and control groups for the purpose of comparison.
Table 2.
Each row shows the mean raw and GEE adjusted PAS scores for all three diagnostic groups. GEE models corrected for differences in age, family SES, and gender, as well as the intercorrelation of PAS scores within families. Adjustments to the raw values were small.
| PAS Raw and GEE Adjusted Mean Scores | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Siblings | Probands | |||||||||||||
| Adjusted | Adjusted | Adjusted | |||||||||||||
| n | Raw | Mean1 | SEM | 95% CI | n | Raw | Mean1 | SEM | 95% CI | n | Raw | Mean1 | SEM | 95% CI | |
| PAS childhood | 260 | .139 | .138 | .009 | .126: .151 | 314 | .175 | .175 | .007 | .162: .190 | 286 | .249 | .284 | .006 | .231: .265 |
| PAS early adolescence | 260 | .153 | .153 | .006 | .142: .164 | 314 | .188 | .188 | .006 | .176: .201 | 277 | .285 | .284 | .008 | .268: .300 |
| PAS late adolescence | 260 | .144 | .145 | .007 | .131: .158 | 314 | .187 | .188 | .007 | .173: .202 | 246 | .330 | .330 | .011 | .309: .351 |
| PAS adulthood | 259 | .113 | .112 | .005 | .102: .123 | 308 | .161 | .159 | .007 | .145: .173 | 184 | .368 | .367 | .015 | .337: .397 |
| PAS general | 260 | .094 | .093 | .005 | .084: .103 | 314 | .125 | .123 | .006 | .111: .135 | 284 | .340 | .341 | .005 | .324: .358 |
GEE Estimated Marginal Mean
Next we sought to evaluate whether poor premorbid adjustment might run in families. To do this, we ran a separate hierarchical regression model for each PAS subtest. Because nesting within families was the focus, rather than a confounder, of this analysis, we elected to use the multiple regression model rather than the General Estimating Equation model. Nonetheless, we used a significance threshold of p=.01 to limit the possibility of type I errors.
In these regression models, we entered age, gender, and family SES in the first step to again eliminate variance in PAS scores attributable to these demographic factors. Next, we regressed PAS scores for the sibling group on their “family proband” scores; we sought to predict sibling PAS scores from their own ill siblings' scores. In the case of multiplex families, we averaged the scores for the multiple participants in the patient group.2 Since the core construct the PAS was developed to assess is premorbid function, each analysis included only those dyads in which the proband(s) had not yet developed psychosis. Thus, for example, if a patient had become ill in late adolescence, data from that family was only included in the childhood and early adolescence analyses.
3. Results
As previously stated, there were significant differences in gender, family SES, age, and education between diagnostic groups. This was determined by conducting a one-way ANOVA with diagnostic status as the independent variable and age as the dependant variable (F(2,857) = 5.417, p = .005). A Levene's test for homogeneity of variance showed that gender had a heterogeneous distribution, so we performed a Kruskal-Wallis test and determined that diagnostic groups differed with respect to gender (χ2 = 83.515, p<.0001). We again posited that education and family SES values would be correlated within families. To investigate whether diagnostic groups differed with respect to these variables, we conducted another set of Generalized Estimating Equation analyses with diagnostic status as the independent variable and years of education, and family SES as the respective dependant variables. (Education: χ2 = 233.122, p<.0001; Family SES: Wald χ2 = 13.722, p=.001). Thus, we included gender, family SES, and age as covariates in our PAS analyses. We decided not to correct for education, because of the likely overlap in causes of poor premorbid function and educational attainment, as well as multi-collinearity between the education and Economic status variables; we thought correcting for education would obscure a real effect.
Estimated marginal means, adjusted in our GEE models for within family nesting and the three covariates, are plotted in figure 1. They are also reported in table 2, along with the raw, unadjusted PAS scores. Because of the large sample sizes, standard errors were small (reflected in the reported confidence intervals), as were the adjustments made to the raw PAS scores. There was a significant main effect of diagnostic status on PAS-Childhood (Wald χ2 = 105.464, p<.0001), PAS-Early Adolescence (Wald χ2 = 178.177, p<.0001), PAS-Late Adolescence (Wald χ2 = 220.036, p<.0001), PAS-Adulthood (Wald χ2 = 253.593, p<.0001), and PAS-General (Wald χ2 = 648.495, p<.001) scores. Results of post-hoc, between groups, pairwise comparisons are reported in table 3. In every instance, controls had significantly lower (i.e. more “normal”) PAS scores than discordant siblings, who had significantly lower PAS scores than probands. All Bonferroni corrected p values were significant at the p<.0001 level, except for the control sibling comparison on the PAS-General scale, which was significant at the p<.001 level. Thus, patients performed more poorly than siblings who performed more poorly than controls at each PAS interval. It is worth noting that that the relatively poorer adjustment in siblings carried over into the Adult and General Ratings.
Figure 1.
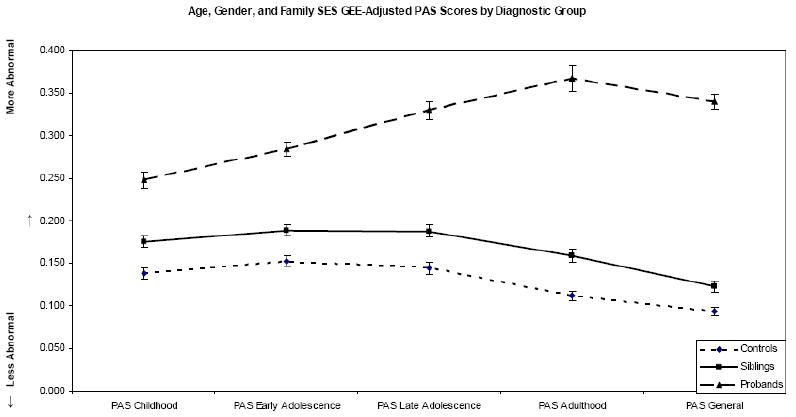
Each line represents the GEE adjusted PAS subscores of controls, siblings, and patients with schizophrenia, respectively. In all cases, variance accounted for by age, gender, and family SES have been partialed out.
Table 3.
After adjustment in GEE analyses, all comparisons of PAS scores between diagnostic groups were significant. Effect sizes are also shown and were medium in magnitude during childhood, but large for all the other subscales
| Planned Comparisons Between Diagnostic Groups; GEE Adjusted | ||||||
|---|---|---|---|---|---|---|
| Controls v. Siblings | Siblings v. Probands | Controls v. Probands | ||||
| Mean Difference | Cohen's d | Mean Difference | Cohen's d | Mean Difference | Cohen's d | |
| PAS- Childhood | 0.037 1 | 0.333 | 0.073 1 | 0.535 | 0.110 1 | 0.864 |
| PAS- Early Adolescence | 0.035 1 | 0.342 | 0.096 1 | 0.77 | 0.132 1 | 1.129 |
| PAS- Late Adolescence | 0.043 1 | 0.353 | 0.142 1 | 0.944 | 0.185 1 | 1.295 |
| PAS- Adulthood | 0.047 1 | 0.426 | 0.208 1 | 1.211 | 0.255 1 | 1.612 |
| PAS- General | 0.030 2 | 0.303 | 0.218 1 | 1.664 | 0.247 1 | 2.094 |
Bonferroni corrected, significant at p < .0001
Bonferroni corrected, significant at p < .001
Effect sizes for the diagnostic group contrasts are also included in table 3. For each PAS subscale, effect sizes were largest for the control v. proband comparisons, followed by the sibling v. proband comparisons, then the control v. sibling comparisons. Furthermore, Cohen's d values for comparisons between the proband group and the other two groups increased as the PAS subscales moved chronologically from childhood through adulthood. Again, in each PAS subscale, the proband group only includes individuals not yet given a Schizophrenia diagnosis. Thus, as seen in Table 2, n's decrease slightly as the subscales move chronologically from childhood through adulthood.
Within-groups effects were also investigated to determine whether the PAS scores of participants in each diagnostic group changed over time. In the proband group, early adolescence scores did not differ from childhood scores [t(515.784) = .000, p = 1.000], but scores did increase significantly for all other epochs thereafter [late adolescence v. early adolescence: t(458.782) = 3.38, p = .001; adulthood v. late adolescence: t(355.876) = 1.989; p = .048], suggesting a decline in adjustment from early adolescence on. Scores for the control and sibling groups did not significantly increase over any developmental epochs. In siblings, PAS early adolescence scores did not differ from childhood scores [t(611.692) = 1.410; p = .159] and late adolescence scores did not differ from early adolescence scores [t(611.692) = .000; p = 1.000]. Similarly, control early adolescent scores did not differ from childhood scores [t(451.247) = 1.387; p = .165] and late adolescence scores did not differ from early adolescence scores [t(506.160) = -.868; p = .385]. However, PAS scores in the sibling group and control group decreased significantly from late adolescence to adulthood [siblings: t(619.942) = -2.929; p = .004; controls: t(468.324) = -3.836; p = .000], suggesting that individuals in these groups became better adjusted between adolescence and adulthood.
After controlling for age, gender, and family SES, proband PAS scores significantly predicted the PAS scores of their own siblings in the Childhood (Beta = .203, p = .0004, simple R2 = .041) and Late Adolescence (Beta = .222, p = .0003, simple R2 = .049) subscales. These results also approached significance in the Early Adolescence (Beta = .129, p = .029, simple R2 = .017) and General (Beta = .128, p = .029, simple R2 = .016) subscales. Results were not significant for the PAS Adulthood subscale (Beta = -.034, p = .632, simple R2 = .001). We also computed simple correlations between patient scores and those of their own discordant siblings, after the same three covariates had been accounted for (r = .205, r = .129, r = .223, r = -.034, and r = .128 for the childhood through adulthood subscales, respectively). In the PAS General subscale, which again includes all patients and targets current general function, the patient/sibling correlation was r =.128. These data are summarized in table 4. Broadly, patient PAS scores appear to predict those of their siblings most strongly in the childhood and late adolescence subscales, uniquely accounting for roughly 4% and 5% of the variance. When including the covariates, the model accounts for roughly 6% and 7% of the variance in sibling childhood and late adolescence scores. Where the model only neared significance, patient scores uniquely accounted for 1.7% of the variability in sibling scores in the early adolescence subscale and 1.6% in the general subscale, while the whole model explained 2.8% and 3.7%, respectively.
Table 4.
Table shows the results of the within family regression analyses. Proband PAS scores in childhood and late adolescence significantly predicted those of their unaffected siblings. This effect approached significance in the Early Adolescence and General subscales. Effect sizes were small for these results.
| Sibling Scores Predicted by their Related Probands1 | ||||||
|---|---|---|---|---|---|---|
| PAS Subscale | n | Beta | p-value | R2 | Simple R2 | Simple Correlation |
| Childhood | 294 | 0.203 | 0.0004 | 0.059 | 0.041 | 0.205 |
| Early Adolescence | 288 | 0.129 | 0.029 | 0.028 | 0.017 | 0.129 |
| Late Adolescence | 257 | 0.222 | 0.0003 | 0.07 | 0.049 | 0.223 |
| Adulthood | 200 | -0.034 | 0.632 | 0.02 | 0.001 | -0.034 |
| General | 293 | 0.128 | 0.029 | 0.037 | 0.016 | 0.128 |
Age, Gender, and Family SES controlled for
4. Discussion
The goal of the current study was to investigate premorbid functioning, using the Premorbid Adjustment Scale, in a group of patients with Schizophrenia and their non-ill siblings. We are unaware of any previous study examining PAS or similar ratings in a large sample of non-twin siblings. Four main findings emerge from our analyses.
First, we found that schizophrenia patients in our large CBDB/NIMH cohort demonstrate robust evidence of impaired premorbid adjustment at all developmental epochs assessed, when compared with healthy control participants. Effect sizes for these differences were all large in size. This finding is consistent with a large body of prior literature.
Second, we found that unaffected siblings of patients showed higher PAS scores than controls, likely as a result of factors (genetic or environmental) shared with their ill family members. These effects were in the small to medium range at all epochs, echoing findings from smaller family samples in prior literature.
Third, mean differences were larger between siblings and probands than between siblings and controls. While proband scores diverged from those of the other two groups between childhood and adulthood, differences stayed relatively constant between siblings and controls. More specifically, we found that proband adjustment scores progressively worsened from early adolescence into adulthood, while both sibling and control participants showed improved adjustment from late adolescence into adulthood, though maintained a statistically significant difference. These data suggest that patient functioning declines and diverges from that of the two unaffected groups as individuals get older. Findings also suggest that siblings maintain a relatively constant impairment in academic and social functioning throughout the lifespan, though do show normative improvements as they move from adolescence into adulthood.
It is worth noting that these findings differ from those recently reported by Walshe and colleagues (2007). These investigators examined ‘familial’ and ‘non-familial’ schizophrenia and showed PAS impairments in discordant siblings, but found that this difference only emerged in adolescence and was likely due to their poorer academic functioning relative to controls. It is very possible that had this benefited from larger sample sizes, they would have observed adjustment differences between the non-affected groups in childhood, as well.
A fourth finding of the present investigation further suggests that there may be a familial component to the aspects of development measured by the PAS. PAS scores in patients were significantly correlated with those of their siblings. Also, results of our regression models showed that patient PAS scores significantly predicted those of their own discordant siblings. Thus, patients with the highest PAS scores tended to have siblings with the highest PAS scores and vice, versa. This effect was significant in childhood and late adolescence, but only neared significance in early adolescence (thus, a relationship may exist there, as well, though further research is needed to substantiate this hypothesis). It was not significant in adulthood. The simple R2's of these predictions were small, accounting for 4%, 1.7%, 4.9%, and .1%, of the variance in sibling PAS scores in the childhood through adulthood subscales, respectively.
The regression model also neared significance in the PAS General subscale (and had a simple R2 of .016). However, this subscale is difficult to interpret in the present context; it compares the current general functioning of two groups originally selected to differ in this particular area. In the present study, all probands were selected because they met diagnostic criteria for schizophrenia—many of these criteria reflect aspects of social and academic/occupational functioning. Conversely, only siblings free of any current illness were selected for this analysis. Therefore, results for the PAS-General subscale are omitted from Table 4 are likely not useful in the within family analyses.
As a corollary to this finding, the predictive ability of patient scores did not maintain itself in adulthood. This lack of correlation in the adult years likely reflects two factors. First, the adult ratings of patients are possibly impacted by developmental and prodromal illness related factors that one would not expect to be shared with well siblings. Second, there is likely a “reciprocal feedback” effect such that poor social and academic function at earlier epochs affects the types of environments patients seek out, which in turn, lead to more abnormal PAS scores. For example, early social problems may lead to interpersonal difficulties and a tendency towards solitary activities that make the development of more advanced social skills difficult. For these reasons, it appears likely that the ratings from relatively early in childhood may offer the least confounded view on the neurodevelopmental features likely to be shared among siblings.
None of the above findings were accounted for by simple differences in socioeconomic circumstances, age, or gender. Combined, they suggest that there are familial components of premorbid adjustment, as assessed by the Premorbid Adjustment Scale. They also suggest that aspects of childhood social and academic adjustment may be worthy of investigation as potential intermediate phenotypes in genetic studies.
Our results have a number of limitations, including the fact that retrospective interviews (using several items that could likely benefit from updating), were used to assess childhood events that occurred in the distant past, with recollection likely colored by subsequent personal and family experience. Second, the current study does not permit conclusions to be drawn about the relative contribution of genetic v. environment factors to the heritability observed in PAS scores. The current study also does not permit conclusions to be drawn about differences in social and academic adjustment, independently. Recent findings by Walshe et al. (2007) suggest that it might be beneficial to differentiate between these two constructs in future investigations of premorbid adjustment, as well as in future uses of the PAS.
Another limitation is that once patients met the threshold for psychosis, they were dropped from chronologically subsequent analyses, resulting in the use of slightly different samples in each subscale analysis. However, had they been included, these post-onset individuals would likely have shown the highest PAS scores. Thus, if anything, their exclusion likely adds a conservative bias to comparisons between probands and the other diagnostic groups. Another limitation emerges in the fact that the PAS rater was not completely blind to the diagnostic status of all participants. The fact that this imprecise tool was able to detect subtle abnormalities in well siblings suggests that more refined measurement approaches and prospectively acquired data might yield an even more robust signal.
Acknowledgments
The authors would like to acknowledge the research and support staff in the Clinical Brain Disorders Branch of the National Institute of Mental Health for their dedication to the Sibling Study. We also thank the NIH Intramural Research Program and Dr. Irwin Waldman at Emory University for statistical consultation.
Role of funding source: The current research was performed at and funded by the National Institute of Mental Health Intramural Research Program. Collection of data is an ongoing process at the Sibling Study in the Genes, Cognition, and Psychosis program of the NIMH.
Footnotes
Our sibling group is slightly larger than our patient group. This discrepancy exists because sibling data remained in our analyses even if it was not possible to collect PAS data from the related patient.
In the current sample, there were 14 families in which there were two participants with schizophrenia.
Conflict of Interest: There are no conflicts of interest surrounding the current work.
Contributors: DI Shapiro performed the statistical analyses and wrote the first draft of the manuscript, JM Gold managed progress of the written product. All authors were involved in aspects of data collection and participated in data analysis, interpretation, and manuscript revisions. DR Weinberger is the director of NIMH GCAPS, the institution at which this research was conducted. All authors have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailer J, Brauer W, Rey ER. Premorbid Adjustment as a Predictor of Outcome in Schizophrenia: Results of a Prospective Study. Acta Psychiatr Scand. 1996;93:368–377. doi: 10.1111/j.1600-0447.1996.tb10662.x. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Gangestad SW, Brown SA, Horan WP. Hedonic Capacity and Schizotypy Revisited: A Taxometric Analysis of Social Anhedonia. J Abnorm Psychol. 2000;109(10):87–95. doi: 10.1037//0021-843x.109.1.87. [DOI] [PubMed] [Google Scholar]
- Brill N, Reichenberg A, Weiser M, Rabinowitz Validity of the Premorbid Adjustment Scale. Schizophr Bull. 2008;34(5):981–3. doi: 10.1093/schbul/sbm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of Premorbid Adjustment in Chronic Schizophrenia. Schizophr Bull. 1982;8(3):470–484. doi: 10.1093/schbul/8.3.470. [DOI] [PubMed] [Google Scholar]
- Childers SE, Harding CM. Gender, Premorbid Social Functioning, and Long-Term Outcome in DSM-III Schizophrenia. Schizophr Bull. 1990;16(2):309–318. doi: 10.1093/schbul/16.2.309. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR, Goldman RS, Tandon R, McGrath-Giroux M, Kim L. Comparison of Indices of Premorbid Function in Schizophrenia. Schizophr Res. 1995;15:283–290. doi: 10.1016/0920-9964(94)e0057-e. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Bernstein G, Kaplansky LM, Lipsitz JD, Rinaldi A, Slater SL, Cornblatt BA, Erlenmeyer-Kimling L. Social Competence and Positive and Negative Symptoms: A Longitudinal Study of Children and Adolescents at Risk for Schizophrenia and Affective Disorder. Am J Psychiatry. 1991;148:1182–1188. doi: 10.1176/ajp.148.9.1182. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative Risk of Attention Deficits in Siblings of Patients with Schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at Risk for Schizophrenia: Sequelae of a Genetic Neurointegrative Defect. Arch Gen Psychiatry. 1992;49:221–235. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Matts CW, Rollmann EA. Sustained Attention Deficits in Relation to Psychometrically Identified Schizotypy: Evaluating a Potential Endophenotypic Marker. Schizophr Res. 2006;82:27–37. doi: 10.1016/j.schres.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical Status of At-Risk Individuals 5 Years Later: Further Validation of the Psychometric High-Risk Strategy. J Abnorm Psychol. 2005;114(1):170–175. doi: 10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Gupta S, Rajaprabhakaran R, Arndt S, Flaum M, Andreason NC. Premorbid Adjustment as a Predictor of Phenomenological and Neurobiological Indices of Schizophrenia. Schizophr Res. 1995;16:189–197. doi: 10.1016/0920-9964(94)00073-h. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Murray GK, Jokelainen J, Croudace T, Jones PB. The Persistence of Developmental Markers in Childhood and Adolescence and Risk for Schizophrenic Psychoses in Adult Life. A 34-Year Follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. 2004;(23):213–25. doi: 10.1016/j.schres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child Development Risk Factors for Adult Schizophrenia in the British 1946 Birth Cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Shenton ME, McCarley RW, Faux SF, Ludwig AS. Premorbid Adjustment in Schizophrenia: Implications for Psychosocial and Ventricular Pathology. Schizophr Res. 1994;12:159–168. doi: 10.1016/0920-9964(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Norton EC, Bieler GS, Ennett ST, Zarkin GA. Analysis of Prevention Program Effectiveness With Clustered Data Using Generalized Estimating Equations. J Consult Clin Psychol. 1996;64(5):919–926. doi: 10.1037//0022-006x.64.5.919. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Rabinowitz J, Weiser M, Mark M, Kaplan Z, Davidson M. Premorbid Functioning in a National Population of Male Twins Discordant for Psychoses. Am J Psychiatry. 2000;157:1514–1516. doi: 10.1176/appi.ajp.157.9.1514. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Caspi A, Knobler HY, Lubin G, Harvey PD, Rabinowitz J, Davidson M. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol. 2006;28(2):193–207. doi: 10.1080/13803390500360372. [DOI] [PubMed] [Google Scholar]
- San L, Ciudad A, Alvarez E, Bobes J, Gilaberte I. Symptomatic Remission and Social/Vocational Functioning in Outpatients With Schizophrenia: Prevalence and Associations in a Cross-Sectional Study. Eur Psychiatry. 2007;22:490–498. doi: 10.1016/j.eurpsy.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Schmael C, Georgi A, Krumm B, Bueger C, Deschner M, Nothen MM, Schulze TG, Rietschel M. Premorbid Adjustment in Schizophrenia—An Important Aspect of Phenotype Definition. Schizophr Res. 2007;92:50–62. doi: 10.1016/j.schres.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Smith CW, Park S, Cornblatt B. Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2006;81(23):211–5. doi: 10.1016/j.schres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Sørensen HJ, Mortensen EL, Parnas J, Mednick SA. Premorbid neurocognitive functioning in schizophrenia spectrum disorder. Schizophr Bull. 2006;32(3):578–83. doi: 10.1093/schbul/sbj040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas NS, Hadjulis M, Vourdas A, Byrne P, Frangou S. The Maudsley Early Onset Schizophrenia Study; Predictors of Psychosocial Outcome at 4-year Follow-up. Eur Child Adolesc Psychiatry. 2007;16(7):465–70. doi: 10.1007/s00787-007-0621-4. [DOI] [PubMed] [Google Scholar]
- Walker E, Lewine RJ. Prediction of Adult-Onset Schizophrenia From Childhood Home Movies of the Patients. Am J Psychiatry. 1990;147:1052–56. doi: 10.1176/ajp.147.8.1052. [DOI] [PubMed] [Google Scholar]
- Walshe M, Taylor M, Schulze K, Bramon E, Frangou S, Stahl D, Kravariti E, Daly E, Fearon P, Murray RM, McDonald C. Familial liability to schizophrenia and premorbid adjustment. Br J Psychiatry. 2007;191:260–261. doi: 10.1192/bjp.bp.106.033688. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Cannon-Spoor E, Potkin SG, Wyatt RJ. Poor Premorbid Adjustment and CT Scan Abnormalities in Chronic Schizophrenia. Am J Psychiatry. 1980;137(11):1410–1413. doi: 10.1176/ajp.137.11.1410. [DOI] [PubMed] [Google Scholar]


