Nanocrystalline materials are of intense and widespread interest because of their promising applications in biological labeling, chemical catalysis, micro/nanoelectronics, and energy production.[1] On the nanometer scale, semiconductors show size-dependent photoluminescence;[2] metals exhibit tunable surface plasmon bands;[1a,b] magnetic materials become superparamagnetic;[3] and chemical catalysts become more active.[1c,4] Recent research has led to high-quality and monodispersed colloidal nanocrystals by using a number of synthetic methods including reactions in reverse micelles,[5] arrested precipitation in aqueous solution,[5a,6] and organic-phase high temperature syntheses using hydrophobic coordinating ligands.[2a,7] The nanocrystals synthesized by these methods have shown various degrees of crystallinity, monodispersity, size-tunability, stability, and processibility. However, a major limitation is that the resulting nanoparticles are soluble only in the reaction solution or in chemically similar media.[8] For example, nanocrystals synthesized in trioctylphosphine oxide or other organic solvents are only soluble in nonaqueous media, whereas nanocrystals prepared in aqueous solution are not compatible with nonpolar solvents that are often used for fabrication of composite materials, device incorporation, and catalytic reactions.[9]
Here we report a new strategy to couple the synthesis and encapsulation of high-quality nanocrystals to yield nearly universal solubility. This method is based on the use of an “amphibious bath” consisting of amphiphilic multidentate ligands in a noncoordinating solvent (such as low-molecular weight polyethylene glycol or PEG). The multidentate polymer ligands (called amphipols[10]) contain aliphatic chains and carboxylic acid functional groups, and are found to act as both a ligand for metal ion precursors and a nanoparticle surface stabilizer. A major finding is that the resulting nanocrystals are instantly soluble in both polar and nonpolar solvents (such as water, acetone, DMF, and chloroform). We demonstrate that this “amphibious bath” method is applicable to a wide variety of technologically important nanocrystals including photoluminescent semiconductors (II-VI and IV-VI quantum dots), catalytic metals (palladium), noble metals (gold and silver), and superparamagnetic materials (iron oxide). This work broadly improves the applicability of nanocrystals in biolabeling, catalysis, and device fabrication.
As depicted in Scheme 1, the multidentate polymer serves as a coordinating ligand for metal ion precursors, replacing traditionally used monovalent ligands such as such as oleic acid or stearic acid. At elevated temperatures (150-280°C), these polymeric carboxylate precursors react with similar chemistry as their monovalent fatty acid analogues.[7,11] Upon nanocrystal nucleation, these multidentate polymers strongly bind to the nanocrystal surface during growth to yield monodisperse, highly stable colloids. As synthesized, the nanocrystals are nonpolar due to directional coordination of the amphiphilic ligand on the crystal surface, allowing solubility in nonpolar solvents. However, metal ions are consumed during nanocrystal growth, releasing free polymer molecules into the reaction solution. When exposed to polar solvents, the multidentate polymer spontaneously encapsulates and solubilises the nanocrystals through the formation of hydrophilic micelles. This strategy is fundamentally different from previous approaches in which nanocrystals with amphibious attributes are coated with amphiphilic polymers such such as polyethyleneimine,[12] or poly[2-(dimethylamino)ethyl] methacrylate.[13] The previous work involved surface modification of pre-synthesized nanocrystals and required removal of the reaction solvent prior to redispersion in a new solvent.
Scheme 1.
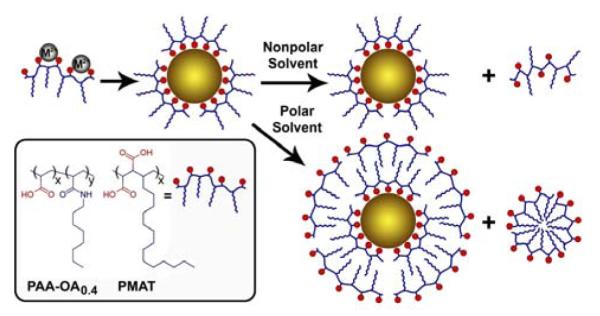
Schematic diagrams showing the use of amphiphilic multidentate ligands to prepare nanocrystals that are instantly soluble in both polar and nonpolar solvents. The resulting nanocrystals are coated with the multidentate polymer, and are soluble in organic solvents. Upon exposure to water or other highly polar solvents, these nanocrystals are spontaneously solubilized by a second layer of the excess multidentate polymer, without any additional materials or steps. The inset shows the structures of two multidentate polymer ligands: octylamine-grafted polyacrylic acid (x = 0.6, y = 0.4, PAA-OA0.4) and hydrolyzed poly(maleic anhydride-alt-1-tetradecene) (PMAT).
Figure 1 shows the optical properties of CdTe nanocrystals in the size range of 2.5 to 7 nm synthesized in an amphibious bath, along with their solubility features in a broad range of solvents. We note that these nanocrystals do not contain a mixture of hydrophilic and hydrophobic surface groups, and are not amphiphilic by themselves. Rather, this broad solubility arises from the amphibious nature of the reaction mixture toward both polar and nonpolar solvents. The nanocrystals purified in nonpolar solvents are no longer soluble in polar solvents unless excess amphipols are again added to the solution. Similarly, once the nanocrystals are solubilised in a polar solvent, they lose their solubility in nonpolar solvents, even if the polar solvent is removed and excess amphipol is added. This indicates that the hydrophilic coating generated in polar solvents is very stable and is essentially irreversible. As a result, the nanocrystals do not aggregate and remain monodispersed, as judged by light scattering and electron microscopy measurements (below), and by the narrow surface plasmon absorption peaks of noble metal nanocrystals (Supporting Figures 1 and 2).
Figure 1.
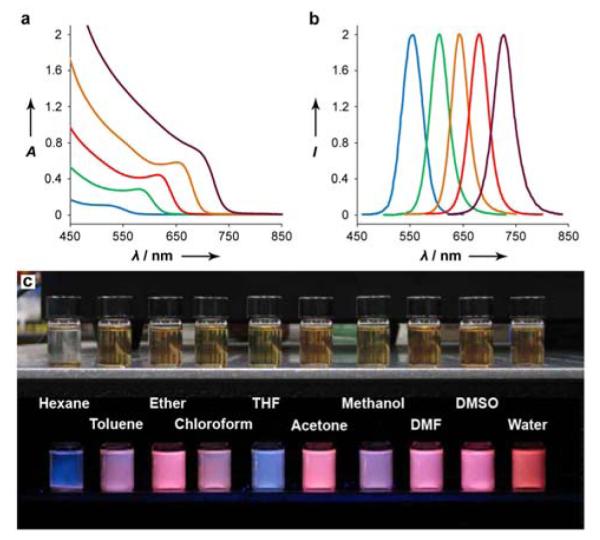
(a) Optical absorption and (b) fluorescence emission spectra of CdTe nanocrystals in the size range of 2.5 – 7 nm (diameter) synthesized in an amphibious bath (a mixture of amphipol and PEG). (c) Fluorescence photograph of red nanocrystals instantly dissolved in a broad range of polar and nonpolar solvents. When the nanocrystal fluorescence is quenched, the amphibious mixture shows a blue hue (see the hexane and THF vials).
In order to further understand the surface coatings of these nanocrystals, we have directly compared them with analogous nanocrystals synthesized with monolavent ligands. We prepared CdTe nanocrystals with a first exciton peak at 550 nm, using both the amphibious reaction bath and a conventional organic ligand method.[14] Theoretically the inorganic nanocrystals comprising these two samples should be essentially the same (3.2 nm diameter) due to their nearly identical optical properties.[15] First we characterized both of these colloids in hexane using dynamic light scattering (DLS). Figure 2a reveals a slightly larger hydrodynamic size for the polymer-coated nanocrystals (6.8 nm) compared to the ones coated with monovalent ligands (5.2 nm). This is due to the steric bulkiness of the amphipol ligand and its larger radius of gyration compared to a small monovalent ligand. In fact, the hydrodynamic thickness value of ∼1.8 nm is consistent with the theoretical prediction of a ‘loops-trains-tails’ binding conformation for a monolayer of this multidentate polymer on the nanocrystal surface.[16] Next, we characterized these same nanocrystals in water. To prepare aqueous dispersions of the conventional CdTe nanocrystals, we encapsulated these nonpolar colloids in micelles composed of the same amphipol that was used for the amphibious nanocrystal synthesis (PAA-OA0.4). Previous research has shown that the resulting hydrophilic nanocrystals are surrounded by a stable hydrophobic bilayer.[8a,17] Once in water, both of these nanocrystals are similar in size (12-13 nm), as determined by DLS and size-exclusion chromatography (Figure 2b). They also have nearly identical electrostatic charges with a zeta potential of about - 35 mV at pH 8.5. Therefore in water, these nanocrystals have similar structures. The thickness of this anionic micellar shell is 4-5 nm, which matches previous measurements of hydrophobic bilayers on nanocrystals.[8a,16a,17a,18]
Figure 2.
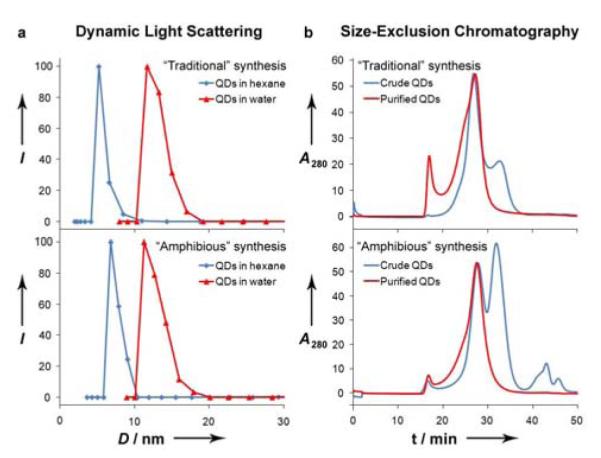
Size characterization of CdTe quantum dots (QDs) in hexane and water. (a) Dynamic light scattering measurements of purified CdTe in hexane (blue) or water (red), prepared using traditional multistep syntheses (top) or the one-step amphibious synthesis (bottom). (b) Size-exclusion chromatograms of aqueous solutions of traditional CdTe QDs encapsulated in a micelle (top) or amphibious nanocrystals (bottom). Chromatograms were obtained on crude reaction mixtures (blue) and nanocrystals isolated via ultracentrifugation (red). Empty amphipol micelles elute at ∼32 minutes and PEG elutes at ∼42 minutes.
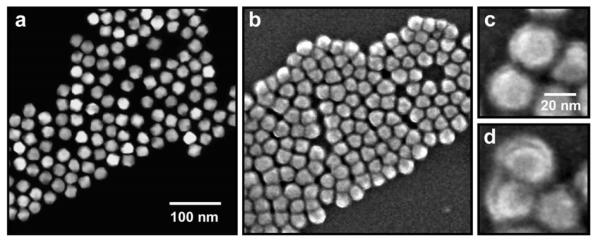
We further examined the self-generated polymeric encapsulation layer by electron microscopy. Transmission electron microscopy (TEM) reveals smaller overall dimensions of the CdTe nanocrystals (Figure 3), compared to their hydrodynamic sizes. This is expected, due to the inability of electron microscopy to resolve surface-associated solvent molecules, and due to compaction of the shell that occurs during the drying process. TEM also confirms that the size of the conventional ligand-coated CdTe nanocrystals matches predictions from their first exciton peak. However the nanocrystals prepared in the one-step amphibious reaction bath are again observed to be larger in size, due to a dense polymeric coating. It is difficult to resolve the interface between the polymeric shell and the nanocrystal surface on such small nanocrystals, but it is more evident on larger nanocrystals. Figure 3 displays images of 22.5 nm aqueous PbSe nanocrysals using both Z-contrast scanning TEM (STEM) and scanning electron microscopy (SEM). Image contrast from STEM is weighted toward electron-dense regions like the nanocrystal core, whereas SEM can resolve surface features like the organic polymer shell. Nearly every individual nanocrystal is found to be coated with a uniform shell with an average dry thickness of 1.6 nm (Figure 4). Some closely packed particles are observed to have organic shells with webbing that connects to adjacent particles, which may indicate interaction between the hydrophobic bilayers upon drying. These structural studies further support the conclusion that a simple single-pot process can be used to synthesize highly ordered micelle-encapsulated nanocrystals that before could only be prepared using a complex and laborious multistep synthesis consisting of nanocrystal synthesis, purification, and encapsulation.
Figure 3.
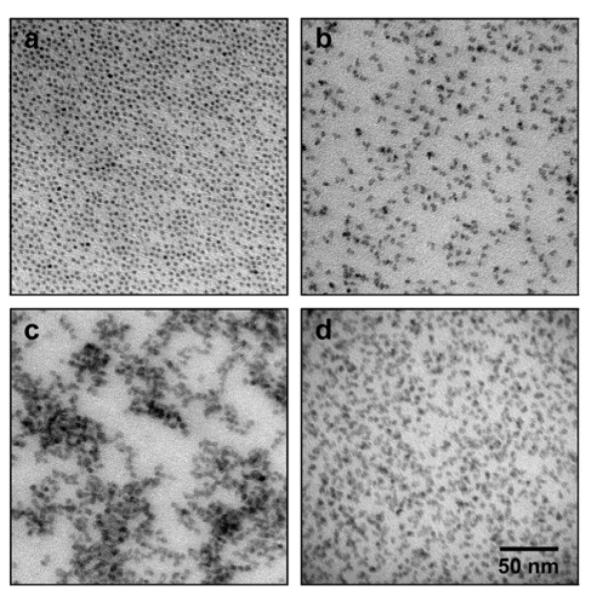
Electron micrographs of purified CdTe nanocrystals cast from a hexane solution, prepared using traditional coordinating ligands (a), or the ‘amphibious’ reaction (b). Also depicted are ‘amphibious’ nanocrystals cast from an aqueous solution before (c) and after purification (d). There was no significant aggregation evident in any of the solvents tested.
Figure 4.
Electron micrographs of PbSe nanocrystals grown in the presence of amphipols, diluted in water, purified, and deposited on a TEM grid. (a) Z-contrast STEM revealed that the nanocrystals are quasi-spherical and highly faceted, with an average diameter of 22.5 ± 1.7 nm. (b) The same grid was imaged via SEM, showing a size of 25.7 ± 2.5 nm. The scale is identical to that of (a). (c) Magnified SEM image, demonstrating an electron-dense core and organic shell. (d) A rare example of nanocrystals in which the polymer shells are seemingly fused together. The scale is identical to that of (c).
This phenomenon of spontaneous encapsulation is related to the nature of the amphiphilic coordinating ligand, as well as the reaction solvent. We have independently evaluated the contributions of both of these reaction components, as summarized in Table 1. The multidentate, amphiphilic structure of the amphipol is crucial for attaining the dual functionality of coordination and encapsulation. Traditional hydrophobic coordinating ligands used in high-temperature nanocrystal reactions, such as oleic acid, can be used to prepare stable, monodisperse colloids in nonpolar solvents. However these ligands are poor surfactants, and cannot stabilize nanocrystals in polar solvents. We have found that achieving efficient encapsulation from a coordinating ligand requires a balanced ratio of coordinating groups to hydrophobic groups. That is, too many coordinating groups yield poor encapsulation efficiency, whereas ligands containing too many hydrophobic domains cannot stabilize the nanocrystals during growth. In general, we find that alkylation ratios of 30-60% work well for aliphatic chain lengths from 8 to 14 carbons. Interestingly, spatial or structural ordering of these domains is not necessary, as amphipols with ordered structures (PMAT, Scheme 1) and randomly grafted structures (PAA-OA0.4) yield nearly identical particles. On the other hand, the use of a linear, graft-like polymer backbone is crucial for the success of this procedure, as it allows directional orientation of the hydrophilic and hydrophobic domains while preventing crosslinking. Performing this same procedure with a di-carboxy PEG ligand resulted in complete precipitation of the nanocrystals after nucleation due to ligand-induced crosslinking.
Table 1.
Solubility data of CdTe nanocrystals synthesized by using multidentate ligands (amphipol) or by using traditional monovalent ligands (oleic acid) in three different solvents (ODE, DOE, or PEG).
| Ligand | Solvent | Spontaneous precipitation |
Chloroform solubility[a] |
Acetone solubility[a] |
Methanol solubility[a] |
Extraction % to polar solvent[b] |
|---|---|---|---|---|---|---|
| Oleic acid | ODE | No | ∼100% | 0% | 0%[d] | 0% |
| DOE | No | ∼100% | 0% | 0% | 0% | |
| PEG | Yes/No[c] | ∼100% | 0% | 0% | 0% | |
| Amphipol | ODE | No | ∼100% | 24.7% | 0%[d] | 0% |
| DOE | No | ∼100% | 72.2% | 72.2% | 0% | |
| PEG | Yes/No[c] | ∼100% | 98% | 98% | ∼100% |
Solubility was assessed as the fraction of nanocrystals stable in solution after dilution of the crude reaction mixture 1:10 in the solvent, and centrifugation at 7000 g for 10 minutes.
Extraction percentage between hexane and methanol, explained further in the experimental section.
Spontaneous precipitation only occurs for dimethoxy-PEG, a liquid at room temperature (<∼500 Da).
ODE is immiscible with methanol.
The capacity to self-generate a micellar surface coating is highly sensitive to the chemical nature of the reaction solvent. Traditional nonpolar solvents, like dioctylether (DOE) and octadecene (ODE) prevent micellar encapsulation of nanocrystals. The use of PEG as a reaction solvent is important because of its ‘amphibious’ nature (that is, soluble in both polar and nonpolar solvents). The only solubility exception for the nanocrystals is hexane (Figure 1b), in which PEG is insoluble. However once PEG is removed from the nanocrystals (see below), they become soluble in aliphatic hydrocarbons. It is thus surprising that the nanocrystals are instantly soluble in diethyl ether, a solvent in which PEG does not disperse. We believe that this feature is a result of the amphiphilicity of amphipol, which can solubilizes a large amount of PEG in ether, even when present in small quantities.
Another interesting finding is that the terminal groups (methoxy or hydroxy) on PEG can influence the colloidal properties of the nanocrystals, even though they do not directly interact with the nanocrystal surface. When the nanocrystals are synthesized in PEG terminated solely by methoxy groups, they spontaneously precipitate out of the reaction mixture when the temperature is cooled below ∼50°C. This observation of temperature-controlled precipitation and dispersion could be exploited to bypass the expensive and laborious purification procedures in large-scale synthesis of various nanocrystals. In contrast to methoxy terminal groups, both monohydroxy- and dihydroxy-terminated PEG solvents result in soluble colloid nanocrystals at room temperature (25°C). As noted above, PEG does not interact with the nanocrystals because (a) the growth kinetics of CdTe nanocrystals are nearly identical when using ODE, DOE, dimethoxy-PEG, monomethoxy-PEG, or dihydroxy-PEG, and (b) the use of a PEG solvent does not increase the hydrophilicity of nanocrystals prepared with monovalent ligands (Table 1). It is thus clear that the strongly binding amphipol ligand is responsible for the amphibious character of the nanocrystals, and PEG is an “adjuvant” that enhances this effect.
The amphibious reaction bath method is broadly applicable to a wide range of nanocrystalline materials (Figure 5). We have synthesized amphibious nanocrystals composed of noble metals (gold and silver, Supporting Figures 1 and 2) that demonstrate discrete surface plasmon bands in various solvents. We have prepared quantum dots with intense, size-tunable photoluminescence (Supporting Figures 3 and 4) that can be used directly in either biological buffers or in devices and composites. In addition, we have prepared catalytic palladium and superparamagnetic iron oxide nanocrystals (Supporting Figure 5). Preliminary studies have shown that the palladium nanocrystals are highly catalytic for cross-coupling reactions between arylboronic acids and aryl halides in both polar and nonpolar solvents. All of these nanocrystals are stable at room temperature for at least 3-4 months after purification in both polar and nonpolar solvents, with no major changes in light scattering measurements and electron micrographs. We attribute this remarkable stability to the strong binding between the multidentate ligand and the nanocrystal surface, as well as the stable micellar coating of amphipols in polar solvents. Indeed, amphipols have previously been used to stabilize nonpolar nanocrystals[17a,19] and integral membrane proteins[10a] in aqueous solution through hydrophobic interactions. Previous studies have found that this hydrophobic binding is essentially irreversible.[8a,20]
Figure 5.
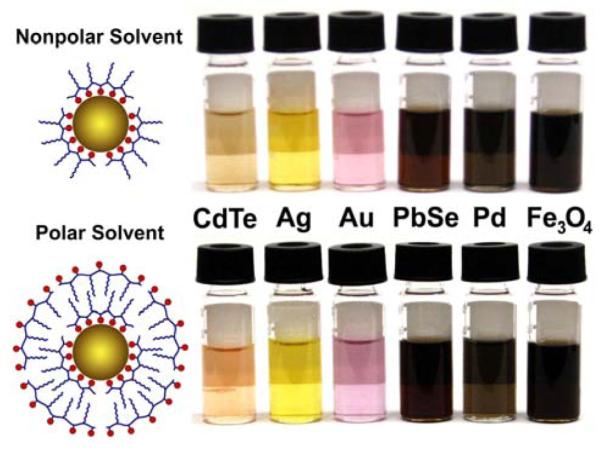
Metal, metal oxide, and semiconductor nanocrystals that are synthesized in an amphibious bath and are instantly soluble in both polar and nonpolar solvents. Shown on the left are schematic structures of self-generated hydrophobic and hydrophilic coatings on the nanocrystal surface.
In summary we have developed a new synthesis strategy for a large variety of nanocrystals that are instantly soluble and stable in both polar and nonpolar solvents. A new finding is that multidentate polymers called amphipols can serve as both coordinating ligands for metal atoms and nanocrystals, as well as micellization agents for nanocrystals encapsulation. To our knowledge, such a highly ordered self-assembly process using a single surfactant for both coordination and hydrophobic encapsulation in a single step has not been reported before. The high temperatures used for crystal growth result in exceptional monodispersity and crystallinity, with reaction yields typically greater than 90%. For applications in nonpolar solvents, these nanocrystals may be spontaneously purified from their reaction solvent, and for use in aqueous solution, greater than 99% of these nanocrystals are stable after dilution in water as carboxyl-functionalized colloids. The long-term stability of these nanocrystals is tremendous due to the strong multidentate coordination by the amphipols and the strong hydrophobic interactions of the micelles in polar solutions, both of which are stabilized through multiple anchor points on the surface. The nanocrystals that we have produced herein have great potential for applications in biological environments, homogeneous catalysis, device incorporation, and for the study of the solvent-dependent characteristics of nanocrystalline materials.
Experimental Section
All nanocrystal syntheses were performed using standard air-free techniques with a Schlenk line and glove box or bag. Detailed syntheses of alkylamine-grafted polyacrylic acid amphipols have been previously reported.[21] A dehydrated form of PMAT was purchased as poly(maleic anhydride-alt-1-tetradecene) (Mn 7300 Da) from Aldrich and was hydrolyzed.[22] For a typical synthesis of CdTe nanocrystals, cadmium(II) acetate hydrate (0.1 mmol, Aldrich, 99.99%), amphipols (0.8 mmol carboxylic acids), and dihydroxy-PEG (4 g, 400 MW, Sigma) were added to a 50 mL flask and evacuated at room temperature to ∼20 Pa. Caution should be used when slowly degassing this extremely sudsy mixture to prevent contamination of the Schlenk line. While under vacuum, the temperature was slowly ramped to 70°C, resulting in vigorous bubbling. After 1 hour of evacuation, the solution was clear, with a slight yellow hue. The reaction was then charged with argon and the temperature was raised to 150°C. Tributylphosphine-telluride precursor was then added, consisting of tellurium (0.05 mmol, Sigma, >99.999%), tributylphosphine (0.4 mmol, Aldrich, 97%), and PEG (2 g), and the reaction temperature was increased to 280°C. The final nanocrystal size was controlled through the amphipol concentration and reaction time. Swift injections of precursors were never required to produce monodisperse nanocrystals. Samples were obtained from the reaction by removing small aliquots, which were diluted in various solvents while the reaction mixture was still warm. Different PEG molecular weights resulted in similar nanocrystals size and monodispersity, although high molecular weights (>2000 Da) were avoided due to their viscosity and very low molecular weights (<250 Da) yielded less efficient micellization.
All other nanocrystal syntheses were performed under similar conditions as the CdTe nanocrystals, with the following modifications of reactants and reaction temperatures. Lead selenide nanocrystals were prepared using lead(II) acetate trihydrate (Fisher, 99%) with tributylphosphine-selenide at 240°C. Iron oxide nanocrystals were prepared using iron(III) acetylacetonate (Aldrich, 99.9%) and 1,2-hexadecanediol (4:1 diol:Fe, Aldrich, 90%) with a small amount of dodecylamine (3:1 amine:Fe, Aldrich, 98%) at 280°C. For the synthesis of Ag and Pd metal nanocrystals, dihydroxy PEG allowed the reduction of metals salts in the absence of an additional reducing agent, likely due to similar mechanisms as polyol syntheses of metal nanocrystals.[23] Silver nanocrystals were prepared using silver(I) acetate (Alfa Aesar, 99%) at a growth temperature of 150°C. Palladium nanocrystals were prepared using palladium(II) acetylacetonate (Aldrich, 99%) at a growth temperature of 150°C. Gold nanocrystals were prepared using gold(III) chloride (Alfa Aesar, 99.99%) with hydroquinone (10:1 hydroquinone:Au, Aldrich, >99%) or other reducing agents at a growth temperature of 280°C. All of the amphipol-metal ion complexes were found to be insoluble in water until nanocrystal nucleation, allowing a convenient means to qualitatively monitor of the progress of the reaction. Nanocrystals were purified in water using ultracentrifugation, size-exclusion chromatography, or dialysis. In nonpolar solvents, nanocrystals were purified by spontaneous precipitation, as described in the text. These particles may also be purified through other various chromatographic or extraction methods.
Extractions described in Table 1 were performed as follows. An aliquot of a crude reaction mixture containing CdTe nanocrystals (1 mL) was diluted in a mixture of hexane (5 mL) and methanol (8 mL). The solution was mixed vigorously and the percentages of nanocrystals in the top hexane phase and the bottom methanolic phase were determined spectrophotometrically.
Dynamic light scattering measurements were performed on a Brookhaven Instruments 90Plus Particle Size Analyzer. Before analysis, nanoparticle samples (1-10 μM) were first centrifuged at 7000 g for 10 minutes and then filtered through a 0.2 μm filter. Photoluminescence spectra were obtained using a spectrofluorometer from Photon Technology International. The excitation source was a xenon lamp, the detector was a photomultiplier tube, and the spectrometer slit widths were typically operated at 4 nm. Absorption spectra were obtained with a Shimadzu spectrophotometer with 1 nm slit widths. Electron microscopy and energy dispersive x-ray spectroscopy (EDS) were performed on a Hitachi HD2000. Size-exclusion chromatography was performed on a Superose 6 10/300 GL column, with 280 nm absorption monitored on an AKTAprime plus (GE Healthcare) system with a 0.5 mL/min flow rate. Zeta potential was measured on a Zetasizer Nano-ZS90 (Malvern Instruments). Ultracentrifugal isolation of aqueous solutions of nanocrystals was performed on a Beckman Coulter Optima TLX Ultracentrifuge, typically at 100,000 rpm for 1 hour.
Supplementary Material
Supporting Figure 1. Left: Absorption spectra of amphibious silver nanocrystals dispersed in water or toluene. Right: Dependence of the surface plasmon peak of amphibious silver nanocrystals on the refractive index of the solvent. Mixtures of benzene-methanol (red squares), as well as 12 other noninteracting solvents (blue circles), were tested.
Supporting Figure 2. Absorption spectra of amphibious gold nanocrystals dispersed in water or toluene.
Supporting Figure 3. Cadmium telluride characterization. Nanocrystals were grown in the amphibious bath to the size of 3.60 ± 0.42 nm, diluted in water, purified, and cast on a TEM grid. (a) High-resolution transmission electron micrographs of the nanocrystals. The dimensions of the image are 75×75 nm. (b) Representative nanocrystal at high magnification with inset showing a fast Fourier transform of the nanocrystal image. The nanocrystal is oriented with a zinc blende {110} plane parallel to the TEM grid. The dimensions of the image are 12×12 nm. (c) Energy dispersive X-ray spectra of the nanocrystals, showing a composition of cadmium and tellurium.
Supporting Figure 4. Lead selenide materials characterization. PbSe nanocrystals were grown in the amphibious bath to a size of 22.5 ± 1.7 nm, diluted in water, purified, and cast on a TEM grid. (a) High-resolution transmission electron micrographs of the nanocrystals. The dimensions of the image are 65×65 nm. (b) Representative nanocrystal at high magnification with inset showing a fast Fourier transform of the nanocrystal image. The nanocrystal is oriented with its rock salt (111) plane parallel to the TEM grid and is significantly faceted along the nonpolar {110} faces. The dimensions of the image are 12×12 nm. (c) Energy dispersive X-ray spectra of the nanocrystals, showing a composition of lead and selenium.
Supporting Figure 5. Iron oxide characterization. Iron oxide nanocrystals in the amphibious bath to a size of 3.03 ± 0.53 nm, diluted in water, purified, and cast on a TEM grid. (a) High-resolution transmission electron micrographs of the nanocrystals. The dimensions of the image are 60×60 nm. (b) Nanocrystal at high magnification with inset showing a fast Fourier transform of the nanocrystal image. The nanocrystal is oriented with its face-centered cubic (100) plane parallel to the TEM grid. The dimensions of the image are 12×12 nm. (c) Energy dispersive X-ray spectra of the nanocrystals, showing a composition of iron.
Footnotes
The authors thank Dr. Hong Yi, Dr. Amar Kumbhar, and Dr. Joan Hudson for electron microscopy assistance. We also acknowledge helpful discussions from Brad Kairdolf and Dr. Xiaohu Gao. This work was funded by xxx
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1] a).Willets KA, Van Duyne RP. Annu. Rev. Phys. Chem. 2007;58:267. doi: 10.1146/annurev.physchem.58.032806.104607. [DOI] [PubMed] [Google Scholar]; b) Qian XM, Nie SM. Chem. Soc. Rev. 2008;37:912. doi: 10.1039/b708839f. [DOI] [PubMed] [Google Scholar]; c) Narayanan R, El-Sayed MA. J. Phys. Chem. B. 2005;109:12663. doi: 10.1021/jp051066p. [DOI] [PubMed] [Google Scholar]; d) Shenhar R, Rotello VM. Acc. Chem. Res. 2003;36:549. doi: 10.1021/ar020083j. [DOI] [PubMed] [Google Scholar]; e) Raimondi F, Scherer GG, Kotz R, Wokaun A. Angew. Chem. 2005;117:2228. doi: 10.1002/anie.200460466. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. Engl. 2005;44:2190. [Google Scholar]; f) Zach M, Hagglund C, Chakarov D, Kasemo B. Curr. Opin. Solid State Mater. Sci. 2006;10:132. [Google Scholar]; g) Lee TH, Gonzalez JI, Zheng J, Dickson RM. Acc. Chem. Res. 2005;38:534. doi: 10.1021/ar040146t. [DOI] [PubMed] [Google Scholar]; h) Cheng MMC, et al. Curr. Opin. Chem. Biol 2006101116418011 [Google Scholar]; i) Daniel MC, Astruc D. Chem. Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]; j) Baca AJ, Ahn JH, Sun Y, Meitl MA, Menard E, Kim HS, Choi WM, Kim DH, Huang Y, Rogers JA. Angew. Chem. 2008;120:5606. doi: 10.1002/anie.200703238. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. Engl. 2008;47:5524. [Google Scholar]; k) Smith AM, Duan HW, Mohs AM, Nie SM. Adv. Drug Delivery Rev. 2008;60:1226. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Alivisatos AP, Gu WW, Larabell C. Annu. Rev. Biomed. Eng. 2005;7:55. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]; m) Penn SG, He L, Natan MJ. Curr. Opin. Chem. Biol. 2003;7:609. doi: 10.1016/j.cbpa.2003.08.013. [DOI] [PubMed] [Google Scholar]; n) Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]; o) Kamat PV. J. Phys. Chem. B. 2007;111:2834. doi: 10.1021/jp070701j. [DOI] [PubMed] [Google Scholar]
- [2] a).Murray CB, Norris DJ, Bawendi MG. J. Am. Chem. Soc. 1993;115:8706. [Google Scholar]; b) Brus LE. J. Chem. Phys. 1984;80:4403. [Google Scholar]; c) Ekimov AI, Onushchenko AA. Soviet Physics Semiconductors-USSR. 1982;16:775. [Google Scholar]
- [3].Krishnan KM, Pakhomov AB, Bao Y, Blomqvist P, Chun Y, Gonzales M, Griffin K, Ji X, Roberts BK. J. Mater. Sci. 2006;41:793. [Google Scholar]
- [4].Narayanan R, El-Sayed MA. Top. Catal. 2008;47:15. [Google Scholar]
- [5] a).Park J, Joo J, Kwon SG, Jang YJ, Hyeon T. Angew. Chem. 2007;119:4714. doi: 10.1002/anie.200603148. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. Engl. 2007;46:4630. [Google Scholar]; b) Wilcoxon JP, Abrams BL. Chem. Soc. Rev. 2006;35:1162. doi: 10.1039/b517312b. [DOI] [PubMed] [Google Scholar]
- [6].Rogach AL, Franzl T, Klar TA, Feldmann J, Gaponik N, Lesnyak V, Shavel A, Eychmuller A, Rakovich YP, Donegan JF. J. Phys. Chem. C. 2007;111:14628. [Google Scholar]
- [7].Yu MW, Peng XG. Angew. Chem. 2002;114:2474. [Google Scholar]; Angew. Chem., Int. Ed. Engl. 2002;41:2368. [Google Scholar]
- [8] a).Smith AM, Duan HW, Rhyner MN, Ruan G, Nie SM. Phys. Chem. Chem. Phys. 2006;8:3895. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]; b) Aldana J, Wang Y, Peng X. J. Am. Chem. Soc. 2001;123:8844. doi: 10.1021/ja016424q. [DOI] [PubMed] [Google Scholar]
- [9].Gaponik N, Talapin DV, Rogach AL, Eychmuller A, Weller H. Nano Lett. 2002;2:803. [Google Scholar]
- [10] a).Sanders CR, Hoffman AK, Gray DN, Keyes MH, Ellis CD. ChemBioChem. 2004;5:423. doi: 10.1002/cbic.200300830. [DOI] [PubMed] [Google Scholar]; b) Popot JL, et al. Cell. Mol. Life Sci. 2003;60:1559. doi: 10.1007/s00018-003-3169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murray CB, Sun SH, Gaschler W, Doyle H, Betley TA, Kagan CR. IBM J. Res. Dev. 2001;45:47. [Google Scholar]
- [12].Nann T. Chem. Commun. 2005:1735. doi: 10.1039/b414807j. [DOI] [PubMed] [Google Scholar]
- [13].Duan HW, Kuang M, Wang DY, Kurth DG, Mohwald H. Angew. Chem. 2005;117:1745. doi: 10.1002/anie.200461023. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. Engl. 2005;44:1717. [Google Scholar]
- [14].Yu WW, Wang YA, Peng XG. Chem. Mater. 2003;15:4300. [Google Scholar]
- [15].Yu WW, Qu LH, Guo WH, Peng XG. Chem. Mater. 2003;15:2854. [Google Scholar]
- [16] a).Smith AM, Nie SM. J. Am. Chem. Soc. 2008 ASAP. [Google Scholar]; b) Chakraborty AK, Golumbfskie AJ. Annu. Rev. Phys. Chem. 2001;52:537. doi: 10.1146/annurev.physchem.52.1.537. [DOI] [PubMed] [Google Scholar]
- [17] a).Pellegrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Radler J, Natile G, Parak WJ. Nano Lett. 2004;4:703. [Google Scholar]; b) Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- [18].Pons T, Uyeda HT, Medintz IL, Mattoussi H. J. Phys. Chem. B. 2006;110:20308. doi: 10.1021/jp065041h. [DOI] [PubMed] [Google Scholar]
- [19].Wu XY, Liu HJ, Liu JQ, Haley KN, Treadway JA, Larson JP, Ge NF, Peale F, Bruchez MP. Nat. Biotechnol. 2003;21:41. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- [20].Zoonens M, Giusti F, Zito F, Popot JL. Biochemistry. 2007;46:10392. doi: 10.1021/bi7007596. [DOI] [PubMed] [Google Scholar]
- [21].Wang KT, Iliopoulos I, Audebert R. Polymer Bulletin. 1988;20:577. [Google Scholar]; Gohon Y, Pavlov G, Timmins P, Tribet C, Popot JL, Ebel C. Anal. Biochem. 2004;334:318. doi: 10.1016/j.ab.2004.07.033. [DOI] [PubMed] [Google Scholar]
- [22].Smith AM, Duan HW, Rhyner MN, Ruan G, Nie SM. Phys. Chem. Chem. Phys. 2006;8:3895. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]
- [23].Wiley B, Sun Y, Mayers B, Xia Y. Chem. Eur. J. 2005;11:454. doi: 10.1002/chem.200400927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1. Left: Absorption spectra of amphibious silver nanocrystals dispersed in water or toluene. Right: Dependence of the surface plasmon peak of amphibious silver nanocrystals on the refractive index of the solvent. Mixtures of benzene-methanol (red squares), as well as 12 other noninteracting solvents (blue circles), were tested.
Supporting Figure 2. Absorption spectra of amphibious gold nanocrystals dispersed in water or toluene.
Supporting Figure 3. Cadmium telluride characterization. Nanocrystals were grown in the amphibious bath to the size of 3.60 ± 0.42 nm, diluted in water, purified, and cast on a TEM grid. (a) High-resolution transmission electron micrographs of the nanocrystals. The dimensions of the image are 75×75 nm. (b) Representative nanocrystal at high magnification with inset showing a fast Fourier transform of the nanocrystal image. The nanocrystal is oriented with a zinc blende {110} plane parallel to the TEM grid. The dimensions of the image are 12×12 nm. (c) Energy dispersive X-ray spectra of the nanocrystals, showing a composition of cadmium and tellurium.
Supporting Figure 4. Lead selenide materials characterization. PbSe nanocrystals were grown in the amphibious bath to a size of 22.5 ± 1.7 nm, diluted in water, purified, and cast on a TEM grid. (a) High-resolution transmission electron micrographs of the nanocrystals. The dimensions of the image are 65×65 nm. (b) Representative nanocrystal at high magnification with inset showing a fast Fourier transform of the nanocrystal image. The nanocrystal is oriented with its rock salt (111) plane parallel to the TEM grid and is significantly faceted along the nonpolar {110} faces. The dimensions of the image are 12×12 nm. (c) Energy dispersive X-ray spectra of the nanocrystals, showing a composition of lead and selenium.
Supporting Figure 5. Iron oxide characterization. Iron oxide nanocrystals in the amphibious bath to a size of 3.03 ± 0.53 nm, diluted in water, purified, and cast on a TEM grid. (a) High-resolution transmission electron micrographs of the nanocrystals. The dimensions of the image are 60×60 nm. (b) Nanocrystal at high magnification with inset showing a fast Fourier transform of the nanocrystal image. The nanocrystal is oriented with its face-centered cubic (100) plane parallel to the TEM grid. The dimensions of the image are 12×12 nm. (c) Energy dispersive X-ray spectra of the nanocrystals, showing a composition of iron.