Abstract
Human breast cancer cells (MDA-MB-435s) secrete a nucleoside diphosphate kinase (NDPK-B) as a phosphoprotein capable of converting diphosphate nucleosides to triphosphate nucleotides for one round in the absence of a phosphoryl donor. Incubation of the partially purified NDPK-B (Nm23-H2 by Western blot) from [γ32P]Pi-labeled cells with non-radioactive ADP results in the formation of [γ32P]ATP (Proc. West. Pharmacol. Soc. 44: 61–63, 2001). The presence of a secreted protein that can maintain ATP levels in the vicinity of capillary and lymph vessels may support cancer metastasis in several ways based on the known actions of ATP at P2Y receptors: facilitate intravasation of breast cancer cells that migrate from a solid tumor, support their extravasation at a distal site, and stimulate angiogenesis. The putative role of angiostatin (AS) as an ATP-synthase inhibitor led us to test the notion that AS blocks NDPK-B activity. Addition of commercial AS (kringles 1–4) did not alter enzyme activity. However, AS produced by us and never lyophilized, blocked NDPK activity in a dose-dependent fashion consistent with the notion that extracellular ATP generation by tumor cells may be important to the development of metastases. The ability of 0.5 mg/ml angiostatin to block NDPK-B activity to ~75% of control activity compared poorly with the polyphenol inhibitors of. The catechin gallates, theaflavins and ellagic acid inhibited NDPK-B completely with the rank order of potency: EA>theaflavins>EGCG>ECG>PAPS. Our results suggest that the biological activity of angiostatin as a putative metastasis inhibitor may be in part the result of nm23 inhibition and that the production, lyophilization, packaging or storage of commercial angiostatin leads to the alteration of its biological activity against NDPK-B. Ellagic acid is a potent (IC50 = 10.5 µM) NDPK-B inhibitor that may prove useful in elucidating the role of cancer-cell secreted NDPK-B in tumor development.
INTRODUCTION
Despite decades of research and significant advances in the detection and treatment of primary breast carcinoma, patients that succumb to the disease do so because of the formation of metastatic tumors [1]. Metastasis is a complex cascade of events involving tumor cell motility, proteolysis, intravasation and extravasation, angiogenesis, and tumor growth [2]. The so-called metastasis sup-pressor gene, nm23, may be an integral mediator of some of these events. Expression of the human isoforms, nm23-H1 and nm23-H2, is thought to be inversely associated with the metastatic potential of a variety of cancers [3–5]. While nm23-H1 is strongly associated with metastasis mechanisms in many tumors, nm23-H2 is not [6–8]. The products of these two genes, NDPK-A and NDPK-B, were named for their function as nucleoside diphosphate (NDP) kinases. These enzymes, in the presence of divalent cations, covalently transfer the terminal γ-phosphate of a nucleoside triphosphate to a nucleoside diphosphate via a high energy phosphohistidine intermediate in a ping-pong fashion. NDPK-B is elaborated into the extracellular environment by the breast carcinoma cell line MDA-MB-435s as well as other cells derived from solid tumors such as colon, lung and prostate [9]. The presence of NDP kinase activity on the surface and external environment of cancer cells that exist in the milieu of apoptosis and necrosis provides an effective mechanism for re-generating extracellular purines. The Nucleotide Axis Hypothesis suggests that extracellular ATP plays a central role in the signaling and responsiveness of vascular endothelium by preserving and amplifying the vasodilatory effects of P2Y dependent stimulation [10]. Localized production of extracellular ATP by tumor-derived NDPK-B may facilitate the process of metastasis as it may support tumor cell transit and intravasation [11]. Based on this hypothesis, inhibitors of secreted NDPK-B may potentiate the suppression of metastasis and thus may be useful agents to use in conjunction with traditional chemotherapy or angiogenesis inhibitors such as bevacizumab (Avastin ®).
Angiostatin, a proteolytic fragment consisting of the first four kringle domains of plasminogen, is produced by human tumors [12,13] and suppresses metastatic growth and neovascularization [14,15]. Presumably this is accomplished, in part, by angiostatin binding the α/β-subunits of ATP synthase which are said to be located on the external surface of endothelial cells [16,17]. However, the downstream effects of angiostatin binding to the synthase have not yet been fully demonstrated [18,19]. Furthermore, the potential that other ATP-production targets for angiostatin might exist in the extracellular environment, and thus defeat the inhibition of the ATP-synthase, has not been investigated. Since both angiostatin and NDPK-B are present in the extracellular milieu, and both compounds have potential effects on metastatic processes, we hypothesized that angiostatin inhibits the transphosphorylation activity of NDPK-B secreted by breast carcinoma cells to the effect of reducing extracellular ATP concentration.
The notion that NDPK-B is secreted from breast cancer cells and acts to produce purine nucleotide extracellularly (eg., ATP), together with our interest in these compounds as vasodilators, angiogenic stimulators and diapedesis enhancers, leads us to wonder if the notion that nm23 gene expression as strictly correlated with low metastasis potential is correct. Indeed, the fact that a number of compounds that are touted as angiogenesis inhibitors (e.g., angiostatin) may interfere with NDPK activity could further implicate extracellular nucleotides in the metastatic process. We have examined a list of compounds implicated in angiogenesis inhibition and find some to be effective NDPK-B inhibitors.
METHODS
Production of NDPK-B
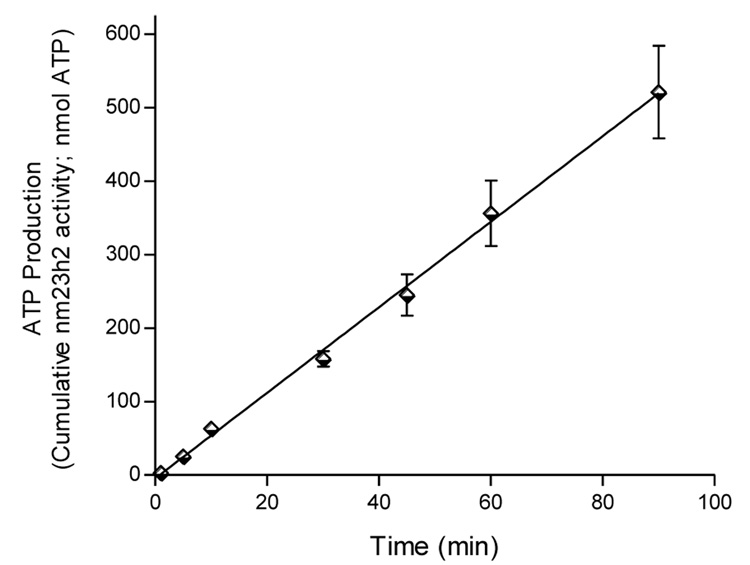
MDA-MB-435s breast ductal carcinoma cells were grown to confluence in Dulbecco’s modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics. Media was replaced with Krebs buffer (25 mM Hepes, pH 7.4) and incubated at 37°C with gentle rocking for 3.5 hr. The supernatant was removed and concentrated using spin filters with a molecular weight cutoff of 8–10,000. NDPK activity of the retentate was assayed and stored at −20°C. The elaboration of NDPK-B activity into the incubation buffer over time is linear. Cells can be returned to growth conditions and re-harvested as secretion is not associated with changes in cell survival.
Production of Angiostatin
Angiostatin was generated by the method of Gately et al. [12]. Briefly, PC-3 prostate adenocarcinoma cells were grown to a confluent monolayer in DMEM with 10% heat-inactivated FBS and antibiotics. Serum-free conditioned media was obtained by incubating the cells for 19–24 hr with DMEM containing antibiotics. The supernatant was removed and briefly centrifuged to remove insoluble debris. Fifty percent of the conditioned media was reserved as a control (PC3-CON). The remainder (PC3-HPg) was incubated for 24 hr with 20 µg/ml human plasminogen at 37° C. After incubation, both fractions of the media were concentrated using spin columns. Bovine serum albumin was added to the retentate (1 mg/ml) which was then stored at 4°C.
Secreted NDPK-B Exhibits Transphosphorylation Activity
Partially purified NDPK-B was incubated for 2 min with GTP (300µM) as a phosphoryl donor and ADP (0–60µM) as substrate. An equal volume of luciferin-luciferase ATP detection buffer (Sigma, St. Louis, MO) was added and a single measurement of luminescence was recorded 10 sec later on a Luminoskan® luminometer. Relative luminescence units (RLU) were corrected from background and converted to ATP by comparison to a standard curve which was linear for three order of magnitude concentration.
Inhibition of NDPK-B activity by PC-3 generated angiostatin
Partially purified NDPK-B was incubated with ADP and GTP in the presence of either commercial angiostatin (Angiogenesis Research, Inc.) or PC-3 derived angiostatin and the resultant ATP measured by luminescence assay.
Western Blot
Immuno blot of PC-3 conditioned media were developed with a polyclonal antibody (Ab-1) against purified human angiostatin protein. Immunopositive bands at 40–45 kDa were present in the conditioned media incubated with plasminogen (PC3-HPg) but absent in control media (PC3-CON), human plasminogen (HPg), and bovine serum albumin (BSA). These bands were identical to those present in commercial angiostatin (AS).
Inhibition Studies
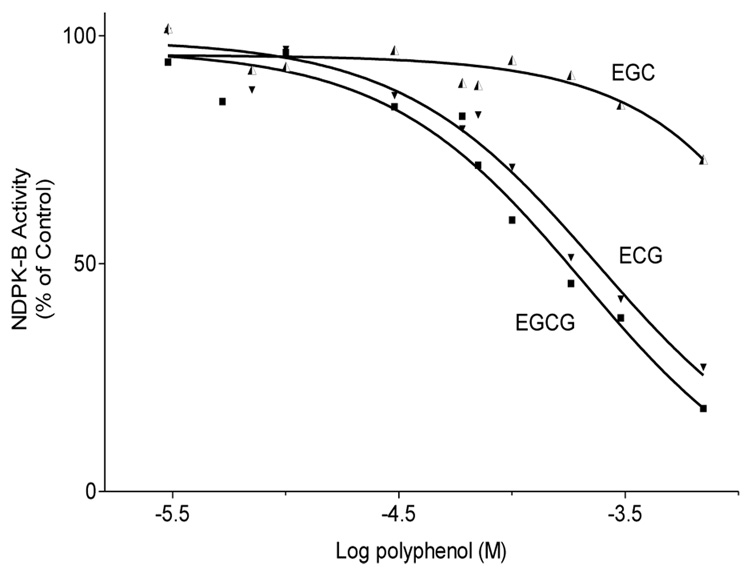
Polyphenols and the nucleoside analog PAPS (adenosine 3’-phosphate 5’-phosphosulfate) were pepared as stocks in in 50:50 aq. EtOH, diluted in nutral buffer and examined for their ability to inhibit NDPK-B activity secreted from MDA-MB-435S cells grown in culture. The addition of each compound with the exception of epigallocatechin (EGC) resulted in a significant reduction in Vmax (p < 0.05) but no change in substrate affinity (KM) which suggests that these compounds act as non-competitive inhibitors. The decreased inhibition by EGC suggests the presence of an additional gallate moiety or its structural equivalent is required for high-affinity interaction with NDPK-B by this series of compounds.
RESULTS
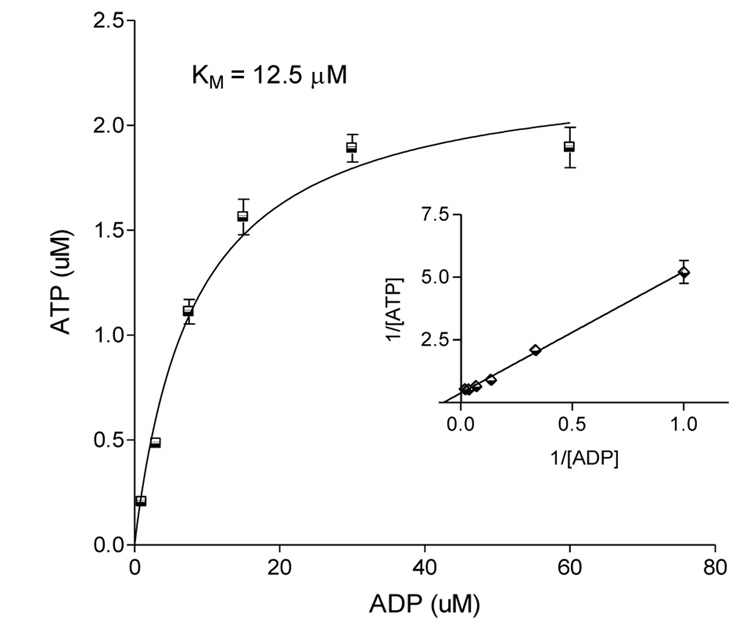
The activity of cancer cell secreted ADP-kinase was determined after concentration of conditioned media as described. Aliquots of conditioned media con-centrate supported ATP production in the presence of GTP as phosphoryl donor and ADP as substrate. The observed affinity was 12.5 µM (Fig. 2).
Figure 2.
Secreted NDPK-B exhibits transphosphorylation activity. Partially purified NDPK-B was incubated for 2 min with GTP (300µM) and ADP (0–60µM). An equal volume of luciferin-luciferase ATP detection buffer (Sigma, St. Louis, MO) was added and, following a 10 s delay, a single measurement of luminescence was recorded by luminometer. Relative luminescence units (RLU) were corrected from background and converted to ATP by comparison to a standard curve. Data are presented as mean ± SEM, n=3.
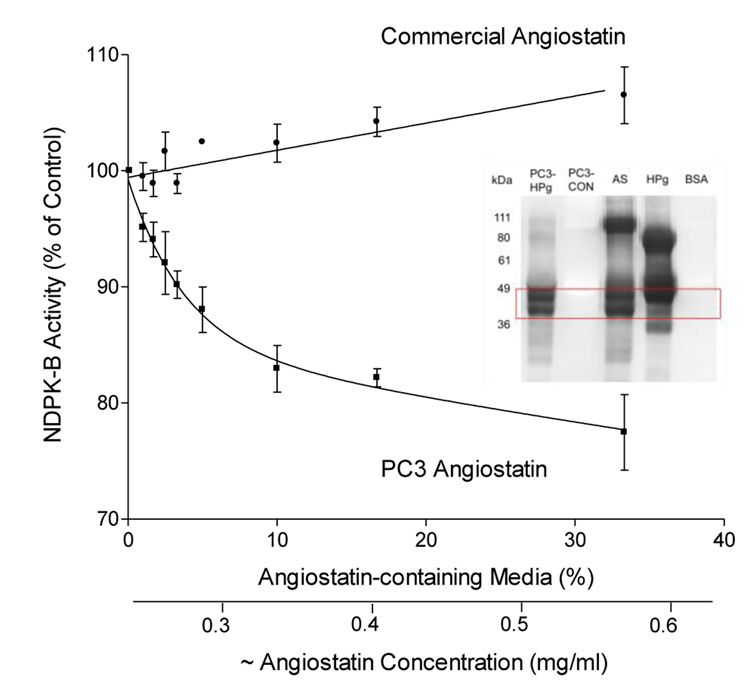
Addition of PC-3 generated angiostatin resulted in a significant reduction in VMAX (p < 0.05) but no change in affinity (Km) which suggests that angiostatin acts as a non-competitive inhibitor (Fig. 3). Neither freeze-thaw nor different lot preparation (we used 3 different lots) of commercial angiostatin could explain the absence of activity. Lyophilization of the PC-3 angiostatin did not deplete activity (not shown).
Figure 3.
Inhibition of NDPK-B activity by PC-3 generated angiostatin but not by commercial angiostatin. Partially purified NDPK-B was incubated with ADP and GTP in the presence of either commercial angiostatin (Angiogenesis Research, Inc.) or PC-3 derived angiostatin and the resultant ATP measured by luminescence assay. Addition of PC-3 angiostatin resulted in a significant reduction in Vmax (p < 0.05) but no change in substrate affinity (Km) which suggests that angiostatin acts as a non-competitive inhibitor. Neither freeze-thaw nor different lot preparation of commercial angiostatin could explain the absence of activity. Data are presented as mean ± SEM, n=3. Inset: Western blot of PC-3 conditioned media with a polyclonal antibody (Ab-1) against purified human angiostatin protein. Immunopositive bands at 40–45 kDa were present in the conditioned media incubated with plasminogen (PC3-HPg) but absent in control media (PC3-CON), human plasminogen (HPg), and bovine serum albumin (BSA). These bands were identical to those present in commercial angiostatin (AS).
Western blot of PC-3 conditioned media with a polyclonal antibody (Ab-1) against purified human angiostatin protein. Immunopositive bands at 40–45 kDa were present in the conditioned media incubated with plasminogen (PC3-HPg) but absent in control media (PC3-CON), human plasminogen (HPg), and bovine serum albumin (BSA). These bands were identical to those present in (AS) commercial angiostatin (Fig 3 inset).
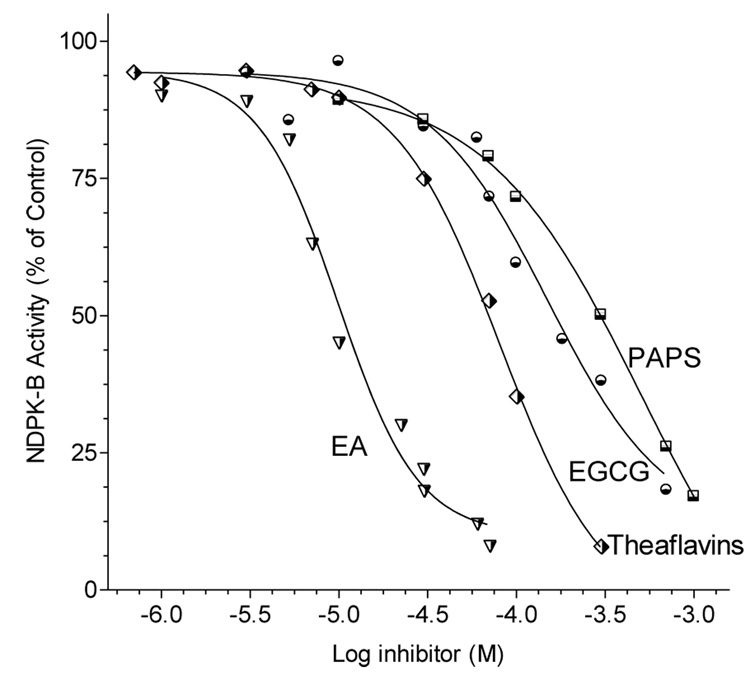
Partially purified NDPK-B was incubated with ADP and GTP in the presence of varying concentrations of NDPK-inhibitors or putative angiogenesis inhibitors and the resulting ATP measured by luminescence assay. The nucleoside analogs PAPS and 8-ClcAMP (not shown ~IC50 = 1 mM) inhibited NDP kinase activity by depressing the Vmax of the enzyme. The polyphenolic tea compounds (theaflavins, EGCG, ECG and ellagic acid) also suppressed ATP production but at higher potency than the nucleoside derivatives (Fig. 5,Fig.6).
Figure 5.
Comparative effect of the green tea polyphenols on NDPK-B activity. NDPK-B was incubated for 4 min with ADP and GTP in the presence of polyphenols and the resultant ATP measured by luminescence assay. Data are presented as mean ± SEM, n=3.
Figure 6.
Inhibition of NDPK-B activity by black tea theaflavins, green tea EGCG, and PAPS. Partially purified NDPK-B was incubated for 4 min with ADP and GTP in the presence of green or black tea polyphenols or PAPS and the resultant ATP measured by luminescence assay. The addition of each compound resulted in a significant reduction in Vmax (p < 0.05) but no change in substrate affinity (Km) which suggests that these compounds act as non-competitive inhibitors. Data are presented as mean ± SEM, n=5.
Breast cancer cells translate nm23H2 as both an ecto- and exoenzyme NDPK-B. The enzyme is secreted as a phosphoprotein and is capable of trasphosphorylation activity in the absence of a phosphoryl donor. This activity may be a mechanism for producing elevated extracellular ATP, particularly in the setting of apoptosis and tumor cell invasion and growth.
Angiostatin, generated from prostate carcinoma cells, inhibits NDPK-B transphosphorylation activity while commercial angiostatin fails to inhibit the enzyme. Nucleoside analogs 8-ClcAMP and PAPS inhibit NDPK-B transphosphorylation activity but with relatively low potency making them unsuitable for tumor inhibition studies. NDPK-B activity is inhibited by the polyphenolic constituents of tea (EGCG, ECG, and theaflavins). These compounds are known to suppress cancer cell proliferation, inhibit invasion into Matrigel®, and inhibit angiogenesis [11,20,21]. The anti-NDP kinase property reported here suggests a novel mechanism by which these compounds may be anti-tumorigenic. Taken together, these findings suggest the hypothesis that inhibition of NDPK-B activity is mechanistically associated with inhibition of metastasis by breast cancer cells.
Figure 1.
Elaboration of NDPK-B into the incubation buffer over time. Aliquots of MDA-MB-435s cell conditioned media concentrate from the indicated times were assayed for ATP production in the presence of VMAX conditions [GTP (300 µM) and ADP (100 µM); 30°C] for 2 min. Data are mean +/− SEM, n = 4.
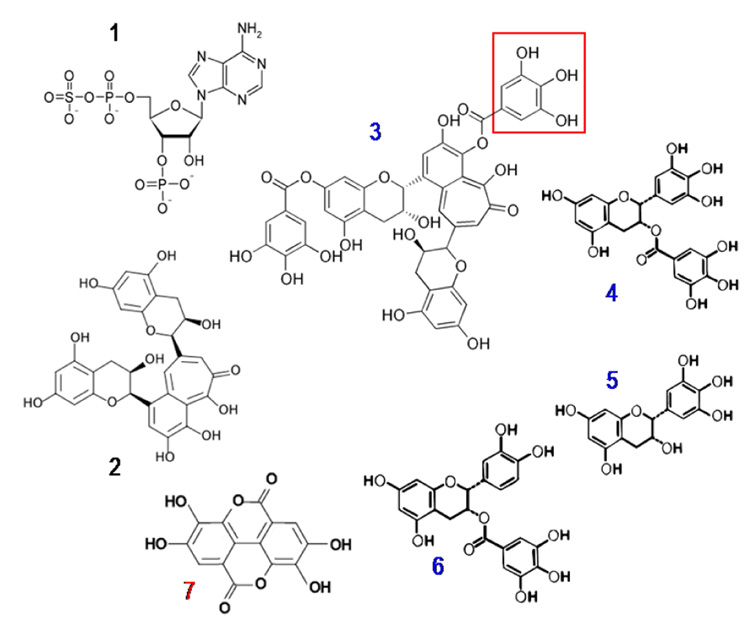
Figure 4.
Compounds tested as NDPK-B inhibitors. Compound 1 = PAPS (adenosine 3’-phosphate 5’-phosphosulfate); 2 = theaflavin; 3 = theaflavin gallate; 4 = EGCG (epigallocatechin gallate; 5 = EGC (epigallocatechin; 6 = ECG (epicatichingallate); 7 = ellagic acid. Compounds numbered in blue (3–6) contain a gallate group as delimited by the red box. For all the polyphenols tested, the presence of the gallate group correlates with inhibition of NDPK. Compounds 1, 2 and 7 are not gallates. Ellagic acid (compound 7) is not a gallate but has a related structure and a symmetry that may improve binding to the enzyme.
REFERENCES
- 1.Yavas O, Hayran M, Ozisik Y. Factors affecting survival in breast cancer patients following bone metastasis. Tumori. 2007;93:580–586. doi: 10.1177/030089160709300611. [DOI] [PubMed] [Google Scholar]
- 2.Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2005;23:9–15. doi: 10.3233/bd-2006-23103. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi A, Urano T, Fushida S, Furukawa K, Nishimura G, Yonemura Y, Miyazaki I, Nakagawara G, Shiku H. Inverse association of nm23-H1 expression by colorectal cancer with liver metastasis. Ann. Surg. 1993:1020–1025. doi: 10.1038/bjc.1993.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazaki H, Fukuda M, Ishijima Y, Takagi Y, Iimura T, Negishi A, Hirayama R, Ishikawa N, Amagasa T, Kimura N. Overexpression of nm23-H2/NDP kinase B in a human oral squamous cell carcinoma cell line results in reduced metastasis, differentiated phenotype in the metastatic site, and growth factor-independent proliferative activity in culture. Clin. Cancer Res. 1999;5:4301–4307. [PubMed] [Google Scholar]
- 5.Steeg PS, Cohn KH, Leone A. Tumor metastasis and nm23: current concepts. Cancer Cells. 1991;3:257–262. [PubMed] [Google Scholar]
- 6.Tschiedel S, Gentilini C, Lange T, Wolfel C, Wolfel T, Lennerz V, Stevanovic S, Rammensee HG, Huber C, Cross M, Niederwieser D. Identification of NM23-H2 as a tumour-associated antigen in chronic myeloid leukaemia. Leukemia. 2008;22:1542–1550. doi: 10.1038/leu.2008.107. [DOI] [PubMed] [Google Scholar]
- 7.Ismail NI, Kaur G, Hashim H, Hassan MS. Nuclear localization and intensity of staining of nm23 protein is useful marker for breast cancer progression. Cancer Cell Int. 2008;8:6. doi: 10.1186/1475-2867-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu CG, Lin LY, Ko JL, Yang SF, Chang H, Lin CY, Tsai HT, Chen SC, Chen SC, Wang PH. High expression of human nonmetastatic clone 23 type 1 in cancer of uterine cervix and its association with poor cell differentiation and worse overall survival. J. Surg. Oncol. 2008 doi: 10.1002/jso.21127. [DOI] [PubMed] [Google Scholar]
- 9.Anzinger J, Malmquist NA, Gould J, Buxton IL. Secretion of a nucleoside diphosphate kinase (Nm23-H2) by cells from human breast, colon, pancreas and lung tumors. Proc. West Pharmacol. Soc. 2001;44:61–63. [PubMed] [Google Scholar]
- 10.Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am. J. Physiol Heart Circ. Physiol. 2001;281:H1657–H1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- 11.Rumjahn SM, Javed MA, Wong N, Law WE, Buxton IL. Purinergic regulation of angiogenesis by human breast carcinoma-secreted nucleoside diphosphate kinase. BR. J. Cancer. 2007;97:1372–1380. doi: 10.1038/sj.bjc.6604019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gately S, Twardowski P, Stack MS, Cundiff DL, Grella D, Castellino FJ, Enghild J, Kwaan HC, Lee F, Kramer RA, Volpert O, Bouck N, Soff GA. The mechanism of cancer-mediated conversion of plasminogen to the angiogenesis inhibitor angiostatin. Proc Natl Acad Sci U S A. 1997;94:10868–10872. doi: 10.1073/pnas.94.20.10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH, Folkman J. Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb. Symp. Quant. Biol. 1994;59:471–482. doi: 10.1101/sqb.1994.059.01.052. [DOI] [PubMed] [Google Scholar]
- 15.Sckell A, Safabakhsh N, Dellian M, Jain RK. Primary tumor size-dependent inhibition of angiogenesis at a secondary site: an intravital microscopic study in mice. Cancer Res. 1998;58:5866–5869. [PubMed] [Google Scholar]
- 16.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-FO ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burwick NR, Wahl ML, Fang J, Zhong Z, Moser TL, Li B, Capaldi RA, Kenan DJ, Pizzo SV. An Inhibitor of the F1 subunit of ATP synthase (IF1) modulates the activity of angiostatin on the endothelial cell surface. J. Biol. Chem. 2005;280:1740–1745. doi: 10.1074/jbc.M405947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahl ML, Moser TL, Pizzo SV. Angiostatin and anti-angiogenic therapy in human disease. Recent Prog. Horm. Res. 2004;59:73–104. doi: 10.1210/rp.59.1.73. [DOI] [PubMed] [Google Scholar]
- 20.Liang YC, Chen YC, Lin YL, Lin-Shiau SY, Ho CT, Lin JK. Suppression of extracellular signals and cell proliferation by the black tea polyphenol, theaflavin-3,3'-digallate. carcinogenesis. 1999;20:733–736. doi: 10.1093/carcin/20.4.733. [DOI] [PubMed] [Google Scholar]
- 21.Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat. Res. 1999;428:339–344. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]