Abstract
Prenylcysteine derivatives are of interest for a variety of different biological reasons, including probing the CaaX protein processing pathway. A solid-phase synthesis protocol for the preparation of prenylcysteines using 2-chlorotrityl chloride resin as a solid support has been developed. A series of novel amide-modified farnesylcysteine analogs were synthesized in both high purity and yield under mild conditions. The farnesylcysteine analogs were evaluated using human isoprenylcysteine carboxyl methyltransferase (Icmt) as a biological target, and several new inhibitors, one with significantly enhanced potency, were identified.
Introduction
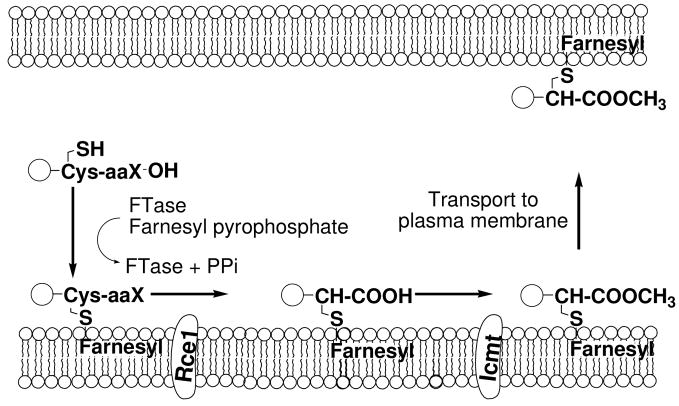
Many proteins contain a C-terminal sequence motif called a -CaaX box, where -C is cysteine, -aa are generally aliphatic residues and -X can be one of several amino acid residues.1 This motif signals many of these proteins for a series of at least three post-translational modifications (Figure 1). The first step is attachment of a 15-carbon farnesyl or 20-carbon geranylgeranyl group to the –CaaX cysteine by either protein farnesyltransferase or protein geranylgeranyltransferase I, respectively. Following prenylation, the last three amino acids are removed proteolytically by the enzyme Rce1 and lastly, the newly exposed α-carboxyl group is methylated by the enzyme isoprenylcysteine carboxyl methyltransferase, Icmt.2 Recent estimates suggest that approximately 120 human proteins are farnesylated or mono-geranylgeranylated, including the Ras family of oncoproteins.3 Importantly, mutations in the ras oncogene are responsible for approximately 15–20% of all human cancers, leading to intense interest in inhibitors of the CaaX processing pathway as potential anti-cancer agents.4
FIGURE 1.
Post-translational processing of –CaaX proteins.
Because of the biological importance of isoprenylated proteins,1 there have been numerous efforts to develop chemical tools to interrogate their function.5 In particular, prenylcysteine analogs6 and prenylated peptides7 have proven to be valuable agents for biological studies. We are interested in developing prenylcysteine analogs as potential Icmt inhibitors. Icmt is a novel potential chemotherapeutic target and inhibitors of Icmt are thus of particular interest as potential cancer chemotherapeutic agents.8 Recently, the Casey laboratory has reported that an Icmt inhibitor, cysmethynil, identified from a compound library, exhibits activity against cancer cells.9
Early studies on the synthesis of prenylated peptides focused on the solid-phase synthesis of non-prenylated peptides followed by the selective prenylation of a free cysteine residue in solution.10 Fragment condensation routes have also been employed.11 Other groups have developed methods for the solid-phase synthesis of prenylated peptides on a variety of linkers such as oxime,12 PTMSEL,13 and sulfonamide.14 However, we wished to develop a method that would allow the on-resin attachment of prenyl groups in order to synthesize diverse libraries of prenylcysteine derivatives. Here we report both the development of such a method and its use to prepare a library of prenylcysteine analogs. One of these analogs exhibits significantly enhanced inhibitory potency against human Icmt.
Results and Discussion
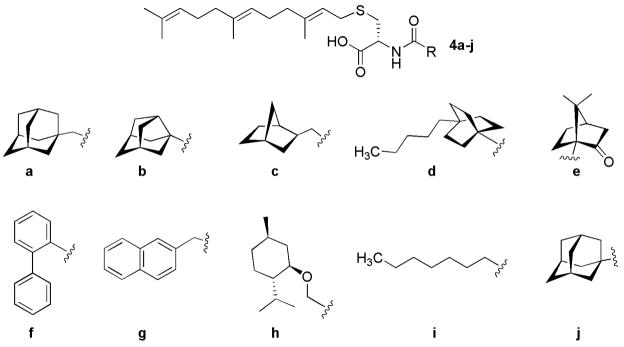
We have previously reported the solution-phase synthesis of a 23-member library of amide-modified farnesylcysteine derivatives (AMFCs).15 This initial library was screened against Icmt, and six inhibitors possessing bulky aromatic and aliphatic carboxylic acids attached to the α-nitrogen of the cysteine were identified. The farnesyl cysteine analog containing an N-1-adamantylcarbonyl group, 4j, (Chart 1) was identified as the most potent inhibitor of Icmt with an IC50 of 12 μM.15 Encouraged by these findings, a method was developed for the solid-phase synthesis of prenylcysteine analogs to facilitate the synthesis of a more comprehensive library of AMFCs. A set of AMFCs based on the previous library and the adamantyl lead (Chart 1) was targeted for synthesis initially.
CHART 1.
Amide-modified farnesylcysteine analogs synthesized on the 2-chlorotritylchloride solid support
The solid-phase synthesis of farnesylcysteine derivatives is complicated by the acid lability of the allylic thioether bond connecting cysteine to the prenyl group. It has been shown that the solid-phase synthesis of lipidated peptides requires mild, non-acidic cleavage conditions. In 2002, the Waldmann group reported an elegant solid-phase method for the synthesis of prenylated peptides terminating in a methyl ester on a hydrazine resin.16 Unfortunately, this method did not work well in our hands for the generation of peptides that end in a free carboxylate, a necessary feature for this class of Icmt inhibitors. Subsequently, Waldmann also reported problems arising from radical side-reactions that occurred during cleavage from the hydrazine resin.14 Thus, for the purposes of synthesizing the desired prenylcysteine library, a new approach to the preparation of prenylcysteine derivatives on solid-phase was developed.
The 2-chlorotrityl chloride resin has proven to be a versatile and useful tool for the synthesis of a variety of peptides,17,18 with certain specific advantages over other solid supports. First, due to the SN1 mechanism of the loading step, attaching the cysteine to the chlorotrityl resin does not require activation of the cysteine carboxyl group, thereby decreasing the likelihood of racemization. Second, the cleavage conditions are very mild and result in the rapid, quantitative release of the modified cysteine from the bead. Third, the resin is commercially available and relatively inexpensive.
Prior to beginning the synthesis of the AMFC set, several steps of the proposed solid-phase synthetic route to prenylcysteine analogs required optimization. These included the loading of the protected cysteine derivative, the deprotection of the cysteine sulfur, and in particular the cleavage of the analog from the resin. We found that using dithiothreitol as a reducing agent, together with diisopropylamine, for cleavage of the cysteine protecting group resulted in fewer side products than the alternative procedure using tributylphosphine and water.19 Interestingly, when collidine was used as a base instead of diisopropylethylamine, we observed no cleavage of the disulfide bond.
A number of methods have been reported for the cleavage of compounds from 2-chlorotrityl resin.20,21 We compared a variety of cleavage cocktails including: 1:4 hexafluoroisopropanol/dichloromethane, 1:2:7 acetic acid/trifluoroethanol/dichloromethane, 0.5:99.5 trifluoroacetic acid/dichloromethane, 1:99 trifluoroacetic acid/dichloromethane, and 3:97 trifluoroacetic acid/dichloromethane. While none of these conditions resulted in significant loss of the farnesyl group from the cysteine, the 0.5:99.5 mixture of trifluoroacetic acid/dichloromethane was chosen for subsequent use due to its speed and nearly quantitative release of the AMFCs from the resin. The 1:4 hexafluoroisopropanol/dichloromethane conditions were attractive, due to the essentially neutral nature of this mixture, but it did not always result in efficient release of the analog from the bead. Additionally, the higher cost of hexafluoroisopropanol combined with the requirement that it be used in higher proportion to TFA made this option less desirable. While the 1:2:7 acetic acid/trifluoroethanol/dichloromethane solution worked well, it was much slower than the TFA solution, which required only 2–3 minutes for complete product release.
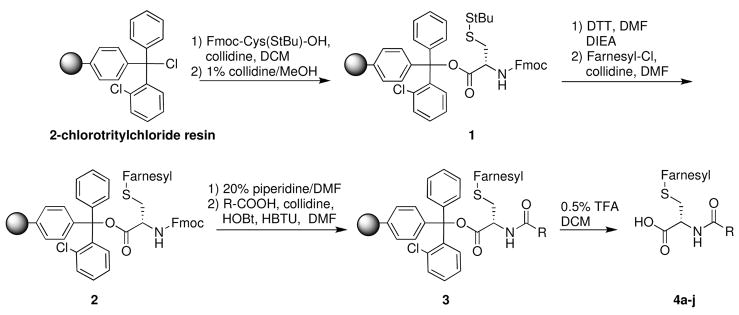
The solid-phase synthetic route to prenylcysteine analogs is outlined in Scheme 1. The commercial 2-chlorotritylchloride resin is coupled to Fmoc-Cys(SStBu)-OH, using 1% collidine in dichloromethane. Any unreacted trityl moieties were capped as methoxides by addition of a 1% collidine/MeOH solution to the reaction mixture. Reductive removal of the dithiotertbutyl protecting group from 1 with dithiothreitol was followed by coupling of the desired farnesyl side chain to the free thiol, using farnesyl chloride as the electrophile and collidine as the base to afford intermediate 2. Next, the Fmoc protecting group was removed with 20% piperidine/DMF. Coupling of the selected carboxylic acid with the resulting free amine was accomplished using HBTU/HOBt.22 The polymer-bound prenylcysteine analog 3 was then released from the resin using our optimized cleavage conditions. Purification by either normal-phase chromatography or C18 reversed-phase chromatography afforded prenylcysteine analogs suitable in purity for characterization and biochemical evaluation, in 45–65% yield based on resin loading.
SCHEME 1.
Solid-phase synthesis of amide-modified farnesylcysteines
The ten prenylcysteines analogs 4a–j (Chart 1) were screened against recombinant human Icmt23 using our recently developed Icmt scintillation proximity assay.14,24 The carboxyl substituents chosen were primarily bulky hydrophobic moieties similar to the lead adamantyl compound 4j. The octanoyl derivative 4i is an exception and was prepared as a control for the effect of hydrophobic moieties on activity. Seven of the analogs, which included our original lead 4j, resulted in a 50% or greater decrease in Icmt activity in the SPA at a concentration of 50 μM. Three of the ten compounds - the norbornyl analog 4c, the 2-napthyl analog 4g, and the octanoyl analog 4i – were inactive in the SPA. The seven active analogs were then further evaluated using a vapor diffusion assay (VDA)21 to determine IC50 values (Table 1). It should be noted that the “hit” rate in this small collection was significantly higher than in our original study of AMFCs as Icmt inhibitors.12
TABLE 1.
Inhibition data for selected AMFC analogs.
| Compound | Human Icmt IC50 (μM) |
|---|---|
| 4a | 18.8 +/−2.7 |
| 4b | 16.7 +/−2.6 |
| 4c | nd |
| 4d | 16.7 +/−3.6 |
| 4e | 15.6 +/−1.3 |
| 4f | 12.1 +/−2.1 |
| 4g | nd |
| 4h | 22.9 +/−2.5 |
| 4i | nd |
| 4j | 12.4 +/−1.0 |
nd: IC50 values not determined due to lack of activity in the initial SPA24 screen.
None of the compounds were more potent than the lead adamantyl analog 4j. However, the ortho-biphenyl analog 4f is equivalent in potency (IC50 = 12.1 μM), and the biphenyl scaffold is significantly more amenable to derivatization than the adamantyl scaffold. Note that the biological findings here further confirm that the inhibitory activity of AMFCs does not correlate simply with the hydrophobicity of these compounds,25 but is related to steric bulk.
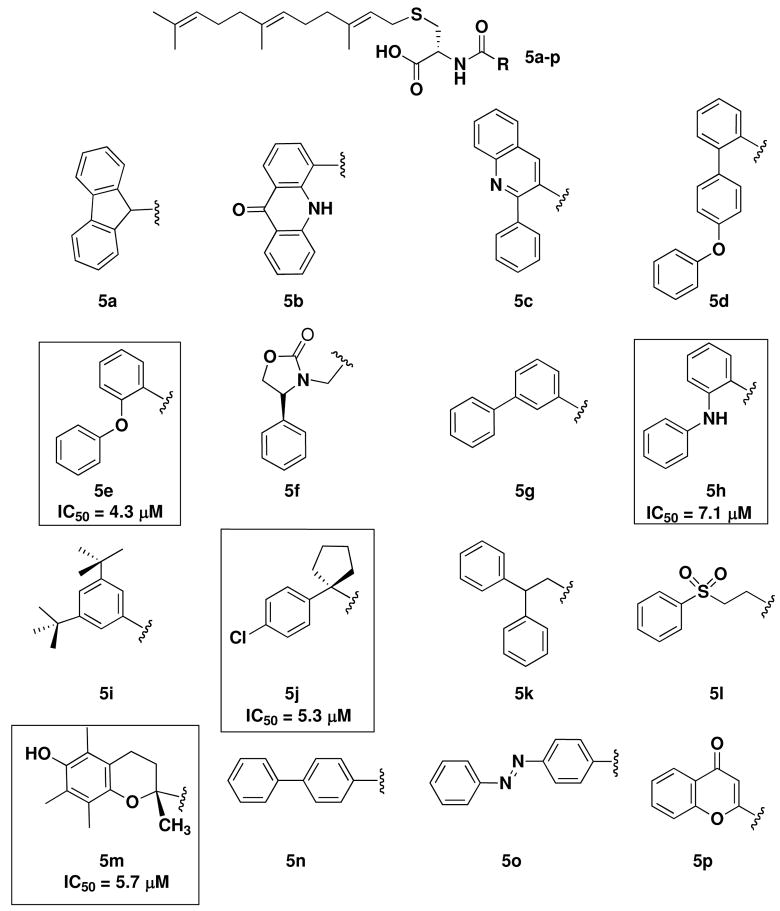
An additional 16 analogs depicted in Chart 2, based primarily on the biphenyl scaffold were synthesized as shown in Scheme 1. A preliminary screen indicated that only five of the sixteen analogs were Icmt substrates. The eleven non-substrates were assayed for inhibitory activity using the VDA and all eleven were found to inhibit Icmt by at least 30% at a concentration of 10 μM. The four indicated compounds in Chart 2 were clearly more potent than the ortho-biphenyl analog 4f. The ortho-phenoxy derivative (5e) inhibited the ability of Icmt to methylate AFC by nearly 80% at 10 μM and is nearly three-fold more potent (IC50 = 4.3 μM) than the previous adamantyl or biphenyl modified lead Icmt inhibitors. Eleven of the sixteen analogs evaluated in the compound library based on the 2-biphenyl scaffold were inhibitors. This result represents a substantial increase in both potency and hit rate from the previous libraries.
CHART 2.
Subsequent AMFC library.
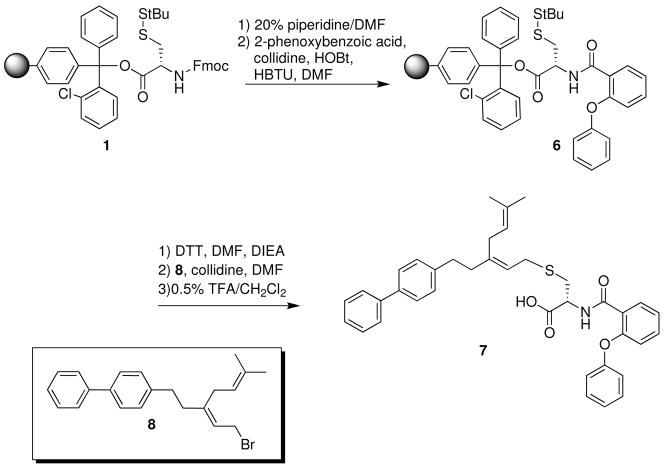
With a suitable lead compound (5e) identified, we next determined if this protocol could be used to generate prenylcysteine analogs modified on both the amide and the prenyl moieties. It was hypothesized that a cysteine compound containing both the 2-phenoxyphenyl N-substitution, and a farnesyl moiety known to lead to a modest inhibition of Icmt23 may be a more potent Icmt inhibitor. The synthesis of this “bidentate” potential inhibitor is shown in Scheme 2. This compound exhibited no substrate activity and inhibited Icmt with an IC50 of 2.5 μM, significantly more potent than the parent o-phenoxyphenyl analog 5e (by ~ 40%). This initial finding suggests that disubstituted cysteines can have increased affinity for Icmt, and thus supports the further investigation of “bidentate” prenylcysteine analog libraries.
SCHEME 2.
Solid-phase synthesis of N,S-disubstituted AMFC analog 7.
In summary, a solid-phase method for the synthesis of amide-modified farnesylcysteine analogs was developed using the 2-chlorotritylchloride resin as a solid support. Using this method, two libraries of AMFCs were synthesized and evaluated as inhibitors of Icmt. The products were obtained in 45–65% yield based on resin loading and in good purity (>90%). The potential to split the prenylcysteine mixture and introduce additional diversity in the penultimate step maximizes the efficiency of the process and greatly accelerates the synthesis of the library. This procedure may also be more broadly useful for the synthesis of other prenylcysteine and prenylpeptide derivatives.
Combining the data from the two solid-phase libraries and the previously described AMFC library,15 trends in activity begin to emerge. Flexible linkers between the amide group and the R-group of the AMFCs tend to lead to Icmt substrates; both compounds 5f and 5l are more efficient Icmt substrates than the prototypical N-acetylfarnesylcysteine. Conversely, AMFCs containing sterically bulky substituents such as quaternary centers adjacent to the amide group tend to lead to inhibitors of Icmt. The most potent AMFC inhibitor was the 2-phenoxyphenyl compound 5e. It exhibits a 3-fold increase in potency over the adamantyl and 2-biphenyl compounds. The 2-anilinophenyl compound 5h was also a potent analog indicating that a spacer between the two phenyl rings confers increased potency to the 2-biphenyl scaffold of Icmt inhibitors. With each of the three libraries synthesized, both the inhibitor hit rate and the potency of the inhibitors identified has increased, indicating the effectiveness of AMFCs as lead Icmt inhibitors. Note that the preparation of the third AMFC library led to four compounds with single digit micromolar IC50s, while the first two libraries contained none.
We have synthesized >50 farnesylcysteines with amide-modifications and identified a lead Icmt inhibitor with an IC50 of 4.3 μM (5e). It was hypothesized that replacing the farnesyl group with a synthetic farnesyl analog might further enhance the potency of 5e. An amide-modified and farnesyl-modified cysteine was synthesized to evaluate the effect of modifications at two positions on the cysteine. The chimeric AMFC contained the 2-phenoxyphenylcarbonyl group on the nitrogen and a previously identified para-biphenyl farnesyl mimic23 on the sulfur of the cysteine. This compound inhibited Icmt with an IC50 of 2.5 μM. This represents a nearly 40% increase in potency over 5e, indicating that disubstituted cysteines (modified on both the N and S) can be more effective inhibitors than cysteine derivatives substituted on either the N or S-substituted alone. In summary, we have developed a new solid-phase route to prenylcysteine analogs, and utilized it to generate an effective Icmt inhibitor.
Experimental Procedures
Solid Phase Synthesis of AMFC Analogs
General procedure for loading cysteine onto resin
2-Chlorotrityl chloride resin (103 mg, 0.114 mmol) was placed in a 25 mL fritted peptide vessel under argon. To this was added 3 mL of anhydrous CH2Cl2. In a separate vial, Fmoc-Cys(StBu)-OH (110 mg, 0.255 mmol) was dissolved in 4 mL anhydrous CH2Cl2 to which was added 2,4,6-collidine (37 μL, 0.281 mmol). After 5 min. the solvent was removed from the resin and the solution of Fmoc-Cys(StBu)-OH was transferred to the resin. The mixture was gently agitated for 3 hours on a peptide shaker under argon. 5 mL of a 1% solution of 2,4,6-collidine in MeOH was then added and the mixture was agitated for an additional 10 minutes. The mixture was drained and the resin was washed 3x each with DMF, CH2Cl2, and DMF. The resin was then dried under high vacuum overnight. By weight, an 80% loading efficiency was achieved.
General procedure for reduction of –StBu group
Dithiothreitol (100 mg, 0.65mmol) was dissolved in a 2% diisopropylethylamine/DMF solution, added to the resin bound cysteine and the reaction vessel was gently agitated overnight. The solvent was drained and the resin washed 3x each with DMF, CH2Cl2, and DMF. A small aliquot of resin was removed, cleaved, and subjected to reverse-phase HPLC analysis, which determined the reaction to be complete.
General procedure for farnesylation of resin-bound Fmoc-cysteine
2,4,6-collidine (95 μL, 0.72 mmol) was added to a solution of farnesyl chloride (0.36 mmol) in 4 mL of CH2Cl2. This solution was added to the resin-bound cysteine under argon, and the reaction vessel was gently agitated for 4 hours at ambient temperature. The solvent was then drained and the resin was washed as before. HPLC analysis following cleavage of a small portion of resin determined the coupling to be quantitative.
Cleavage of N-Fmoc group from cysteine
4 mL of a 20% solution of piperidine in DMF was added to the resin-bound farnesylcysteine and the vessel was agitated for 10 min. The solvent was drained, rinsed once with DMF and the process was repeated. The solvent was drained and the resin rinsed as before.
General procedure for coupling of carboxylic acids
1-adamantaneacetic acid, hydroxybenzotriazole (HOBt), and HBTU (0.36 mmol of each), were dissolved in 4 mL of DMF and 2,4,6-collidine (0.40 mmol) was then added. After 5 min. this solution was then added to the resin and agitated for 3 hours. The solvent was drained and the resin rinsed as before.
General procedure for cleavage of AMFC from resin
The resin-bound 4b was treated twice with a 0.5% solution of trifluoroacetic acid in CH2Cl2 for 3 minutes. The solution was drained into a round-bottom flask, and the resin was washed twice with anhydrous CH2Cl2. In selected cases, the crude mixture was analyzed by HPLC (C8 reversed phase analytical columm; 1.0 mL/min flow rate; 90%A/10%B to 100%B over 20 minutes (A: 0.025% trifluoroacetic acid/water; B: acetonitrile)); the crude AMFC was obtained in good purity (70–90%). The solvent was removed in vacuo and the crude material was loaded directly onto a silica column (mobile phase 0.5% MeOH, 0.5% AcOH, 99% CH2Cl2) and purified to give 28 mg of 4b, 65% (based on starting cysteine derivative loaded onto the resin). Alternatively, in certain cases, the AMFC was purified by C18 reversed-phase sep pak syringe cartridges (Fisher Scientific), using a gradient of 20:80 acetonitrile:water to 100% acetonitrile (with 0.5% (v/v) trifluoroacetic acid).
N-1-adamantylacetylcarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4a)
This compound was synthesized in 65% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-3-noradamantanecarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4b)
This compound was synthesized in 58% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-((2R,4S)-bicyclo[2.2.1]heptan-2-yl)acetyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4c)
This compound was synthesized in 55% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-4-pentylbicyclo[2.2.2]octane-1-carbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4d)
This compound was synthesized in 60% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-(1S)-7,7-dimethyl-2-oxobicyclo[2.2.1]heptane-1-carbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4e)
This compound was synthesized in 62% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-phenylbenzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4f)
This compound was synthesized in 48% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-1-napthylacetylcarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4g)
This compound was synthesized in 52% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy)acetyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4h)
This compound was synthesized in 65% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-octylcarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4i)
This compound was synthesized in 62% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-adamantylcarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (4j)
This compound was synthesized in 60% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-9-fluorenylcarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5a)
This compound was synthesized in 47% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-9,10-dihydro-9-oxoacridine-4-carbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5b)
This compound was synthesized in 48% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-phenyl-quinolinoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5c)
This compound was synthesized in 53% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-(4-phenoxyphenyl)-benzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5d)
This compound was synthesized in 55% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-(2-oxyphenyl)benzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5e)
This compound was synthesized in 62% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-((S)-4-phenyl-2-oxo-oxazolidin-3-yl)acetyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5f)
This compound was synthesized in 60% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-3-phenylbenzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5g)
This compound was synthesized in 58% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-(aminophenyl)benzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5h)
This compound was synthesized in 47% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-3,5-ditertbutylbenzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5i)
This compound was synthesized in 55% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-1-(4-chlorophenyl)cyclopentanecarbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5j)
This compound was synthesized in 46% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-3,3-diphenylpropanoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5k)
This compound was synthesized in 65% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-3-(phenylsulfonyl)propanoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5l)
This compound was synthesized in 60% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-(R)-6-hydroxy-2,5,7,8-tetramethylchroman-2-oyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5m)
This compound was synthesized in 47% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-4-phenylbenzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5n)
This compound was synthesized in 63% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-4-phenylazobenzoyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5o)
This compound was synthesized in 57% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-4-oxo-4H-chromene-2-carbonyl-L-cysteine[S-(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]-OH (5p)
This compound was synthesized in 51% yield following the general procedure for the solid-phase synthesis of AMFCs.
N-2-Phenoxybenzoyl-S-3-(3-methyl-but-2-enyl)-5-(4-biphenyl)pent-2-enyl-L-cysteine (7)
This compound was synthesized in 40% yield following the general procedure for the solid phase synthesis o AMFCs, with the exception that farnesyl chloride was replaced as the prenylating reagent with the previously prepared bromide 8.23 1H NMR (500 MHz, CDCl3) δ1.64 (s, 3 H), 1.69 (s, 3 H), 2.29 (app t, 2 H), 2.70 (app t, 2 H), 2.75 (d, 1 H, J~7Hz), 2.95 (dd, 1H, J~14Hz, 6.5Hz), 3.00 (dd, 2 H, J~14Hz, 5.2Hz), 3.17 (m (complex AB pattern), 2 H), 4.98 (m, 2 H), 5.16 (t, 1 H, J~7.5Hz), 6.86 (d, 1 H, J~8.5Hz), 7.10 (d, 2 H, J~8.0Hz), 7.19 (m, 2 H), 7.21 (d, 2 H, J~8.0Hz), 7.33 (t, 1 H, J~7.0Hz), 7.37 (app t, 3 H), 7.43 (t, 2 H, J~8.0Hz), 7.51 (d, 2 H, J~8.0Hz), 7.58 (d, 2 H, J~7.0Hz), 8.15 (bs, 1 H), 8.27 (d, 1 H, J~7.0Hz), 8.75 (d, 1 H, J~7.0Hz) 13C NMR (125 MHz, CDCl3) δ17.9, 25.7, 29.2, 29.7, 32.6, 34.1, 38.6, 52.8, 117.9, 119.8, 120.1, 121.7, 122.4, 123.4, 124.8, 126.9, 127.0, 128.7, 128.7, 130.0, 132.6, 133.3, 138.7, 141.0, 141.1, 142.9, 155.1, 156.0, 165.4, 174.3 HRMS for C38H39NO4SNa calc. 628.2497, found 628.2503.
Determination of IC50 Values
The compounds were assayed for Icmt inhibition as previously described.23 Briefly, in a 60 μL reaction, yeast membranes expressing hIcmt (5 μg) were incubated in 100 mM Tris-HCl in the presence of 25 μM AFC, 20 μM 14C-SAM and various concentrations of AMFC analog dissolved in DMSO. The assay mixtures were mixed gently and incubated for 30 min at 30°C. Reactions were terminated with the addition of 50 μL 1 M NaOH/1% SDS solution (50 μL). The reaction mixture (100 μL) was spotted onto a folded piece of filter paper. The filter paper was lodged in the neck of a scintillation vial containing 10 mL of scintillation fluid. The vials were capped and allowed to sit for 2.5 h to allow diffusion of the [14C]methanol into the scintillation fluid. After 2.5 h, the filter paper was removed, the vials were shaken, and then counted in a scintillation counter. Icmt activity (% control) was fitted versus AMFC concentration to determine the reported IC50 values.
Supplementary Material
Acknowledgments
This work was supported in part by the US Army CDMRP (NF020054; subcontract to RAG), by a grant from the National Pancreas Foundation to CAH, and by the NIH (RO1CA112483 (RAG) and P30CA21368 (Purdue Cancer Center Support Grant)).
Footnotes
Supporting Information Available: Synthetic experimental details and spectroscopic characterization of 4a-j, 5a–p, and 7. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.McTaggert SJ. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter-Vann AM, Casey PJ. Nat Rev Cancer. 2005;5:405–411. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- 3.Reid TS, Terry KL, Casey PJ, Beese LS. J Mol Biol. 2004;343:417–423. doi: 10.1016/j.jmb.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Basso AD, Kirshmeyer PT, Bishop WR. J Lipid Res. 2006;46:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Krzysiak AJ, Rawat DS, Scott SA, Pais JE, Handley M, Harrison ML, Fierke CA, Gibbs RA. ACS Chem Biol. 2007;2:385–389. doi: 10.1021/cb700062b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloog Y, Cox AD. Semin Cancer Biol. 2004;14:253–261. doi: 10.1016/j.semcancer.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Watzke A, Brunsveld L, Durek T, Alexandrov K, Rak A, Goody RS, Waldmann H. Org Biol Chem. 2005;3:1157–1164. doi: 10.1039/b417573e. [DOI] [PubMed] [Google Scholar]
- 8.Bergo MO, Gavino BJ, Hong C, Beigneux AP, McMahon M, Casey PJ, Young SG. J Clin Invest. 2004;113:539–550. doi: 10.1172/JCI18829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter-Vann AM, Baron RA, Wong W, de la Cruz J, York JD, Gooden DM, Bergo MO, Young SG, Toone EJ, Casey PJ. Proc Natl Acad Sci USA. 2005;102:4336–4341. doi: 10.1073/pnas.0408107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naider FR, Becker JM. Biopoly. 1997;43:3–14. doi: 10.1002/(SICI)1097-0282(1997)43:1<3::AID-BIP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Xie H, Shao Y, Becker JM, Naider F, Gibbs RA. J Org Chem. 2000;65:8552–8563. doi: 10.1021/jo000942m. [DOI] [PubMed] [Google Scholar]
- 12.Dolence EK, Dolence JM, Poulter CD. J Comb Chem. 2000;2:522–536. doi: 10.1021/cc000026m. [DOI] [PubMed] [Google Scholar]
- 13.Lumbierres M, Palomo JM, Kragol G, Waldmann H. Tetrahedron Lett. 2006;47:2671–2674. [Google Scholar]
- 14.Palomo JM, Lumbrierres M, Waldmann H. Angew Chem Int Ed. 2006;45:477–481. doi: 10.1002/anie.200503298. [DOI] [PubMed] [Google Scholar]
- 15.Donelson JL, Hodges HB, MacDougall DD, Henriksen BS, Hrycyna CA, Gibbs RA. Bioorg Med Chem Lett. 2006;16:4420–4423. doi: 10.1016/j.bmcl.2006.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludolph B, Eisele F, Waldmann H. J Am Chem Soc. 2002;124:5954–5955. doi: 10.1021/ja025768t. [DOI] [PubMed] [Google Scholar]
- 17.Sohma Y, Hayashi Y, Kimura M, Chiyomori Y, Taniguchi A, Sasaki M, Kimura T, Kiso Y. J Pep Sci. 2005;11:441–451. doi: 10.1002/psc.649. [DOI] [PubMed] [Google Scholar]
- 18.Kitagawa K, Aida C, Fujiwara H, Yagami T, Futaki S. Tetrahedron Lett. 1997;38:599–602. [Google Scholar]
- 19.Ludolph B, Waldmann H. Chem Eur J. 2003;9:3683–3691. doi: 10.1002/chem.200304822.Other workers have had difficulties with the tributylphosphine/water protocol for cysteine disulfide deprotection: Rijkers DTS, John AW, Kruijtzer JAW, Killian JA, Liskamp RMJ. Tetrahedron Lett. 2005;46:3341–3345.
- 20.Barlos K, Chatzi O, Gatos D, Stavroppoulos G. Int J Pep Prot Res. 1991;37:513–520. [PubMed] [Google Scholar]
- 21.Bollhagen R, Schmiedberger M, Barlos K, Grell E. J Chem Soc, Chem Comm. 1994;22:2559–2560. [Google Scholar]
- 22.Dourtoglou V, Bernard G, Lambropoulou V, Zioudrou C. Synth. 1984;7:572–574. [Google Scholar]
- 23.Anderson JA, Henriksen BS, Gibbs RA, Hrycyna CA. J Biol Chem. 2005;280:29454–29461. doi: 10.1074/jbc.M504982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whyte D, McGuirk M, Nunez-Oliva I, Hockenberry T, Pai J. Ras Converting Endoprotease (RCE) and Methods. 2001 U.S. Patent No. 6,261,793. [Google Scholar]
- 25.a) Using data from our previously published set of AMFCs,15 we have compared IC50 values for seven analogs with their calculated LogP values, and found no significant correlation (R2 = 0.18). b) We have not tested these compounds versus FTase; however, note that the prenylcysteine peptide product of this enzyme binds very poorly to it: Cassidy PB, Poulter CD. J Am Chem Soc. 1996;118:8761–8762.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.