Abstract
The objectives of this study were to characterize the active and passive contributions to joint kinetics during walking in healthy young and older adults, and assess whether isokinetic ankle strength is associated with ankle power output during walking. Twenty healthy young (18–35 years) and 20 healthy older (65–85 years) adults participated in this study. We measured subject-specific passive-elastic joint moment-angle relationships in the lower extremity and tested maximum isokinetic ankle strength at 30deg/sec. Passive moment-angle relationships were used to estimate active and passive joint moment, power, and work quantities during walking at 80, 100, and 120% of preferred walking speed. There were no significant differences in walking speed, step length, or cadence between the older and young adults. However, the older adults produced significantly more net positive work at the hip but less net positive work at the ankle at all walking speeds. Passive contributions to hip and ankle work did not significantly differ between groups, inferring that the older adults generated the additional hip work actively. Maximum isokinetic ankle strength was significantly less in the older adults, and correlated with peak positive plantar-flexor power at both the preferred and fast walking speeds. The results of this study suggest that age-related shifts in joint kinetics do not arise as a result of increased passive hip joint stiffness, but seem to be reflected in plantar-flexor weakness.
Keywords: gait, aging, lower extremity, joint powers, passive moments
INTRODUCTION
Aging is associated with a general decline in walking speed and step length (Winter et al., 1990; Judge et al., 1996; Kerrigan et al., 1998). Even after accounting for these spatio-temporal changes, older adults demonstrate an increased reliance on proximal, rather than distal, muscles for power generation (Winter et al., 1990; Judge et al., 1996; Kerrigan et al., 1998; DeVita and Hortobagyi, 2000). In particular, older adults exhibit reduced ankle plantar-flexor power (Winter et al., 1990; Judge et al., 1996; Kerrigan et al., 1998; DeVita and Hortobagyi, 2000) with corresponding increases in either hip extensor or hip flexor power (Judge et al., 1996; Kerrigan et al., 1998; DeVita and Hortobagyi, 2000; McGibbon, 2003), when compared to young adults. An understanding of the biomechanical factors that underlie this increased reliance on proximal power production is important to help distinguish impairments from normal aging, and thus develop intervention programs aimed to maintain gait and functional independence with age.
Declines in muscle strength could induce changes in lower extremity joint kinetics. Reductions in skeletal muscle strength of 20–40% by age 70 have been well documented (Larsson et al., 1979; Murray et al., 1985; Winegard et al., 1996). It is thus possible that older adults may be unable to produce adequate ankle power during push-off, particularly at faster walking speeds (Judge et al., 1996; Kerrigan et al., 1998). The power generation requirements about the hip tend to be less than at the ankle, relative to maximal capacity. Thus, hip musculature may have the capacity to compensate for distal weakness by increasing power output. Alternatively, age-related increases in joint stiffness may alter joint kinetics. Increased hip joint stiffness could facilitate the development of additional flexor power passively, rather than actively, and thus contribute to the increased hip flexor power commonly observed in the gait of older adults (McGibbon, 2003). Furthermore, an increase in hip flexor stiffness, due to static contractures, could limit hip extension during late stance, potentially limiting plantar-flexor push-off (Winter et al., 1990; Judge et al., 1996; Kerrigan et al., 2001; Riley et al., 2001). In this case, reduced ankle plantar-flexor power would be secondary to increased hip flexor stiffness. We recently introduced a technique for measuring and modeling passive-elastic properties about the joints of the lower extremity (Silder et al., 2007). Applying these models to gait demonstrated that the passive hip flexor moment allowed for substantial energy storage during terminal stance and subsequent release during pre-and initial swing in healthy young adults (Whittington et al., 2007).
The objective of the present study was to characterize the contribution of passive and active mechanisms to joint kinetics during walking in healthy young and healthy older adults. We hypothesized that older adults would exhibit an increased reliance on passive stiffness to generate hip flexor power and demonstrate decreased active ankle power generation during walking. We also investigated the relationship between maximum isokinetic ankle strength and plantar-flexor power during walking to evaluate whether strength could be a limiting factor in ankle power generation during walking. Finally, we performed a comparison of kinematic measures between age groups to enable a more comprehensive evaluation of age-associated changes during gait.
METHODS
Subjects
Twenty healthy young adults aged 18–35 and 20 healthy older adults aged 65–85 participated in this study (Table 1). Exclusion criteria were self-reported via an initial phone screening and included current or history of orthopedic diagnosis, joint pain, or known cardiac, neurologic, or gait or balance impairment. Older adults were further screened by a geriatrician and excluded from the study if they obtained a score less than 24 in a cognitive test (the Mini Mental State Exam, MMSE) or were unable to perceive a 5.07 Semmes-Weinstein (10-g) monofilament in a plantar sensation test. Prior to gait analysis, each older adult completed a self-reported physical activity questionnaire (Community Healthy Activities Model Program for Seniors, CHAMPS, (Stewart et al., 2001)). Each subject gave informed consent according to a protocol approved by the University of Wisconsin’s Health Sciences Institutional Review Board.
Table 1.
Mean (SD) demographics of the young and older adults included in this study.
| Young Adults (n=20) | Older Adults (n=20) | |||
|---|---|---|---|---|
| 9 men | 11 women | 7 men | 13 women | |
| Age (years) | 26 (4) | 26 (3) | 73 (4) | 72 (6) |
| Body Mass (kg) | 79 (8) | 59 (13) | 74 (8) | 67 (11) |
| Height (m) | 1.82 (0.10) | 1.66 (0.10) | 1.75 (0.07) | 1.65 (0.09) |
Measurement and Analysis
The dominant limb, defined as the limb the subject would select to kick a ball, was used to measure passive joint stiffness and isokinetic ankle strength. All kinematic data were collected at 100Hz using an 8-camera passive motion capture system (Motion Analysis Corporation, Santa Rosa, CA) and processed using motion capture software (EVaRT v5.0). Analog signals were recorded at 2000Hz using a 12 bit A/D converter interfaced with the collection computer.
Gait analysis
Each subject performed five over-ground walking trials each at 80, 100, and 120% of preferred walking speed across a 10m walkway. Whole body motion was tracked using 23 anatomical markers placed on identifiable landmarks and 19 additional markers to aid in tracking and minimize skin motion artifact (Cappozzo et al., 1995). Ground reaction forces were synchronously recorded using three imbedded forceplates (Model BP400600, MiniAmp Amplifier, AMTI, Watertown, MA).
Passive joint stiffness
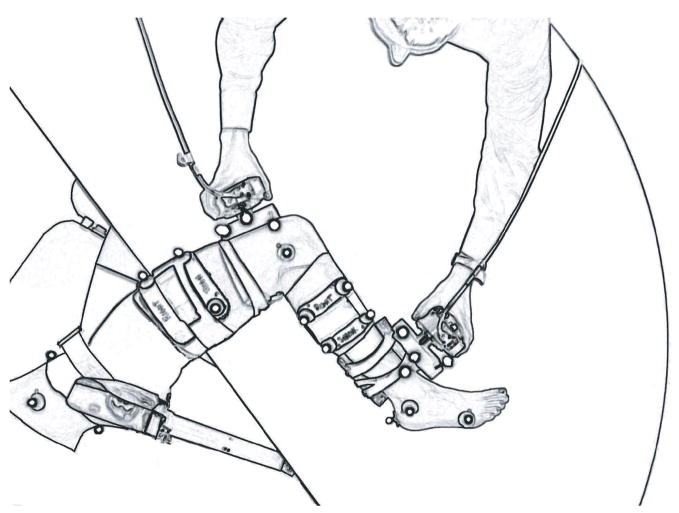
Passive hip, knee, and ankle joint stiffness was measured for each subject. Individuals were positioned side-lying with their dominant limb supported on a table via low-friction carts placed under the medial side of the thigh and leg (Fig 1). The pelvis was secured to a padded brace to restrict movement during testing. Lower extremity motion was tracked using a subset of the markers used in gait analysis, consisting of the markers on the pelvis and dominant lower extremity. A physical therapist slowly manipulated the limb using two hand-held three-dimensional load cells (model 30E15A4, range 200lbs; JR3 Inc., Woodland, CA). Fifteen unique trials were performed which decoupled the contributions of the uni-articular tissues and bi-articular muscles of the hip, knee, and ankle (Silder et al., 2007). Three-dimensional kinematics of the lower extremity and load cells were recorded simultaneously with load cell forces and moments
Fig 1.
Passive joint stiffness was measured with the subject positioned side-lying and their dominant limb supported on a table via low-friction carts (top-view). The hip, knee, and ankle were slowly manipulated through full ranges of motion to elicit the passive stretch of both uni- and bi-articular tissues. Reprinted with permission from Silder et al. (2007).
Electromyographic (EMG) signals were recorded from seven muscles of the dominant lower extremity during the passive testing (vastus lateralis, rectus femoris, medial hamstrings, biceps femoris, tibialis anterior, medial gastrocnemius, and soleus). Pre-amplified single differential surface electrodes (DE-2.1, DelSys, Inc, Boston, MA), with a fixed 10mm inter-electrode distance, were interfaced with a Bagnoli-16 amplifier/processor unit (CMRR>84 dB at 60Hz; input impedance>100MΩ) and coated with conducting gel prior to application. Electrode locations were prepared by shaving the skin and cleaning with alcohol. EMG signals were visually monitored to ensure that the muscles were indeed inactive. Any trials with visual muscle activity were repeated.
Ankle strength testing
Each subject performed concentric isokinetic ankle strength tests (30deg/sec) at knee angles of 0, 30, and 60 degrees of flexion. The dominant foot was secured to a dynamometer (Biodex Multi-Joint System 2, Biodex Medical Systems, Inc.), with the talocrural joint aligned with the dynamometer axis. The seat was adjusted so that the tibia was horizontal to the floor; the knee angle was measured with a goniometer. Leg motion other than ankle rotation was restricted using straps over the foot and tibia. Subjects were asked to push through their available range of ankle motion and verbally encouraged to perform at maximum effort. Subjects performed two sets of three repetitions at each knee angle. The testing order of the knee angles was randomized across subjects. Voltages corresponding to footplate angular position and ankle torque were continuously recorded. Angular position and torque data were digitally low-pass filtered at 5Hz. Maximum ankle strength for each subject was defined as the peak plantar-flexor torque that the subject was able to generate across any of the tests.
Biomechanical model
A scaled, 18 degree of freedom (dof), seven segment, model was used to represent the pelvis and lower extremity for each subject (Delp et al., 1990). The pelvis was the base segment with six dof; the hip was represented as a spherical joint with three dof; the knee was represented as a one dof joint in which non-sagittal rotations and tibiofemoral and patellofemoral translations were computed as a function of the sagittal knee angle (Walker et al., 1988); the ankle and subtalar joints were represented as pin joints aligned with the anatomical axes (Delp et al., 1990). An upright calibration trial was used to define body segment coordinate systems, tracking marker locations, and segment lengths for each individual. A functional hip joint center identification algrorithm (Piazza et al., 2004) was implemented using pelvis and thigh kinematics from trials in which the subject circumducted his/her right and left limbs.
A low-pass Butterworth filter was used to filter kinematic (4th order, gait at 6Hz and passive at 2Hz) and forceplate (4th order, 40Hz) data. A global optimization inverse kinematics routine was used to compute pelvis position, pelvis orientation, and lower extremity joint angles at each time step in the trials (Lu and O’Connor, 1999). Body segment kinematics, anthropometric data (de Leva, 1996), and load cell forces (passive testing) or forceplate measures (gait analysis) were used in conjunction with inverse dynamics analysis to subsequently compute lower extremity joint moments. SIMM Pipeline (Musculographics Inc., Motion Analysis Corp, Santa Rosa, CA) was used with SD/FAST (Parametric Technology Corporation, Waltham, MA) to perform the inverse kinematics and inverse dynamics analyses.
A passive stiffness model, consisting of eight exponential functions, was used to describe the relationships between passive hip, knee, and ankle moments and corresponding joint angles measured in the passive testing. Uni-articular tissues were described by two parameters, an offset angle and a stiffness parameter. Bi-articular functions included a third parameter to represent the ratio of moment-arms between neighboring joints. A least squares approach was used to estimate the model parameters using subject-specific measurements. Additional details about the model and parameter estimation procedure are described in Silder et al. (2007).
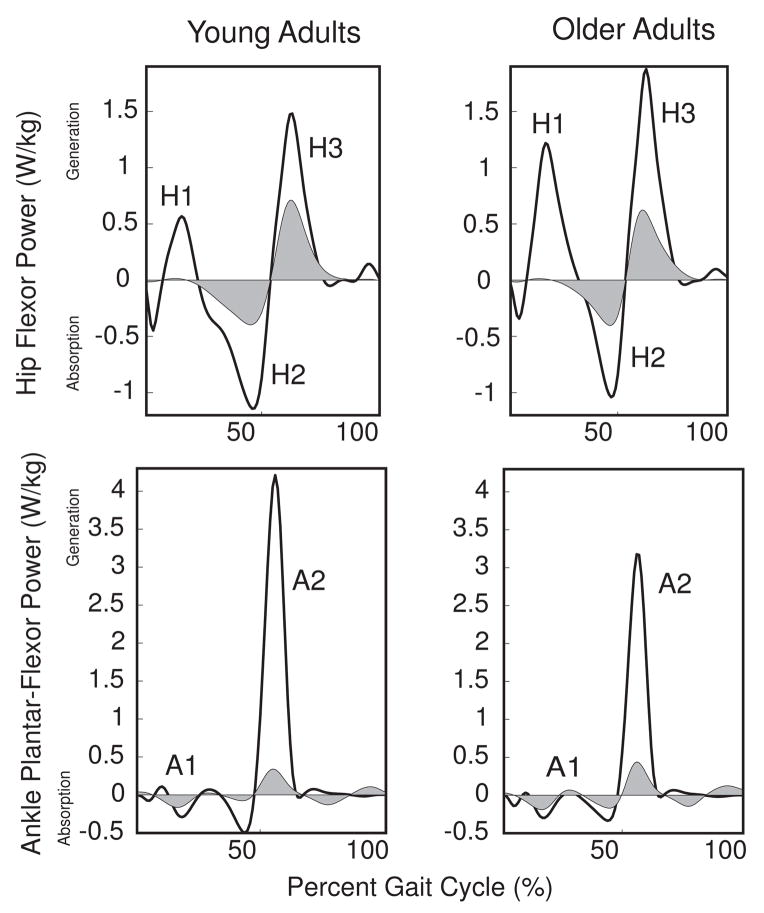
Hip, knee, and ankle joint angles during gait were used as inputs into the passive stiffness model to estimate sagittal plane passive joint moments during walking. Passive moments were multiplied by corresponding joint angular velocities during walking to compute passive joint powers. Sagittal plane hip and ankle powers were defined using terminology adopted from Winter (1991) (Fig 3). Power absorption occurs via the ankle dorsi-flexors (A1) and hip flexors (H2), while power generation occurs via the ankle plantar-flexors (A2), hip extensors (H1) and hip flexors (H3). Power curves were integrated over specific power bursts to estimate the net work, and the work attributable to active and passive mechanisms. Joint moment, power, and work quantities were subsequently normalized to body mass.
Fig 3.
Net and passive group ensemble averaged power curves for the hip and ankle at fast walking speed. Shaded regions represent passive contributions to net joint power. Both young and older adults generated a substantial portion of the H2 and H3 powers from passive-elastic mechanisms. Positive power refers to energy generation while negative power refers to energy absorption.
Statistical analyses
Demographic and spatio-temporal gait variables were compared between age groups using unpaired t-tests. Kinematic and kinetic measures were compared using 2-factor (group by walking speed) repeated measures ANOVAs. Significant interactions and main effects were further analyzed using Tukey’s HSD. The relationship between ankle strength and ankle power during walking was assessed using Pearson’s Correlation. Statistical significance was defined at p<0.05.
RESULTS
Twenty older adults and 20 young adults completed the entire testing procedure (Table 1). However, nine of the older adults exhibited reflex activity in the tibialis anterior during trials in which the ankle was passively dorsi-flexed. The ankle torque data for these subjects were therefore not included in our analysis of passive contributions to joint kinetics. The older adults in this study scored 11772±220 on the CHAMPS questionnaire (Stewart et al., 2001).
Spatio-temporal Measures
There was no significant difference in preferred walking speed, and hence slow or fast walking speeds, between groups (Table 2). Young and older adults walked with similar step length, cadence, and double limb support at all speeds.
Table 2.
Mean (SD) spatio-temporal measures of gait for the young and older adults at the three walking speeds tested.
| Young Adults | Older Adults | |||||
|---|---|---|---|---|---|---|
| Slow | Preferred | Fast | Slow | Preferred | Fast | |
| Walking Velocity (m/s) | 1.06 (0.10) | 1.33 (0.13) | 1.59 (0.14) | 1.06 (0.10) | 1.32 (0.13) | 1.58 (0.16) |
| Step Length (normalized to height) | 0.75 (0.06) | 0.83 (0.07) | 0.90 (0.07) | 0.75 (0.06) | 0.82 (0.06) | 0.90 (0.08) |
| Cadence (steps/min) | 99 (10) | 112 (10) | 122 (10) | 100 (6) | 115 (7) | 125 (8) |
| Double Limb Support (%) | 30 (3) | 28 (3) | 26 (4) | 31 (4) | 28 (3) | 26 (3) |
Kinematics
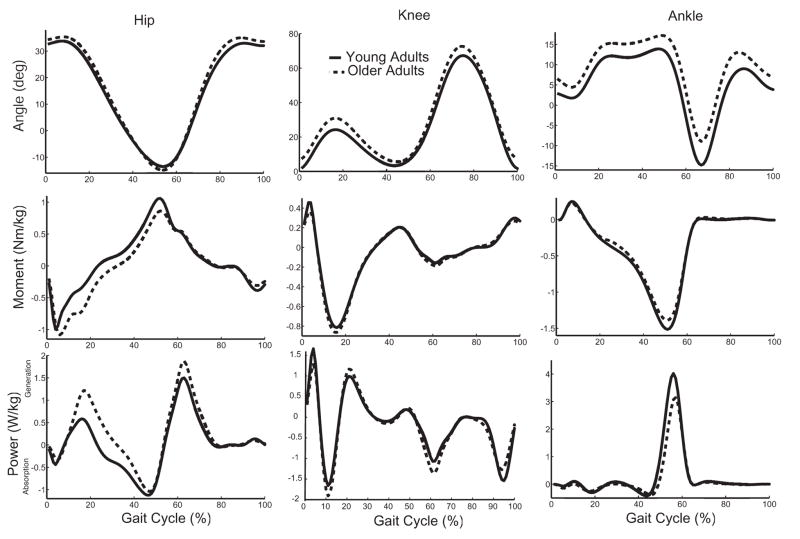
Both groups exhibited significantly increased peak hip flexion, peak hip extension, hip excursion, and peak ankle plantar-flexion as walking speed increased (Table 3). Older adults exhibited significantly greater hip excursion, greater peak ankle dorsi-flexion, and reduced peak ankle plantar-flexion at all walking speeds when compared to the young adults.
Table 3.
Mean (SD) peak joint angles for the young and older adults at each of the walking speeds tested. Significant group differences are indicated.
| Slow | Preferred | Fast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Young | Old | p-value | Young | Old | p-value | Young | Old | p-value | |
| Hip Flexion | 32 (5) | 34 (5) | 33 (5) | 35 (5) | 35 (5) | 37 (6) | |||
| Hip Extension | −10 (5) | −12 (5) | −13 (5) | −14 (5) | −14 (5) | −15 (5) | |||
| Hip Excursion | 43 (4) | 45 (4) | <0.05 | 46 (5) | 49 (5) | <0.05 | 49 (4) | 53 (5) | <0.05 |
| Dorsi-Flexion | 16 (4) | 21 (5) | <0.01 | 16 (4) | 20 (5) | <0.01 | 16 (4) | 20 (5) | <0.01 |
| Plantar-Flexion | −14 (5) | −7 (8) | <0.01 | −16 (6) | −8 (8) | <0.01 | −16 (6) | −9 (8) | <0.01 |
| Ankle Excursion | 31 (5) | 28 (5) | 31 (6) | 28 (5) | 32 (5) | 29 (5) | |||
Peak Joint Powers and Net Work
A significant group-by-speed effect for peak A2, H1, and H3 powers was observed (Table 4). Young and older adults increased peak H1 power by 78% and 97% between slow and fast speeds, respectively, and peak H3 power by 95% and 111%, respectively. The increase in peak A2 power between slow and fast speeds was less for the older adults (54%) compared with the young adults (75%). Older adults exhibited greater peak H1 power at all three speeds. Net work quantities showed even larger age-related changes, with older adults generating significantly less negative H2 work and more positive H1 and H3 work at all speeds.
Table 4.
Mean (SD) peak powers, net work, active work, and passive work normalized to body mass for young and older adults across all three speeds. Passive joint work was estimated using subject-specific passive stiffness models in conjunction with gait measures. Active work was calculated as net work minus passive work. Significant group differences are indicated.
| Slow | Preferred | Fast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Young | Old | p-value | Young | Old | p-value | Young | Old | p-value | |
|
Joint Power (Watts/kg) | |||||||||
| A1 | −0.64 (0.22) | −0.68 (0.17) | −0.74 (0.29) | −0.87 (0.32) | −0.80 (0.38) | −0.84 (0.35) | |||
| A2 | 2.56 (0.61) | 2.34 (0.59) | 3.49 (0.92) | 3.09 (0.72) | 4.48 (1.06) | 3.61 (0.86) | <0.01 | ||
| H1 | 0.37 (0.16) | 0.68 (0.22) | <0.01 | 0.54 (0.22) | 1.01 (0.40) | <0.01 | 0.66 (0.27) | 1.34 (0.43) | <0.01 |
| H2 | −0.57 (0.21) | −0.45 (0.11) | −0.86 (0.36) | −0.72 (0.19) | −1.26 (0.44) | −1.15 (0.35) | |||
| H3 | 0.86 (0.34) | 0.96 (0.32) | 1.19 (0.41) | 1.46 (0.42) | 1.68 (0.55) | 2.03 (0.54) | |||
|
| |||||||||
|
Net Joint Work (J/kg) | |||||||||
| A1 | −0.09 (0.03) | −0.12 (0.03) | −0.07 (0.03) | −0.10 (0.03) | −0.06 (0.04) | −0.06 (0.03) | |||
| A2 | 0.24 (0.06) | 0.20 (0.05) | 0.29 (0.07) | 0.24 (0.06) | 0.34 (0.08) | 0.27 (0.07) | <0.01 | ||
| H1 | 0.04 (0.03) | 0.11 (0.04) | <0.01 | 0.05 (0.02) | 0.14 (0.06) | <0.01 | 0.06 (0.03) | 0.16 (0.06) | <0.01 |
| H2 | −0.13 (0.06) | −0.07 (0.02) | <0.01 | −0.15 (0.07) | −0.09 (0.03) | <0.01 | −0.19 (0.06) | −0.13 (0.04) | <0.01 |
| H3 | 0.09 (0.03) | 0.13 (0.04) | <0.01 | 0.12 (0.04) | 0.17 (0.04) | <0.01 | 0.16 (0.04) | 0.22 (0.06) | <0.01 |
|
| |||||||||
|
Active Joint Work (J/kg) | |||||||||
| A1 (old n=11) | −0.08 (0.03) | −0.09 (0.03) | −0.06 (0.03) | −0.08 (0.03) | <0.05 | −0.05 (0.03) | −0.05 (0.04) | ||
| A2 (old n=11) | 0.19 (0.06) | 0.16 (0.05) | <0.05 | 0.25 (0.07) | 0.21 (0.06) | <0.05 | 0.28 (0.09) | 0.23 (0.07) | <0.01 |
| H1 | 0.05 (0.03) | 0.12 (0.05) | <0.01 | 0.05 (0.03) | 0.15 (0.06) | <0.01 | 0.06 (0.03) | 0.16 (0.07) | <0.01 |
| H2 | −0.06 (0.05) | −0.03 (0.02) | <0.01 | −0.07 (0.05) | −0.05 (0.03) | <0.01 | −0.10 (0.06) | −0.07 (0.04) | <0.01 |
| H3 | 0.05 (0.03) | 0.06 (0.03) | 0.07 (0.04) | 0.09 (0.04) | <0.05 | 0.11 (0.05) | 0.13 (0.05) | <0.05 | |
|
| |||||||||
|
Passive Joint Work (J/kg) | |||||||||
| A1 (old n=11) | −0.02 (0.01) | −0.03 (0.02) | −0.02 (0.01) | −0.03 (0.02) | −0.02 (0.01) | −0.01 (0.01) | |||
| A2 (old n=11) | 0.03 (0.02) | 0.04 (0.02) | 0.03 (0.02) | 0.04 (0.02) | 0.03 (0.02) | 0.04 (0.01) | |||
| H1 | 0.00 (0.00) | −0.01 (0.01) | 0.00 (0.00) | −0.01 (0.01) | 0.00 (0.00) | −0.01 (0.01) | |||
| H2 | −0.04 (0.03) | −0.03 (0.02) | −0.05 (0.03) | −0.05 (0.02) | −0.06 (0.03) | −0.06 (0.03) | |||
| H3 | 0.06 (0.03) | 0.06 (0.02) | 0.07 (0.04) | 0.08 (0.03) | 0.08 (0.04) | 0.09 (0.03) | |||
Passive and Active Contributions
Both young and older adults utilized passive-elastic mechanisms to generate a substantial portion of the H2 and H3 power bursts (Fig 3). However, the absolute magnitudes of the passive contributions to hip and ankle joint work quantities did not differ significantly between the groups (Table 4). Because net work was significantly different between groups, active contributions to joint work quantities were also significantly different between groups.
Ankle Strength
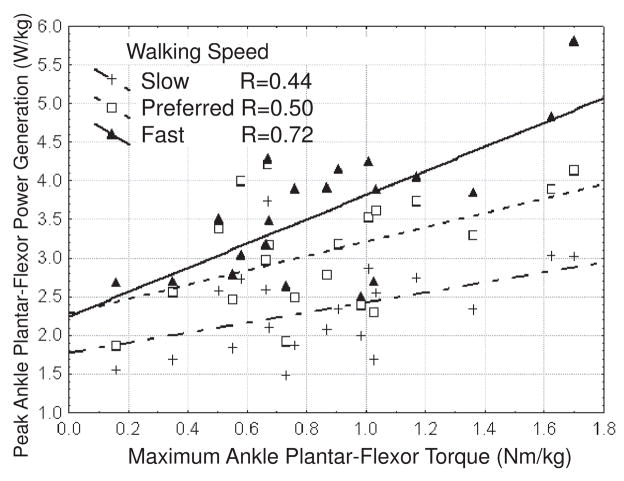
The older adults produced significantly less isokinetic ankle plantar-flexor torque than the young adults (young: 1.39±0.32Nm/kg; old: 0.87±0.39Nm/kg). Isokinetic strength was significantly correlated with peak A2 power at both the preferred and fast walking speed for the older adults (preferred speed: r=0.50, p<0.05; fast speed: r=0.71, p<0.01), but not for the young adults (Fig 4).
Fig 4.
Maximum isokinetic ankle plantar-flexor torque was significantly correlated to peak ankle plantar-flexor power at preferred (r=0.50, p<0.05) and fast (r=0.72, p<0.01) walking speeds for the older adults, as shown here, but not the young adults (not shown).
DISCUSSION
The objectives of this study were to characterize the contribution of passive and active mechanisms to joint kinetics during walking in healthy young and healthy older adults, and to investigate the relationship between isokinetic ankle strength and plantar-flexor power output during walking. Unlike other studies (Winter et al., 1990; Judge et al., 1996; Kerrigan et al., 1998; Zijlstra, 2004), we did not observe any age-group differences in preferred gait speed. This may, in part, be attributable to the high activity level of the participating subjects as compared to typical active older adults (CHAMPS score, 3386±219 (Stewart et al., 2001)). Nevertheless, the older adults generated less positive work about the ankle and more positive work about the hip when compared to the young adults, supporting the idea that a distal to proximal shift in joint kinetics during walking occurs with normal aging (DeVita and Hortobagyi, 2000; McGibbon, 2003).
Passive-elastic mechanisms provide for energy storage and release about the hip from terminal stance through initial swing, thereby reducing the need for active power generation during the H3 power burst (Whittington et al., 2007). This mechanism involves stretch-shortening of the uni-articular hip flexors and the bi-articular rectus femoris, which transfers energy proximally from the knee to the hip (Whittington et al., 2007). Contrary to our hypothesis, the older adults were no more reliant on passive structures to generate the H2 and H3 power bursts than young adults (Table 4). Thus, we conclude that the additional net work done by older adults during the positive hip flexor power burst was likely produced actively rather than passively. Similar to previous findings (Whittington et al., 2007), the passive contributions of the uni-articular hip extensors and hamstrings were minimal during the H1 power burst for both groups (Fig 3, Table 4), suggesting that the additional hip extensor power in the older adults was also produced actively. Additionally, the older adults did significantly less negative hip flexor (H2) work, which was most likely attributable to a slightly prolonged H1 power burst (Fig 1). It is important to recognize that the older adults in this study were very active individuals and therefore were less likely to exhibit hip flexion contractures than impaired older adults (McGibbon, 2003). Further study is needed to determine if impaired and/or more sedentary older adults exhibit an increased reliance on passive mechanisms to generate hip power.
Ankle weakness may have played a role in the age-related reduction in plantar-flexor power observed at fast walking speed with the young adults increasing peak ankle plantar-flexor power by 75% between slow and fast walking speeds and the older adults producing only 54% more power. Additionally, isokinetic ankle plantar-flexor strength proved to be a good predictor of ankle power during walking in older adults, particularly as walking speed increased. This provides further support for the idea that limitations at the ankle may be a primary cause of age-related gait changes in otherwise healthy older adults (Judge et al., 1996). Isokinetic ankle strength testing was performed at a slower angular velocity (30deg/sec) than is observed during the stance phase of walking (~60deg/sec, (Winter 1991)), which precludes a direct quantitative comparison of the ankle power generated in the two conditions. However, isokinetic strength measured at different speeds have been shown to be highly correlated for individual subjects (Abernethy and Jurimae, 1996), such that low-speed strength may serve as a surrogate measure for higher-speed power capacity.
Nine of the older adults exhibited reflex activity in the tibialis anterior during passive dorsi-flexion of the ankle, which could not be voluntarily suppressed. This precluded the use of these subjects’ passive ankle data in the present study. Further research is needed to ascertain what triggers this reflex activity and whether it may play a functional role in generating co-contraction activity in the tibialis anterior during gait, thereby decreasing net ankle power production. Visual inspection of EMG activities in the remaining muscles indicated that these muscles were indeed relaxed during the passive testing. However, the relaxed state of deeper muscles (e.g. iliopsoas) could not be verified using surface electromyography (McGill et al., 1996). Other superficial muscles (e.g. lateral gastrocnemius, vastus lateralis) were not directly monitored but were assumed to be relaxed.
We only analyzed passive contributions to sagittal plane joint powers, and therefore cannot conclude what age-related changes or contributions exist, if any, to joint powers in the frontal or transverse planes. Furthermore, our methodology (Silder et al., 2007) inherently assumes that passive properties are additive with the active components during walking. This is likely a reasonable assumption for fully passive structures such as the joint capsule, ligaments, and skin. However, the interaction of passive and active components in muscle remains an area of active research (Rassier et al., 2005), making it more challenging to quantify precisely how passive components are utilized during movement. Nevertheless, similar assumptions were applied to the data of both groups.
The results of this study suggest that normal age-related changes in joint kinetics during walking likely do not arise as a result of increased passive hip joint stiffness. Alternatively, reduced plantar-flexor strength was correlated with a decrease in plantar-flexor power during walking, and both were observed in conjunction with increased positive hip power generation. Thus, ankle joint function is important to consider when using gait analysis to help distinguish older adults at risk for disablement.
Fig 2.
Group ensemble averaged angles, moments, and powers for the sagittal plane hip, knee, and ankle at a fast walking speed for both young and older adults.
Acknowledgments
We gratefully acknowledge Ben Whittington, MS, for his assistance with data collection and analysis. We also acknowledge Jane Mahoney, MD, for her help in subject screening and enrollment. This research was supported by the National Institutes of Health (grant no. AG24276) and a National Science Foundation pre-doctoral fellowship (AS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abernethy PJ, Jurimae J. Cross-sectional and longitudinal uses of isoinertial, isometric, and isokinetic dynamometry. Medicine and Science in Sports and Exercise. 1996;28(9):1180–1187. doi: 10.1097/00005768-199609000-00015. [DOI] [PubMed] [Google Scholar]
- Cappozzo A, Catani F, Croce UD, Leardini A. Position and orientation in space of bones during movement: anatomical frame definition and determination. Clinical Biomechanics (Bristol, Avon) 1995;10(4):171–178. doi: 10.1016/0268-0033(95)91394-t. [DOI] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. Journal of Biomechanics. 1996;29(9):1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37(8):757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. Journal of Applied Physiology. 2000;88(5):1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Judge JO, Davis R, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. Journal of Gerontology. 1996;51(6):M303–M312. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Lee LW, Collins JJ, Riley PO, Lipsitz LA. Reduced hip extension during walking: healthy elderly and fallers versus young adults. Archives of Physical Medicine and Rehabilitation. 2001;82(1):26–30. doi: 10.1053/apmr.2001.18584. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Archives of Physical Medicine and Rehabilitation. 1998;79(3):317–322. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. Journal of Applied Physiology. 1979;46(3):451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Lu TW, O’Connor JJ. Bone position estimation from skin marker coordinates using global optimisation with joint constraints. Journal of Biomechanics. 1999;32(2):129–134. doi: 10.1016/s0021-9290(98)00158-4. [DOI] [PubMed] [Google Scholar]
- McGibbon CA. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exercise and Sport Science Review. 2003;31(2):102–108. doi: 10.1097/00003677-200304000-00009. [DOI] [PubMed] [Google Scholar]
- McGill S, Juker D, Kropf P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. Journal of Biomechanics. 1996;29(11):1503–1507. doi: 10.1016/0021-9290(96)84547-7. [DOI] [PubMed] [Google Scholar]
- Murray MP, Duthie EH, Jr, Gambert SR, Sepic SB, Mollinger LA. Age-related differences in knee muscle strength in normal women. Journal of Gerontology. 1985;40(3):275–280. doi: 10.1093/geronj/40.3.275. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Erdemir A, Okita N, Cavanagh PR. Assessment of the functional method of hip joint center location subject to reduced range of hip motion. Journal of Biomechanics. 2004;37(3):349–356. doi: 10.1016/s0021-9290(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Rassier DE, Lee EJ, Herzog W. Modulation of passive force in single skeletal muscle fibres. Biology Letters. 2005;1(3):342–345. doi: 10.1098/rsbl.2005.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley PO, DellaCroce U, Kerrigan DC. Effect of age on lower extremity joint moment contributions to gait speed. Gait and Posture. 2001;14(3):264–270. doi: 10.1016/s0966-6362(01)00133-3. [DOI] [PubMed] [Google Scholar]
- Silder A, Whittington B, Heiderscheit B, Thelen DG. Identification of passive elastic joint moment-angle relationships in the lower extremity. Journal of Biomechanics. 2007;40(12):2628–2635. doi: 10.1016/j.jbiomech.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Walker PS, Rovick JS, Robertson DD. The effects of knee brace hinge design and placement on joint mechanics. Journal of Biomechanics. 1988;21(11):965–974. doi: 10.1016/0021-9290(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Whittington B, Silder A, Heiderscheit B, Thelen DG. The contribution of passive-elastic mechanisms to lower extremity joint kinetics during human walking. Gait Posture. 2007 doi: 10.1016/j.gaitpost.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegard KJ, Hicks AL, Sale DG, Vandervoort AA. A 12-year follow-up study of ankle muscle function in older adults. Journal of Gerontology A Biological Sciences Medical Sciences. 1996;51(3):B202–207. doi: 10.1093/gerona/51a.3.b202. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla A, Frank J, Walt S. Biomechanical walking pattern changes in the fit and healthy elderly. Physical Therapy. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- Zijlstra W. Assessment of spatio-temporal parameters during unconstrained walking. European Journal of Applied Physiology. 2004;92(1–2):39–44. doi: 10.1007/s00421-004-1041-5. [DOI] [PubMed] [Google Scholar]