Abstract
Objectives
Positive airway pressure (PAP) is considered a standard treatment for moderate-to-severe obstructive sleep apnea (OSA) patients. However, compliance with PAP treatment is suboptimal because of several types of discomfort experienced by patients. This study investigated compliance with PAP therapy, and affecting factors for such compliance, in OSA patients.
Methods
We performed a survey on 69 patients who engaged in PAP therapy between December 2006 and November 2007. After diagnostic polysomnography and manual titration, patients trialed PAP using the ResMed instrument and explored autoadjusting PAP (APAP), continuous PAP (CPAP), and flexible PAP (using expiratory pressure relief [EPR]) at least once every week for 1 month. Compliance measures were mean daily use (hr), percentage of days on which PAP was used, and percentage of days on which PAP was used for >4 hr. Data were obtained at night using the software Autoscan version 5.7® of the ResMed Inc. We obtained data on anthropometric (age, BMI, neck circumflex, Epworth sleepiness scale, Pittsburgh Sleep Quality Index, hypertension, alcohol intake), polysomnographic data (severity of apnea-hypopnea index [AHI], proportion of nonsupine sleep time, position dependence of sleep), PAP mode and AHI during PAP use for affecting factors.
Results
After 1 month, 41 of the 69 patients (59.4%) were pleased with PAP therapy and purchased instruments. Twenty-four patients (34.7%) used PAP for more than 3 months. The percentage of days on which PAP was used was statistically higher in patients with hypertension than in normotensive patients (P=0.003). There were negative correlations 1) between nonsupine position sleep time and percentage of days on which PAP was used (r=-0.424, P=0.039), and 2) between the AHI during PAP use and the percentage of days on which PAP was used for >4 hr (r=-0.443, P=0.030). There were no statistical differences between AHI, BMI, PAP pressure, or other measured parameters, on the one hand, and compliance, on the other.
Conclusion
The affecting factors for PAP use were hypertension history, sleep posture (shorter nonsupine sleep time), and lower AHI during PAP use.
Keywords: Obstructive sleep apnea, Continuous positive airway pressure, Compliance, Hypertension, Posture
INTRODUCTION
Positive airway pressure (PAP) is the most effective treatment for patients with moderate-to-severe obstructive sleep apnea (OSA) (1). However, compliance with continuous positive airway pressure (CPAP) treatment is suboptimal because of patient discomfort and various complications (2). Many investigators have sought methods by which PAP use might be enhanced (3).
In contrast to the fixed pressure level used in conventional CPAP therapy, autoadjusting positive airway pressure (APAP) devices adjust air pressure within a predetermined range according to patient need, thereby delivering the lowest effective pressure during the night.
Flexible PAP (using expiratory pressure relief [EPR], ResMed Inc., Sydney, Australia) is a recent variant of PAP developed to improve patient comfort and increase duration of use by lowering the pressure below the prescribed PAP in early exhalation with a return to the prescribed level at the end of expiration (4, 5).
No study has addressed the compliance of patients who have opportunities to select preferred PAP modalities. We introduced APAP, CPAP, and EPR to OSA patients during a trial period and allowed them to choose and purchase their preferred instrument. The aims of the study were to discover such preferences, to explore compliance, and to determine factors influencing compliance.
MATERIALS AND METHODS
Materials
Sixty-nine patients (62 males and 7 females), who complained of snoring, sleep apnea, or excessive daytime sleepiness, were recruited from the Asan Medical Center Snoring and Sleep Apnea Clinic between December 2006 and November 2007. All patients were diagnosed with OSA based on full polysomnography and all patients tested therapeutic PAP. Mean patient age was 46.1 yr (range 23 to 67 yr).
Full polysomnography was performed from 10 pm on the day of admission to 6 am on the following morning in a neurology center. Procedures included electroencephalography (C3/A1, C4/A2, O1/A1, O2/A2); electro-oculography; electromyography of the chin and the anterior tibialis; electrocardiography; respiratory flow measurement (using a nasal cannula/pressure transducer); measurement of thoracic or abdominal movements (inductive plethysmography); and arterial oxygen saturation measurement (pulse oximetry). Apnea was defined as cessation of airflow for at least 10 sec and hypopnea was defined as blood oxygen desaturation of 4% or greater and 30% reduction in airflow for more than 10 sec. The apnea-hypopnea index (AHI) was the number of apneas and hypopneas per hour of sleep.
OSA was diagnosed when a patient had an AHI greater than 5 and symptoms of excessive daytime sleepiness, or an AHI greater than 15 regardless of daytime symptoms, in line with the 2007 American Academy of Sleep Medicine recommendations. Severity of OSA was judged from AHI data, and graded as mild OSA (5≤AHI<15/hr), moderate OSA (15≤AHI<30/hr), and severe OSA (AHI>30/hr). When we decided to use PAP therapy, optimal fixed pressures for PAP were determined by manual titration during the second night in the clinic.
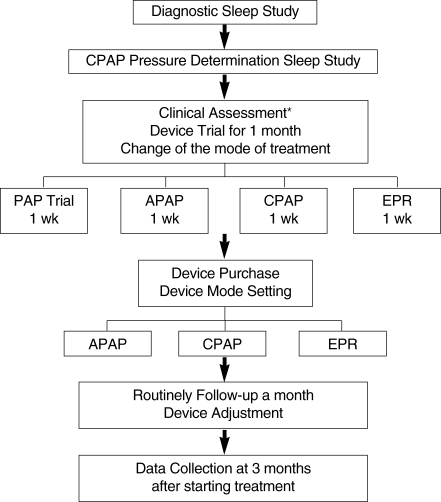
Study design (Fig. 1)
Fig. 1.
Summary of the study protocol.
*Clinical assessment baseline characteristics (age, sex, body mass index, neck circumflex, Friedman stage, hypertension status, alcohol use, smoking, sleep study data, optimal pressure in treatment study, Epworth Sleepiness Score, Pitt- sburgh Sleep Quality Index).
APAP: autoadjusting positive airway pressure; CPAP: continuous positive airway pressure; EPR: expiratory pressure relief (flexible CPAP).
Patients considering PAP therapy tested a device (ResMed Inc.) for 1 month. After an adaptation period of 1 week, they experimented with APAP (S8 AutoSet Spirit™), CPAP (S8 Elite™), and EPR (S8 Elite™, EPR) at least once every week. In choosing their preferred form of PAP therapy after the 1-month trial period, patients purchased instruments after consideration of usage information recorded by the test device software, and also considered convenience of use. Those who experienced no difference between APAP and CPAP were recommended to purchase a CPAP-mode instrument. Patients with congestive heart failure, patients with chronic obstructive pulmonary disease, those expected to have nocturnal arterial desaturation arising from conditions other than OSA (such as obesity hypoventilation syndrome), patients who did not snore (either naturally or as a result of palate surgery), and patients who showed central sleep apnea syndromes, were not candidates for APAP.
Patients were considered as a group, and we obtained data on body mass index (BMI, kg/m2); neck circumflex (cm); palatine tonsil size; palate position according to Friedman's classification; hypertension defined as systolic blood pressure (BP) >140 mm Hg or diastolic BP >90 mm Hg in three independent measurements using a conventional sphygmomanometer (6); alcohol intake classified levels of it into none and alcohol drinker; scores on the Epworth sleepiness scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Apnea Index (AI), AHI, apnea-arousal Index; minimum SaO2 values (%); proportions of nonsupine-position sleep time (nonsupine sleep time/total sleep time), position dependence of sleep (nonsupine AHI/supine AHI); and dependence of rapid eye movement (REM) sleep on AHI (REM AHI/non-REM AHI).
Patients have visited our clinic every month since the trial period; we have assisted them to overcome any discomfort and we collected data recorded in their devices. Results from patients who used PAP for more than 3 months were analyzed using Autoscan version 5.7® software from ResMed Inc. The data monitoring system recorded AHI, the pressure profile (95th percentiles of pressure, median pressure, and maximal pressure), air leakage, and device use time (when used, hours of daily use, and days used per month). From these data, we calculated:
Mean daily use (hr)=(total hr when CPAP used)/(total number of follow-up days)
Percentage of days CPAP was used=(number of days when ≥1 hr of use was recorded)/(total number of follow-up days)
Percentage of days that PAP was used for >4 hr =(number of days when >4 hr of use was recorded)/(total number of follow-up days)
Commonly used definitions of adequate compliance are PAP usage of >4 hr per night for 70% of days (3) or more than 5 days per week and for more than 4 hr per day of use (7). But other reports presented compliance as the duration of nightly use of the device (8), the amount of time that the device is switched on and being worn correctly so as to deliver effective therapeutic pressure (9) and percentage of days CPAP was used, mean daily use (hr) and mean daily use on days CPAP was used (10). In other words, because there is no standardized definition of CPAP compliance, compliance was judged by mean daily use (hr), percentage of days on which PAP was used, and percentage of days on which PAP was used for >4 hr.
The mean follow-up period was 8.4 months.
Statistical analysis
Numerical variables such as anthropometric and polysomnographic data are expressed as means±SDs. Compliances according to hypertension, alcohol intake, severity of AHI, and proportion of nonsupine sleep time, were calculated using the Mann-Whitney test. Calculations of significant differences between manual titration pressure and auto-titration pressure according to PAP mode, and assessment of compliance according to mode, were made using the Kruskal-Wallis test. Correlation coefficients between compliance on the one hand, and BMI, neck circumflex, Friedman's classification, ESS score, PSQI score, AI score, AHI score, apnea-arousal index scores, minimum SaO2 (%) values, proportion of nonsupine sleep time, position dependence of sleep, position dependence of REM sleep, AHI during PAP use, PAP pressure, and air leakage during PAP, on the other, were calculated using the Pearson correlation test. To determine important affecting factors of compliance with PAP, we used a stepwise multiple regression model to select out significant variables; only those variables that produced a P-value <0.05 were included in the final model (P-values of <0.05 were considered statistically significant). Data analyses were performed using SPSS for Windows, ver. 15.0 (SPSS, Chicago, IL, USA).
RESULTS
Sixty-nine patients engaged in PAP therapy, including 3 (4.4%) with mild OSA, 13 (18.8%) with moderate OSA, and 53 (76.8%) with severe OSA.
Preference
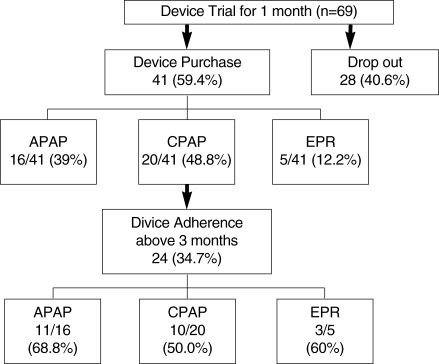
Forty-one (59.4%) of the 69 patients purchased a device after the 1-month trial; 28 patients (40.6%) dropped out. Of the 41 patients who purchased PAP devices, 16 (39%) chose APAP, 20 (48.8%) CPAP, and 5 (12.2%) EPR. Twenty-four patients (34.7%), 11 of the 16 (68.8%) who purchased APAP devices; 10 of 20 (50.0%) who purchased CPAP instruments; and 3 of 5 (60%) who purchased EPR devices, used PAP for more than 3 months (Fig. 2).
Fig. 2.
Compliance of patients.
APAP: autoadjusting positive airway pressure; CPAP: continuous positive airway pressure; EPR: expiratory pressure relief (flexible CPAP).
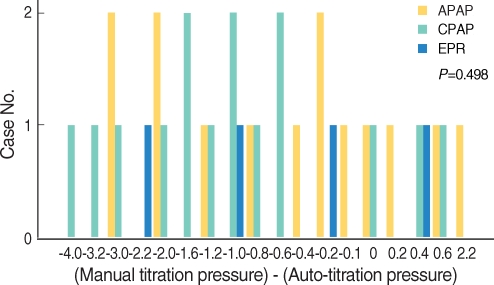
When we analyzed differences between manual titration pressure and auto-titration pressure according to PAP mode in 37 patients (so excluding 4 patients for whom fixed PAP pressures were not available), no statistically significant difference was seen. The means were -0.71±1.43 cm H2O in APAP users, -1.31±1.31 cm H2O in CPAP patients, and -0.75±1.12 cm H2O in EPR users (P=0.498) (Fig. 3).
Fig. 3.
Difference between manual titration pressure and auto-titration pressure according to PAP mode.
APAP: autoadjusting positive airway pressure; CPAP: continuous positive airway pressure; EPR: expiratory pressure relief (flexible CPAP).
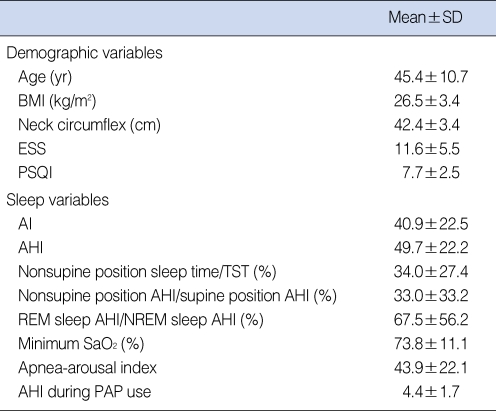
Of the 24 long-term (at least 3 months) PAP users, 23 were male and 1 was female, of mean age 45.4 yr. Three patients were of stage II and 17 of stage III on Friedman's classification (so excluding 4 patients for Friedman's classification were not recorded). Thirteen had hypertension and 12 were consumers of alcohol. Table 1 shows other demographic and sleep variables.
Table 1.
Demographic and sleep variables in PAP users (n=24)
PAP: positive airway pressure; BMI: body mass index; ESS: Epworth sleepiness scale; PSQI: Pittsburgh sleep quality index; AI: apnea index; AHI: apnea-hypopnea index; TST: total sleep time; REM: rapid eye movement; NREM: non-rapid eye movement.
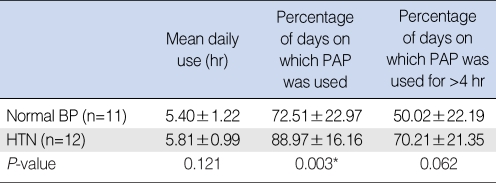
Compliance according to hypertension status
Patients with hypertension showed a statistically significant higher percentage of PAP usage days than did normotensives (P=0.003). The percentage of days on which PAP was used for >4 hr was slightly higher in hypertensives than in normotensives but this was not statistically significant (P=0.062) (Table 2).
Table 2.
Compliance according to hypertension status
*P<0.05.
BP: blood pressure; HTN: hypertension; PAP: positive airway pressure.
Compliance according to alcohol consumption
The mean daily use (hr) (P=0.354), the percentage of days on which PAP was used (P=0.413), and the percentage of days on which PAP was used for >4 hr (P=0.477) did not differ with statistical significance between alcohol consumers and those who did not drink alcohol.
Compliance according to severity of AHI
When 4 patients with moderate OSA (16.7% of total) and 20 patients with severe OSA (83.3%) were compared, the mean daily use (hr) (P=0.337), percentage of days on which PAP was used (P=0.681), and the percentage of days on which PAP was used for >4 hr (P=0.140) showed no statistically significant differences.
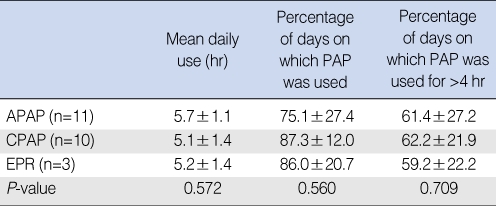
Compliance according to PAP mode
When APAP, CPAP, and EPR data were compared, mean daily use (hr) (P=0.572), percentage of days on which PAP was used (P=0.560), and percentage of days on which PAP was used for >4 hr (P=0.709), did not significantly differ (Table 3).
Table 3.
Compliance according to PAP mode
APAP: autoadjusting positive airway pressure; CPAP: continuous positive airway pressure; EPR: expiratory pressure relief.
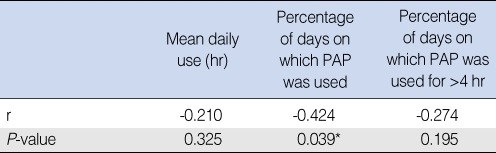
Compliance according to proportion of nonsupine sleep time
There was an obvious negative correlation between nonsupine sleep time as shown by standard polysomnography and percentage of days on which PAP was used (P=0.039, correlation coefficient=-0.424) (Table 4).
Table 4.
Compliance according to nonsupine position sleep time
*P<0.05.
r: Spearman's Rho correlation coefficient; PAP: positive airway pressure.
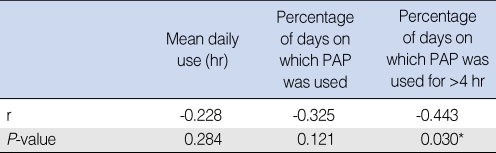
Compliance according to AHI during PAP use
There was an obvious negative correlation between AHI during PAP use and percentage of days on which PAP was used for >4 hr (P=0.030, correlation coefficient=-0.443) (Table 5).
Table 5.
Compliance according to AHI during PAP use
AHI: apnea-hypopnea index; PAP: positive airway pressure; r: Spearman's Rho correlation coefficient.
None of age, gender, BMI, neck circumflex, ESS score, PSQI score, palatine tonsil size, palate position according to Friedman's classification, AI score, AHI score, minimum SaO2 (%), apnea-arousal index score, position dependence of sleep, AHI dependence on REM sleep, PAP pressures (95th percentile pressure, median pressure, maximal pressure), or air leakage during PAP, was related to compliance.
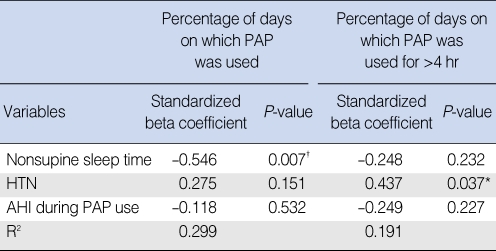
In the final stepwise multiple regression analysis showed that nonsupine sleep time was independently associated with percentage of days on which PAP was used (beta=-0.546, P=0.007), but hypertension was independently associated with percentage of days on which PAP was used for >4 hr (beta=0.437, P=0.037) (Table 6).
Table 6.
Multiple regression analysis with variables associated with the percentage of days on which PAP used and percentage of days on which PAP was used for >4 hr*
*Mean daily use (hr) was not included in the multiple regression model.
†P<0.05.
AHI: apnea-hypopnea index; HTN: hypertension; PAP: positive airway pressure.
DISCUSSION
OSAS has been managed with medication, positional therapy, oral appliances, or upper airway surgery, but PAP is the most effective treatment (1). Therefore, PAP must be considered the treatment of choice in patients with moderate-to-severe OSA (AHI≥15/hr) or in patients with mild OSA (5≤AHI<15/hr) associated either with symptoms (daytime sleepiness, insomnia, impaired cognition, mood disorders) or comorbidities (hypertension, previous cerebrovascular accident, ischemic heart disease) (1, 11, 12).
However, a limitation of PAP therapy is poor compliance and patients frequently cease therapy. One study reported that only 46% of all CPAP-treated patients with OSA used therapy for at least 4 hr on at least 70% of all nights (13). Although recent work has suggested that extensive education and regular feedback can achieve CPAP compliance rates of 65-89% over 6 months (10), over 50% of patients commencing on CPAP in the general population may not be using CPAP 1 yr later (14). Also, in the present study, 59.4% of PAP-trial patients commenced therapy but only 34.7% of all PAP-trial patients used devices for more than 3 months. Lack of education on the benefits and side-effects of PAP are principally responsible for poor compliance. Thus, clinicians must educate OSA patients and explain, in full, how improvements in symptoms and objective sleep variables, and reductions in risks of comorbidities, may be achieved using PAP. Furthermore, advisors should emphasize during follow-up that pressure intolerance during PAP can be solved by changes in PAP mode, and that discomfort arising from mask use, or nasal symptoms such as obstruction, congestion, and epistaxis, can be overcome by optimization of mask design and humidification. Regular patient observation is necessary.
One method for improving compliance with PAP is the use of alternative PAP modes. Theoretically, using APAP to deliver the lowest effective pressure might improve PAP acceptance or compliance. Compliance studies comparing APAP with CPAP have yielded conflicting results. A study found that use of APAP did not increase compliance compared to CPAP, but air leakage was lower and satisfaction higher with APAP (15). A randomized study comparing the efficacy of and compliance with APAP and CPAP reported that both parameters were similar with use of either modality and there was no correlation between leakage and compliance (8).
EPR (flexible CPAP) is similar to bilevel PAP in that expiratory pressure is reduced, and patient comfort increased, by allowing early expiration pressure to fall below the prescribed PAP, with a return to the prescribed level at the end of exhalation, when the need for applied positive-pressure therapy is most critical (5, 16). A prospective, randomized, crossover study compared polysomnographic data and compliance in sleep apnea patients receiving CPAP and flexible CPAP for 7 weeks, and flexible CPAP was more effective in terms of decreasing AHI, lengthening device use time, and reducing mouth dryness. Thus, flexible CPAP was effective in low-compliance patients and in patients requiring CPAP >9 cm H2O or who experienced dry mouth using CPAP (17). Another study demonstrated that a change to flexible PAP improved compliance in patients persistently non-compliant with CPAP even after standard interventions, including mask optimization, heated humidification, topical nasal therapy, and sleep apnea education (18).
Our study allowed patients to trial all three modes of APAP, CPAP, and EPR, and to choose a preferred mode. The use of manual titration pressure or auto-titration pressure did not affect the choice of mode. Patients selecting CPAP showed higher autotitration pressure levels than manual titration pressure levels, compared with those using APAP. In 24 patients employing PAP for more than 3 months, preference for APAP was similar to that for CPAP and there was no difference in compliance by PAP mode. Consequently, a choice of preferred mode after trialing each mode may improve patient compliance.
Studies on factors predicting compliance showed that continuing use of CPAP correlated with higher AHI, higher BMI, higher ESS, and greater patient benefit (2, 3). In our study, significant determinants of PAP use were hypertension, sleep posture, and lowered AHI with PAP use. None of BMI, AI, AHI, ESS, or other parameters examined, affected compliance.
As it is known that long-term CPAP reduces blood pressure in patients with hypertension and sleep apnea, and that good CPAP compliance offers significant reduction in blood pressure (19), patients with hypertension seem to show better compliance with PAP. We suggest that health concerns may be an important factor explaining this observation.
During PAP use, patients must wear facial devices, including nasal, oronasal (full face), or total face masks; nasal prongs; or pillows. Consequently, longer nonsupine sleep times lower mask compliance, and PAP use time falls. As AHI fell during PAP use, symptoms were relieved and use times seemed to increase. Although PAP has a high success rate when used to treat sleep apnea, if compliance is only 34.7% as in this study, it is impossible to state that PAP is more successful than surgery.
The limitation of our study is the imperfect crossover design in that patients trialed different mode during only a single week, so we cannot conclude that they will maintain their mode preference indefinitely. Therefore, we will investigate facial device details, and side-effects of such devices, and identify solutions for perceived problems. We will research why patients cease therapy, using the ESS and PQSI to measure improvement in subjective symptoms, and determine how these factors affect compliance. Future studies are needed to assess long-term mode preference and compliance over 3 yr.
CONCLUSION
In this study, 59.4% of OSA patients with indications for PAP therapy started using PAP after a trial period and 34.7% of patients continued to use PAP for more than 3 months. Factors influencing compliance were hypertension, sleep posture (shorter nonsupine sleep time), and AHI reduction during PAP use. The PAP pressure modality selected did not affect mode choice or compliance.
To improve compliance with PAP, and either a comfortable mask or changes in sleep posture should be developed. Use of a preferred mode after trial of each mode is preferable.
References
- 1.Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment for adult obstructive sleep apnea patients: a consensus statement. Chest. 1999 Mar;115(3):863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 2.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999 Apr;159(4 Pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 3.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea/hypopnea syndrome. Sleep Med Rev. 2003 Feb;7(1):81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 4.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007 Sep;132(3):1057–1072. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 5.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005 Jun;127(6):2085–2093. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Pepin JL, Krieger J, Rodenstein D, Cornette A, Sforza E, Delguste P, et al. Effective compliance during the first 3 months of continuous positive airway pressure: a European prospective study of 121 patients. Am J Respir Crit Care Med. 1999 Oct;160(4):1124–1129. doi: 10.1164/ajrccm.160.4.9802027. [DOI] [PubMed] [Google Scholar]
- 8.Galetke W, Anduleit N, Richter K, Stieglitz S, Randerath WJ. Comparison of automatic and continuous positive airway pressure in a night-by-night analysis: a randomized, crossover study. Respiration. 2008;75(2):163–169. doi: 10.1159/000097767. [DOI] [PubMed] [Google Scholar]
- 9.Marshall NS, Neill AM, Campbell AJ. Randomised trial of compliance with flexible (C-Flex) and standard continuous positive airway pressure for severe obstructive sleep apnea. Sleep Breath. 2008 Nov;12(4):393–396. doi: 10.1007/s11325-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 10.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002 Feb;121(2):430–435. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 11.Gay P, Weaver T, Loube D, Iber C, et al. Positive Airway Pressure Task Force; Standards of Practice Committee. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006 Mar;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 12.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep related breathing disorders. Sleep. 2006 Mar 01;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 13.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993 Apr;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 14.Stepnowsky CJ, Jr, Moore PJ. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003 Jun;54(6):599–605. doi: 10.1016/s0022-3999(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 15.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep. 2004 Dec;27(8):1512–1517. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002 Jul;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 17.Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006 Oct;130(4):1018–1024. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 18.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007 Dec 15;3(7):706–712. [PMC free article] [PubMed] [Google Scholar]
- 19.Campos-Rodriguez F, Perez-Ronchel J, Grilo-Reina A, Lima-Alvarez J, Benitez MA, Almeida-Gonzalez C. Long-term effect of continuous positive airway pressure on BP in patients with hypertension and sleep apnea. Chest. 2007 Dec;132(6):1847–1852. doi: 10.1378/chest.07-1478. [DOI] [PubMed] [Google Scholar]