Abstract
Both steroids and growth factors stimulate proliferation of steroid-dependent tumor cells, and interaction between these signaling pathways occurs at several levels. Steroid receptors are classified as ligand-activated transcription factors, and steps by which they activate target gene transcription are well understood. Several steroid responses have now been functionally linked to other intracellular signaling pathways, including c-Src or tyrosine kinase receptors. Steroids such as 17β-estradiol (E2), via binding to cytoplasmic or membrane-associated receptors, were also shown to rapidly activate intracellular signaling cascades such as ERK, PI3K and STATs. These E2-stimulated phosphorylations can then contribute to altered tumor cell function. ER-positive breast cancer cells, in which proliferation is stimulated by E2 and suppressed by antiestrogens, have been of particular interest in dissecting nuclear and cytoplasmic roles of estrogen receptors (ER). In some cell contexts, ER interacts directly with the intracellular tyrosine kinase c-Src and other cytoplasmic signaling and adaptor molecules, such as Shc, PI3K, MNAR, and p130 Cas. Although the hierarchy among these associations is not known, it is clear that c-Src plays a fundamental role in both growth factor and E2-stimulated cell growth, and this may also require other growth factor receptors such as those for EGF or IGF-1. STAT transcription factors represent one pathway to integrate E2 cytoplasmic and nuclear signaling. STAT5 is phosphorylated in the cytoplasm at an activating tyrosine in response to E2 or EGF, and then is translocated to the nucleus to stimulate target gene transcription. E2 stimulates recruitment of STAT5 and ER to the promoter of several proliferative genes, and STAT5 knockdown prevents recruitment of either protein to these promoters. STAT5 activation by E2 in breast cancer cells requires c-Src and EGF receptor, and inhibition of c-Src or EGFR, or knock-down of STAT5, prevents E2 stimulation of several genes and breast cancer cell proliferation. Hyperactivation of the growth factor receptor-c-Src pathway can in some contexts decrease growth responses to E2, or render cells and tumors resistant to suppressive actions of endocrine therapies. Crosstalk between growth factors and steroids in both the cytoplasm and nucleus may thus have a profound impact on complex biological processes such as cell growth, and may play a significant role in the treatment of steroid-dependent breast cancers.
1. Introduction
Most cells and tissues respond to growth factors with cell proliferation, and a subset of these also respond to the sex steroid 17β-estradiol (E2). E2 exerts its actions through two members of the nuclear receptor superfamily, estrogen receptor (ER)α and ERβ, and a recently discovered G protein-coupled membrane receptor, GPR30 (Fig. 1) [1, 2]. Mechanisms by which ERα and ERβ bind ligand, dimerize, associate with coactivators or corepressors, and regulate gene transcription through binding to target genes, are well-known and are typically referred to as “genomic” actions [1]. As a consequence, transcription of E2-responsive genes increases and proliferation of steroid-sensitive tissues such as the mammary gland and uterus is augmented. The binding of growth factors to their tyrosine kinase transmembrane receptors initiates multiple cellular signaling cascades. As a result, activation of the intracellular tyrosine kinase c-Src or enzymes such as MAPK (ERK1/2), Akt, and others results in phosphorylation of numerous cytoplasmic enzymes, transcription factors such as STATs, and other targets including steroid receptors and coregulatory molecules themselves, which ultimately regulate both cell proliferation and survival [3,4]. More recently, E2 and other steroids have been found to have rapid, cytoplasmic actions as well [4,5], and the mechanisms and relative role of these actions in proliferation is discussed in this work. Our focus will be primarily on ER-positive breast cancer cells, as an intensively studied and biologically relevant example of E2-dependent proliferation.
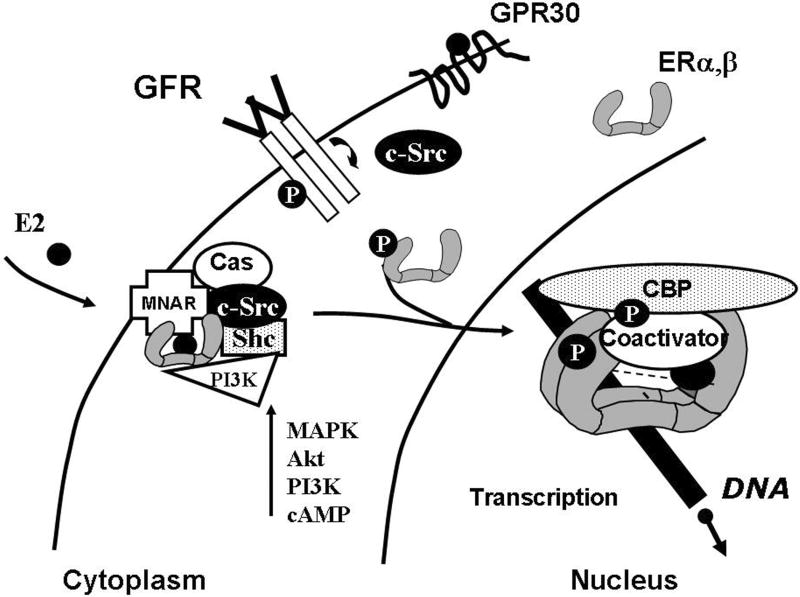
Figure 1.
E2 stimulates cytoplasmic and nuclear signaling. E2 ligand binds to estrogen receptors (ER), stabilizes ER dimers, and stimulates direct interaction with growth factor receptors (GFR), association with c-Src and adaptor molecules (Shc, MNAR, Cas), and stimulation of common cytoplasmic signaling pathways. ER can also initiate gene transcription in the absence of E2 via phosphorylation (circled P) and activation of receptors and coactivators by growth factor signaling cascades, or by a ligand-stimulated mechanism.
2. Interdependence and Cooperation of Growth Factor and E2 signaling pathways for proliferation
Steroids hormones, 17β-estradiol (E2) and progesterone, along with growth factors such as epithelial growth factor (EGF) or insulin-like growth factor-1 (IGF-1), play a key role in the development of the mammary gland and other reproductive tissues during embryogenesis, puberty, pregnancy and lactation [1]. E2 and EGF are required for the proliferation of ductal epithelial cells in the breast. Similarly, E2 and IGF-1 stimulate proliferation of uterine epithelial cells [6,7]. Both tissues require E2 and growth factors for maximal proliferative effects, and treatment with E2 and EGF or IGF-1 is synergistic [1,4-9]. Dysregulation, by overexpression or hyperactivation, of these signaling molecules results in uncontrolled proliferation and survival. E2 can stimulate expression of some EGF and IGF-1 ligands and receptors in rodent tissues and human cell lines, and EGF may also regulate ER and receptor coregulator protein expression [6,10,11]. Additional interdependence between the growth factor and E2 responses are noted in studies in which ER or growth factor receptors are ablated. Knock-out of the EGFR or IGF-1R in mice decreases or abolishes the E2 proliferative response, and inhibition of EGFR activity or introduction of mutant EGFR suppresses E2-stimulated signaling and proliferation in breast cancer cells [9,12,13]. Conversely, knock-down of ER in breast cancer cells by small interfering RNA, or inhibition by antiestrogens, prevents both E2 and EGF stimulation of DNA-synthesis [14].
3. Rapid membrane and cytoplasmic responses to E2 in breast cancer cells – cognate or novel ERs?
It is now appreciated that, in addition to modulation of gene expression, E2 can rapidly stimulate cytoplasmic signaling pathways, including many of those shared by growth factors, including MAPK, PI3K and Akt [4,5]. Although most ERα and ERβ proteins reside in the nucleus, a population of ER molecules is localized to the cytosolic and membrane compartments. This population is especially important in ER-positive breast cancers (40-70% of tumors), where ERα protein is overexpressed up to 10-fold over levels in normal breast tissue [10]. ER can become associated directly with the cytoplasmic membrane via a palmitic acid covalently associated with a specific cysteine in the ligand binding domain (C447 in hERα) [15]. In this situation, more ER is available for crosstalk with membrane and cytoplasmic signaling molecules, and may thus have additional impact to modulate the rate of proliferation. E2 binding to ER in this compartment is fundamental to the initiation of ER activation and rapid ER signaling. The recently described GPR30, a bona fide E2-binding G-protein coupled receptor, acts via G protein βγ subunits and also has the potential to modulate E2-stimulated changes in PKA or MAPK activity [2]. Intriguingly, GPR30 is expressed in many tissues and cell types and is present in a subset of breast cancer cells that do not express the cognate ERα and ERβ proteins. Its pharmacological profile differs from nuclear ER, and it can often be stimulated by antiestrogens such as tamoxifen and the “pure” antagonist ICI 182,780 [2]. Currently, it appears that although GPR30 may play a role in certain biological processes or even proliferation of certain cell types, E2-stimulated proliferation of breast cancer cells has not been associated with this protein.
Most rapid actions of E2 in breast cancer cells, and ultimately cell proliferation, appear to be associated with the cognate nuclear ERs. E2 stimulation of MAPK in breast cancer cells occurs within 3-15 min, and can be inhibited with antiestrogens such as ICI 182,780, or knockdown of ERα [16,17]. Cells from ERα/ERβ-KO mice cannot support rapid E2 cytoplasmic signaling, and introduction of ERα or ERβ into ER-negative breast cancer or other cell lines confers E2 signaling [5,12,17]. Finally, introduction of siRNAs for ERα and/or ERβ abrogates rapid E2 signaling and cell proliferation in breast cancer cells, whereas introduction of GPR30 siRNA and knockdown of this protein does not [17-20]. However, results may be cell-context specific, and GPR30 could play a significant role in non-genomic E2 effects and steroid-regulated proliferation in other cell types [21].
4. Scaffold proteins in E2-stimulated c-Src association and cytoplasmic signaling
Because ERα and ERβ do not have kinase activity, other molecules must transmit information from E2-ER binding to stimulate cytoplasmic signaling. The intracellular tyrosine kinase c-Src, which associates directly with many growth factor receptors and signaling molecules, has a central role in E2 cytoplasmic signaling (Fig. 2) [3,4]. E2-stimulated proliferation and activation of intracellular pathways such as MAPK and STATs are severely inhibited in the presence of c-Src inhibitors or kinase-dead c-Src [13,16,22]. It has been difficult to demonstrate direct association of purified ER and c-Src, although ER directly associates with the p85 subunit of PI3K [23], and several adaptor or scaffolding molecules have now been proposed to serve a role in enabling or stabilizing ER-c-Src associations and signaling, including Shc, MNAR, and Cas. Because c-Src activity is restricted by intramolecular interactions, association of ER-c-Src and scaffold proteins may serve to stimulate c-Src activity and activate signaling through a number of intracellular signaling pathways, including PI3K and Shc-Grb/Sos/Raf/Ras to MAPK.
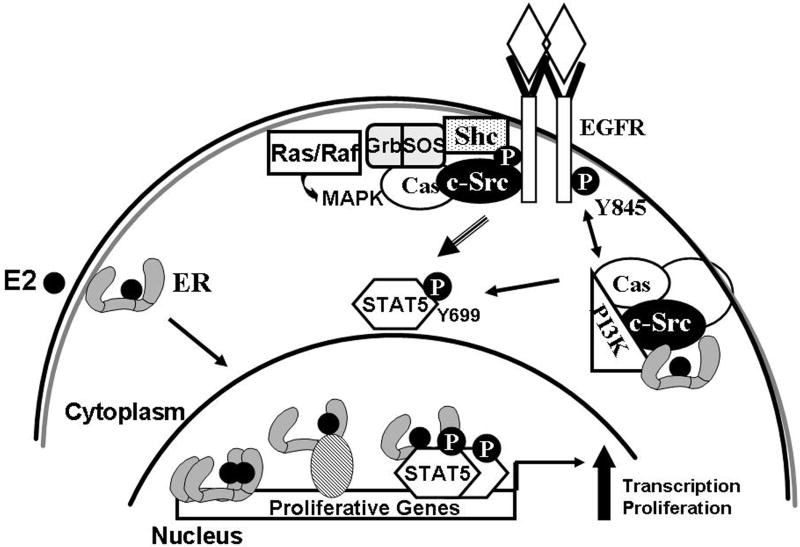
Figure 2.
Functional interactions between estrogen receptors and growth factors. In the cytoplasm, both ER and growth factor receptors (GFR) can act via c-Src-stimulated pathways that include Shc, Cas and other adaptors, which impinge on the Ras/Grb/Sos pathway to MAPK and the PI3K pathway to Akt. Cytoplasmic stimulation of STAT5 phosphorylation by ER or GFR results in activated STAT5 in the nucleus, which can stimulate genes individually, or in cooperation with activated ER.
MNAR (Modulator of Non-genomic Action of estrogen Receptor) is a scaffold protein that associates with PI3K via a phosphorylated tyrosine, with the ER ligand binding domain through a coactivator-like LXXLL sequence, and with c-Src through a proline-rich motif; phosphorylation of ER at tyrosine 537 has also been proposed to be involved in MNAR interactions (reviewed in [24]). These associations may serve to bring ER and c-Src or PI3K into proximity and increase the efficiency of ER signaling to these molecules. Altered MNAR expression by overexpression or knockdown can enhance or partially attenuate rapid ER signaling in breast cancer cells, but effects on proliferation are unknown. The adaptor protein Shc (Src-homology and collagen homology) can also associate with both c-Src and ERα, although in this case the ER N-terminal domain is involved, and ERα tyrosine 537 is not important [16]. In breast cancer cells, E2 rapidly stimulates Shc phosphorylation and MAPK activation in a process that requires both ERα and c-Src activity. The IGF-1R has been shown to act as a docking protein for the binding of the ER-Shc complex to membranes, and both Shc and IGF-1R protein and activity were required for E2-stimulated MAPK [18]. A third adaptor/scaffold protein, p130Cas (Cas), can also be found in cellular complexes containing ER, c-Src and PI3K, and siRNA for Cas attenuates E2-stimulated c-Src and MAPK activity in breast cancer cells [25,26]. EGFR activity and c-Src activation, as assessed by pharmacological inhibitors and mutant proteins, are required for E2-stimulated proliferation of breast cancer cells [13,26,27], but it is not clear if there is a particular hierarchy or requirement for specific adaptor molecules for c-Src activation and proliferation. Additional mechanisms for E2 action via the EGFR also exist in breast cancer cells. For example, transactivation/phosphorylation of EGFR by E2 in MCF7 cells can also involve the rapid liberation of heparin-binding EGF via matrix metalloproteinases; this protein binds to EGF receptor and stimulates cytoplasmic pathways which can be blocked by antibodies to this ligand for EGFR [27].
5. Resistance to steroids and steroid antagonists- convergence with growth factor signaling pathways
Proliferation of ER-positive breast cancer cells or tumors can be inhibited with antiestrogens such as tamoxifen, or aromatase inhibitors that prevent synthesis of E2. However, up to 50% of patients with ER-positive tumors either initially do not respond or become resistant to these drugs, and ER-positive cells in culture may also become resistant [26, 28-32]. In many tumors, antiestrogen resistance is associated with overexpression or hyperactivation of proteins involved in growth factor and ER signaling, including EGFR family members such as HER2, IGF-1R, and c-Src [3,28]. In tumors, overexpression of Cas, a c-Src binding protein, correlates with reduced survival and intrinsic resistance to tamoxifen [29]. In preclinical xenograft models using human breast cancer cells, resistance to aromatase inhibitors is similarly associated with increased expression or activity of growth factor receptor pathways, particularly for HER2 expression, and HER2 and MAPK activation [30]. Tamoxifen-resistant ER-positive breast cancer cells developed from the MCF7 or T47D cell lines have been found to have increased expression of EGFR receptor family members, and/or increased c-Src activity [26, 31-33]. In one model of tamoxifen resistance, developed by long term treatment of MCF7 cells with tamoxifen, increased ERα localization to the cytoplasm/membrane, correlating with increased cytoplasmic signaling, was observed [32]. Inhibition of c-Src activity resulted in restoration of growth inhibition with tamoxifen, and the loss of increased membrane/cytoplasm ER localization [32]. In another model of resistance, in vitro overexpression of Cas, which activates c-Src, resulted in failure of tamoxifen to inhibit proliferation and apoptosis [26]. Direct association of Cas with c-Src was required for this resistance. Under these conditions, tamoxifen continued to suppress ERE-mediated transcription. These data suggest that either increased GFR-c-Src activity or increased ER-c-Src activity in the cytoplasm can overcome inhibition of ER. They also suggest that growth factor and ER-mediated pathways of proliferation may have some common intermediaries, which include but are not limited to MAPK and Akt signaling, and members of the STAT family of transcription factors.
Activated EGFR or HER family members bind to Grb2 and Sos, resulting in the activation of the Ras/Raf pathway and phosphorylation of mitogen-activated protein kinases (MAPK) [3-5]. Phosphorylated MAPKs translocate to the nucleus and phosphorylate a number of transcription factors involved in proliferation. Activated EGFR also binds and activates PI3K, which in turn activates Akt. This pathway is involved in cell growth, apoptosis, invasion and migration (Fig. 2). Both EGFR-stimulated and c-Src-stimulated pathways can result in phosphorylation and activation of the transcription factor STAT5 [34]. Overexpression of members of the human EGF receptor family is not sufficient to induce breast cell tumorigenesis, but co-expression with c-Src highly increases the rate of transformation and tumorigenesis [3]. Co-expression of c-Src and EGFR in breast cancer cell lines results in novel phosphorylations within the catalytic domain of EGFR, including tyrosine 845 (Y845); this modification is not required for catalytic activity, but highly increases proliferation, transformation and tumor formation in vivo [35]. Mutation of Y845 severely suppresses tumorigenesis caused by co-overexpression of EGFR and c-Src. Because Y845 is not required for EGFR catalytic activity, it has been proposed to be involved in protein-protein interactions. Mutation of this residue to phenylalanine, which cannot be phosphorylated (Y845F), did not affect EGF-stimulated phosphorylation of phospholipase C, MAPK, or STAT3, but completely suppressed phosphorylation of STAT5b at its activating tyrosine, Y699 [36].
The c-Src-induced phosphorylation of EGFR plays a role in both resistance to tamoxifen and ER-E2 signaling pathways to promote proliferation [13,26]. In the Cas-overexpression breast cancer cell model of tamoxifen resistance, overexpression of wild-type Cas, but not a mutant that cannot bind c-Src, resulted in increased phosphorylation of two c-Src targets, EGFR at Y845, and STAT5b [26]. Introduction of EGFR-Y845F restored tamoxifen suppression of proliferation and stimulation of apoptosis. Interestingly, EGFR-Y845F also prevented E2-stimulated proliferation in MCF7 cells overexpressing Cas. Stimulation of STAT5b activity alone may be sufficient to confer tamoxifen resistance, as introduction of a constitutively active mutant of STAT5b into T47D cells with endogenous Cas levels prevented tamoxifen-induced inhibition of DNA-synthesis and cell proliferation [13].
6. STAT transcription factors as a mechanism to integrate cytoplasmic and nuclear E2 actions
The STAT family of transcription factors is involved in proliferation, differentiation, and oncogenesis [37]. Seven members have been identified, including STAT 1-4, 5a, 5b, and 6. Under non-stimulated conditions, STATs are primarily or exclusively cytoplasmic (Fig. 2). Upon stimulation, phosphorylation of specific tyrosine residues in their C-terminal domains results in dimerization and translocation to the nucleus, where they associate with DNA and alter gene transcription. ERα co-immunoprecipitates with STAT5 and STAT3 in mammary epithelial cells and ER-positive MCF7 and T47D human breast cancer whole cell extracts [38-40]. E2 treatment of these cells phosphorylates STATs on their activating tyrosines, resulting in STAT-activated transcription; for STAT5b this process requires ER, EGFR, and c-Src activity [13, 38-40]. ER and STAT5b, but not STAT1 or STAT3, are necessary for E2-induced proliferation of T47D and MCF7 breast cancer cells [13]. Knock-down of STAT5b by siRNA abolishes E2-induced transcription of proliferative genes, such as cyclin D1 and c-myc [13], and introduction of a dominant-negative STAT5b prevents E2 stimulation of breast cancer cell growth in culture or xenografts [26,41].
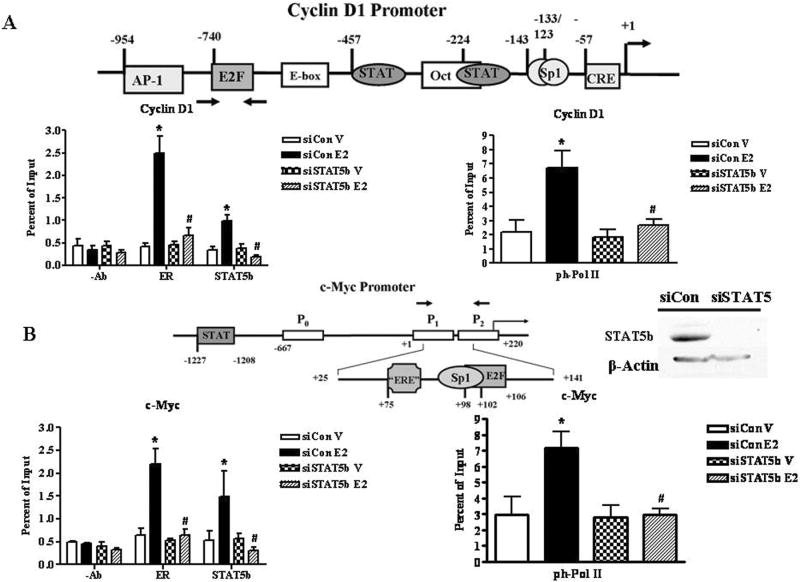
To investigate the mechanism by which STAT5b cooperates with ERα to modulate genes that regulate E2-stimulated breast cancer proliferation, chromatin immunoprecipitation assays were performed in the ER-positive human breast cancer T47D cell line (Fig. 3) according to established protocols [42-44]. The cyclin D1 and c-Myc promoters, which are both stimulated by E2, contain defined binding sites for STAT5, and either composite (c-Myc) or tethered association sites for ER (cyclin D1). For example, ERα has been reported to associate with the cyclin D1 promoter by interacting with Sp1 proteins at GC-rich promoter sequences (-143 bp and -133/-123 bp), with c-Jun/ATF-2 at a variant cAMP response element (CRE) (-57 bp), and with Fos and Jun at an AP-1 site (-954 bp) [45-47]. The c-Myc regulatory region contains three promoters (P0, P1, and P2), of which P2 is the major transcription start site in humans [48]. The E2-responsive region of the c-Myc promoter is 116 bp 5’ of the P2 promoter between +25 bp and +141 bp [49]. ERα associates with the c-Myc promoter through a Sp1 site (+96 bp) adjacent to an ERE half site (+75 bp) [48,49].
Figure 3.
E2 Induces ERα, STAT5b, and Phosphorylated RNA Polymerase II Recruitment to the Cyclin D1 and c-Myc Promoters. STAT5b Knockdown Inhibits E2-induced Recruitment of ERα and Phosphorylated RNA Polymerase II. A-B, T47D cells were nucleofected with 2.5 μg of 20 μm control or STAT5b siGENOME SMARTpool siRNA (Dharmacon) per reaction, grown in E2-free conditions for three days, then treated with control media containing ethanol (V) or 10 nm E2 for 30 min. ChIP assays were performed using either no antibody (-Ab) or antibodies directed against ERα, STAT5b, and the phosphorylated RNA Pol II CTD. The immunoprecipitated DNA was quantitated by real-time PCR with the use of primer sets directed towards the E2-responsive regions of the cyclin D1 (A) and c-Myc (B) promoters. The data represent the mean ± SEM of four experiments done in triplicate. Two-way ANOVA was used to determine statistical significance for –Ab, ERα, and STAT5b. *, P < 0.05 vs. V; #, P < 0.01 vs. E2-stimulated siCon cells. Student’s t test was used to determine statistical significance for ph-Pol II. *, P < 0.05 vs. V; #, P < 0.05 vs. E2-stimulated siCon cells. Inset, Western blot verifying STAT5b knockdown prior to 30 min E2 treatment. T47D cells were nucleofected with 2.5 μg of 20 μm control or STAT5b siRNA and lysed after 72 h. Proteins were separated by 8% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies specific for the STAT5 SH2 domain or β-Actin.
Chromatin immunoprecipitation (ChIP) is a powerful technique to detect the binding of endogenous transcription factors to promoters in vivo in the presence of native chromatin under different physiological conditions, as well as associations of coregulators or other proteins binding to tethered transcription factors, and epigenetic modifications [42-44]. ChIP studies show that E2 induces both ERα and STAT5b recruitment to the cyclin D1 and c-Myc promoters within 30 min (Fig. 3), thus suggesting that STAT5b phosphorylation induced by E2 [13], which occurs in the cytoplasm, results in stimulated association of activated nuclear STAT5b with proliferative genes. Although STAT3 and/or STAT5 have previously been demonstrated to bind to the promoters of these genes [49-51], and STAT binding to chromatin is stimulated by peptides such as prolactin and leptin [50,51], these studies show that steroids such as E2 can also activate STAT5 by this mechanism. The increase in promoter occupancy by ERα and STAT5b correlates with enhanced association of the phosphorylated (i.e., active) form of the RNA Polymerase II CTD with both promoters (Fig. 3). These data suggest that ERα and STAT5b promoter occupancy correlates with de novo transcription, and results in the E2-stimulated increase in mRNA levels for these genes [13]. Interestingly, knockdown of STAT5 in this system abolishes recruitment of both E2-induced ERα and phosphorylated RNA Polymerase II to the cyclin D1 and c-Myc promoters (Fig. 3), suggesting that STAT5b signaling in the nucleus is required for the E2-induced regulation of these endogenous proliferative genes. This may occur because STAT5b helps tether ERα to the promoter, because STAT5 plays an integral role in communicating with the transcriptional machinery, or because of other roles in the transcription of these genes. Nevertheless, this pathway demonstrates that the integration of both cytoplasmic (E2-ER stimulation of STAT5b phosphorylation via a c-Src-EGFR-requiring pathway) and nuclear (ERα and STAT5 association in the nucleus) E2-stimulated pathways results in breast cancer cell proliferation.
7. Cell context helps define the role of cytoplasmic pathways
With the wealth of new information emerging on rapid cytoplasmic responses to steroids, it is becoming clear that cell context plays the critical role in determining the relative biological role and importance of these pathways in a given system. For example, E2 modulation of STAT5 activity is cell-context dependent, and may depend on the cellular stoichiometry of important signaling molecules such as ER, c-Src, and EGFR [52-54]. In general, in cells expressing endogenous ER and low levels of HER family receptors, E2 appears to stimulate STAT activation and STAT5-mediated transcription [13,26,39-41]. STAT5b is required for E2-induced breast cancer proliferation in at least some of these cell lines [13]. In contrast, E2 negatively regulates STAT5 activation in breast cancer cell lines engineered to artificially express ER, and which typically express high levels of growth factor receptors [40,52]. Under these conditions, E2-ER may actually interfere with the more efficient growth factor pathways. Similarly, the relative roles of specific pathways and signaling adaptors may be different in different cell types. For example, MNAR (PELP) knockdown has modest effects on ERα cytoplasmic signaling in breast cancer cells, but profound suppressive effects on the growth of ovarian cancer cell xenografts [54]. GPR30 does not appear to have a major influence on rapid E2 signaling or proliferation in breast cancer cells [17-20], but it may in other cell types [21,55]. Finally, although the use of an exclusively membrane-acting form of E2 (E2 dendrimer [56]) or ERα tethered to the cytoplasmic membrane [57] stimulated MAPK and Akt, but were insufficient to initiate all the genes and processes necessary for proliferation for the breast cancer cell lines tested, these cytoplasmic signaling pathways clearly play an integral role in steroid-stimulated growth [13, 16-19], and may play even larger roles in other cells or tissues, such as the vascular system.
What may be the determinants in deciding the role of steroid-initiated cytoplasmic signaling in a given cell or tissue? One component may be the strength and duration of the cytoplasmic signal, including influx of ions, MAPK, Akt, and STAT activation, or stimulation of other pathways. This will be influenced by number and identity of receptors for both growth factors and steroids, as well as their location - increased cytoplasmic receptors for the steroids should result in more robust signaling. The number and identity of adaptor molecules – and the relative hierarchy with which they associate with steroid receptors or other cytoplasmic signaling molecules-may also influence the efficiency of a response. Finally, the existence of other competing or limiting molecules (phosphatases) or pathways (progesterone and estrogen; GPR30 and ER) may influence overall biological responses.
8. Conclusions
E2 actions to promote cell proliferation and rapidly activate intracellular signaling cascades such as ERK, PI3K and STATs, particularly in breast cancer cells, have been shown to occur by both nuclear and cytoplasmic pathways. These E2-stimulated phosphorylations can then contribute to altered tumor cell function. ER alone does not activate these pathways, but instead works in cooperation with adaptor molecules and growth factor receptors, with the intracellular tyrosine kinase c-Src playing a central role. Context and networks of signaling molecules are critical, and the relative presence and ratios of participating molecules can determine the direction and importance of responses. Many new tools, including new specific ligands and inhibitors, as well as knockout animals, will continue to help uncover the role of these pathways in individual biological responses.
Acknowledgments
The authors gratefully acknowledge the support of the National Institutes of Health (NIH DK57082 to MAS), the Women’s Oncology Research Program of the University of Virginia Cancer Center (MAS), and the UVA Cancer Center support grant (NCI-P30 CA44579).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 2.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–3. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;18(23):7906–9. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 4.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–89. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 5.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–17. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 6.Kapur S, Tamada H, Dey SK, Andrews GK. Insulin-like growth factor-I (IGF-I) and its receptor in the peri-implantation mouse uterus, and cell-specific regulation of IGF-I gene expression by estradiol and progesterone. Biol Reprod. 1992;46:208–19. doi: 10.1095/biolreprod46.2.208. [DOI] [PubMed] [Google Scholar]
- 7.Richards RG, DiAugustine RP, Petrusz P, Clark GC, Sebastian J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci USA. 1996;93:12002–7. doi: 10.1073/pnas.93.21.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner RM, Verner G, Kirkland JL, Stancel GM. Regulation of uterine epidermal growth factor (EGF) receptors by estrogen in the mature rat and during the estrous cycle. J Steroid Biochem. 1989;32:339–43. doi: 10.1016/0022-4731(89)90205-7. [DOI] [PubMed] [Google Scholar]
- 9.Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, et al. Requirement of Estrogen Receptor-in Insulin-like Growth Factor-1 (IGF-1)-induced Uterine Responses and in Vivo Evidence for IGF-1/Estrogen Receptor Cross-talk. J Biol Chem. 2002;277:8531–7. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 10.Fox EM, Davis RJ, Shupnik MA. ERbeta in breast cancer--onlooker, passive player, or active protector? Steroids. 2008;73:1039–51. doi: 10.1016/j.steroids.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulds MH, Olsen H, Helguero LA, Gustafsson JA, Haldosen LA. Estrogen receptor functional activity changes during differentiation of mammary epithelial cells. Mol Endocrinol. 2004;18:412–21. doi: 10.1210/me.2003-0290. [DOI] [PubMed] [Google Scholar]
- 12.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–72. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 13.Fox M, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells. Mol Endocrinol. 2008;22:1781–96. doi: 10.1210/me.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, de Falco A, et al. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65:10585–93. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- 15.Hammes SR, Levin E. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 16.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, et al. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–27. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 17.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 18.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–81. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahola TM, Alkio N, Manninen T, Ylikomi T. Progestin and G protein-coupled receptor 30 inhibit mitogen-activated protein kinase activity in MCF-7 breast cancer cells. Endocrinology. 2002;143:4620–6. doi: 10.1210/en.2002-220492. [DOI] [PubMed] [Google Scholar]
- 21.Vivacqua A, Bonofiglio D, Recchnia AG, Musti AM, Picard D, Ando S, et al. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–46. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 22.Castoria G, Barone MV, Di Domenico M, Bilanico A, Anetrano D, Migliaccio A, et al. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 1999;18:2500–10. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheskis BJ, Greger J, Cooch N, McNally C, McIarney S, Lam HS, et al. MNAR plays an important role in ERα activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–5. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Cabodi S, Moro L, Bai G, Smeriglio M, Di Stefano P, Gippone S, et al. p130Cas interacts with estrogen receptor alpha and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci. 2004;117:1603–11. doi: 10.1242/jcs.01025. [DOI] [PubMed] [Google Scholar]
- 26.Riggins RB, Thomas KS, Ta HQ, Wen J, Davis RJ, Schuh NR, et al. Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66:7007–15. doi: 10.1158/0008-5472.CAN-05-3952. [DOI] [PubMed] [Google Scholar]
- 27.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–12. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 28.Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 29.van der Flier S, Brinkman A, Look MP, Kok EM, Meijer-van Gelder ME, Klijn JGM, et al. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst. 2000;92:120–7. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 30.Macedo LF, Sabnis G, Brodie A. Preclinical modeling of endocrine response and resistance: focus on aromatase inhibitors. Cancer. 2008 Feb 1;112(3 Suppl):679–88. doi: 10.1002/cncr.23191. [DOI] [PubMed] [Google Scholar]
- 31.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–44. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 32.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–60. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 33.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–46. [PubMed] [Google Scholar]
- 34.Silva CM, Shupnik MA. Integration of steroid and growth factor pathways in breast cancer: focus on signal transducers and activators of transcription and their potential role in resistance. Mol Endocrinol. 2007;21:1499–512. doi: 10.1210/me.2007-0109. [DOI] [PubMed] [Google Scholar]
- 35.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 36.Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278:1671–9. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Jove R. The stats of cancer - new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Matsuda T, Junicho A, Kishi H, Saatcioglu F, Muraguchi A. Cross-talk between signal transducer and activator of transcription 3 and estrogen receptor signaling. FEBS Lett. 2000;486:143–8. doi: 10.1016/s0014-5793(00)02296-1. [DOI] [PubMed] [Google Scholar]
- 39.Björnström L, Sjöberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16:2202–14. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- 40.Faulds MH, Pettersson K, Gustafsson JA, Haldosen LA. Cross-talk between ERs and signal transducer and activator of transcription 5 is E2 dependent and involves two functionally separate mechanisms. Mol Endocrinol. 2001;15:1929–40. doi: 10.1210/mend.15.11.0726. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita H, Iwase H, Toyama T, Fujii Y. Naturally occurring dominant-negative Stat5 suppresses transcriptional activity of estrogen receptors and induces apoptosis in T47D breast cancer cells. Oncogene. 2003;22:1638–52. doi: 10.1038/sj.onc.1206277. [DOI] [PubMed] [Google Scholar]
- 42.Ferris H, Walsh HE, Stevens J, Fallest PC, Shupnik MA. Luteinizing hormone beta promoter stimulation by adenylyl cyclase and cooperation with GNRH1 in transgenic mice and LBetaT2 cells. Biol Reprod. 2007;77:1073–80. doi: 10.1095/biolreprod.107.064139. [DOI] [PubMed] [Google Scholar]
- 43.Shang YF, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–52. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 44.Wen J, Lu Y, Li R, Shupnik MA. Decreased BRCA1 confers tamoxifen resistance in breast cancer cells by altering Estrogen Receptor-coregulator interactions. Oncogene. doi: 10.1038/onc.2008.405. submitted? Accepted? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro-Rivera E, Samudio I, Safe S. Estrogen-regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J Biol Chem. 2001;276:30853–61. doi: 10.1074/jbc.M103339200. [DOI] [PubMed] [Google Scholar]
- 46.Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–60. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 47.Sabbah M, Courilleau D, Mester J, Redeuilh G. Estrogen induction of the cyclin D1 promoter: Involvement of a cAMP response-like element. Proc Natl Acad Sci U S A. 1999;96:11217–22. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubik D, Shiu RPC. Mechanism of estrogen activation of c-Myc oncogene expression. Oncogene. 1992;7:1587–94. [PubMed] [Google Scholar]
- 49.Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and sp1 sites by estrogen receptors alpha and beta. J Biol Chem. 2005;280:347–54. doi: 10.1074/jbc.M407879200. [DOI] [PubMed] [Google Scholar]
- 50.LeBaron MJ, Xie JW, Rui H. Evaluation of genome-wide chromatin library of Stat5 binding sites in human breast cancer. Mol Cancer. 2005;4:6–24. doi: 10.1186/1476-4598-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–25. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boerner JL, Gibson MA, Fox EM, Posner ED, Parson SHJ, Silva CM, et al. Estrogen negatively regulates epidermal growth factor (EGF)-mediated signal transducer and activator of transcription 5 signaling in human EGF family receptor-overexpressing breast cancer cells. Mol Endocrinol. 2005;19:2660–70. doi: 10.1210/me.2004-0439. [DOI] [PubMed] [Google Scholar]
- 53.Hitosugi T, Sasaki K, Sato M, Suzuki Y, Umezawa Y. Epidermal growth factor directs sex-specific steroid signaling through Src activation. J Biol Chem. 2007;282:10697–706. doi: 10.1074/jbc.M610444200. [DOI] [PubMed] [Google Scholar]
- 54.Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–9. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- 55.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 56.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 57.Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–17. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]