Abstract
An increase in intracellular Ca2+ is one of the initiating events in T cell activation. A calcium-mediated signaling cascade in T cells involves activation of calcineurin and the dephosphorylation and translocation of Nuclear Factor of Activated T-cells (NFAT), resulting in the transcriptional activation of target genes such as IL-2. In the present study, we found that increased intracellular calcium leads to induction of the antioxidant protein ferritin H. We previously reported that the ferritin H gene is transcriptionally activated under oxidative stress conditions through an antioxidant responsive element (ARE). The facts that the ferritin H ARE contains a composite AP1 site, and that NFAT collaborates with AP1 transcription factors, led us to test whether calcium-activated NFAT is involved in the ferritin H induction through the ARE. Treatment of Jurkat T cells with the calcium ionophore, ionomycin, increased ferritin H mRNA and protein expression. Though NFAT translocated to the nucleus and bound a consensus NFAT sequence located in the IL-2 promoter following ionomycin treatment, it did not activate ferritin H transcription despite the presence of a putative NFAT binding sequence in the ferritin H ARE. In addition, the calcineurin inhibitor cyclosporin A treatment blocked ionomycin-mediated NFAT nuclear translocation but failed to abrogate the increase in ferritin H mRNA. Analysis of mRNA stability following actinomycin D treatment revealed that ionomycin prolongs ferritin H mRNA half-life. Taken together, these results suggest that ionomycin-mediated induction of ferritin H may occur in an NFAT-independent manner but through posttranscriptional stabilization of the ferritin H mRNA.
Keywords: ferritin, ionomycin, calcium, NFAT, calcineurin, ARE
INTRODUCTION
An elevation in intracellular calcium incites a signaling cascade, leading to the activation of T cells in the immune response [1]. Increased calcium is responsible for the activation of calcineurin [2], a calcium-calmodulin dependent phosphatase that is involved in transcriptional regulation of cytokine genes [3]. Studies have shown that calcineurin is integrally involved in diverse signaling pathways, which correlate with those controlled by calcium concentration, especially pathways that dictate lymphocyte function [2]. The major substrate of calcineurin is nuclear factor of activated T cells (NFAT) that was originally described for its role in T cell activation [4]. Classically, NFAT binds a highly conserved promoter sequence originally found in the upstream region of IL-2 gene; however, recently it has been shown to bind many other promoter targets, including a number of other interleukins, GM-CSF, IFN-gamma, TNF-alpha and e-selectin [3] through interaction with Activator Protein 1 (AP1) family transcription factors [5, 6]. Thus, various NFAT-AP1 composite binding sites have been identified in those NFAT-regulated genes [3].
During T cell activation, the population of T cells increases and reactive oxygen species (ROS) are produced; however, following these events a large proportion of the population dies via an apoptotic pathway [7]. Calcium may lead directly to activation of the apoptotic pathway [8]. Recently, several lines of evidence have shown that calcium may be released from the ER or taken up through channels during cellular stress response [9–11]. It is thought that this calcium increase in the cell may be the compulsory signal for the cell to die [12]. It has also been proposed that ROS produced during T cell activation may sensitize T cells to undergo apoptosis [13].
ROS are potentially damaging to the cells, but because they are ubiquitous, cells have evolved antioxidant systems to convert them into more benign molecules. In response to exposure to reactive metabolites and oxidative stress, phase II detoxification enzymes are induced and activated. At the transcriptional level, such phase II genes as glutathione-S-transferase [14], NADPH:quinone-oxidoreductase-1 [15, 16], and heme oxygenase-1 [17], are induced via a conserved cis-element in their promoter regions, aptly named antioxidant responsive element (ARE) [14]. Recently, our studies revealed an ARE in the 5’ region of the human ferritin H promoter [18] that is responsible for activation of transcription in response to oxidative stress inducing agents such as hydrogen peroxide, tert-butylhydroquinone (t-BHQ) and hemin [18–20]. The ferritin H ARE is composed of two bidirectional AP1 motifs, to which AP1 and Maf/Nrf2 family members bind [18–21].
Ferritin H serves a cytoprotective role against iron-catalyzed formation of ROS under conditions of oxidative stress, where labile Fe(II) participates in the production of the hydroxyl radical, which causes the most deleterious effect in the cells [22]. Ferritin is an iron storage protein that sequesters free intracellular iron before it may become toxic to the cells. In vertebrates, there are 2 subunits of ferritin, heavy and light, which assemble in 24 subunit collections to create a channel that encloses the iron [23]. The heavy, or H, subunit has ferroxidase activity, and thereby oxidizes Fe(II) to Fe (III) and aggregates the iron inside the core [22, 23]. Ferritin H functions to protect cells against iron-mediated oxidative stress [24–28]. We demonstrated that the mouse and human ferritin H genes are transcriptionally activated through the conserved ARE sequences [18, 21] and the ARE activation is mediated by AP1 family member transcription factors, including JunD [18, 20]. Ferritin translation by iron, in contrast, is controlled by a well known translational mechanism involving the binding of an iron regulatory protein (IRP) to an iron responsive element (IRE) in the 5’-UTR of ferritin mRNA, in which binding of IRP to IRE blocks translation, and occurs under conditions of iron deficiency [29, 30].
Very little is known about the responses that allow cells to survive the concurrent intracellular calcium concentration and ROS production during T cell activation, which may prevent apoptosis in selected cells in the population. In this study, we tested our hypothesis that elevated calcium levels may induce the antioxidant protein, ferritin H, and it may be regulated by calcium-activated NFAT through a composite NFAT-AP1 site in the ferritin H ARE. Indeed, our results showed that ferritin H was upregulated in response to elevated intracellular calcium. However, the enhanced ferritin H expression was independent of NFAT activity, and occurred through a transcription-independent pathway. Instead, increased calcium levels conferred ferritin H mRNA stabilization.
MATERIALS and METHODS
Cell culture
NIH3T3 and Jurkat E6-1 cells were purchased from American Type Culture Collection and cultured in Dulbecco’s Modified Eagle's Medium with 10% Bovine Calf Serum (Hyclone) and RPMI1640 with 10% Fetal Bovine Serum (Mediatech), 0.45% glucose, 1 mM sodium pyruvate, and penicillin/streptomycin, respectively. Cells were maintained in a humidified, 5% CO2 incubator at 37°C. Ionomycin (free acid) and actinomycin D (Calbiochem), tert-butylhydroquinone (t-BHQ, Sigma Aldrich) and 12-O-tetradecanoylphorbol-13-acetate (TPA, Sigma Aldrich) were dissolved in DMSO. Cyclosporin A (Calbiochem) was dissolved in ethanol.
Promoter reporter constructs and DNA transfection
pBluescript SK(-) human ferritin H ARE-luciferase reporter plasmids were previously described [18]. pGL3-mouse ferritin H ARE-Luc and −0.22kb mouse-ferritin H-Luc were constructed by Sma I digestion of pGL3 - 4.8 kb mouse ferritin H followed by either self ligation or insertion of double stranded ARE oligo: 5’-TACCCCCTCCATGACAAAGCACTTTTGGAGCCCAACCCCTCCAAAGGAG CAGAATGCTGAGTCACGGTGGAACAA-3’ [21]. Jurkat cells were transiently transfected via electroporation (BioRad Gene Pulser X-Cell) utilizing a manufacturer presetting condition. As an internal control for transfection efficiency, 0.1 ug of pRL-EF (elongation factor promoter-renilla luciferase reporter) was simultaneously cotransfected. Following electroporation of luciferase promoter-reporter constructs into 1×107 Jurkat cells, they were seeded at an initial density of 4–5 × 105 cells per 35 mm dish containing 2 ml culture medium. After incubation for 48 h, the cells were treated with the indicated reagents for 24 h. Preparation of cell extracts and luciferase assays were performed using Dual Luciferase Assay Reagents (Promega). Firefly luciferase expression driven by the ARE of the ferritin H gene was normalized to the Renilla luciferase activity.
Western blotting
Western blots were performed using either whole cell lysates prepared using Reporter Lysis Buffer (Promega) or Lysis Buffer A Solution containing 10 mM Na2HPO4, 150 mM NaCl, 1% Triton-X100, 0.5% sodium deoxycholate, 0.1% SDS, and 0.2% Na azide, pH 7.4. Cytosolic and nuclear extracts were obtained using Nuclear Extract Kit (Active Motif, Carlsbad, CA). Unless otherwise noted, 50 ug total protein was subjected to SDS-PAGE, and all primary antibodies were incubated overnight at 4°C. NFAT family antibody (k-18X, Santa Cruz Biotechnology) and NFATc1 specific antibody (7a6X, Santa Cruz Biotechnology) were used with a working dilution of 1:500 in Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% skim milk. Ferritin H specific antibody (H-53, Santa Cruz Biotechnology) was diluted 1:3000. Secondary antibodies (anti-rabbit IgG and anti-mouse IgG from Alpha Diagnostic, anti-Goat IgG from Calbiochem) were used at 1:5000 dilutions. Signals were visualized via ECL or ECL Advance Western Blotting Detection Reagents (Amersham-GE Healthcare). Rainbow molecular weight marker (Amersham-GE Healthcare) or Prosieve prestained protein marker (Cambrex) was used for protein size markers of SDS-PAGE.
Northern blotting
NIH3T3 or Jurkat cells were treated for 24 h with 0, 1, 5 uM ionomycin, or an equimolar amount of DMSO vehicle. In some experiments, cells were pretreated with 1 ug/ml cyclosporin A for 1 hour. Total RNA was isolated using TRIzol (Invitrogen). 5–15 ug total RNA was applied to a 1.1% agarose formaldehyde-containing gel. The separated RNA was blotted to a 0.45 micron nitrocellulose Protran BA85 membrane (Whatman, Schleicher & Schuell) and ferritin H mRNA was detected using an α-32P-dCTP labeled 0.9 kb fragment of ferritin H human cDNA as a probe.
Gel Retardation Assay
Nuclear and cytosolic extracts were prepared using the nuclear extraction kit from Active Motif. Binding reaction and separation of retarded bands by polyacrylamide gel electrophoresis was described previously [31]. NFAT antibody along with NFAT consensus oligonucleotide were obtained from Santa Cruz Biotechnology.
35S Translabeling /ferritin immunoprecipitation
Jurkat cells were treated with either 2.5 uM ionomycin for 0–24 h, or 1– 5 uM ionomycin or 10 uM t-BHQ for 24 h. After treatment, cells were pelleted at 1000 rpm for 5 min, and normal growth media was replaced with DMEM/methione/cysteine deficient media. Simultaneously, 10 uCi/ml of 35S methionine/cysteine (PRO-MIX 35S-Cell Labeling Mix, > 1000Ci/mmole, ICN) was added to each cell suspension and incubated for 1 hour. Total cell lysates were prepared with Lysis Buffer A Solution, and cleared using non-immunized rabbit serum (CAPEL) and protein A agarose (Calbiochem) overnight at 4°C. 35S incorporation was measured by TCA precipitation and scintillation counting. The amount of input for each immunoprecipitation was determined by adding equal radioactive counts to each immunoprecipitation reaction. 6 ul of anti-ferritin antibody (DAKO, A133) and 20 ul of protein A agarose were used for overnight immunoprecipitation at 4°C. Finally, the resulting immunoprecipitates were subjected to SDS-PAGE as described above, and the dried gel was exposed to film at −86°C for 2–5 days.
RESULTS
Calcium ionophore increases ferritin H expression in Jurkat cells
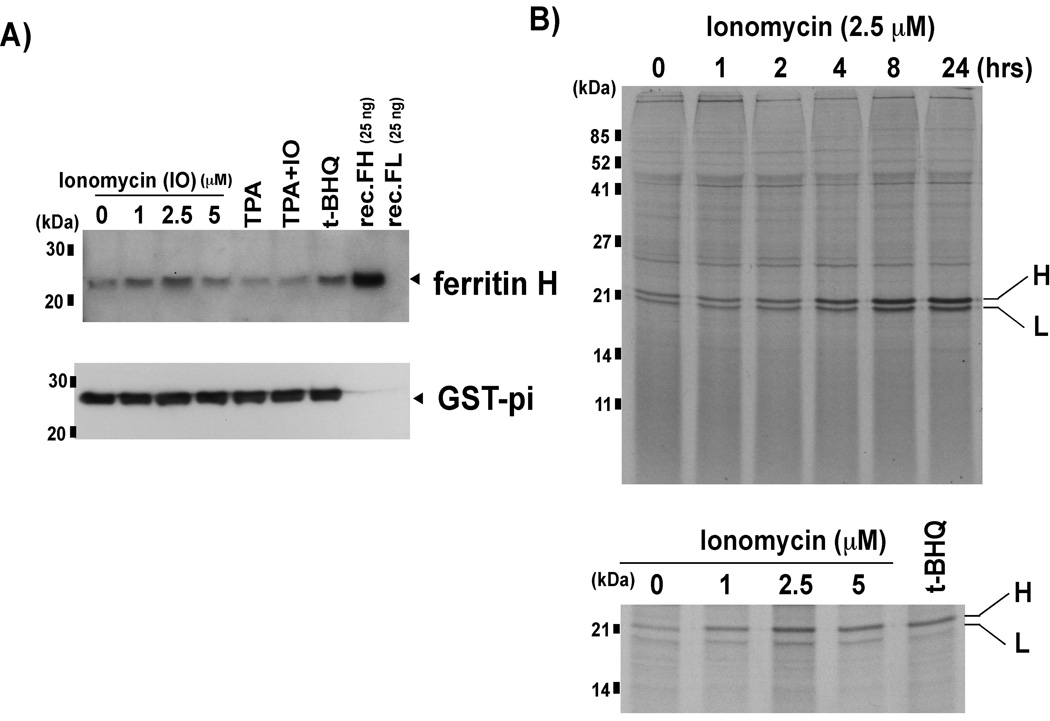
We previously demonstrated that ferritin H was induced by various oxidative stress-inducing agents [18–21]. Because T cells are exposed to high levels of ROS during activation and also may subsequently undergo apoptosis [32, 33], we investigated whether an increase in intracellular calcium levels, which is the initiating signal for T cell activation, may induce ferritin H. Jurkat acute leukemic T cells were treated with the calcium ionophore, ionomycin, 12-O-tetradecanoylphorbol-13-acetate (TPA), or TPA and ionomycin together. TPA is a tumor promoter that activates PKC signaling [34]. Co-treatment of TPA and ionomycin is a prototypical model for T cell activation [35]. Treatment of Jurkat cells with 1 and 2.5 uM ionomycin resulted in a dose-dependent increase in ferritin H protein (Fig. 1A). The highest dose of ionomycin treatment still resulted in a modest increase in ferritin H protein synthesis (Figs. 1A and 1B bottom). This may be due to some toxicity observed at higher concentrations of ionomycin. Neither TPA nor TPA/ ionomycin induced ferritin H protein; rather ferritin H protein expression appeared to be slightly decreased compared to control. Expression of glutathione S-transferase-pi (GST-pi), another phase II antioxidant enzyme, was unaffected by treatment with ionomycin, TPA, or ionomycin plus TPA (Fig. 1A), suggesting that ferritin H induction by ionomycin was not the result of the general antioxidative response of cells.
Fig. 1. Ferritin protein expression is increased by calcium ionophore treatment.
A) Jurkat cells were treated with 0, 1, 2.5 and 5 uM ionomycin (IO), 50 ng/ml TPA, 50 ng/ml TPA+ 2.5 uM IO, or 10 uM t-BHQ (as a positive control for ferritin H induction, [18]) for 24 h, and whole cell extracts were subjected to Western blotting using anti-Ferritin H antibody. Recombinant ferritin H and L (recFH, recFL) were included to assess the specificity of the antibody. Ferritin H band is indicated with an arrowhead. The membranes were re-probed with anti-GST-pi antibody. B) In vivo 35S methionine/cysteine labeling was performed following incubation with 2.5 uM ionomycin for 0–24 h (top), or 24 h incubation with 0–5 uM ionomycin (bottom). Lysates equaling 1×107 TCA insoluble counts were subjected to immunoprecipitation with anti-ferritin antibody. Ferritin H and L bands are indicated (H, L).
Ferritin is regulated by iron at the translational level via iron regulatory proteins (IRPs) [29, 30]. We previously reported that H2O2 transiently represses ferritin H protein synthesis for 2 h after H2O2 treatment through activation of the IRPs [21]. To examine whether the regulation of ferritin protein synthesis by ionomycin is similar to that by H2O2, we performed 35S methionine translabeling and ferritin immunoprecipitation. Treatment of Jurkat cells for 1, 2, 4, 8 and 24 h resulted in increased ferritin protein synthesis without transient inhibition between 1–2 h (Fig. 1B, top), which was different from that previously observed after H2O2 treatment [21]. We also observed that ferritin H protein appears to be more upregulated in comparison to ferritin L (Fig. 1B, top). In addition, treatment with increasing concentrations of ionomycin resulted in a dose-dependent increase in ferritin H protein synthesis (Fig. 1B, bottom). The amount of newly synthesized ferritin H following ionomycin treatment was similar to that of t-BHQ (Fig. 1B, bottom), a prototypical phase II gene activator [14].
Ferritin H mRNA induction following ionomycin treatment is independent of NFAT-mediated transcriptional activation via the ARE
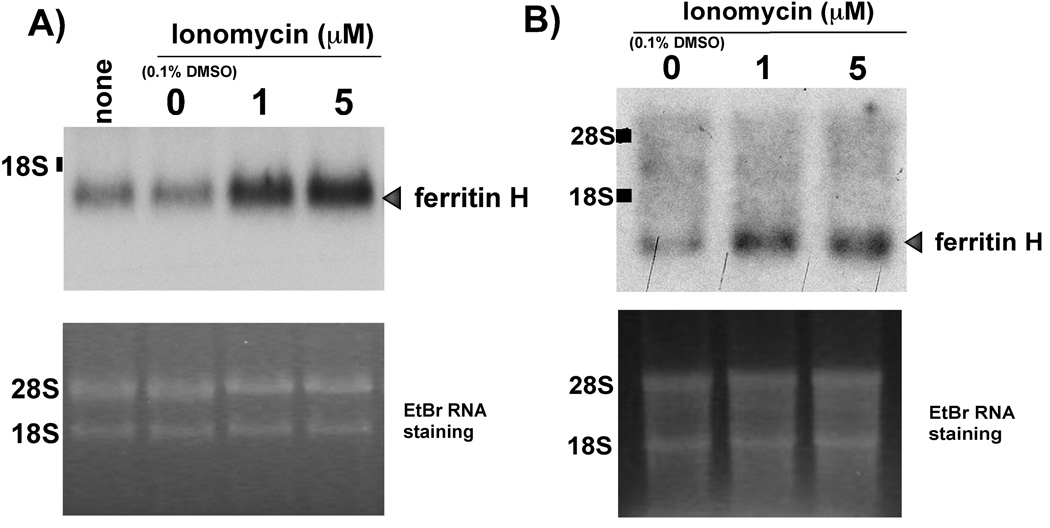
In addition to ferritin translational regulation, the ferritin H gene can also be regulated at the transcriptional level [36]. To address whether or not ionomycin treatment may regulate the expression of ferritin H mRNA, we exposed both Jurkat and NIH3T3 cells to either 1 or 5 uM ionomycin or vehicle control for 24 h and assessed ferritin H mRNA by Northern blotting. The results in Fig. 2 show that ferritin H mRNA was increased by ionomycin treatment in both Jurkat (Fig. 2A) and NIH3T3 cells (Fig. 2B).
Fig. 2. Calcium ionophore treatment increases ferritin H mRNA.
Jurkat (A) or NIH3T3 cells (B) were treated for 24 h with 0, 1, 5 uM ionomycin, or an equimolar concentration of DMSO vehicle was added. Northern blots were performed using total RNA and hybridized with α32P-dCTP random primer labeled human ferritin H cDNA probe. The ferritin H specific band and migration of the 18S and 28s ribosomal RNA subunits are indicated. Ethidium bromide (EtBr) staining of RNA was performed to assess equal loading. Representative images of 3 independent experiments are shown for both (A) and (B).
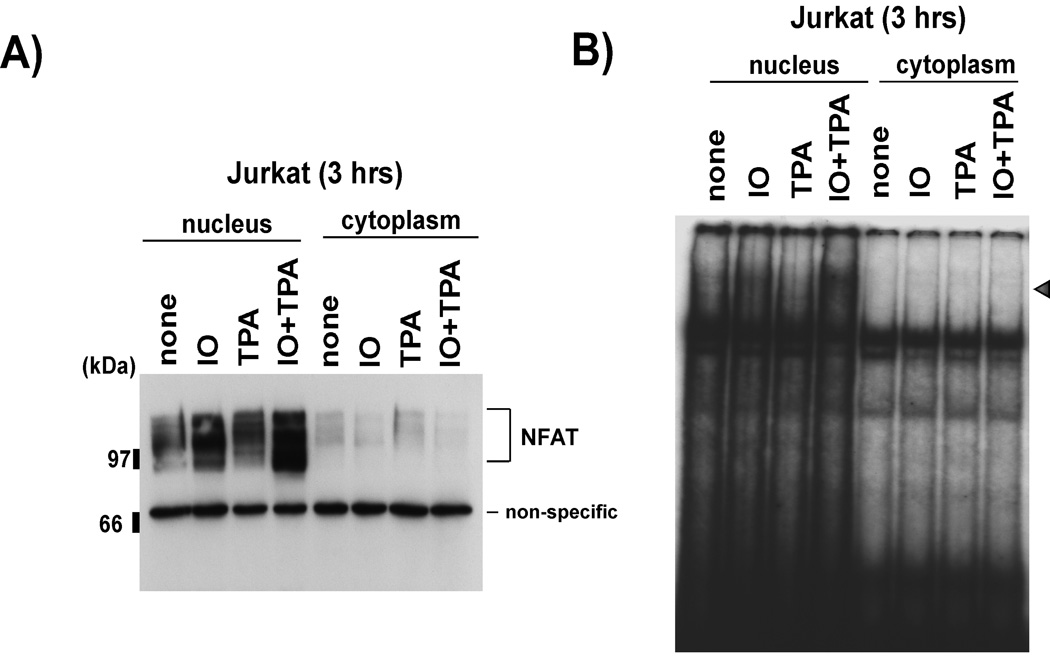
Since 1) NFAT is one of the major transcription factors activated by increased intracellular calcium during T cell activation [3, 4, 37], 2) NFAT associates with AP1 and binds a composite NFAT-AP1 site [3, 5, 6] and 3) the ferritin H ARE contains a putative NFAT-AP1 composite site (Fig. 4), we hypothesized that the increase in ferritin H mRNA by ionomycin may be due to NFAT-mediated transcriptional activation of the ferritin H gene via the ARE. First, we tried to confirm that NFAT was activated by ionomycin treatment in Jurkat cells. Nuclear and cytoplasmic fractions of Jurkat cells treated with ionomycin, TPA, or TPA/ionomycin were subjected to Western blotting to detect the translocation of NFAT to the nucleus. As shown in Fig. 3, both ionomycin and, to a greater extent, TPA/ionomycin treatment resulted in an increase in the amount of NFAT detected in the nuclear fraction, suggesting that NFAT was activated and available to bind to DNA. To reveal the ability of translocated NFAT to bind its target sequence, the same cellular fractions were subjected to gel retardation assay using an NFAT consensus sequence (derived from the IL-2 promoter). A shifted band was detected in the ionomycin and TPA/ ionomycin treated nuclear fractions (Fig. 3B), suggesting that the translocated NFAT was capable of binding to specific DNA target elements.
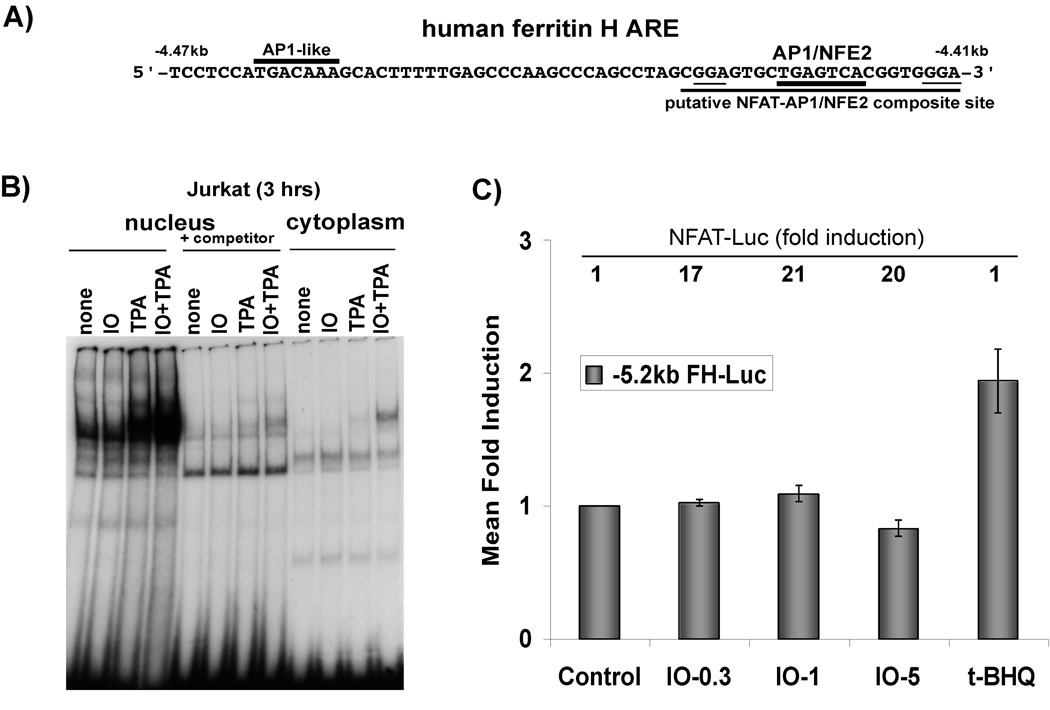
Fig. 4. NFAT is not involved in ferritin H transcriptional activation by calcium ionophore.
A) Schematic of the human ferritin H ARE sequences, including critical AP1-like, AP1/NFE2 sites, and putative NFAT binding sites (underlined). B) Nuclear and cytosplasmic extracts were prepared from cells treated with 2.5 uM ionomycin (IO), 50 ng/ml TPA, or 2.5 uM IO + 50ng/ml TPA for 3 h. 30 ug of each fraction was utilized for gel retardation assay with a γ32P-ATP labeled extended AP-1/NFE2 probe containing the putative NFAT site (underlined in A). +Competitor indicates the addition of the unlabeled NFAT oligonucleotide in 50-fold excess to the binding reaction. C) Jurkat cells were transiently transfected with −5.2 kb human FH-Luc and were allowed 24 hr recovery period. Then they were treated with DMSO control or 0.3, 1, or 5 uM ionomycin (IO), or t-BHQ (10 uM). Cell lysates were prepared and luciferase activity was assessed via luminometry with dual luciferase reagents (Promega). Luciferase activity in DMSO-treated cells was set to 1. S.E.M are shown, n=6 independent experiments with duplicate samples. The relative activity of NFAT-Luc (mean fold induction) for each treatment is indicated above each respective bar at the top of the graph.
Fig. 3. NFAT is activated by calcium ionophore treatment.
Nuclear and cytoplasmic fractions were prepared from Jurkat cells treated with 2.5 uM ionomycin (IO), 50 ng/ml TPA, or 2.5 uM ionomycin + 50 ng/ml TPA (IO+TPA) for 3 h. A) Nuclear and cytoplasmic fractions were used for Western blotting with anti-NFATc1 antibody (Santa Cruz Biotechnology sc-7294). B) The same fractions were used for gel retardation assay with a γ32P-ATP labeled NFAT consensus binding sequence as a probe (see materials and methods).
Then we tested our hypothesis that NFAT may target the ferritin H promoter for activation following ionomycin treatment. Upon examination of the human ferritin H promoter sequence, we identified two putative NFAT binding sites adjacent to the AP1/NFE2 sites in the ferritin H ARE (Fig. 4A). Though the putative NFAT binding site was not an exact match to the consensus NFAT binding sequence, the presence of a contiguous AP-1 binding site increases the likelihood of NFAT binding because NFAT cooperatively binds and functions with AP1 family transcription factors. Therefore, we examined whether or not NFAT binds to the ARE sequence and it is increased following ionomycin treatment. A probe containing the putative NFAT site and AP-1/NFE2 site of the ARE was used for gel retardation assay with nuclear extracts of Jurkat cells treated with ionomycin. We observed no increase in the amount of protein binding to the putative NFAT-AP1/NFE2 site after ionomycin treatment compared to the no treatment control (Fig. 4B). TPA and TPA/Ionomycin stimulation both resulted in a strong increase in the amount of protein bound to the putative NFAT-AP1/NFE2 site in the ferritin H ARE (Fig. 4b). This is likely due to the stimulation of AP1 transcription factor binding by TPA. These results suggest that NFAT activated by ionomycin treatment may not be involved in the ferritin H mRNA induction via the ARE activation.
Next, we asked whether or not the ferritin H enhancer/promoter is activated by ionomycin. We employed 5.2 kb of the upstream region of the human ferritin H promoter containing the ARE fused to a luciferase reporter for transient transfection [18]. The results in Fig 4C shows that ionomycin treatment failed to activate the ferritin H promoter in Jurkat cells, while it strikingly induced an NFAT-Luciferase promoter reporter construct (Fig. 4C). The antioxidant t-BHQ, a positive control of the ARE activation used in this study, induced expression of luciferase driven by the ferritin H promoter (Fig. 4C). We also employed a wild type ARE consensus oligonucleotide inserted into a TATA-luciferase reporter for transient transfection assay. Similarly, the ARE was also not activated by ionomycin treatment, though it was induced by t-BHQ (data not shown).
To further assess that the ferritin H mRNA induction following ionomycin treatment is NFAT-independent, we employed cyclosporin A. Cyclosporin A is an immunosuppressant that blocks the activation of calcineurin and also prevents downstream calcium mediated signaling events [38]. To confirm that cyclosporin A abrogates ionomycin-mediated activation of NFAT, we examined the amount of NFAT present in the nuclear fraction following treatment. NFAT was increased in the nuclear fraction after ionomycin treatment alone, but the addition of cyclosporin A completely blocked NFAT translocation (Fig. 5A). When ferritin H mRNA levels were examined, cyclosporin A treatment rather slightly enhanced basal expression, and it did not abrogate ionomycin-mediated increase in ferritin H mRNA (Fig. 5B). It also show that ionomycin or cyclosporine A had marginal effect on ferritin L mRNA (Fig. 5B). Taken together, we concluded that induction of ferritin H mRNA by ionomycin treatment was not involved in the transcriptional activation of the ferritin H gene by the activated NFAT.
Fig. 5. Inhibition of NFAT by cyclosporin A does not block ionomycin-mediated ferritin H mRNA induction.
A) Nuclear and cytoplasmic fractions were prepared from Jurkat cells treated with either 2.5 uM ionomycin (IO) or 2.5 uM ionomycin + 1ug/ml cyclosporin A (CsA). Western blotting for NFAT was performed to assess nuclear translocation of NFAT using an NFAT1 specific antibody (Santa Cruz Biotechnology sc-7296). B) Jurkat calls were treated with 0, 1, or 5 uM ionomycin with or without 1ug/ml CsA for 24 h. Total RNA was harvested and ferritin mRNA levels were assessed by Northern blotting, using 32P-dCTP labeled human ferritin H or ferritin L cDNA probe. Ethidium bromide (EtBr) staining was performed to assess RNA loading (bottom). Representative images of 3 experiments are shown.
Ionomycin mediated induction of ferritin H occurs via a posttranscriptional mechanism involving mRNA stabilization
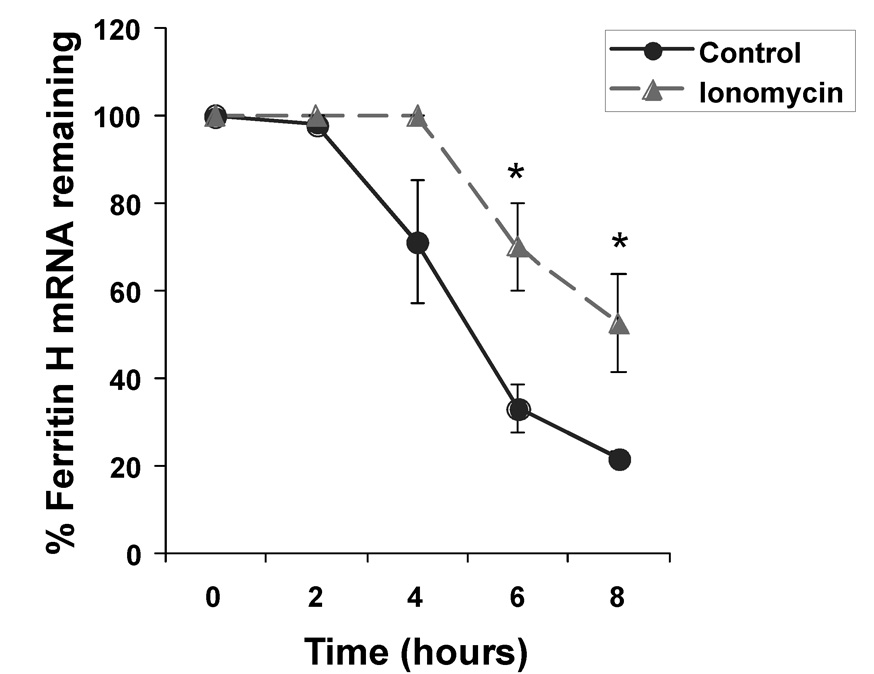
Our results suggested that ferritin H was not transcriptionally activated by ionomycin, and indicated that ferritin H induction was independent of NFAT activation. We then asked whether or not the increase in ferritin H mRNA following ionomycin treatment may be due to a posttranscriptional mechanism. Enhanced mRNA stability is one of the major posttranscriptional mechanisms that can increase mRNA levels. One of the main mechanisms of mRNA stabilization is the binding of RNA binding proteins to target sequences in the mRNA, which allows them to modulate the stability of the transcript [39]. To test whether or not ferritin H mRNA half-life is enhanced by ionomycin treatment, Jurkat cells were treated with either vehicle or ionomycin, and then treated for up to 8 h with the transcription inhibitor, actinomycin D. Total RNA was isolated from these Jurkat cells for Northern blotting to measure the half-life of ferritin H mRNA. The results in Fig. 6 show that ionomycin treatment appeared to induce a delay in the onset of ferritin H mRNA decay, ultimately resulted in prolonged ferritin H mRNA half-life from 5 h to 8 h and conferred mRNA stabilization.
Fig. 6. Ionomycin treatment results in the stabilization of ferritin H mRNA.
Jurkat cells treated with 0 or 1 uM ionomycin for 16 h were exposed to 5 ug/ml actinomycin D for up to 8 h. At the indicated time points, RNA was harvested. 2–5 ug RNA was subjected to Northern blotting with a ferritin H cDNA probe. The resulting ferritin H mRNA signal was analyzed by densitometry (Image J) and normalized to RNA ethidium bromide staining. S.E.Ms are shown of n=3 experiments, and * is defined as p<0.05, as determined by Student’s t-test.
DISCUSSION
T cells are subjected to elevated intracellular calcium and increased ROS during activation [37, 40]. Our previous studies have demonstrated that ferritin H, an iron sequestering protein, is transcriptionally activated by a number of oxidative stress inducing compounds through an ARE in the far upstream region of the promoter [18, 20, 21]. In this study we tested our hypothesis that elevated intracellular calcium may induce ferritin H in the mechanism of ARE activation similar to that evoked under oxidative stress conditions. We observed that, indeed, ferritin H mRNA and protein were increased following treatment with calcium ionophore, ionomycin (Fig. 1, Fig. 2), however, the potent T cell activating stimulus of co-treatment with TPA and ionomycin failed to induce ferritin H (Fig. 1). This may be a result of TPA-induced activation of proteins that bind the AP1/NFE2 site in the ferritin H ARE and occupy the ARE (Fig. 4). The increased binding of proteins to the AP1/NFE2 site may compete binding to the ARE with major ARE binding proteins such as Nrf2/Maf transcription factors, thereby resulting in the decreased expression of ferritin H by TPA alone or ionomycin plus TPA treatment (Fig. 1).
NFAT is a critical transcription factor involved in the activation of cytokine genes during T cell activation [4, 37]. We found a putative NFAT binding site adjacent to the AP1/NFE2 binding site in the ferritin H ARE (Fig. 4). The presence of a composite AP1 binding site appeared to increase the potential for NFAT binding and transactivation because Fos and Jun family AP1 transcription factors was shown to associate with NFAT and enhance NFAT-mediated transcriptional activation in cytokine genes [5, 6]. Though we confirmed the translocation and transactivating potential of NFAT on a consensus binding site derived from its classical target, the IL-2 promoter, following ionomycin treatment, neither the 5.2kb upstream region of the ferritin H promoter (Fig. 4) nor the wild-type ARE inserted into a TATA-luciferase promoter reporter (data not shown) was activated in our transient transfection. Furthermore, cyclosporin A blocked NFAT activation by ionomycin, but it failed to block induction of ferritin H mRNA by ionomycin (Fig. 5). Cyclosporin A rather slightly increased ferritin H mRNA levels (Fig. 5), in which it is likely that the inhibition of the phosphatase, calcineurin, may prevent the dephosphorylation of some transcription factors that positively regulate ferritin H transcription. Cyclosporin A may also affect the stability of ferritin H mRNA by a similar mechanism to the increase in parathyroid hormone mRNA stability, in which cyclosporine A inhibits calcineurin leading to modifications of phosphorylation status of the AU-rich element binding factor, AUF1 [41]. Indeed, we found that sequences of the 3’UTR of the human ferritin H mRNA contain AU-rich elements (see below). Calcineurin was reported to be inhibited by ROS [42], suggesting that inhibition of phosphatase activity is a pan-activating action that allows for the activation or maintenance of activity of many DNA and RNA binding proteins that are important to a stress response. In the case of NFAT, it is positively regulated by dephosphorylation by calcineurin [2, 43]. Our results indicate that the ionomycin-mediated induction of ferritin H mRNA appears to occur through a pathway distinct from calcineurin-NFAT mediated transcriptional activation mechanism.
Since ferritin H was not transcriptionally induced by NFAT that was activated by ionomycin treatment, we investigated whether or not it is posttranscriptionally regulated. When we examined the stability of ferritin H mRNA following ionomycin exposure and actinomycin D treatment, the overall degradation of ferritin H mRNA was delayed and the ultimate half-life of ferritin H mRNA was extended from 5 h to 8 h by ionomycin treatment (Fig. 6). The relatively long half life of the human ferritin H mRNA observed in this study appears to be consistent with the previous report that TPA induced ferritin H mRNA in human monocytic THP-1 cells via stabilization of mRNA from 7 h to 12 h [44]. The mechanism of the delay in the onset of ferritin H mRNA decay following ionomycin treatment is not clear, however, we found that sequences of the 3’UTR of the human ferritin H mRNA contain two contiguous AU-rich elements (5’-UAUUUGUAUUUAUUA-3’), suggesting that RNA binding proteins may target ferritin H mRNA for posttranscriptional regulation. AU-rich elements are localized in the UTRs of mRNA of many short-lived transcripts, including those of immediate early genes and cytokines [45, 46]. Of the RNA-binding proteins that are involved in mRNA stability and that target AU-rich elements, most are involved in the destabilization of mRNA [39]. It was demonstrated that IL-2 mRNA is stabilized by ionomycin treatment [47]. Recent studies have indicated that mRNA stabilization may be one event in calcium signaling through an AU-rich element [48, 49]. RNA binding proteins HuR and AUF1 have been implicated to play a role in stabilizing mRNAs through the binding to the AU-rich elements [41, 50]. In addition, the p38 mitogen-activated protein kinase (MAP kinase) pathway was shown to be involved in the stabilization of many AU-rich transcripts following lipopolysaccharide treatment in the human monocytic THP-1 cells [51].
We observed that ionomycin also increased ferritin L protein synthesis, to a lesser extent than ferritin H, in a dose dependent manner (Fig. 1B) in the absence of its mRNA induction (Fig. 5B). The lack of ferritin L mRNA induction by ionomycin treatment may be explained by the absence of the AUUUA sequence in the 3’UTR of the ferritin L mRNA, although it also contains high AU-sequences such as 5’-AAAUAAAGCUUUUUGAU-3’. Ferritin L also contains an ARE [52]; however, many prooxidants preferentially induce ferritin H [53–55]. Furthermore, ferritin H, but not ferritin L, confers cytoprotection against oxidative stress [56]. Upregulation of ferritin H by calcium ionophore may be part of a cytoprotective response to the concomitant ROS production or subsequent apoptotic signaling. Indeed, we observed that knocking down ferritin H by siRNA enhanced accumulation of ROS and susceptibility to apoptosis that were induced by the mitochondrial complex I inhibitor rotenone (unpublished observation).
The mRNA stability of the transferrin receptor, another major protein involved in iron transport, is increased at low iron concentrations via the enhanced interaction between IRPs and IREs in the 3’-UTR of the transferrin receptor mRNA, leading to more transferrin receptor expression and iron uptake [30]. In the same conditions, the increased IRP-IRE interactions in the 5’-UTR of ferritin H and L mRNA inhibit ferritin translation initiation, resulting in lower capacity of iron storage in the cells that allows incorporated iron readily available [30]. The mechanism by which ferritin H transcripts are stabilized following ionomycin treatment is unclear, however, this study suggests a new posttranscriptional mechanism of the ferritin H mRNA regulation by calcium. The poly-A tail does provide a degree of protection from degradation machinery, and deadenylation-dependent and independent pathways have been proposed to be involved in the destabilization and degradation of mRNA [39]. Further studies that elucidate the responsible RNA binding protein and mechanism of enhanced ferritin H mRNA stability will provide more insight into this unique pathway of posttranscriptional regulation of the ferritin H gene in response to increased intracellular calcium.
ACKNOWLEDGEMENTS
This work was supported by NIH grant DK-60007 to Y. Tsuji.
Abbreviations footnote
- AP1
activator protein 1
- ARE
antioxidant responsive element
- CsA
cyclosporin A
- DMSO
dimethylsulfoxide
- ER
endoplasmic reticulum
- EtBr
ethidium bromide
- GM-CSF
granulocyte macrophage-colony stimulating factor
- GST
glutathione S-transferase
- IFN
interferon
- IL-2
interleukin-2
- IO
ionomycin
- IRE
iron responsive element
- IRP
iron regulatory protein
- NFAT
nuclear factor of activated T-cells
- PKC
protein kinase C
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- t-BHQ
tert-butylhydroquinone
- TNF
tumor necrosis factor
- TPA
12-O-tetradecanoylphorbol-13-acetate
- UTR
untranslated region
REFERENCES
- 1.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 2.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 3.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- 5.Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 6.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 7.Owen JJ, Jenkinson EJ. Apoptosis and T-cell repertoire selection in the thymus. Ann. N Y Acad. Sci. 1992;663:305–310. doi: 10.1111/j.1749-6632.1992.tb38673.x. [DOI] [PubMed] [Google Scholar]
- 8.Jambrina E, Alonso R, Alcalde M, del Carmen Rodriguez M, Serrano A, Martinez AC, Garcia-Sancho J, Izquierdo M. Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J. Biol. Chem. 2003;278:14134–14145. doi: 10.1074/jbc.M211388200. [DOI] [PubMed] [Google Scholar]
- 9.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 10.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 11.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 12.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell. Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 13.Tripathi P, Hildeman D. Sensitization of T cells to apoptosis--a role for ROS? Apoptosis. 2004;9:515–523. doi: 10.1023/B:APPT.0000038033.14925.02. [DOI] [PubMed] [Google Scholar]
- 14.Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 15.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 16.Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J. Biol. Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 17.Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J. Biol. Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji Y. JunD activates transcription of the human ferritin H gene through an antioxidant response element during oxidative stress. Oncogene. 2005;24:7567–7578. doi: 10.1038/sj.onc.1208901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki K, Hailemariam K, Tsuji Y. PIAS3 interacts with ATF1 and regulates the human ferritin H gene through an antioxidant-responsive element. J. Biol. Chem. 2007;282:22335–22343. doi: 10.1074/jbc.M701477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki K, Mackenzie EL, Hailemariam K, Sakamoto K, Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol. Cell. Biol. 2006;26:2845–2856. doi: 10.1128/MCB.26.7.2845-2856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji Y, Ayaki H, Whitman SP, Morrow CS, Torti SV, Torti FM. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol. Cell. Biol. 2000;20:5818–5827. doi: 10.1128/mcb.20.16.5818-5827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 23.Theil EC, Matzapetakis M, Liu X. Ferritins: iron/oxygen biominerals in protein nanocages. J. Biol. Inorg. Chem. 2006;11:803–810. doi: 10.1007/s00775-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 24.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin Heavy Chain Upregulation by NF-kappaB Inhibits TNFalpha-Induced Apoptosis by Suppressing Reactive Oxygen Species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem. J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 27.Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vitro role of ferritin ferroxidase activity. J. Biol. Chem. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- 28.Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont C, Cabantchik ZI. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood. 1999;94:3593–3603. [PubMed] [Google Scholar]
- 29.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 30.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji Y, Akebi N, Lam TK, Nakabeppu Y, Torti SV, Torti FM. FER-1, an enhancer of the ferritin H gene and a target of E1A-mediated transcriptional repression. Mol. Cell. Biol. 1995;15:5152–5164. doi: 10.1128/mcb.15.9.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekkat C, Dornand J, Gerber M. Oxidative phenomena are implicated in human T-cell stimulation. Immunology. 1988;63:431–437. [PMC free article] [PubMed] [Google Scholar]
- 33.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic. Biol. Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 35.Delia D, Greaves M, Villa S, DeBraud F. Characterization of the response of human thymocytes and blood lymphocytes to the synergistic mitogenicity of 12-O-tetradecanoylphorbol-13-acetate (TPA)-ionomycin. Eur. J. Immunol. 1984;14:720–724. doi: 10.1002/eji.1830140809. [DOI] [PubMed] [Google Scholar]
- 36.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 37.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 38.McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, Ho AM, Burgeon E, Lane WS, Lambert JN, Curran T, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 39.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 41.Bell O, Gaberman E, Kilav R, Levi R, Cox KB, Molkentin JD, Silver J, Naveh-Many T. The protein phosphatase calcineurin determines basal parathyroid hormone gene expression. Mol. Endocrinol. 2005;19:516–526. doi: 10.1210/me.2004-0108. [DOI] [PubMed] [Google Scholar]
- 42.Lee JE, Kim H, Jang H, Cho EJ, Youn HD. Hydrogen peroxide triggers the proteolytic cleavage and the inactivation of calcineurin. J. Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04340.x. [DOI] [PubMed] [Google Scholar]
- 43.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 44.Pang JH, Wu CJ, Chau LY. Post-transcriptional regulation of H-ferritin gene expression in human monocytic THP-1 cells by protein kinase C. Biochem. J. 1996;319:185–189. doi: 10.1042/bj3190185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winzen R, Gowrishankar G, Bollig F, Redich N, Resch K, Holtmann H. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol. Cell. Biol. 2004;24:4835–4847. doi: 10.1128/MCB.24.11.4835-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein N, Curatola AM, Schneider RJ. Calcium-induced stabilization of AU-rich short-lived mRNAs is a common default response. Gene Expr. 1999;7:357–365. [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CY, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 48.Ruth JH, Esnault S, Jarzembowski JA, Malter JS. Calcium ionophore upregulation of AUUUA-specific binding protein activity is contemporaneous with granulocyte macrophage colony-stimulating factor messenger RNA stabilization in AML14.3D10 cells. Am. J. Respir. Cell. Mol. Biol. 1999;21:621–628. doi: 10.1165/ajrcmb.21.5.3694. [DOI] [PubMed] [Google Scholar]
- 49.Misquitta CM, Chen T, Grover AK. Control of protein expression through mRNA stability in calcium signalling. Cell Calcium. 2006;40:329–346. doi: 10.1016/j.ceca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Peng SS, Chen CY, Xu N, Shyu AB. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO. J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frevel MA, Bakheet T, Silva AM, Hissong JG, Khabar KS, Williams BR. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 2003;23:425–436. doi: 10.1128/MCB.23.2.425-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hintze KJ, Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc. Natl. Acad. Sci. U S A. 2005;102:15048–15052. doi: 10.1073/pnas.0505148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torti SV, Kwak EL, Miller SC, Miller LL, Ringold GM, Myambo KB, Young AP, Torti FM. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J. Biol. Chem. 1988;263:12638–12644. [PubMed] [Google Scholar]
- 54.Tsuji Y, Miller LL, Miller SC, Torti SV, Torti FM. Tumor necrosis factor-alpha and interleukin 1-alpha regulate transferrin receptor in human diploid fibroblasts. Relationship to the induction of ferritin heavy chain. J. Biol. Chem. 1991;266:7257–7261. [PubMed] [Google Scholar]
- 55.Jennings-Gee JE, Tsuji Y, Pietsch EC, Moran E, Mymryk JS, Torti FM, Torti SV. Coordinate inhibition of cytokine-mediated induction of ferritin H, manganese superoxide dismutase, and interleukin-6 by the adenovirus E1A oncogene. J. Biol. Chem. 2006;281:16428–16435. doi: 10.1074/jbc.M600038200. [DOI] [PubMed] [Google Scholar]
- 56.Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, Arosio P. Analysis of the biologic functions of H-and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103:2377–2383. doi: 10.1182/blood-2003-06-1842. [DOI] [PubMed] [Google Scholar]