SUMMARY
Chemokines (chemoattractant cytokines) are fundamental regulators of immune cell movement from the bloodstream into tissues. Regulating expression of chemokines might, therefore, alleviate inflammation in autoimmune diseases and transplant rejection, or augment immune responses in cancer and immunodeficiency. RANTES (regulated upon activation, normal T cell expressed and secreted [also known as CCL5]) is a model chemokine of relevance to a myriad of diseases. Regulation of RANTES expression is complex. In fibroblasts and monocytes, rel proteins alone suffice to induce transcription of RANTES. By contrast, expression of RANTES in T lymphocytes 3–5 days after activation requires the development of a molecular complex (enhancesome) including KLF13 (Krueppel-like factor 13), rel proteins p50 and p65, and scaffolding proteins. This complex recruits enzymes involved in acetylation, methylation and phosphorylation of chromatin, and ultimately in the expression of RANTES. In addition, KLF13—the lynchpin for recruitment of this molecular complex—is itself translationally regulated. Such complex regulation of biological systems has major implications for the rational design of drugs aimed at increasing or decreasing inflammatory responses in patients.
Keywords: CCL5, KLF13, RANTES, transcription, translation
INTRODUCTION
Chemokines—chemoattractant cytokines—are fundamental to the initiation and maintenance of inflammation.1 Immune cells are drawn from the blood vessels into sites of damage or ‘danger’ by a myriad of soluble mediators released from a variety of cell types. Chemokines are recognized by membrane-spanning G-protein-coupled receptors, which relay signals into the cells that express them. Chemokines can bind one or more type of receptor, in various combinations and affinity hierarchies. The end result—movement of immune cells along a chemokine gradient from low to high concentrations—is called chemotaxis. Many soluble mediators also attract immune cells on a solid matrix, such as the endothelial cell wall. This movement, called haptotaxis, underlies the multistep process of immune cell exit from blood vessels into tissue (Figure 1).2 Chemokines, their receptors, and the initiation and maintenance of inflammation have all been recently reviewed.1,3,4
Figure 1.
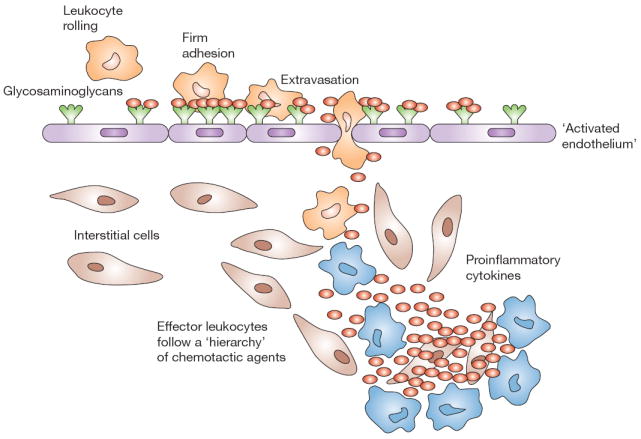
RANTES (red ovals) attracts immune cells from the peripheral blood to sites of inflammation. Leukocytes ‘roll’ along the vascular endothelium via interactions with selectins. Chemokines, such as RANTES, attract immune cells via haptotaxis, inducing firm adhesion and diapedesis. RANTES induces expression of integrins (e.g. CD11a/CD18 [lymphocyte function-associated antigen-1]) involved in adhesion, and metalloproteinases involved in movement through the vascular wall basement membranes and tissues. The immune cells follow the chemotactic gradient to the site of inflammation. T cells, once activated by specific antigen, express RANTES within 3–5 days, amplifying the immune response in time and space.
We first identified the RANTES gene (regulated upon activation, normal T cell expressed and secreted; also known as CC-chemokine ligand 5 [CCL5]) in a search for genes expressed by human T lymphocytes at a ‘late’ stage (3–5 days) after activation through their T-cell receptors.5 RANTES is of broad clinical importance in an array of human diseases including AIDS, cancer, atherosclerosis, asthma, transplantation, and autoimmune diseases such as arthritis, diabetes and glomerulonephritis;6 Box 1 lists a variety of renal diseases in which expression of RANTES is involved. RANTES binds to three types of chemokine receptor; CCR5 (CC-chemokine receptor 5), a major RANTES receptor, is also important as a co-receptor for HIV uptake into immune cells.7 For all of these reasons, RANTES and its receptors are important pharmaceutical targets.
Box 1 RANTES expression in renal disease.
Renal injury
Acute renal failure25
Nephrotoxic serum nephritis26
Interstitial nephritis14,27,28
HIV nephropathy29
Pyelonephritis30
Glomerulonephritis/nephrotic syndrome
Systemic lupus erythematosus37,38
Hepatitis C-associated glomerulonephritis39
Focal segmental glomerulosclerosis42
Membranous nephropathy45
Nephrotic syndrome46
Chronic kidney disease/progression
Chronic kidney disease50
Glomerulosclerosis of aging54
Renal transplant rejection
Renal cancer
Most chemokines are expressed as ‘immediate early’ genes in monocytes, epithelial cells and fibroblasts within minutes of cell damage. This expression is regulated through JAK/STAT pathways and involves rel proteins of the NFκB (nuclear factor kappa-B) family of transcription factors.8 By contrast, RANTES is expressed 3–5 days after T cell activation.5 This unusual kinetic profile is important for maintenance of inflammation, facilitating expansion of the inflammatory infiltrate in both space and time. As RANTES is broadly chemoattractive for T lymphocytes, monocytes, natural killer cells, basophils and eosinophils, and can also activate immune cells, the mechanism of late expression is interesting from both a biological and pharmaceutical perspective.9 Increasing RANTES expression might have therapeutic value in diseases such as HIV and cancer. Decreasing RANTES expression could prove to be therapeutic for atherosclerosis, transplantation and autoimmune diseases. Detailed evaluation of the mechanism of expression of RANTES in T lymphocytes at late postactivation stages has, therefore, been undertaken.10
IDENTIFICATION AND CHARACTERIZATION OF RANTES
RANTES was identified by subtractive hybridization screening for genes expressed by human T lymphocytes 3–5 days after activation but never expressed by human B cells.5 RANTES and a cytolytic molecule, granulysin,11,12 were identified using this screening method. The importance of RANTES in renal disease was first apparent in a study of renal transplants undergoing rejection.13 Rejecting grafts exhibited large amounts of RANTES bound to the vascular endothelium while controls did not. We now know that RANTES released from stromal cells in damaged tissues binds to glycosaminoglycans on the endothelium, where it serves as a ‘sign-post’ for recruitment of immune cells (Figure 2). Expression of high levels of RANTES is associated with a wide range of immune-mediated diseases including glomerulonephritides and interstitial nephritides.14
Figure 2.
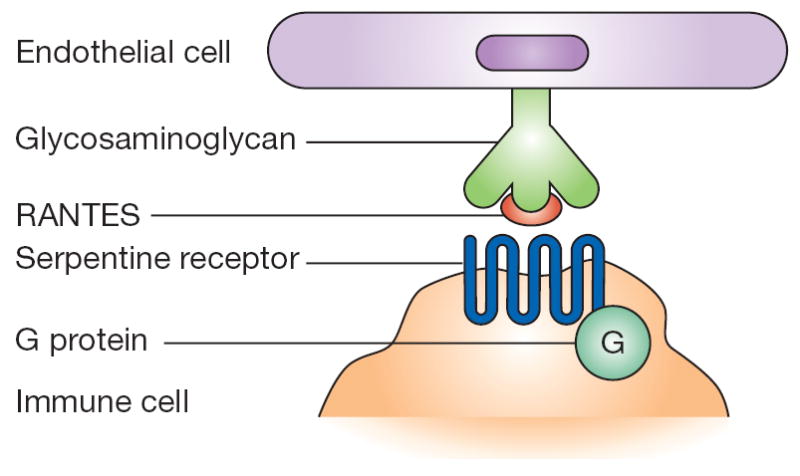
RANTES activity involves a tetramolecular complex. Glycosaminoglycans on the endothelium bind RANTES on the basis of charge (nonsignaling interaction). In turn, RANTES is presented to serpentine, seven-membrane-spanning, G-protein-associated receptors on immune cells (signaling interaction).
TRANSCRIPTIONAL REGULATION OF RANTES EXPRESSION
Electrophoretic mobility gel shift and reporter gene assays were used to identify transcription factors regulating RANTES expression (Figure 3).15 A ‘developmental switch’ in T lymphocytes was described.16,17 Known transcription factors, including nuclear factor of activated T cells 1 (NFAT1) and nuclear factor of interleukin 6 (NF-IL6), are expressed within the first 2 days of T cell activation, and their expression declines over 2–3 days. By contrast, novel transcription factors that bound to the RANTES promoter showed ‘late’ kinetics, mirroring RANTES expression. Some transcription factors, such as RFLAT-1 (RANTES factor of late activated T lymphocytes 1, now known as Krueppel-like factor 13 [KLF13]), are expressed ‘late’ after T cell activation and their levels remain elevated in long-term T cell lines, and in memory and cytotoxic T lymphocytes. Other transcription factors, such as a factor that binds the C region of the RANTES promoter, are expressed late but only transiently; expression returns to baseline levels 5–7 days after T cell activation. RANTES promoter polymorphisms associated with asthma, eczema and resistance to HIV have been described.6,9
Figure 3.
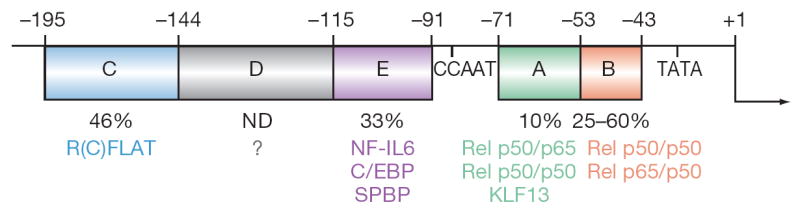
The RANTES promoter was identified by reporter gene and electrophoretic mobility shift assays. Regions A to E were named in order of discovery. Nucleotide positions relative to the transcription start site are shown. The percentages under each region indicate the fraction of activity remaining in reporter gene assays after deletion or replacement of that region. The R(A)FLAT is KLF13 and R(E)FLAT is SPBP. Abbreviations: C/EBP, CCAAT/enhancer binding protein; KLF13, Krueppel-like factor 13; ND, not determined; NF-IL6, nuclear factor of interleukin 6; RFLAT, RANTES factor of late activated T lymphocytes; SPBP, stromelysin 1 PDGF-responsive element binding protein.
REGULATION OF RANTES EXPRESSION IN T LYMPHOCYTES BY KLF13
Rel proteins are sufficient for transcriptional induction of RANTES expression in stromal cells such as fibroblasts.15 Reporter gene assays demonstrated that a very short segment of the upstream region of the RANTES promoter was required for ‘late’ expression in T lymphocytes. The assays also showed that this expression was dependent upon rel proteins binding the A and B regions of the RANTES promoter, and on a novel factor binding the A region. Song et al. used expression cloning to identify the novel factor binding the A site and regulating RANTES expression in T lymphocytes.15 This factor, now known as KLF13, binds to the A site of the RANTES promoter. If this region is replaced or deleted, late expression of RANTES in T lymphocytes does not occur. Reporter gene assays demonstrated that the rel proteins p50 and p65, as homodimers and heterodimers, are sufficient to induce RANTES expression in fibroblasts or other stromal cell types, but that the late expression evident in T lymphocytes depended on the combination of rel proteins and KLF13.
TRANSLATIONAL REGULATION OF KLF13
Remarkably, although KLF13 messenger RNA is present in a variety of cell types and at all time points after T cell activation, the cell and tissue distribution of KLF13 protein is much more limited.15 KLF13 protein is expressed only 3–5 days after T cell activation, coincident with RANTES expression.15 A series of studies showed that KLF13 is translationally regulated, like another member of the KLF family, BTEB, and many other factors involved in cell growth and activation (Box 2).18,19 Thus, memory T lymphocytes normally express messenger RNA for RANTES but not RANTES protein. After activation with growth factors, such as interleukin 2, or through mitogen-activated protein (MAP) kinase ‘stress’ pathways, initiation factors are expressed in T lymphocytes, leading to the translation of the KLF13 messenger RNA and de novo expression of RANTES protein. This rapid process is responsible for a rheostat effect whereby RANTES protein can be quickly expressed and released after stimulation of a T cell that has already been exposed to antigen. This is in contradistinction to the 3–5 days required to induce RANTES expression in a naive T cell, as described above.
Box 2 Translationally regulated messenger RNAs.
Cell cycle regulators
CDK4, p53, cyclin D1, Bcl-2, p27Kip1, Bax, MDM-2, Fas
Growth factors and cytokines
FGF-2, TGF-β, ILGF-2, VEGF, PDGF, IL-15
Hormone receptors
Androgen receptor, retinoic acid receptor β2
Protein kinases
Lck, Pim-1, C-mos
Transcription factors
c-fos, BTEB, c-myc, C/EBP-β (NF-IL6)
Translational machinery
Ribosomal proteins, elongation factors, eIF-4E, eIF-4G
REGULATION OF RANTES EXPRESSION IN T LYMPHOCYTES BY ENHANCESOMES
Recent studies indicate that a complex of multiple factors that bind to and modify DNA is involved in RANTES expression.20 Expression libraries screened for messenger RNA encoding proteins that bind the RANTES promoter identified high mobility group protein HMG-1/HMG-Y (HMG-I[Y]), rel proteins and RFLAT-2.10 RFLAT-2 is identical to nuclear factor SPBP (stromelysin 1 PDGF-responsive element binding protein; R Gantzos et al., personal communication).21 This transcription factor regulates the expression of matrix metalloproteinase-9. This regulation is important because RANTES induces metallo proteinase expression, metalloproteinases are expressed at high levels at sites of inflammation, and metalloproteinases are required to facilitate movement of cells through extracellular matrix along chemokine gradients to sites of inflammation.22 SPBP functions as a coactivator (R Gantzos et al., personal communication). It binds to the RANTES promoter E site, but its binding is not required to increase RANTES expression. SPBP binds KLF13 and other factors as part of an enhancesome on the RANTES promoter (Figure 3).20,23 SPBP, like CBP/p300 (cyclic AMP response element binding protein/p300), can function as a scaffold protein, binding several factors that might or might not also interact directly with DNA in the RANTES promoter.
CHROMATIN REMODELING
Proteins in the transcription complex contribute to chromatin remodeling; acetylation, phosphorylation and methylation of chromatin are involved in ‘late’ expression of RANTES in T lymphocytes.20 The observed binding of CBP/p300 to the RANTES promoter indicated that chromatin remodeling might be important in ‘late’ expression of RANTES in T lymphocytes. A number of enzymes with acetylase, methylase and phosphorylase activities that bind to transcription factor complexes and modify chromatin structure have been described. KLF13 is a ‘lynchpin’ for recruitment of numerous factors involved in chromatin remodeling. These factors open RANTES promoter DNA, permitting transcription (Figure 4).
Figure 4.
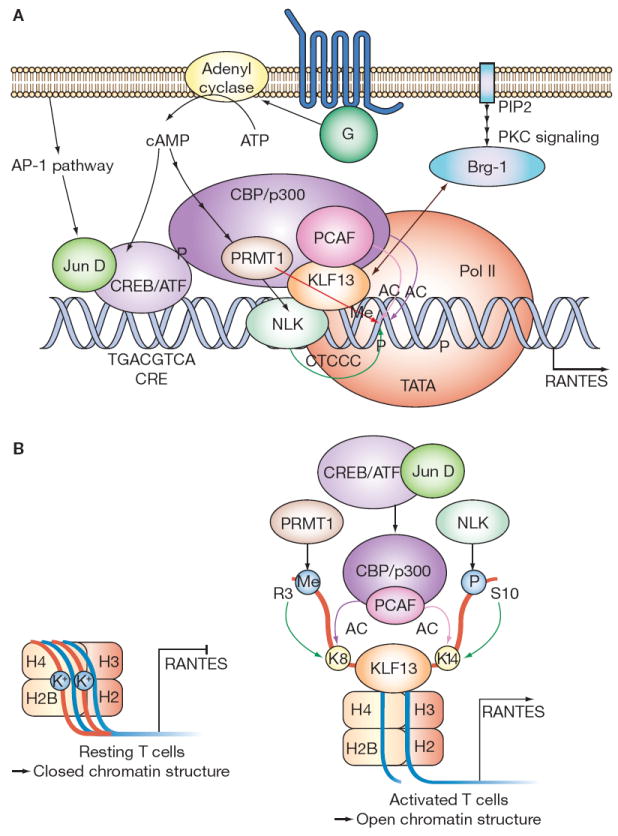
Model of molecular interactions involved in RANTES transcription. See text for details. (A) Signal transduction and the RANTES enhancesome. (B) Chromatin state in resting and activated T lymphocytes. Abbreviations: AC, acetylation; AP-1, activator protein 1; Brg-1, Brahma-related gene 1 protein (ATPase); cAMP, cyclic AMP; CBP/p300, cyclic AMP response element binding protein/p300; CRE, cyclic AMP response element; CREB/ATF, phospho-cAMP response element binding protein/activation transcription factor; G, G protein; H, histone; Jun D, transcription factor jun-D; K+, potassium; K8, lysine at amino acid 8; K14, lysine at amino acid 14; KLF13, Krueppel-like factor 13; Me, methylation; NLK, Nemo-like kinase; P, phosphorylation; PCAF, p300/CBP-associated factor; PIP2, phosphoinositol phosphate 2; PKC, protein kinase C; Pol II, polymerase II; PRMT1, protein arginine N-methyltransferase 1; RANTES, regulated upon activation, normal T cell expressed and secreted; R3, arginine at amino acid 3; S10, serine at amino acid 10.
Approximately 3 days after activation of T lympho cytes via their antigen-specific T cell receptors in the context of co-stimulation,24 KLF13 binds its core binding element on the RANTES promoter. A MAP kinase, NLK (Nemo-like kinase), binds KLF13 and phosphorylates KLF13 and chromatin. The CREB/ATF/jun-D (phospho-cAMP response element binding protein/activation transcription factor/jun-D) complex binds a nearby CRE (cyclic AMP response element) on the RANTES promoter and recruits CBP/p300. PCAF (p300/CBP-associated factor)20 and PRMT1 (protein arginine N-methyltransferase 1) are in turn recruited to the RANTES promoter by CBP/p300. The ATPase BRG-1 (Brahma-related gene protein 1) associates with the RANTES promoter via interaction with KLF13, resulting in recruitment of polymerase II to the TATA box and initiation of RANTES transcription (Figure 4A).
In resting T lymphocytes, RANTES chromatin is in closed conformation (heterochromatin) and the promoter is transcriptionally silent (Figure 4B, left panel). After T lymphocyte activation, the series of molecular interactions described above leads to phosphorylation of histone H3 by NLK, acetylation of histone H3 by CBP/p300, methylation of histone H4 by PRMT1, and acetylation of histone H4 by PCAF (Figure 4B, right panel). This initiates ATP-dependent chromatin remodeling at the A site of the RANTES promoter in association with KLF13 and BRG-1. ATP-dependent remodeling twists and/or deforms chromatin into an open conformation (euchromatin) that is transcriptionally active, permitting binding of polymerase II to the TATA box and transcription of RANTES.
CONCLUSIONS
The ‘new biology’ applied to identification and development of novel pharmaceuticals depends upon a linear understanding of signal transduction. Studies of the regulation of RANTES have revealed complex molecular interactions and biological pathways. Single agents intended to interrupt binding between any two molecules might not be efficacious in such pathways. Most of the drugs in current use are ‘biologics’ (drugs first identified in living organisms, e.g. steroids, ciclosporin, tacrolimus and sirolimus) that act at several levels within cellular pathways. Biologics undoubtedly have more-complex mechanisms of action than are presently understood, and exert effects at several different points in signal transduction pathways. New computer-based approaches to systems biology, and appreciation of the multifactorial nature of cell signaling and function-associated pathways, might be required for rational design of new drugs to interrupt or mimic components of biological systems.
The studies detailed here are part of a new chapter in the search for novel therapies. Their immediate relevance to clinical disease in general, and renal disease in particular, is as a contribution to a basic understanding of the role of epigenetic mechanisms in regulating gene expression. Nonheritable modifications—including methylation, acetylation, and phosphorylation of histone—contribute to gene expression and silencing. As epigenetic alterations are reversible, drugs can and have been designed to impact gene expression and effector function. Histone deacetylase inhibitors are already emerging as potential therapeutics for cancer and inflammation. New drugs are in the pipeline; their ultimate utility as therapeutics will depend upon their safety and specificity of action.
KEY POINTS.
Manipulating expression of chemokines—key regulators of immune cell movement—such as RANTES (CCL5) might prove beneficial for a range of renal diseases such as acute renal failure, nephritis, nephropathy of various etiologies, and transplant rejection
Key regulators of RANTES (CCL5) expression include rel proteins (in fibroblasts and monocytes), and an ‘enhancesome complex’ comprising KLF13 (Krueppel-like factor 13), rel proteins and scaffolding proteins (in T lymphocytes)
In T lymphocytes, acetylation, phosphorylation and methylation of chromatin are key regulators of RANTES (CCL5) expression
Understanding of the complex mechanisms that control expression of chemokines such as RANTES (CCL5) is driving the rational design of new therapeutics
Acknowledgments
This work was supported by NIH R37 DK35008-23.
Footnotes
Competing interests AM Krensky holds patents involving RANTES transcription and KLF13. See the article online for full details. Y-T Ahn declared he has no competing interests.
References
- 1.Ebert LM, et al. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Wiedermann CJ, et al. Monocyte haptotaxis induced by the RANTES chemokine. Curr Biol. 1993;3:735–739. doi: 10.1016/0960-9822(93)90020-o. [DOI] [PubMed] [Google Scholar]
- 3.Johnson Z, et al. Multi-faceted strategies to combat disease by interference with the chemokine system. Trends Immunol. 2005;26:268–274. doi: 10.1016/j.it.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Coelho AL, et al. Chemokines provide the sustained inflammatory bridge between innate and acquired immunity. Cytokine Growth Factor Rev. 2005;16:553–560. doi: 10.1016/j.cytogfr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Schall TJ, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 6.Nelson PJ, Krensky AM. Chemokines, chemokine receptors, and allograft rejection. Immunity. 2001;14:377–386. doi: 10.1016/s1074-7613(01)00118-2. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PJ, Krensky AM. Chemokines, lymphocytes and viruses: what goes around, comes around. Curr Opin Immunol. 1998;10:265–270. doi: 10.1016/s0952-7915(98)80164-7. [DOI] [PubMed] [Google Scholar]
- 8.Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol. 2003;15:5–14. doi: 10.1016/s1044-5323(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 9.Krensky AM. Biology and therapeutic implications of the chemokine RANTES. ACI International. 1999;11:16–21. [Google Scholar]
- 10.Song A, et al. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol Rev. 2000;177:236–245. doi: 10.1034/j.1600-065x.2000.17610.x. [DOI] [PubMed] [Google Scholar]
- 11.Jongstra J, et al. The isolation and sequence of a novel gene from a human functional T cell line. J Exp Med. 1987;165:601–614. doi: 10.1084/jem.165.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayberger C, Krensky AM. Granulysin. Curr Opin Immunol. 2003;15:560–565. doi: 10.1016/s0952-7915(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 13.Pattison J, et al. RANTES chemokine expression in cell-mediated transplant rejection of the kidney. Lancet. 1994;343:209–211. doi: 10.1016/s0140-6736(94)90992-x. [DOI] [PubMed] [Google Scholar]
- 14.Zheng G, et al. The role of tubulointerstitial inflammation. Kidney Int. 2005;(Suppl 94):S96–S100. doi: 10.1111/j.1523-1755.2005.09423.x. [DOI] [PubMed] [Google Scholar]
- 15.Song A, et al. RFLAT-1: a new zinc finger transcription factor that activates RANTES gene expression in T lymphocytes. Immunity. 1999;10:93–103. doi: 10.1016/s1074-7613(00)80010-2. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz BD, et al. Kinetics of transcription factors regulating the RANTES chemokine gene reveal a developmental switch in nuclear events during T-lymphocyte maturation. Mol Cell Biol. 1996;16:202–210. doi: 10.1128/mcb.16.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz BD, et al. Switching gears during T-cell maturation: RANTES and late transcription. Immunol Today. 1997;18:468–471. doi: 10.1016/s0167-5699(97)01128-6. [DOI] [PubMed] [Google Scholar]
- 18.Nikolcheva T, et al. A translational rheostat for RFLAT-1 regulates RANTES expression in T lymphocytes. J Clin Invest. 2002;110:119–126. doi: 10.1172/JCI15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imataka H, et al. Cell-specific translational control of transcription factor BTEB expression: the role of an upstream AUG in the 5′-untranslated region. J Biol Chem. 1994;269:20668–20673. [PubMed] [Google Scholar]
- 20.Ahn Y-T, et al. Dynamics of chromatin remodeling regulate late expression of the chemokine RANTES. Mol Cell Biol. 27:253–266. doi: 10.1128/MCB.01071-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rekdal C, et al. The nuclear factor SPBP contains different functional domains and stimulates the activity of various transcriptional activators. J Biol Chem. 2000;275:40288–40300. doi: 10.1074/jbc.M006978200. [DOI] [PubMed] [Google Scholar]
- 22.Xia M, et al. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol. 1996;156:160–167. [PubMed] [Google Scholar]
- 23.Miyamoto NG, et al. Interleukin-1β induction of the chemokine RANTES promoter in the human astrocytoma line CH235 requires both constitutive and inducible transcription factors. J Neuroimmunol. 2000;105:78–90. doi: 10.1016/s0165-5728(00)00195-8. [DOI] [PubMed] [Google Scholar]
- 24.Krensky AM, et al. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med. 1990;322:510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 25.Li S, et al. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289:F469–F480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 26.Schadde E, et al. Expression of chemokines and their receptors in nephrotoxic serum nephritis. Nephrol Dial Transplant. 2000;15:1046–1053. doi: 10.1093/ndt/15.7.1046. [DOI] [PubMed] [Google Scholar]
- 27.Roson MI, et al. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats. Kidney Int. 2006;70:1439–1446. doi: 10.1038/sj.ki.5001831. [DOI] [PubMed] [Google Scholar]
- 28.Danoff TM. Chemokines in interstitial injury. Kidney Int. 1998;53:1807–1808. doi: 10.1046/j.1523-1755.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 29.Kimmel PL, et al. Upregulation of MHC class II, interferon-α and interferon-γ receptor protein expression in HIV-associated nephropathy. Nephrol Dial Transplant. 2003;18:285–292. doi: 10.1093/ndt/18.2.285. [DOI] [PubMed] [Google Scholar]
- 30.Hertting O, et al. Enhanced chemokine response in experimental acute Escherichia coli pyelonephritis in IL-1β-deficient mice. Clin Exp Immunol. 2003;131:225–233. doi: 10.1046/j.1365-2249.2003.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crisman JM, et al. Chemokine expression in the obstructed kidney. Exp Nephrol. 2001;9:241–248. doi: 10.1159/000052618. [DOI] [PubMed] [Google Scholar]
- 32.Vielhauer V, et al. Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- 33.Anders HJ, et al. CC chemokine ligand 5/ RANTES chemokine antagonists aggravate glomerulonephritis despite reduction of glomerular leukocyte infiltration. J Immunol. 2003;170:5658–5666. doi: 10.4049/jimmunol.170.11.5658. [DOI] [PubMed] [Google Scholar]
- 34.Anders HJ, et al. Chemokine and chemokine receptor expression during initiation and resolution of immune complex glomerulonephritis. J Am Soc Nephrol. 2001;12:919–931. doi: 10.1681/ASN.V125919. [DOI] [PubMed] [Google Scholar]
- 35.Furuichi K, et al. Distinct expression of CCR1 and CCR5 in glomerular and interstitial lesions of human glomerular diseases. Am J Nephrol. 2000;20:291–299. doi: 10.1159/000013603. [DOI] [PubMed] [Google Scholar]
- 36.Cockwell P, et al. In situ analysis of C–C chemokine mRNA in human glomerulonephritis. Kidney Int. 1998;54:827–836. doi: 10.1046/j.1523-1755.1998.00053.x. [DOI] [PubMed] [Google Scholar]
- 37.Chan RW, et al. Messenger RNA expression of RANTES in the urinary sediment of patients with lupus nephritis. Nephrology (Carlton) 2006;11:219–225. doi: 10.1111/j.1440-1797.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 38.Ye DQ, et al. Polymorphisms in the promoter region of RANTES in Han Chinese and their relationship with systemic lupus erythematosus. Arch Dermatol Res. 2005;297:108–113. doi: 10.1007/s00403-005-0581-9. [DOI] [PubMed] [Google Scholar]
- 39.Wornle M, et al. Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol. 2006;168:370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagrowska-Danilewicz M, et al. CC chemokines and chemokine receptors in IgA nephropathy (IgAN) and in non-IgA mesangial proliferative glomerulonephritis (MesProGN): the immunohistochemical comparative study. Pol J Pathol. 2005;56:121–126. [PubMed] [Google Scholar]
- 41.Lim CS, et al. Th1/Th2 predominance and proinflammatory cytokines determine the clinicopathological severity of IgA nephropathy. Nephrol Dial Transplant. 2001;16:269–275. doi: 10.1093/ndt/16.2.269. [DOI] [PubMed] [Google Scholar]
- 42.Strehlau J, et al. Activated intrarenal transcription of CTL-effectors and TGF-β1 in children with focal segmental glomerulosclerosis. Kidney Int. 2002;61:90–95. doi: 10.1046/j.1523-1755.2002.00090.x. [DOI] [PubMed] [Google Scholar]
- 43.Mlynarski WM, et al. Risk of diabetic nephropathy in type 1 diabetes is associated with functional polymorphisms in RANTES receptor gene (CCR5): a sex-specific effect. Diabetes. 2005;54:3331–3335. doi: 10.2337/diabetes.54.11.3331. [DOI] [PubMed] [Google Scholar]
- 44.Wang SN, et al. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–1014. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 45.Mezzano SA, et al. Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy. Kidney Int. 2000;57:147–158. doi: 10.1046/j.1523-1755.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- 46.Le Berre L, et al. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005;68:2079–2090. doi: 10.1111/j.1523-1755.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 47.Torheim EA, et al. Increased expression of chemokines in patients with Wegener’s granulomatosis—modulating effects of methylprednisolone in vitro. Clin Exp Immunol. 2005;140:376–383. doi: 10.1111/j.1365-2249.2005.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Y, et al. Relative importance of CCR5 and antineutrophil cytoplasmic antibodies in patients with Wegener’s granulomatosis. J Rheumatol. 2003;30:1541–1547. [PubMed] [Google Scholar]
- 49.Coulomb-L’Hermine A, et al. Expression of the chemokine RANTES in pulmonary Wegener’s granulomatosis. Human Pathol. 2001;32:320–326. doi: 10.1053/hupa.2001.22757. [DOI] [PubMed] [Google Scholar]
- 50.Liu B-C, et al. Application of antibody array technology in the analysis of urinary cytokine profiles in patients with chronic kidney disease. Am J Nephrol. 2006;26:483–490. doi: 10.1159/000096871. [DOI] [PubMed] [Google Scholar]
- 51.Pawlak K, et al. Hepatitis intensified oxidative stress, MIP-1β and RANTES plasma levels in uraemic patients. Cytokine. 2004;28:197–204. doi: 10.1016/j.cyto.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Pawlak K, et al. Oxidative stress influences CC-chemokine levels in hemodialyzed patients. Nephron Physiol. 2004;96:105–112. doi: 10.1159/000077381. [DOI] [PubMed] [Google Scholar]
- 53.Corsi MM, et al. RANTES and MCP-1 chemokine plasma levels in chronic renal transplant dysfunction and chronic renal failure. Clin Biochem. 1999;32:455–460. doi: 10.1016/s0009-9120(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 54.Zheng F, et al. The glomerulosclerosis of aging in females: contribution of the proinflammatory mesangial cell phenotype to macrophage infiltration. Am J Pathol. 2004;165:1789–1798. doi: 10.1016/S0002-9440(10)63434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruster M, et al. Differential expression of beta-chemokines MCP-1 and RANTES and their receptors CCR1, CCR2, CCR5 in acute rejection and chronic allograft nephropathy of human renal allografts. Clin Nephrol. 2004;61:30–39. doi: 10.5414/cnp61030. [DOI] [PubMed] [Google Scholar]
- 56.Falkensammer C, et al. IL-4 inhibits the TNF-α induced proliferation of renal cell carcinoma (RCC) and cooperates with TNF-α to induce apoptotic and cytokine responses by RCC: implications for antitumor immune responses. Cancer Immunol Immunother. 2006;55:1228–1237. doi: 10.1007/s00262-006-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kondo T, et al. High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. J Urol. 2004;171:2171–2175. doi: 10.1097/01.ju.0000127726.25609.87. [DOI] [PubMed] [Google Scholar]


