Abstract
The skeletal system, while characterized by a hard tissue component, is in fact an extraordinarily dynamic system, with disparate functions ranging from structural support, movement and locomotion and soft-organ protection, to the maintenance of calcium homeostasis. Amongst these functions, it has long been known that mammalian bones house definitive hematopoiesis. In fact, several data demonstrate that the bone microenvironment provides essential regulatory cues to the hematopoietic system. In particular, interactions between the bone forming cells, or osteoblasts, and the most primitive hematopoietic stem cells (HSC) have recently been defined. This review will focus mainly on the role of osteoblasts as HSC regulatory cells, discussing the signaling mechanisms and molecules currently thought to be involved in their modulation of HSC behavior. We will then review additional cellular components of the HSC niche, including endothelial cells and osteoclasts. Finally, we will discuss the potential clinical implications of our emerging understanding of the complex HSC microenvironment.
Examples of Stem Cell Niches
The concept of a regulatory environment determining stem cell fate choices was initially proposed in the 1970’s, when Schofield hypothesized a structure that housed stem cells and provided regulation of self-renewal and proliferation, since transplantation of HSC into recipient animals resulted in only limited HSC expansion [1]. Although the idea of the niche was proposed for HSC, the first stem cell niches characterized were in Drosophila gonads and in Caenorhabditis elegans [2; 3; 4]. Studies of the ovary and testis of Drosophila determined that a stem cell niche functions to protect stem cells from differentiation, apoptotic and proliferation signals that would deplete the reservoir of stem cells or cause excessive production, leading to tumorigenesis. Early studies in Drosophila demonstrated that the niche is composed of cells that physically contact the germline stem cells through adhesion molecules that are able to transduce signals to the stem cells to regulate their behavior [4; 5]. Further, it was noted that the Drosophila ovary is capable of reprogramming cells to become stem cells [2; 6], underscoring the regulatory effect of the niche on its resident cells.
Besides the bone marrow niche for HSC, other stem cells niches have been defined in mammals, such as the intestinal crypts and the hair follicle. The intestinal crypt structure houses intestinal stem cells (ISC), which can regenerate the entire villus, consisting of 4 cells types, every 3–5 days [7]. Within the crypt, the ISC are located in a niche near the base, where they are regulated by paracrine factors secreted from surrounding mesenchymal cells [8]. Asymmetric cell division of the ISC generate progeny cells, the Transit Amplifying cells, which begin to migrate upwards and are committed to differentiating into a single lineage [9]. Thus, intestinal stem cells and their progeny integrate spatial cues with signals from the microenvironment to regulate their behavior.
The hair follicle provides a niche for hair follicle stem cells (HFSC) that are located in the outer root sheath, called the bulge, and generate hair and sebaceous glands [10]. In the hair follicle, HFSC are surrounded by epidermal and dermal cells, and it is thought that dermal cells provide most of the regulatory signals for the stem cells. Like ISC, HFSC receive spatial and temporal cues, and with each hair cycle, the follicle rearranges to bring the HFSC closer to the dermal papillae which activates the stem cells to generate a new hair structure [11]. The highly conserved Wnt and Bone Morphogenic Protein (BMP) signaling pathways play important roles in regulating the quiescence and activation of HFSC [9].
Barriers to HSC niche identification
Currently, HSC are the best characterized stem cells and were the first to be used routinely for therapeutic purposes. Clinical experience has shown that HSC number is an important limiting factor in treatment success [12; 13]. Strategies to expand HSC are of great clinical appeal since they would improve therapeutic use of these cells in stem cell transplantation and in conditions of bone marrow failure. Current technology enables identification and prospective isolation of HSC utilizing surface markers and flow cytometric analysis and sorting. Virtually all HSC activity is contained in the subset of cells that are negative for lineage markers (lin−), positive for Sca-1 and c-Kit, or LSK cells [14; 15; 16; 17]. HSC can also be quantified in vitro using a long term co-culture technique referred to as Long Term Culture-Initiating Cells (or LTC-ICs) Assay. The gold standard of HSC identification is the competitive repopulation assay, in which the ability of HSC to reconstitute all hematopoietic lineages is tested in vivo. Unfortunately, detailed description of the microenvironment that regulates HSC has been difficult due to the complex anatomy of bone and challenges in histological analysis. Moreover, identification of HSC has relied upon well-established but cumbersome in vivo or in vitro assays, or prospective isolation by flow cytometric analysis. While flow cytometric analysis and sorting have been extraordinarily effective in improving our understanding of HSC biology, until recently they have required a combination of markers for HSC identification. As discussed above, this method is not feasible for in situ identification of HSC in the bone marrow microenvironment. In spite of these hurdles, there have been many advances in the past few years that have shed light on the cellular and molecular components of the HSC niche.
Osteoblast as HSC regulatory cell
The intimate physical association between hematopoietic stem cells and osteoblasts prompted several groups to investigate whether osteoblasts could provide regulatory support to hematopoietic cells. Human osteoblasts were shown to produce several hematopoietic cytokines such as G-CSF, GM-CSF and LIF [18; 19] and to be able to support hematopoietic cells in vitro and maintain LTC-ICs [20]. Further, co-transplantation of osteoblasts purified from murine long bones, but not dendritic cells, with lin− bone marrow cells enhanced engraftment and lymphohematopoietic reconstitution in lethally irradiated mice [21]. These data, combined with imaging of labeled transplanted HSC showing that engraftment of the most primitive HSC occurred near the endosteum [22], suggested that osteoblasts lining the endosteal surface of the trabecular bone could serve as the primary support for HSC.
Our laboratory used transgenic mice with a constitutively active Parathyroid Hormone Receptor (PTH1R) under control of the 2.3 kb fragment of the Iα(1) collagen gene promoter (Col1-caPTH1R mice) to demonstrate the importance of osteoblasts in the HSC niche. Col1-caPTH1R mice had increased osteoblast number and trabecular bone and also a parallel increase in HSC number. Likewise, systemic intermittent treatment with Parathyroid Hormone (PTH) expanded HSC in vivo. Since the PTH1R is not expressed in HSC [23], this effect also implies a PTH-dependent niche effect. In vitro, the addition of PTH to stromal cultures increased the number of osteoblasts and their ability to support hematopoietic cells. Further, treatment of mice with PTH after bone marrow transplantation conferred a survival advantage compared to vehicle-treated mice [24]. At the same time, Zhang et al. demonstrated that conditional inactivation of BMP receptor IA, which is not expressed in HSC, also resulted in an increase in osteoblast and long-term HSC [25]. In vivo labeling in this study suggested that HSC may be in direct contact with spindle shaped N-cadherin+ osteoblasts lining the endosteal surface. These two genetic models further strengthened the idea that osteoblasts could serve as the regulatory component of the HSC niche. Additionally, ablation of osteoblasts via gancyclovir treatment of transgenic mice expressing herpes virus thymidine kinase under the Iα collagen gene promoter caused loss of osteoblasts, followed by a marked decrease in bone marrow cellularity with a loss of myeloid, lymphoid and erythroid progenitors and a switch to extra-medullary hematopoiesis in the spleen [26]. Although in this latter study the massive number of dying osteoblasts may have had non-specific toxic effects on hematopoietic cells in the local microenvironment, these results provide further support for the role of osteoblasts in the hematopoietic stem cell niche.
While these data support the role of osteoblastic cells as HSC regulators, the differentiation stage of this HSC-supporting osteoblast is currently unknown, and it is certainly possible that osteoblastic precursor cells may also be important niche components. In fact, recent data suggest that certain human mesenchymal stem cells may expand ex vivo neonatal cord blood HSC [27] and adult human peripheral blood progenitor cells [28].
Mesenchymal stem cells can differentiate into many cell types including chondrocytes, osteoblasts and adipocytes [29]. The regulation of this differentiation will alter the osteoblastic pool, and may preferentially expand other differentiated cell types, which are abundantly present in the bone marrow microenvironment, such as adipocytes. The role of adipocytes in HSC regulation is still poorly understood, however, emerging data suggest that adipocytes may produce essential chemokines capable of regulating HSC behavior [30]. Further studies are required to determine whether the adipocytic population in the bone marrow provides regulation of HSC.
Other cellular components
Thus far, we have discussed compelling evidence for osteoblasts serving as the main regulatory component in the HSC niche. However, in the complex and heterogeneous bone marrow microenvironment, it is possible and even likely that other cellular components may modulate the activity of HSC. It is clear that HSC can be supported by cells other than osteoblasts, since hematopoiesis occurs in other sites during embryogenesis [31] and extramedullary hematopoiesis can occur in pathologic situations in adults [32].
While few HSC are generally found circulating in the blood, they can be rapidly mobilized within minutes of treatment with cytokines, and it has been suggested that the proximity of the HSC to endothelial cells may determine this phenomenon [33]. Several studies now lend support to the importance of the endothelial cell in regulating stem cells. For instance, neural stem cells are located near endothelial cells in two different niches within the brain [34], [35] and endothelial cells can activate Notch in neural precursors [36]. In terms of HSC regulation, Kiel et al. used cell surface receptors of the SLAM family, CD150, CD244 and CD48 to identify different subsets of HSC in tissue sections and found that 60% of the HSC identified in bone were associated with sinusoidal endothelium whereas only 14% were attached to the endosteum [37]. However, proximity does not necessarily equal functional interaction and regulation. Additionally, in vivo confocal imaging of mouse bone marrow in order to determine the microenvironment that supports malignant metastasis demonstrated that normal HSC and hematopoietic progenitors could also localize to microdomains of the endothelium that expressed certain adhesion molecules [38], rather than at endosteal sites. One caveat of these results is highlighted by a recent study illustrating restriction of engraftment of transplanted hematopoietic cells by the endogenous host cells occupying HSC niches, and that clearance with antibodies to c-kit can increase engraftment rates [39]. Thus, it is possible that the endogenous HSC were occupying the endosteal niches in the study by Sipkins et al., causing the transplanted HSC to subsequently home to the endothelial niche. Although HSC can clearly be found in close vicinity to endothelial cells and more mature hematopoietic progenitors are supported by the vasculature, it still remains to be determined if endothelial cells are actually regulating the function of HSC.
Another means for the organism to exert control over HSC is through cells of the nervous system. Katayama et al. noted that mice which lack the ability to make galactocerebrosides, components of myelin sheaths, not only had defects in nerve conduction but also had no egress of HSC in response to G-CSF. This prompted investigation into the role of the nervous system in mobilization of hematopoietic cells, and norepinephrine signaling via the sympathetic nervous system was found to be responsible for G-CSF induced suppression of osteoblasts and subsequent decrease in expression of the chemokine CXCL12 (previously known as SDF-1), resulting in HSC mobilization [40]. Thus, it appears that the sympathetic nervous system modulates the egression of hematopoietic cells from the bone marrow which provides an intriguing opportunity for intervention in order to increase the efficiency of mobilization.
Osteoclasts also serve as potential HSC regulators. A study by Kollet et al. demonstrated that activation of osteoclasts by RANKL or a stress response resulted in Cathepsin K-meditated cleavage of CXCL12 causing mobilization of immature progenitor cells into the circulation [41]. Thus, activation of osteoclasts could increase the efficiency of mobilization by cytokine treatment. Interestingly, treatment with strontium, a bone anabolic agent which increases osteoblastic number and inhibits osteoclasts, delayed hematopoietic recovery in mice receiving bone marrow transplantation [42]. These data point out that global osteoblastic expansion is not sufficient to expand HSC, and may actually suggest a role for osteoclastic regulation of HSC.
While much data have recently been collected defining the HSC niche in murine models, its human counterpart had not been explored. However, recent xenograft experiments have suggested that human bone marrow derived CD146+ cells may be essential for initiation of the hematopoietic microenvironment [43]. Further studies are now necessary to fully elucidate the existence and characteristics of the human HSC niche.
Signaling within the niche
Regulation of the stem cell niche entails creating a balance between self-renewal and differentiation in order to establish a reservoir of stem cells while still supplying adequate numbers of mature hematopoietic cells to the organism. One potential way in which the osteoblast could modulate this balance is through Notch signaling. The increase in HSC in the Col1-caPTH1R mice may be Notch dependent, since activation of Notch1 was observed in LSK cells and treatment with a γ-secretase inhibitor abrogated the expansion of stem cells [24]. Weber et al. demonstrated that treatment of mice with intermittent PTH caused an increase in the Notch ligand Jagged1 in specific populations of osteoblasts, namely the trabecular and endosteal osteoblasts and spindle shaped cells in the bone marrow cavity, but not periosteal osteoblasts or osteocytes. Although the G-protein coupled PTH1R is linked to both the Adenylate Cyclase/Protein Kinase A (AC/PKA) and Protein Lipase C/Protein Kinase C (PLC/PKC) signaling pathways, PTH stimulation of Jagged1 occurs mainly through the AC/PKA pathways [44].
Notch signaling has long been implicated in decision making between self-renewal and differentiation, and Notch activation by its ligands results in transcriptional repression of genes that are necessary for differentiation [45], [46]. Specifically, Notch signaling has been defined as an important signaling pathway in HSC self-renewal [47; 48; 49; 50; 51; 52]. We focused on Notch and on the Notch ligand Jagged1, which is sufficient for stroma-dependent expansion of human HSC [53]. Osteoblasts, through activation of PTH1R and increased production of Jagged1, could induce Notch signaling in HSC, and thus influence HSC to self-renew in order to maintain a steady pool of stem cells. However, Notch ligands and receptors are expressed in both stromal/osteoblastic and hematopoietic components of the bone marrow microenvironment. In fact, ongoing studies in our laboratory and others have begun to elucidate the importance of Notch signaling in osteoclastic and osteoblastic cells [54; 55; 56; 57] [44; 58; 59; 60; 61]. It is therefore possible that local changes in Notch signaling may affect both the stromal/osteoblastic and the hematopoietic component of the bone marrow microenvironment. Hence, further studies are required to define the likely complex role of specific Notch signaling partners in niche and stem cells in order to fully elucidate their involvement in the cellular and molecular mechanisms mediating the osteoblastic regulation of HSC behavior.
In addition to Notch signaling, other pathways such as Wnt signaling provide a mechanism for modulation of the stem cell niche. It has been well established that Wnt signaling plays an important role in self-renewal of HSC [62], [63]. The source of these Wnt proteins that transduce signals in HSC could be multiple. One recent study demonstrates how osteoblasts use Wnt signaling to control the differentiation fate of mesenchymal progenitor cells [64]; thus it is plausible that osteoblasts or other cells within the niche could be exerting a similar type of control on HSC through Wnt signaling. In addition, Wnt signaling could modify osteoblastic differentiation thus modifying their ability to support HSC.
Recent data have also demonstrated that non-canonical Wnt 5a, by antagonizing the canonical Wnt pathway, can regulate HSC and enhance repopulation [65], suggesting a major role also for non-canonical Wnt signaling in HSC regulation imposed by the microenvironment. Similarly, in vivo treatment of mice transplanted with human hematopoietic repopulating cells (or SCID-repopulating cells) with Wnt-5a conditioned medium showed a 3-fold enhancement in engraftment of human cells compared to control treatment, and the most primitive repopulating cells were preferentially expanded [66].
Data supporting the importance of both Notch and Wnt signaling in the self-renewal of HSC indicate that these pathways may act either synergistically or in distinct ways to increase HSC numbers. Duncan et al. established that the two aspects of self-renewal, namely proliferation and inhibition of differentiation, can be separated. Their data demonstrated that Notch signaling plays a dominant role in the process by inhibiting differentiation, and that Wnt signaling could maintain the undifferentiated cells in a Notch-dependent manner [67].
As described above, osteoblasts or other stromal cells appear to direct self-renewal of HSC through the Notch and Wnt signaling pathways. This is important for maintaining an adequate supply of stem cells to maintain the entire hematopoietic lineage for the life of the organism. However, unchecked proliferation of primitive cells may result in tumorigenesis. Therefore, mechanisms must exist to restrain the self-renewal signals that HSC receive. Indeed, the BMP pathway has been shown to play a role in limiting cell number of both hematopoietic stem cells and intestinal stem cells. For intestinal stem cells, BMP signaling acts directly by attenuating Wnt/β-catenin signals [68]. In the case of HSC, BMP signaling is thought to work indirectly by limiting the number of niches available to support HSC [68],[69] [25]. Figure 1 summarizes some of the cellular interactions and signaling pathways that have been identified in HSC/osteoblastic interactions.
Figure 1. Summary of the major signaling pathways involved in regulating self-renewal and differentiation of HSC.
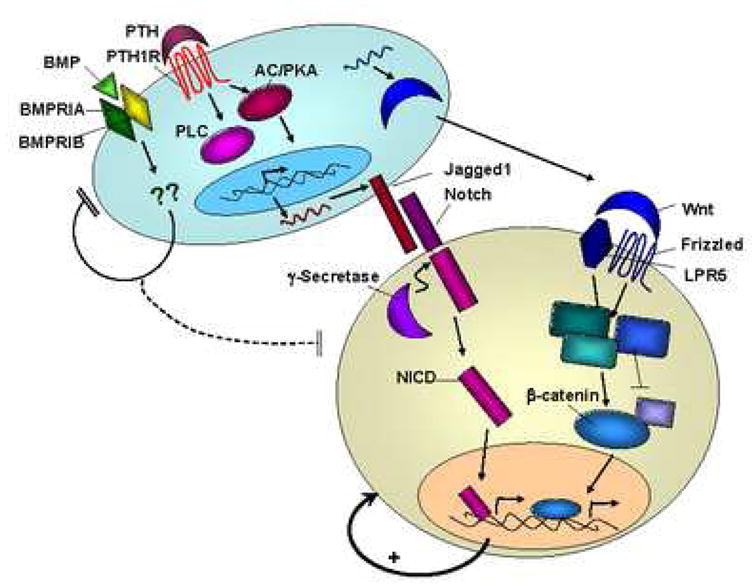
PTH signaling through the PTH1R on osteoblasts (blue cell) acts via PKA and PLC to increase expression of the Notch ligand Jagged1, which can then interact with Notch receptors on HSC (tan cell). Notch signaling within the HSC causes cleavage of the Notch intracellular domain (NICD) which translocates to the nucleus and results in self-renewal of the HSC. Wnt proteins produced by osteoblasts interact with receptors on HSC such as Frizzled and LPR5 and also induce self-renewal via β-catenin. In contrast to these self-renewal signals, BMP signaling in osteoblasts is thought to indirectly inhibit proliferation of HSC by limiting the niche cells available to support HSC.
Molecules produced by osteoblasts that may contribute to HSC regulation
In addition to the signaling pathways described above, HSC also respond to bone-specific signals from osteoblasts such as the matrix glycoprotein osteopontin (OPN). OPN is produced by osteoblasts, especially in the endosteum and in areas of active bone remodeling, in amounts which vary with the activation state of the cell [70]. HSC express several of the receptors that OPN interacts with, namely CD44, α4 and α5β1 integrins [71], [72]. Mice deficient in OPN and stimulated with PTH demonstrated an accentuated increase in HSC, indicating that OPN normally acts to negatively constrain the amount of HSC in the stem cell niche [73]. Further confirming this role, Nilsson et al. demonstrated a marked increase in Bromodeoxyuridine (BrdU) incorporation into HSC from OPN−/−mice compared to wild type, and a suppression of cycling during exogenous treatment with OPN in vitro [74]. Taken together, these data underscore the importance of extracellular matrix components as a functional part of the HSC niche.
Annexin II (Anxa2) may provide another protein adhesive interaction between osteoblasts and HSC. Jung et al. found that Annexin II is expressed by both osteoblasts and HSC and that the N-terminal portion of the peptides interact to adhere the cells together. Further, Anxa2−/− mice have fewer HSC in the bone marrow and exhibited less efficient engraftment and survival of irradiated mice transplanted with whole bone marrow [75]. Thus, Anxa2 appears to play a critical role in HSC homing and may function in the maintenance of HSC in the niche.
The niche is responsible for both attracting HSC to the bone marrow, during fetal development and during bone marrow transplantation, and maintaining the cells there once they arrive. The receptor tyrosine kinase Tie2 and its ligand Angiopoietin-1 (Ang-1) are important in these processes. Tie2 is expressed in HSC, and long-term repopulating activity was found to be in the Tie2+ population of HSC in the fetal liver [76]. Mice lacking Tie1 and Tie2 receptors did not have any fetal hematopoiesis defects, but were unable to maintain stem cells in the adult bone marrow [77]. Ang-1 is produced by osteoblasts [78] and the interaction of Tie2 on HSC with Ang-1 induces HSC quiescence and increases their adherence to bone, resulting in enhanced myeloprotection from 5-fluorouracil treatment [79]. Signaling through Tie2/Ang-1 induces expression of β1 integrin and N-cadherin in Tie2+ HSC [80], which, as discussed below, are interactions that promote quiescence. Thus, Tie2/Ang-1 signaling appears to be critical for maintaining a protected and quiescent stem cell population in the bone marrow.
Non-cellular components of the niche
Structural molecules in the niche must also be involved in tightly binding HSC to the bone lining and in releasing HSC from the microenvironment into the circulation. Integrins, which are heterodimeric molecules composed of an α and a β subunit, have long been known to function in engraftment and mobilization of hematopoietic cells [81]. It was observed that mouse β1−/− hematopoietic progenitors are unable to seed the fetal liver, but that these progenitors function normally in colony-forming unit assays in vitro, suggesting that loss of β1 does not cause a defect in hematopoietic cell function, but rather a defect in homing of the cells [82]. Wagers et al. investigated the expression of different integrins on Lin−/loThy1.1loSca-1+c-kit+ HSC in the bone marrow and in the circulation after mobilization treatment and found that, in general, HSC express high levels of most β1 integrin subunits and that mobilization downregulated α2 and upregulated α5. Further, mobilized cells did not home as well to the bone marrow as did non-mobilized cells, suggesting that these integrin molecules are indeed important in recruiting cells and retaining them in the bone marrow [83].
Zhang et al. defined the osteoblasts that constitute the support cell of the niche as N-cadherin-positive and claimed that N-cadherin is also expressed in a portion of HSC and forms an adherens complex with β-catenin between the osteoblast and HSC [25]. However, two aspects of this hypothesis have recently been highly debated. First, these HSC were identified as BrdU-retaining cells based on the theory that stem cells are more quiescent and divide asymmetrically, thus retaining the BrdU longer than other cells [84], while more recent studies do not support this theory [85]. In addition, further data have challenged the importance of N-cadherin in maintaining HSC within the niche by suggesting that bone marrow cells with HSC activity do not express N-cadherin [86]. This latter observation is in contrast to a number of studies which have found N-Cadherin in cells of the hematopoietic lineage [25; 79; 87]
Also important in HSC homing to the bone marrow and promoting quiescence are CXCL12 and its receptor, CXCR4. CXCL12 is produced by osteoblasts in the bone marrow [88]. CXCL12/CXCR4 are important in HSC retention in the niche [41; 89] and are regulated in osteoblasts by PTH [24, 90]. Mice lacking CXCL12 have fetal hematopoietic defects [91; 92]. CXCL12 and CXCR4 are necessary for hematopoietic repopulation, since treatment of primitive CD34+ human bone marrow cells with an antibody to CXCR4 prevented their engraftment in SCID mice [93]. Further, treatment with a CXCR4 antagonist caused rapid mobilization of mouse long-term repopulating (LTR) cells and human CD34+ cells, along with more mature hematopoietic cells, strongly suggesting that CXCL12/CXCR4 interactions retain HSC in the bone marrow microenvironment [94]. Interestingly, Sugiyama et al. illustrated that conditional deletion of CXCR4 decreased HSC and actually slightly increased more mature hematopoietic progenitors, underscoring the specific role that CXCL12 plays in primitive HSC. In addition, they found that an increased proportion of HSC exited G0 and entered the cell cycle in CXCR4 conditionally deficient mice. Intriguingly, chimeric mice generated from donor CD34−c-kit+Sca-1+ bone marrow cells with the floxed CXCR4 and competitor CD34−c-kit+Sca-1+ bone marrow cells with wild-type CXCR4 had a decreased percentage of HSC in G0 not only in the CXCR4 deficient donor cells, but also in the wild-type competitor cells, suggesting a microenvironmental effect [95].
In contrast to the results of prior studies, Sugiyama et al. analyzed the expression of CXCL12 in the bone marrow using CXCL12 knock-in mice and found a population of reticular cells within the intertrabecular space that abundantly expressed CXCL12, which they named CXCL12-abundant reticular (CAR) cells. Although it was previously reported that osteoblasts lining the trabeculae expressed CXCL12 [88], they found only low levels of expression in osteocalcin positive osteoblasts. However, CAR cells were shown to express high levels of Jagged1, which was previously detected in osteoblasts that supported HSC expansion in Col1-caPTH1R mice [24]. Interestingly, identifying stem cells as Lin−c-kit+sca-1+ or with SLAM receptor markers, shown previously to identify cells enriched for HSC activity [37], demonstrated that most HSC were adjacent to CAR cells. These CAR cells are surrounded by endothelial cells, which could mean that CAR cells are regulating a niche supported by endothelial cells. However, CAR cells have not been fully characterized, nor has their cell of origin been identified.
Additional non-cellular components of the HSC niche
Within the niche, it is clear that osteoblasts and possibly other cells regulate HSC behavior. However, the niche concept is complex, and it involves variables such as extracellular fluid concentrations of electrolytes and local chemical mediators. Unexpectedly, the ability of HSC to sense ambient calcium is crucial to their homing and retention to the bone marrow, suggesting that ionized calcium is also an important HSC regulator. The calcium-sensing receptor (CaR) is present on HSC and HSC lacking the CaR do not properly home. Interestingly, bone is a unique organ in that it has a much higher extracellular calcium concentration than normally found in serum, especially near osteoclasts that are resorbing bone [96]. HSC may have exploited this characteristic and depend on their CaR to properly home and engraft into the endosteal niche [97]. Fluctuations in the calcium concentration within the bone marrow during different physiologic or pathologic states could also influence the activity of HSC via this receptor.
Prostaglandins are arachidonic acid derivatives produced through cyclooxygenase 1 and 2 (COX-1 and COX-2) activation in a variety of tissues, where they are involved in a number of local physiologic and pathologic processes. Osteoblasts produce prostaglandins [98]. Interestingly, PTH is one of the principal stimulators of prostaglandin E2 (PGE2) in osteoblastic cells mainly through COX-2 [99]. Recently, ex vivo treatment of zebrafish bone marrow with chemicals that stimulate production of PGE2 caused an increase in HSC numbers and had a myeloprotective effect on the kidney marrow [100]. Additionally, in vitro and in vivo effects of PGE2 treatment were also observed in murine models (reviewed in [101]), thus PGE2 may act as a local mediator of the HSC niche. Therefore, locally produced PGE2 could mediate some of the effects of PTH-dependent HSC expansion, through niche activation by PGE2, and/or through direct PGE2 effects on the HSC.
Modifying the HSC niche
Understanding the components and regulation of the HSC niche allows for its specific manipulation for therapeutic purposes. This is desirable in situations of bone marrow failure and hematologic malignancies which require bone marrow transplantation. The cellular components, adhesion molecules and signaling pathways discussed above could all serve as potential targets for modifying the bone marrow microenvironment and subsequently the biologic activity of HSC within the niche. For example, intermittent treatment of mice with PTH targets osteoblasts causing not only an anabolic effect on bone but also a parallel increase in HSC [24]. The effect of intermittent PTH on bone metabolism is well known in humans (reviewed in [102]), and clinical studies are now being initiated to investigate whether the hematopoietic effects demonstrated in mice are also present in humans.
As we uncover more components of the HSC niche, we may learn that multiple factors are activated concurrently. For example, PTH increases osteoblastic numbers, potentially increasing HSC niches. It increases local production of the Notch ligand Jagged1, and of CXCL12 [24; 44], and it increases osteoclastic numbers [98]. OPN is also increased [103], and osteoblastic production of PGE2 is stimulated by upregulation of osteoblastic COX-2 [99]. Some of these potential mediators of PTH action on osteoblastic regulation of HSC are summarized in Figure 2. Such level of complexity, especially if these factors contribute similarly to HSC regulation, may make genetic analysis of these interactions particularly challenging.
Figure 2. Summary of potential differential effects of PTH signaling in the HSC niche.
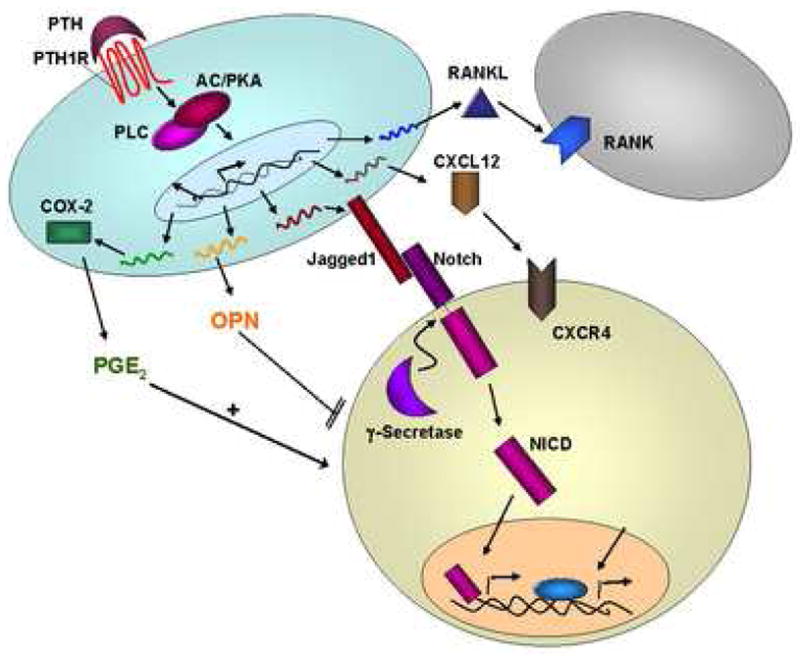
Signaling through the PTH1R on osteoblasts causes an increased production in RANKL, which in turn recruits osteoclasts (gray cell). PTH also increases CXCL12 which can interact with CXCR4 on HSC. Jagged1 expression is upregulated with PTH1R stimulation, which activates Notch signaling in the HSC. Prostaglandin E2 production also increases with PTH signaling, mostly through upregulation of COX-2. Osteopontin (OPN) produced by osteoblasts interacts with receptors on HSC and negatively regulates the number of HSC in the niche.
As more is understood about the molecular and cellular events that regulate the functions of the niche, novel methods of modifying it therapeutically should be revealed. We have used PTH to activate the osteoblasts and expand HSC. A recent study exploited a known interaction between c-kit and Stem Cell Factor and showed that disruption via treatment with an antibody to c-kit caused clearance of endogenous HSC from niches and allowed for more efficient engraftment of cells transplanted into SCID mice [39].
Clinical implications
HSC were the first type of stem cell to be used therapeutically and they are still commonly and successfully used for treating hematologic malignancies and restoring normal hematopoiesis after bone marrow failure. However, challenges in bone marrow transplantation still exist, such as sufficient mobilization for harvesting of HSC in autologous transplants and delayed recovery of mature hematopoietic cell numbers after myeloablation. Further definition of the HSC microenvironment is likely to uncover specialized niches for HSC subsets and studies have already suggested the existence of these niches [35]. Since less quiescent HSC (Short-Term HSC and Multi Potent Progenitors) can more rapidly generate progeny, methods to stimulate specific niches to amplify these cells may expedite recovery from toxic or iatrogenic myeloablation.
In addition to the clinical implications of the niche supporting normal HSC, the niche may be of critical importance in malignant conditions as well. The cancer stem cell (CSC) hypothesis is gaining momentum [104], and there is ample evidence that cancer cells respond to microenvironmental clues [105]. Since strong evidence, reviewed above, supports the role of the niche in stem cell regulation, there is interest in understanding whether a specific CSC niche exists, and whether such a niche shares any characteristics with a normal stem cell niche. The HSC niche may be housing CSC and modification of the niche could prove to be critical in eradication of disease. Alternatively, since the niche can clearly modulate the biological activity of the cells that reside in it, it is possible that it could modify the differentiation state of its resident cells and contribute to the development of malignancy. Further understanding of the CSC may be essential for the management and cure of metastatic disease to bone and bone marrow.
Given our recent progress in understanding the HSC niche, and abundant evidence supporting the existence of Leukemic Stem Cells (LSC) [106], there is currently much interest in determining the differences, if any, between the normal HSC niche and the LSC niche. For example, it is now known that CD44 expression on leukemic cells allows them to attach to the stroma of the bone marrow and that targeting of CD44 can eliminate human Acute Myelogenous Leukemia cells [107], [108]. If the microenvironmental requirements of the LSCs were revealed, these could provide exciting new targets for manipulation of the LSC niche. In addition, as we manipulate the niche to expand HSC, it is essential to consider potential effects on CSC and LSC behavior, since HSC expansion is often required after cancer and leukemia treatment.
Also of clinical importance are the parallels between HSC homing to the niche and tumor metastasis to bone. One such parallel is outlined by recent studies defining the role of CXCL12, a known osteoblastic and endothelial product which regulates HSC homing to the bone marrow, in breast and prostate cancer cells homing to the bone [109; 110]. These data provide exciting new therapeutic venues for metastatic cancer treatment based on a more precise understanding of HSC niche and homing mechanisms.
In conclusion, the extraordinary complexity of the bone marrow microenvironment is beginning to come into focus. As cellular and non-cellular components of the HSC niche are identified, new biological interactions are discovered, which are likely to revolutionize our approach to stem cell manipulation for therapeutic purposes.
Acknowledgments
This work was supported by the National Institutes of Health (K08 DK064381 to L.M.C.) and the Pew Foundation (L.M.C.). R.L.P is a trainee in the Medical Scientist Training Program, NIH T32 GM-07356.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 3.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science. 2001;294:2542–2544. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 4.Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–663. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- 5.Tulina N, Matunis E. Control of stem cell selfrenewal in Drosophila spermatogenesis by JAK–STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 6.Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 8.Leedham SJ, Brittan M, McDonald SA, Wright NA. Intestinal stem cells. J Cell Mol Med. 2005;9:11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 10.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci. 2003;100(Supplement 1):11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 13.Grewal SS, Barker JN, Davies SM, Wagner JE. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood. 2003;101:4233–4244. doi: 10.1182/blood-2002-08-2510. [DOI] [PubMed] [Google Scholar]
- 14.Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CL, Johnson GR. Murine hematopoietic stem and progenitor cells: I. Enrichment and biologic characterization Blood. 1995;85:1472–1479. [PubMed] [Google Scholar]
- 17.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1998;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 18.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marusi A, Kalinowski JF, Jastrzebski S, Lorenzo JA. Production of leukemia inhibitory factor mRNA and protein by malignant and immortalized bone cells. J Bone Miner Res. 1993;8:617–624. doi: 10.1002/jbmr.5650080513. [DOI] [PubMed] [Google Scholar]
- 20.Taichman RS, Reilly MJ, Emerson SG. Human Osteoblasts Support Human Hematopoietic Progenitor Cells in In Vitro Bone Marrow Cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- 21.El-Badri NS, Wang BY, Cherry, Good RA. Osteoblasts promote engraftment of allogeneic hematopoietic stem cells. Exp Hematol. 1998;26:110–116. [PubMed] [Google Scholar]
- 22.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 23.Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 24.Calvi LM, Adams GB, Weibrecht W, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng J, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 26.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 27.Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, Gu JH, McNiece IK, Wang JF. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16:579–585. doi: 10.3727/000000007783465073. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve JF, Stoltz JF, Eljaafari A. Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol. 2007;35:507–515. doi: 10.1016/j.exphem.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- 31.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 33.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;284:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 34.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 35.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 37.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature Medicine. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 42.Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- 43.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Weber JM, Forsythe SR, Christianson CA, Frisch BJ, Gigliotti BG, Jordan CT, Milner LA, Guzman ML, Calvi LM. Parathyroid hormone stimulates expression of the Notch ligand Jagged1 in osteoblastic cells. Bone. 2006;39:485–493. doi: 10.1016/j.bone.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 46.Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999;93:2431–2448. [PubMed] [Google Scholar]
- 47.Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, Bhatia M. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karanu FN, Murdoch B, Miyabayashi T, Ohno M, Koremoto M, Gallacher L, Wu D, Itoh A, Sakano S, Bhatia M. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97:1960–1970. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 49.Karanu FN, Yuefei L, Gallacher L, Sakano S, Bhatia M. Differential response of primitive human CD34- and CD34+ hematopoietic cells to the Notch ligand Jagged-1. Leukemia. 2003;17:1366–1374. doi: 10.1038/sj.leu.2402973. [DOI] [PubMed] [Google Scholar]
- 50.Ohishi K, Varnum-Finney B, Bernstein ID. The notch pathway: modulation of cell fate decisions in hematopoiesis. Int J Hematol. 2002;75:449–459. doi: 10.1007/BF02982106. [DOI] [PubMed] [Google Scholar]
- 51.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nature Medicine. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 52.Shojaei F, Trowbridge J, Gallacher L, Yuefei L, Goodale D, Karanu F, Levac K, Bhatia M. Hierarchical and ontogenic positions serve to define the molecular basis of human hematopoietic stem cell behavior. Dev Cell. 2005;8:651–663. doi: 10.1016/j.devcel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Milner LA, Deng Y, Iwata M, Banta A, Graf L, Marcovina S, Friedman C, Trask BJ, Hood L, Torok-Storb B. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8:43–55. doi: 10.1016/s1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- 54.Pereira RM, Delany AM, Durant D, Canalis E. Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem. 2002;85:252–258. doi: 10.1002/jcb.10125. [DOI] [PubMed] [Google Scholar]
- 55.Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 56.Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S, Shindo H, Yamaguchi A. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005;280:15842–15848. doi: 10.1074/jbc.M412891200. [DOI] [PubMed] [Google Scholar]
- 57.Shindo K, Kawashima N, Sakamoto K, Yamaguchi A, Umezawa A, Takagi M, Katsube K, Suda H. Osteogenic differentiation of the mesenchymal progenitor cells, Kusa is suppressed by Notch signaling. Exp Cells Res. 2003;290:370–380. doi: 10.1016/s0014-4827(03)00349-5. [DOI] [PubMed] [Google Scholar]
- 58.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. doi: 10.1074/jbc.M508370200. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe N, Tezuka Y, Matsuno K, Miyatani S, Morimura N, Yasuda M, Fujimaki R, Kuroda K, Hiraki Y, Hozumi N, Tezuka K. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Miner Metab. 2003;21:344–352. doi: 10.1007/s00774-003-0428-4. [DOI] [PubMed] [Google Scholar]
- 60.Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 61.Fiúza UM, Arias AM. Cell and molecular biology of Notch. J Endocrinology. 2007;194:459–74. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 62.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 63.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JRr, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors Wnt3a. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 64.Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through WNT signaling. J Cell Biochem. 2007 doi: 10.1074/jbc.M702687200. [DOI] [PubMed] [Google Scholar]
- 65.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, Moon RT, Bhatia M. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci. 2003;100:3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 68.He XC, Zhang J, Li L. Cellular and molecular regulation of hematopoietic and intestinal stem cell behavior. Ann N Y Acad Sci. 2005;1049:28–38. doi: 10.1196/annals.1334.005. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Li L. BMP signaling and stem cell regulation. Developmental Biology. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 70.McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res. 1993;8:485–496. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]
- 71.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 73.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. JEM. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 75.Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu HC, Ema H, Osawa M, Nakamura Y, Suda T, Nakauchi H. Hematopoietic stem cells express Tie-2 receptor in the murine fetal liver. Blood. 2000;96:3757–3762. [PubMed] [Google Scholar]
- 77.Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195:1549–1563. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc Med. 2005;15:75–79. doi: 10.1016/j.tcm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 82.Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require b1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 83.Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin(−/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol. 2002;30:176–185. doi: 10.1016/s0301-472x(01)00777-9. [DOI] [PubMed] [Google Scholar]
- 84.Takano H, Ema H, Sudo K, Nakauchi H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kiel MJ, Radice GL, Morrison SJ. Lack of Evidence that Hematopoietic Stem Cells Depend on N-Cadherin-Mediated Adhesion to Osteoblasts for Their Maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, McCauley LK, Taichman RS. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 92.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 94.Broxmeyer HE, Orschell CM, lapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 96.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cells Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 97.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 98.Rodan SB, Rodan GA, Simmons HA, Walenga RW, Feinstein MB, Raisz LG. Bone resorptive factor produced by osteosarcoma cells with osteoblastic features is PGE2. Biochem Biophys Res Commun. 1981;102:1358–1365. doi: 10.1016/s0006-291x(81)80161-1. [DOI] [PubMed] [Google Scholar]
- 99.Tetradis S, Pilbeam CC, Liu Y, Herschman HR, Kream BE. Parathyroid hormone increases prostaglandin G/H synthase-2 transcription by a cyclic adenosine 3′,5′-monophosphate-mediated pathway in murine osteoblastic MC3T3-E1 cells. Endocrinology. 1997;138:3594–3600. doi: 10.1210/endo.138.9.5391. [DOI] [PubMed] [Google Scholar]
- 100.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lord AM, North TE, Zon LI. Prostaglandin E2: Making More of Your Marrow. Cell Cycle. 2007;6:3054–3057. doi: 10.4161/cc.6.24.5129. [DOI] [PubMed] [Google Scholar]
- 102.Hamann KL, Lane NE. Parathyroid hormone update. Rheum Dis Clin North Am. 2006;32:703–719. doi: 10.1016/j.rdc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bruce WR, Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 105.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Path. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 106.Jordan CT, Guzman ML, Noble M. Cancer Stem Cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 107.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12 doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 108.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 109.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 110.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]


