SUMMARY
Bursts of spikes triggered by sensory stimuli in midbrain dopamine neurons evoke phasic release of dopamine in target brain areas, driving reward-based reinforcement learning and goal-directed behavior. NMDA-type glutamate receptors (NMDARs) play a critical role in the generation of these bursts. Here we report LTP of NMDAR-mediated excitatory transmission onto dopamine neurons in the substantia nigra. Induction of LTP requires burst-evoked Ca2+ signals amplified by preceding metabotropic neurotransmitter inputs in addition to the activation of NMDARs themselves. PKA activity gates LTP induction by regulating the magnitude of Ca2+ signal amplification. This novel form of plasticity is associative, input specific, reversible, and depends on the relative timing of synaptic input and postsynaptic bursting in a manner analogous to the timing rule for cue-reward learning paradigms in behaving animals. NMDAR plasticity may thus represent a potential neural substrate for conditioned dopamine neuron burst responses to environmental stimuli acquired during reward-based learning.
INTRODUCTION
The appropriate association between environmental cues and motivational valence is crucial for the brain to accurately guide behavior. Dopamine (DA) neurons, located in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA), are thought to assign positive values to objects and experiences in order to effectively influence decision-making strategies (Montague et al., 2004). In vivo experiments in non-human primates and rodents coupled with human functional imaging and computational modeling studies have suggested that this occurs through changes in DA neuron firing rate, which encode reward prediction errors (D’Ardenne et al., 2008; Montague et al., 1996; Pan et al., 2005; Schultz, 1998). As such, DA neurons transition from tonic single-spike firing (1–5 Hz) to burst firing (2–10 spikes at 10–50 Hz) in response to the unexpected presentation of primary rewards. Intriguingly, the burst response shifts in time to reward-predicting cues after conditioning with repeated cue-reward pairing. However, the locus of neural plasticity responsible for this conditioned DA neuron response remains elusive.
Glutamatergic inputs activating NMDA receptors (NMDARs) have been shown to drive the transition from slow, tonic firing to burst firing in DA neurons (Chergui et al., 1994; Morikawa et al., 2003; Overton and Clark, 1997; Tong et al., 1996; Zweifel et al., 2009), although AMPA receptors (AMPARs) may also play a role (Blythe et al., 2007). Therefore, potentiation of NMDAR-dependent excitation of DA neurons may contribute to the development of the conditioned burst response. Despite numerous studies describing the plasticity of AMPARs in DA neurons (Jones and Bonci, 2005; Kauer and Malenka, 2007), synaptic activity-dependent plasticity of NMDAR-mediated transmission has yet to be demonstrated [but see Borgland et al. (2006), Schilstrom et al. (2006), and Ungless et al. (2003) for enhancement of NMDAR function caused by metabotropic receptor agonists].
Ca2+ signaling, triggered by either postsynaptic action potentials (APs) or local synaptic events, is implicated in the plasticity of synapses throughout the CNS (Linden, 1999; Sjostrom and Nelson, 2002). We have previously shown that AP-evoked Ca2+ signals can be amplified by the activation of metabotropic glutamate receptors (mGluRs) and other neurotransmitter receptors coupled to phosphoinositide (PI) hydrolysis in DA neurons (Cui et al., 2007). This amplification results from an elevation in cytosolic inositol trisphosphate (IP3) levels, leading to enhanced Ca2+-induced Ca2+ release (CICR) through IP3 receptors (IP3Rs) located on intracellular Ca2+ stores. IP3, generated by activation of PI-coupled neurotransmitter receptors, and Ca2+, provided by AP-induced influx, thus synergistically coactivate IP3Rs (Taylor and Laude, 2002). In this study, we asked if this synergistic Ca2+ signaling could drive plasticity of NMDAR-mediated transmission onto DA neurons. We found that repeated pairing of sustained synaptic stimulation with burst firing results in long-term potentiation (LTP) of NMDAR EPSCs. The induction of LTP requires PI-coupled receptor-mediated facilitation of burst-induced Ca2+ signals and NMDAR activation. LTP induction is also gated by protein kinase A (PKA), which regulates IP3R sensitivity. We further show that NMDAR LTP is input specific, requires appropriately timed presynaptic and postsynaptic activity, and can be reversed by repetitive presynaptic stimulation without postsynaptic firing.
RESULTS
Activity-Dependent LTP of NMDAR EPSCs in DA Neurons
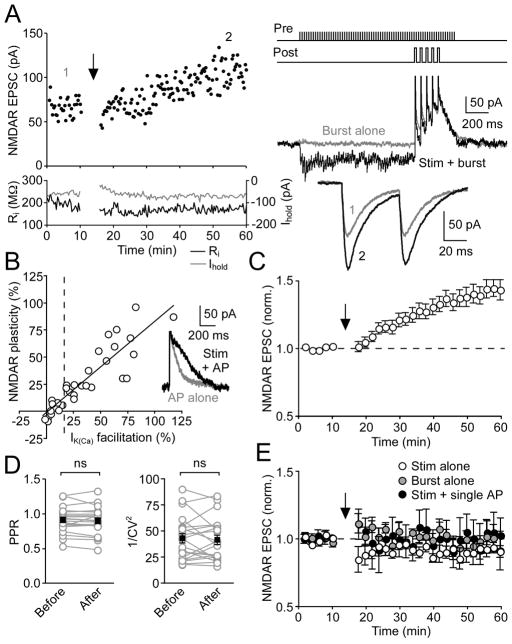
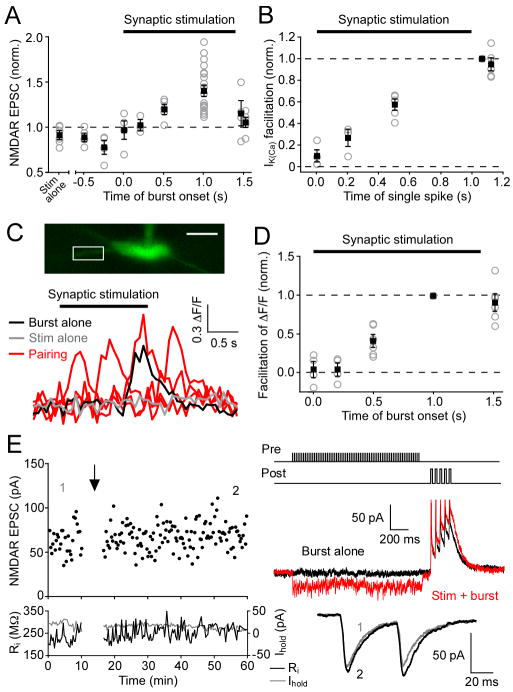
Whole-cell voltage-clamp recordings were made from DA neurons in the SNc (~90%) and VTA (~10%) using rat midbrain slices. Previous studies examining the conditioning of DA neuron responses in behaving animals, in which larger number of neurons were sampled from the SNc than the VTA, have reported similar response profiles in these two areas (Mirenowicz and Schultz, 1996; Pan et al., 2005; Schultz, 1998). A bipolar stimulating electrode was placed 50–150 μm rostral to the recorded neuron. Pharmacologically isolated NMDAR EPSCs were recorded at −62 mV in low Mg2+ (0.1 mM) to remove blockade of NMDARs. After 10 min of baseline EPSC recording, we delivered an LTP induction protocol consisting of a train of presynaptic stimulation (70 stimuli at 50 Hz) paired with a burst of 5 postsynaptic unclamped APs at 20 Hz, which mimics burst firing observed in behaving rats (Hyland et al., 2002). The onset of the burst was delayed by 1 s from that of the synaptic stimulation train. We found that repetitive synaptic stimulation-burst pairing (10 times every 20 s) resulted in LTP of NMDAR EPSCs in some but not all neurons tested (Figure 1A). The pattern of synaptic stimulation used in the induction protocol can augment AP-induced Ca2+ signals via activation of PI-coupled receptors, mainly mGluR1 (Cui et al., 2007). To address the role of AP-evoked Ca2+ signals in LTP induction, we measured small-conductance Ca2+-sensitive K+ (SK) currents (IK(Ca)) activated by unclamped APs (see Experimental Procedures). Immediately before induction, we tested each neuron for facilitation of IK(Ca) following synaptic stimulation by evoking a single AP at 60 ms after the offset of a 1-s stimulation train (example traces shown in inset of Figure 1B). The magnitude of NMDAR LTP, determined 30–40 min after the induction, was positively correlated with that of IK(Ca) facilitation (n = 31, r2 = 0.80) (Figure 1B). However, NMDAR LTP was not correlated with the size of IK(Ca) itself or with baseline EPSC amplitude (Figure S1). On average, NMDAR EPSCs were potentiated by 43% ± 6% in 21 neurons that exhibited IK(Ca) facilitation >15%, whereas no LTP was observed when IK(Ca) facilitation was <15% (1% ± 2% change, n = 10) (Figure 1C). The paired-pulse ratio (PPR, 50-ms interstimulus interval, expressed as EPSC2/EPSC1) and the coefficient of variation (CV, expressed as 1/CV2) of EPSCs were not significantly changed in 21 neurons that exhibited LTP (Figure 1D), suggesting a postsynaptic locus of LTP expression (Malinow and Tsien, 1990; Zalutsky and Nicoll, 1990). Repeated delivery of postsynaptic burst firing alone failed to induce LTP of NMDAR EPSCs (0% ± 3% change, n = 6), while synaptic stimulation alone produced a small but significant LTD (−8% ± 4% change, n = 6) (Figure 1E). Furthermore, LTP was not observed when the burst was replaced with a single AP during pairing (−1% ± 10% change, n = 5). Together, these results suggest that synaptic facilitation of burst-induced Ca2+ signaling is involved in the induction of NMDAR LTP. Due to the correlation between IK(Ca) facilitation and LTP in our initial finding, subsequent experiments were conducted in neurons that exhibited >15% IK(Ca) facilitation unless otherwise stated (see Table S1).
Figure 1. Repeated Synaptic Stimulation-Burst Pairing Induces LTP of NMDAR EPSCs in DA Neurons.
(A) Representative experiment showing LTP of NMDAR EPSCs. Left: Time graph of NMDAR EPSC amplitude, input resistance (Ri, black), and holding current (Ihold, gray). The LTP induction protocol, which consisted of 10 synaptic stimulation-burst pairings (illustrated at top right), was delivered at the time indicated by the arrow. Middle right: Current traces evoked by burst alone (gray) and synaptic stimulation-burst pairing (black). Bottom right: Traces of EPSCs (averaged over 10 min) at times indicated by numbers in the EPSC time graph.
(B) Relationship between the magnitude of NMDAR LTP and facilitation of AP-evoked IK(Ca) by preceding synaptic stimulation for 31 neurons. Solid line is a linear fit to the data. Dashed vertical line indicates 15% IK(Ca) facilitation. Inset at right shows traces of IK(Ca) for a single AP alone (gray) and an AP following synaptic stimulation (black) from the same neuron as in (A).
(C) Summary time graph of NMDAR LTP for neurons that exhibited >15% IK(Ca) facilitation (n = 21). Each symbol represents mean normalized EPSC amplitude from a 2-min window.
(D) PPR (left) and 1/CV2 (right) were not significantly altered after LTP induction for the 21 neurons in (C). Black squares indicate mean.
(E) Summary time graph showing that synaptic stimulation alone (n = 6), burst alone (n = 6), and pairing synaptic stimulation with a single AP (n = 5) all failed to induce NMDAR LTP. Note that a small LTD was induced with synaptic stimulation alone.
Error bars indicate SEM.
To confirm that this form of plasticity could be induced in physiological Mg2+, we recorded NMDAR EPSCs in 1.2 mM Mg2+ at slightly depolarized holding potentials (−47 to −62 mV) using Cs+-based internal solution to enhance the resolution of small NMDAR EPSCs (23 ± 3 pA, n = 8). Measurable IK(Ca) was not observed in these experiments, most likely due to the low permeability of SK channels to Cs+ (Shin et al., 2005). Pairing presynaptic stimulation with postsynaptic bursting produced LTP >10% in 6 out of 8 neurons tested in physiological Mg2+ (23%± 4% change, n = 6) (Figure S2).
We also examined the effect of the burst pairing protocol on AMPAR-mediated transmission. Here, AMPAR EPSCs were recorded at −62 or −77 mV in 1.2 mM Mg2+ with NMDARs intact, while synaptic stimulation-burst pairing was delivered at −62 mV. This resulted in LTD of EPSCs (−29% ± 3% change, n =5) (Figure S3). The magnitude of LTD showed no correlation with IK(Ca) facilitation by synaptic stimulation (r2 = 0.0003) (Figure S3C). Furthermore, there was no difference (p > 0.5) in the amount of LTD expressed when postsynaptic burst firing was omitted and neurons received the synaptic stimulation train alone (−26% ± 3% change, n = 3) (Figures S3B and S3C). It should be noted that the intracellular machinery responsible for the induction of AMPAR LTP may be “washed-out” during whole-cell recordings in DA neurons (Bonci and Malenka, 1999).
Induction of NMDAR LTP Requires PI-Coupled Receptor Activation and Release of Ca2+ from Internal Stores
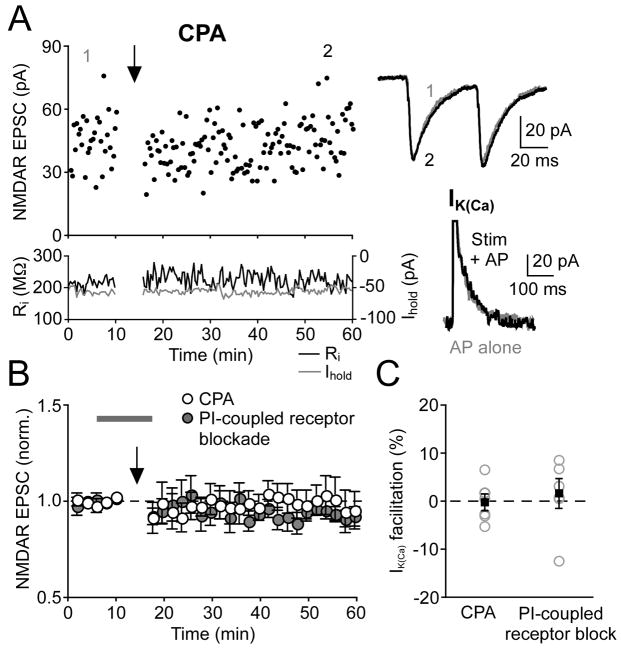
Activation of PI-coupled receptors facilitates AP-evoked Ca2+ signals in DA neurons via an increase in IP3 levels, which enhances IP3R-dependent CICR from intracellular stores (Cui et al., 2007). We thus examined the role of this Ca2+ signaling cascade in NMDAR LTP. Treatment of slices with cyclopiazonic acid (CPA, 10 μM), which depletes intracellular Ca2+ stores (Seidler et al., 1989), eliminated the facilitation of IK(Ca) by synaptic stimulation (0% ± 2%, n = 6) as well as the induction of NMDAR LTP (2% ± 4% change, n = 6) (Figure 2). Pharmacological blockade of mGluR1 together with muscarinic acetylcholine and α1-adrenergic receptors, other major PI-coupled neurotransmitter receptors expressed in DA neurons (Fiorillo and Williams, 2000; Paladini and Williams, 2004), also abolished IK(Ca) facilitation (2% ± 3%, n = 6) and NMDAR LTP (−6% ± 4% change, n = 6) (Figures 2B and 2C). We further confirmed that intracellular BAPTA (100 μM) blocked IK(Ca) facilitation (4% ± 1%, n = 6) and NMDAR LTP (−1% ± 5% change, n = 6). Together with the data presented in Figures 1B and 1E, these results demonstrate that Ca2+ store-dependent enhancement of burst-induced Ca2+ signals is critical for LTP induction.
Figure 2. PI-Coupled Receptor Activation and Release of Ca2+ from Internal Stores Is Necessary for NMDAR LTP Induction.
(A) Time graph of a representative experiment conducted in the presence of CPA (10 μM). Sample traces to the right show average NMDAR EPSCs before (1) and after (2) synaptic stimulation-burst pairing (top traces) and IK(Ca) with (black) and without (gray) synaptic stimulation (bottom traces). Note the lack of facilitation of IK(Ca) by synaptic stimulation.
(B) Summary time graph of experiments conducted in the presence of CPA (n = 6) and experiments where PI-coupled receptors were blocked during the induction, as indicated by the gray bar, with a cocktail containing the mGluR1 antagonist CPCCOEt (50–75 μM), the muscarinic acetylcholine receptor antagonist scopolamine (100 nM), and the α1-adrenergic receptor antagonist prazosin (1 μM) (n = 6).
(C) Summary graph depicting lack of significant facilitation of IK(Ca) by synaptic stimulation in CPA or during PI-coupled receptor blockade. Gray open circles indicate individual experiments; black squares represent mean.
Error bars indicate SEM.
PKA Regulates PI-coupled Receptor-Mediated Facilitation of Ca2+ Signals and Induction of NMDAR LTP
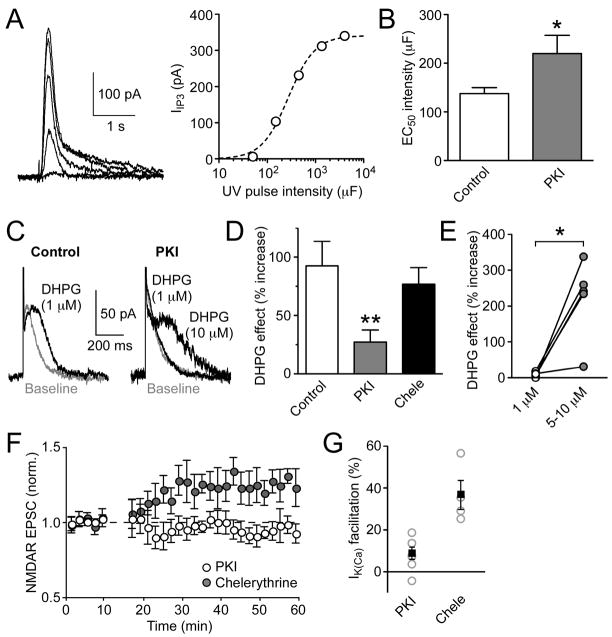
IP3 sensitivity of IP3Rs can be increased by PKA-mediated phosphorylation (Tang et al., 2003; Wagner et al., 2008). To examine the role of PKA, we loaded recorded neurons with the specific PKA inhibitor PKI (100–200 μM) through the whole-cell pipette. We first tested the effect of PKI on IP3 sensitivity of IP3Rs by performing flash photolysis of caged IP3 using different UV pulse intensities (expressed in μF; see Experimental Procedures) to vary the concentration of IP3 released and measured the resulting SK-mediated outward current (IIP3) (Figure 3A). Intracellular PKI significantly increased the UV pulse intensity producing half maximal IIP3 amplitude (138 ± 12 μF in control, n = 5 vs. 220 ± 37 μF in PKI, n = 7, p < 0.05) (Figure 3B), suggesting that IP3 sensitivity is enhanced by tonic PKA activity. Although PKA is not known to modulate SK channel function, recent evidence indicates that PKA phosphorylation can regulate surface expression of SK2 channels (Lin et al., 2008; Ren et al., 2006). However, PKI failed to alter the maximal IIP3 amplitude (data not shown). This may be due to the predominant expression of SK3 channels in DA neurons (Wolfart et al., 2001).
Figure 3. PKA Regulates IP3 Sensitivity and NMDAR LTP Induction.
(A) Left: Traces of IIP3 evoked with different UV pulse intensities in a PKI-loaded neuron. Right: IIP3 amplitude is plotted versus UV pulse intensity, expressed in terms of the capacitance (μF) of the flash photolysis system, in the same neuron. Dotted line represents fit to a logistic equation. EC50 intensity was 275 μF in this neuron. (B) Bar graph showing that PKI (n = 7) significantly increased the EC50 intensity to produce IIP3. *p < 0.05 vs. control internal solution (n = 5). (C) Representative traces illustrating the effects of DHPG on single AP-evoked IK(Ca) recorded with a control internal solution (left) or PKI (200 μM; right). (D) Summary bar graph demonstrating that PKI (n = 10), but not chelerythrine (n = 6), significantly reduced the effect of DHPG (1 μM) on IK(Ca). **p < 0.01 vs. control internal solution (n = 13). (E) The effects of DHPG at 1 μM vs. 5–10 μM are plotted in 5 PKI-loaded neurons. *p < 0.05. (F) Summary time graph showing that PKI (n = 7), but not chelerythrine (n = 4), blocked NMDAR LTP. (G) Summary graph showing the magnitude of IK(Ca) facilitation by synaptic stimulation in PKI and chelerythrine. Gray open circles indicate individual experiments; black squares represent mean.
Error bars indicate SEM.
PKI also significantly reduced the magnitude of IK(Ca) facilitation caused by bath perfusion of the mGluR agonist DHPG (1 μM) (92% ± 21% in control, n = 13 vs. 27% ± 10% in PKI, n = 10, p < 0.01) (Figures 3C and 3D). In 5 PKI-loaded neurons that exhibited <20% IK(Ca) facilitation (9% ± 3%) in response to 1 μM DHPG, higher concentrations of DHPG (5–10 μM), which should further elevate cytosolic IP3 levels, produced significantly larger IK(Ca) facilitation (221% ± 51%, p < 0.05) (Figure 3E), consistent with the idea that PKI reduced the IP3 sensitivity of IP3Rs.
We next tested the effect of PKI on NMDAR LTP. Intracellular PKI suppressed IK(Ca) facilitation by synaptic stimulation (9% ± 3%, n = 7) as well as the induction of LTP (−3% ± 6% change, n = 7) (Figures 3F and 3G). In contrast, intracellular dialysis with the PKC inhibitor chelerythrine (10 μM), which has been shown to block NMDAR LTP in the hippocampus (Kwon and Castillo, 2008), had no significant effect on IK(Ca) facilitation or NMDAR LTP (Figures 3D, 3F, and 3G). Together, these data demonstrate that PKA activity regulates the induction of NMDAR LTP by augmenting PI-coupled receptor-mediated facilitation of Ca2+ signals.
NMDAR LTP is DA-Independent
DA neuron bursts are thought to provide a plasticity signal in projection areas via phasic DA release, thus driving reward-based learning (Schultz, 1998). DA neuron bursts may also trigger Ca2+-dependent dendritic release of DA in the SNc (Beckstead et al., 2004; Chen and Rice, 2001). Furthermore, activation of DA D1/5 receptors can produce potentiation of NMDAR EPSCs (Schilstrom et al., 2006), raising the possibility that DA may play a role in LTP induction. However, significant NMDAR LTP was observed (38% ± 9% change, n = 5) even when the DA D1/5 receptor antagonist SCH 23390 (1 μM) was present during induction (Figure S4). The DA D2 receptor antagonist eticlopride (100–200 nM) was always present in the extracellular solution in this study to block D2 receptor-mediated IPSCs (Beckstead et al., 2004). Thus, burst-induced DA release is not involved in the induction of NMDAR LTP in DA neurons.
NMDAR Activation Is Necessary for Induction of NMDAR LTP
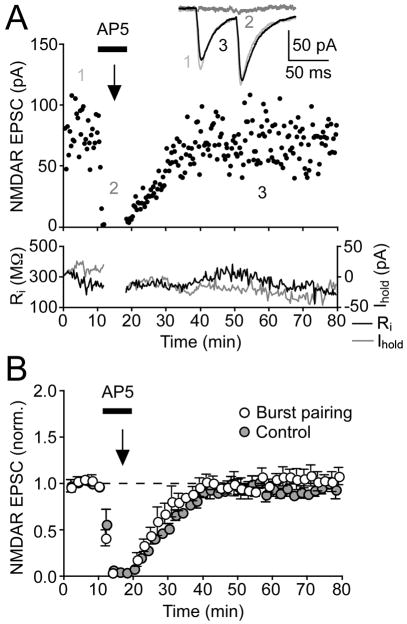
Recent studies on the plasticity of NMDARs at hippocampal mossy fiber synapses indicate that activation of NMDARs, in addition to activation of mGluRs, is required during LTP induction (Kwon and Castillo, 2008; Rebola et al., 2008). In order to test this possibility in DA neurons, we acutely blocked NMDARs with the NMDAR antagonist DL-AP5 (50–100 μM) during induction (Figure 4). Perfusion of DL-AP5 after 10 min of baseline recording rapidly and completely blocked NMDAR EPSCs (from 44 ± 11 pA to 2 ± 1 pA, n = 4), and the burst pairing protocol was delivered thereafter. DL-AP5 was washed out immediately after induction. Despite robust facilitation in all neurons tested (56% ± 13%, n = 4), none exhibited LTP of NMDAR EPSCs (μ1% ± 2% change) (Figure 4B). We confirmed that the washout of DL-AP5 (100 μM) was complete in ~30 min when no burst pairing protocol was delivered (n = 3). Therefore, the induction of NMDAR LTP requires the activation of NMDARs themselves.
Figure 4. NMDAR LTP Requires NMDAR Activation during Synaptic Stimulation-Burst Pairing.
(A) Transiently blocking NMDARs with DL-AP5 (100 μM) during delivery of the induction protocol, as indicated by the black bar, prevented the development of LTP in this example experiment. Average NMDAR EPSCs taken at the times indicated are shown in inset for control (1), in AP5 (2), and after LTP induction and AP5 washout (3).
(B) Summary time graph of experiments where LTP was blocked by DL-AP5 (50–100 μM) perfused during the induction (n = 4). Summary of control experiments is also shown, where DL-AP5 (100 μM) was perfused and washed out without delivery of the induction protocol (n = 3). Error bars indicate SEM.
NMDAR Plasticity Is Induced in a Burst Timing-Dependent Manner
We next examined if LTP induction is dependent on the relative timing between synaptic stimulation and burst firing. In our routine induction protocol, there is a 1-s delay between the onset of the 1.4-s synaptic stimulation train and that of the burst. When this delay was omitted, i.e., when the onset of the burst was shifted forward to coincide with that of synaptic stimulation, no LTP was induced (−3% ± 10% change, n = 4) (Figure 5A). Similarly, no significant LTP was observed when the burst was elicited with a delay of 200 ms after the onset of synaptic stimulation (3% ± 5% change, n = 3). However, sizable LTP was induced when the burst occurred with a 500-ms delay during the pairing protocol (20% ± 6% change, n = 5), although reduced in magnitude compared to the LTP induced with a 1-s burst delay. In line with these observations, we found that the magnitude of IK(Ca) facilitation gradually increased during 1-s synaptic stimulation in these neurons tested for the burst-timing dependence of LTP induction (Figure 5B), most likely reflecting gradual increases in cytosolic IP3 levels. In a separate series of experiments, we performed fluorescence imaging of burst-induced Ca2+ signals using fluo-5F (50 μM) as a Ca2+ indicator and examined the burst-timing dependence of facilitation produced by a 1.4-s synaptic stimulation train. The magnitude of facilitation of burst-evoked fluorescence change also gradually increased as the delay between the onset of synaptic stimulation and that of the burst was prolonged up to 1 s in 6 neurons tested (Figures 5C and 5D). Synaptic stimulation increased burst-evoked fluorescence change by 35% ± 5% (n = 6) at 1-s delay. This increase was abolished by CPCCOEt (75 μM, n = 2), consistent with the role of mGluR1 in synaptic facilitation (Cui et al., 2007), but was unaffected by DL-AP5 (50–100 μM, n = 4) (Figure S5). No AP5-sensitive fluorescence change was observed with synaptic stimulation alone, indicating that NMDAR-mediated Ca2+ influx was not detected with our imaging system.
Figure 5. Burst-Timing Dependence of NMDAR Plasticity.
(A) Summary graph depicting the burst-timing dependence of NMDAR plasticity. The magnitude of LTP/LTD is plotted versus time of burst onset relative to the onset of 1.4-s synaptic stimulation (black bar) during the induction protocol. Individual experiments are shown as gray open circles; black squares represent mean. Data for the 1-s delay (n = 21) are from control LTP experiments with IK(Ca) facilitation >15% shown in Figure 1B, while the data for synaptic stimulation alone are from Figure 1E.
(B) Summary graph illustrating the timing dependence of IK(Ca) facilitation assessed using 1-s synaptic stimulation. Data are from neurons shown in (A) (21 neurons in the control LTP experiments with 1-s delay are not included). In order to measure IK(Ca) facilitation, a single AP was evoked at the indicated time relative to 1-s synaptic stimulation. The amount of IK(Ca) facilitation thus obtained was normalized to that measured at 60 ms after the offset of synaptic stimulation in each neuron. Therefore, data in the “60 ms after offset” group are all normalized to unity. Gray open circles represent data from individual experiments, while black squares indicate mean.
(C) Example experiment imaging burst-evoked Ca2+ signals at various synaptic stimulation-burst timing intervals. Fluorescence changes were measured at the ROI indicated in the confocal fluorescence image of a DA neuron filled with fluo-5F (scale bar: 20 μm). Black and gray traces represent burst alone and synaptic stimulation alone, respectively, while red traces represent synaptic stimulation-burst pairing, in which the burst was evoked at onset, 500 ms after onset, 1 s after onset, and 120 ms after offset of 1.4-s synaptic stimulation (black bar).
(D) Summary graph showing the timing dependence of synaptic facilitation of burst-evoked Ca2+ signals. Facilitation is plotted versus time of burst onset relative to the onset of 1.4-s synaptic stimulation (black bar). The magnitude of facilitation was normalized to that produced when burst was elicited 1 s after onset of synaptic stimulation in each neuron. Gray open circles represent data from individual experiments, while black squares indicate mean.
(E) Example experiment in which the burst was delayed by 120 ms after the offset of synaptic stimulation train, as illustrated at top right. Middle right: Sample traces show the response to postsynaptic burst alone (black) and synaptic stimulation-burst pairing with a 120-ms delay (red). Bottom right: Average EPSCs before (1) and after (2) 120-ms delay pairing taken at the times indicated.
Error bars indicate SEM.
Next, we delayed the burst until after the offset of the synaptic stimulation train. This resulted in a significant decrease in LTP with an interval of 60 ms (13% ± 14% change, n = 4) and near complete lack of LTP with a 120-ms interval (6% ± 5% change, n = 5) (Figures 5A and 5E). In the 5 neurons in which LTP induction was attempted with a 120-ms interval, facilitation of IK(Ca) at 120 ms after the offset of synaptic stimulation was indistinguishable from that at 60 ms, the interval routinely used to assess IK(Ca) facilitation (Figure 5B). Furthermore, in Ca2+ imaging experiments, facilitation of burst-evoked fluorescence change was not significantly reduced when the burst was elicited at 120 ms after the offset of the 1.4-s synaptic stimulation train (Figures 5C and 5D), indicating that the decrease in LTP is not due to a reduction in synaptic facilitation of Ca2+ signaling. Indeed, IP3-mediated enhancement of Ca2+ signals has been shown to last for hundreds of milliseconds because of prolonged lifetime of IP3 binding to IP3Rs (Sarkisov and Wang, 2008). In contrast, NMDAR EPSCs evoked by synaptic stimulation decayed by 80% ± 4% at 60 ms after the offset of stimulation in the 4 neurons tested at 60-ms interval for LTP induction, while the decay of NMDAR EPSCs was almost complete (96% ± 1%) at 120 ms in the 5 neurons tested for 120-ms interval. This implies that the burst may need to occur while NMDARs are activated during induction. Therefore, the burst-timing dependence of LTP induction described here is consistent with the requirement of both PI-coupled receptor-mediated facilitation of burst-induced Ca2+ signals (Figure 2) and activation of NMDARs (Figure 4).
Finally, we evoked burst firing before the onset of synaptic stimulation during induction. Interestingly, sizable NMDAR LTD was observed when the onset of the burst was placed 250 ms before that of the synaptic stimulation train (−22% ± 7% change, n = 4) (Figure 5A). There was no significant change in either PPR or 1/CV2 (0.84 ± 0.07 vs. 0.86 ± 0.06 and 41 ± 12 vs. 39 ± 10, respectively; p > 0.5 for both parameters), suggesting a postsynaptic locus of LTD expression as for LTP. When the interval between burst onset and synaptic stimulation was increased to 500 ms, where burst-induced Ca2+ rise had minimal overlap, if any, with synaptic stimulation, the magnitude of LTD was reduced to a level comparable to that induced by presynaptic stimulation alone (500 ms before onset: −10% ± 4% change, n = 4 vs. synaptic stimulation alone: −8% ± 4% change, n = 6, p > 0.5). Together, these results demonstrate that the relative timing between presynaptic stimulation and postsynaptic burst firing determines the direction and the magnitude of NMDAR plasticity.
NMDAR LTP Is Input Specific
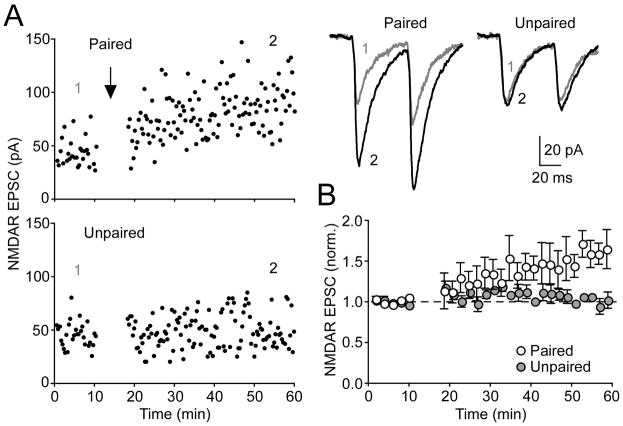
The involvement of NMDAR activation in the induction of NMDAR LTP raises the possibility that NMDARs may be potentiated specifically at those synapses stimulated during induction. To test this possibility, we placed two stimulating electrodes >100 μm apart from each other. After confirming the independence of the two pathways (see Experimental Procedures), we monitored NMDAR EPSCs in each pathway for 10 min. Once a stable baseline was established, one pathway received sustained synaptic stimulation paired with burst firing while the other pathway was held silent (Figure 6). This produced LTP selectively in the paired pathway (paired pathway: 65% ± 16% change vs. unpaired pathway: 2% ± 2% change, n = 4, p < 0.05), demonstrating that NMDAR LTP can be input specific.
Figure 6. Input Specificity of NMDAR LTP.
(A) Time graphs of a representative experiment where two independent pathways were alternately stimulated via two extracellular electrodes. During the induction, only one pathway was stimulated in conjunction with postsynaptic bursting (top, “paired”), while the other pathway was left unstimulated (bottom, “unpaired”). Sample traces show average NMDAR EPSCs taken at the times indicated for the paired (left) and unpaired (right) pathways.
(B) Summary time graph of NMDAR LTP in paired versus unpaired pathways in 4 neurons. Error bars indicate SEM.
NMDAR LTP Is Unlikely to Be Associated with a Change in Subunit Composition
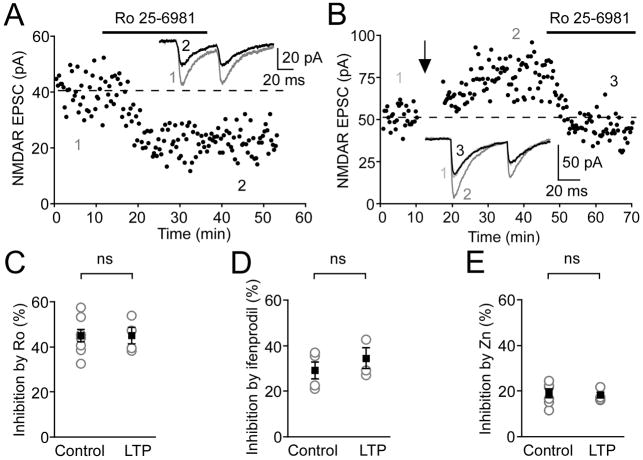
It has been shown that bath application of orexin A or DA D1/5 receptor agonists produces long-lasting increases in NMDAR EPSCs via changes in the composition of NR2 subunits of NMDARs in DA neurons (Borgland et al., 2006; Schilstrom et al., 2006). An activity-dependent switch in NR2 subunit composition has also been reported at neonatal hippocampal synapses (Bellone and Nicoll, 2007). We therefore tested if burst-dependent LTP of NMDARs in DA neurons is also associated with a change in the subunit composition by comparing the effects of NMDAR subunit specific antagonists on control NMDAR EPSCs versus potentiated EPSCs after successful LTP induction. We used three different NR2 subtype-specific antagonists: Ro 25–6981 (1 μM) and ifenprofil (3 μM), NR2B-containing receptor antagonists, and Zn2+ (100 nM), an NR2A-containing receptor antagonist (Fischer et al., 1997; Paoletti et al., 1997; Williams, 1993). None of these antagonists showed differential effects on control versus potentiated NMDAR EPSCs (Figure 7), suggesting that the burst pairing protocol induces NMDAR LTP without a change in the subunit composition of NMDARs.
Figure 7. NMDAR LTP Is Unlikely to Be Expressed via a Change in NR2 Subunit Composition.
(A) Representative time graph showing the effect of Ro 25–6981 (1 μM) on control NMDAR EPSCs. Ro 25–6981 was perfused during the time indicated by the black bar. Average EPSCs before (1) and after Ro 25–6981 application (2) are shown in inset.
(B) Representative time graph depicting the effect of Ro 25–6981 (1 μM) on NMDAR EPSCs after successful induction of LTP. Burst pairing protocol was delivered at the arrow, while Ro 25–6981 was perfused during the time indicated by the black bar. Inset shows average EPSCs before (1) and after LTP induction (2), together with the average EPSC after Ro 25–6981 application (3).
(C–E) Summary of the effects of Ro 25–6981 (1 μM; C), ifenprodil (3 μM; D), and Zn2+ (100 nM; E) on control NMDAR EPSCs (n = 6, n = 4, and n = 6, respectively) and potentiated EPSCs after LTP induction (n = 4, n = 3, and n = 4, respectively). Gray circles indicate data from individual neurons, while black squares indicate mean ± SEM.
NMDAR LTP Is Reversible
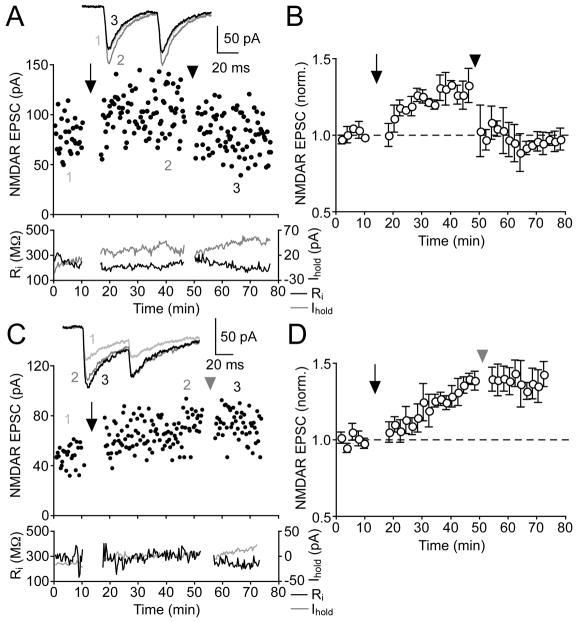
Synaptic plasticity induced by correlated presynaptic and postsynaptic activity can be reversed by presynaptic stimulation in the absence of postsynaptic activation (Bellone and Nicoll, 2007; Massey and Bashir, 2007). To examine if NMDAR LTP can be reversed (depotentiated) in DA neurons, we repeatedly delivered synaptic stimulation alone (10 times every 20 s) 30 min after inducing LTP of NMDAR EPSCs (30% ± 6% change, n = 4) (Figures 8A and 8B). This depotentiation protocol rapidly depressed previously potentiated NMDAR EPSCs back towards baseline levels in all 4 neurons tested (baseline: 59 ± 9 pA, LTP: 75 ± 10 pA, post-depotentiation: 57 ± 9 pA). Depotentiation was not associated with a change in either PPR or 1/CV2 (0.99 ± 0.10 vs. 1.00 ± 0.10 and 19 ± 4 vs. 17 ± 4, respectively; p > 0.5 for both parameters). It should be noted that the same procedure, i.e., delivery of synaptic stimulation alone, also induced a small but rapid LTD of control EPSCs that had not undergone LTP induction (−8% ± 4% change, n = 6) (Figure 1E).
Figure 8. NMDAR LTP Can Be Reversed.
(A) Representative experiment showing that repeated synaptic stimulation can depotentiate previously potentiated NMDAR EPSCs. The arrow indicates LTP induction by synaptic stimulation-burst pairing, while the arrowhead indicates the delivery of the depotentiation protocol consisting of synaptic stimulation alone. Average NMDAR EPSCs taken at the times indicated are shown in inset for control (1), after LTP (2), and after depotentiation (3).
(B) Summary time graph of depotentiation experiments (n = 4). Burst pairing protocol was delivered at the arrow to induce LTP, while the depotentiation protocol was applied at the arrowhead.
(C) Time course of a representative experiment in which pairing synaptic stimulation with single postsynaptic APs during the depotentiation protocol prevented reversal of previously induced NMDAR LTP. LTP was induced at the arrow, while the synaptic stimulation-single AP pairing protocol was applied at the gray arrowhead. Average NMDAR EPSCs are shown in inset for control (1), after LTP (2), and after single-AP pairing (3).
(D) Summary time graph of experiments attempting depotentiation with single-AP pairing (n = 4).
Error bars indicate SEM.
We next inserted a single AP into the depotentiation protocol at 1 s after the onset of synaptic stimulation, i.e., at the same timing as the burst in the burst pairing protocol (Figures 8C and 8D). Surprisingly, paring synaptic stimulation with a single AP completely prevented depotentiation in 4 out of 4 neurons (baseline: 53 ± 3 pA, LTP: 71 ± 3 pA, post-single AP pairing: 71 ± 3 pA). The same protocol also produced no change in control NMDAR EPSCs (1% ± 8% change, n = 5) (Figure 1E). Thus, synaptic stimulation-single AP pairing had no effect on NMDAR EPSCs regardless of whether they had been previously potentiated or not. Together, these results demonstrate that NMDAR LTP can be reversed by repetitive synaptic stimulation in the absence of postsynaptic firing activity.
DISCUSSION
Here we have demonstrated that repetitive pairing of sustained synaptic stimulation with burst firing induces LTP of NMDAR EPSCs in midbrain DA neurons. The induction of NMDAR LTP requires 1) synaptic facilitation of burst-evoked Ca2+ signals via mGluRs and other PI-coupled receptors generating IP3, and 2) activation of NMDARs. The burst-timing dependence of LTP induction is consistent with these two requirements in that 1) the burst needs to occur with a certain delay (~0.5–1 s) after the onset of synaptic stimulation, reflecting the time required for synaptic stimulation to cause a rise in IP3 levels, and 2) the burst also has to take place prior to or immediately (within tens of milliseconds) after the termination of synaptic stimulation so that NMDARs are activated at the time of the burst. Intriguingly, LTD of NMDAR EPSCs is induced when the burst precedes synaptic stimulation during the induction protocol, although the exact cellular mechanisms underlying bidirectionality of NMDAR plasticity remain to be determined (Harney et al., 2006). The activity-dependent plasticity of NMDARs in this study represents a novel mechanism for long-term regulation of DA neuron output that may also integrate with other forms of synaptic plasticity dependent on NMDAR activation (Engblom et al., 2008; Nugent et al., 2007; Zweifel et al., 2008).
Induction Mechanisms of NMDAR LTP in DA Neurons
It is well established that Ca2+ signals triggered by postsynaptic APs play a critical role in the induction of synaptic plasticity (Linden, 1999; Sjostrom and Nelson, 2002). APs can propagate and trigger Ca2+ influx in DA neuron dendrites with high efficiency (Hausser et al., 1995; Wilson and Callaway, 2000). Interestingly, the induction of NMDAR LTP in this study requires burst firing, as pairing synaptic stimulation with a single AP was ineffective at driving plasticity. In addition, burst-induced Ca2+ signals need to be amplified by preceding activation of PI-coupled receptors, which recruits CICR via IP3Rs on intracellular stores, to effectively induce LTP. Why are Ca2+ transients resulting from burst-induced Ca2+ influx insufficient to drive plasticity by themselves? Perhaps the mechanism is similar to that described for localized Ca2+ signaling and LTD of AMPAR EPSCs at parallel fiber synapses on cerebellar Purkinje neurons (Sarkisov and Wang, 2008; Wang et al., 2000). Here, climbing fiber activation and subsequent dendritic Ca2+ spike generation do not evoke large enough Ca2+ transients in dendritic spines to reach the threshold for plasticity induction unless CICR is triggered by parallel fiber inputs activating mGluRs and producing local IP3 increases in spines. The main difference between NMDAR LTP in DA neurons and AMPAR LTD in Purkinje neurons is the involvement of NMDAR activation in the induction. At parallel fiber-Purkinje neuron synapses, which lack NMDARs, chemical compartmentalization offered by dendritic spines restricts IP3 and Ca2+ signaling to individual spines, thereby mediating synapse specificity of plasticity (Nimchinsky et al., 2002; Wang et al., 2000). However, such compartmentalization of IP3-dependent Ca2+ signaling may not be easily attained at glutamatergic synapses on DA neurons, which are mostly formed on dendritic shafts [(Carr and Sesack, 2000; Charara et al., 1996), but also see (Sarti et al., 2007)]. Indeed, synaptic activation of mGluRs augments burst-induced Ca2+ transients throughout individual dendrites in DA neurons (Cui et al., 2007). Therefore, the localized signal underlying the input specificity of NMDAR LTP is presumably provided by NMDARs causing Ca2+ influx only at activated synapses, which would be below the spatial resolution of the confocal imaging system used in the present study. In support of this idea, synaptic activation of ionotropic glutamate receptors (i.e, Ca2+-permeable AMPARs) has been shown to produce highly localized (~1 μm) Ca2+ transients in aspiny dendrites mediating input-specific Ca2+ signaling and plasticity (Goldberg et al., 2003; Soler-Llavina and Sabatini, 2006). The requirement for coactivation of NMDARs and mGluRs, together with the dependence on intracellular Ca2+ stores, is in line with recent studies demonstrating input-specific LTP of NMDAR EPSCs at hippocampal mossy fiber synapses (Kwon and Castillo, 2008; Rebola et al., 2008). It should also be noted that Ca2+ transients resulting from NMDAR-induced Ca2+ influx can be amplified via an mGluR- and IP3-dependent CICR mechanism at Schaffer collateral synapses on hippocampal CA1 pyramidal neurons (Dudman et al., 2007).
A number of studies have reported LTP of NMDAR-mediated transmission in the hippocampus (Bashir et al., 1991; Bellone and Nicoll, 2007; Harney et al., 2008; Kwon and Castillo, 2008; Rebola et al., 2008), yet none of these studies have addressed the role of postsynaptic APs in LTP induction. A delayed NMDAR LTP has been observed in cortical pyramidal neurons, which is induced by simultaneous presynaptic and postsynaptic burst firing and is dependent on preceding AMPAR LTP (Watt et al., 2004). In the present study, NMDAR LTP required a delay between the onset of presynaptic stimulation and postsynaptic burst firing and was independent of AMPARs. Thus, NMDAR LTP in DA neurons represents a form of Hebbian plasticity of NMDAR-mediated transmission that has not been previously described.
Ample evidence indicates the important role of PKA in regulating different aspects of synaptic plasticity (Nguyen and Woo, 2003). In particular, PKA has been shown to gate the induction of AMPAR LTP by modulating CaMKII and SK2 channels in the hippocampus and amygdala (Blitzer et al., 1998; Faber et al., 2008). Our data show that PKA gates the induction of NMDAR LTP in DA neurons through enhancement of IP3R function. LTP induction may also be affected by PKA regulation of NMDAR-mediated Ca2+ influx (Skeberdis et al., 2006).
Burst-dependent potentiation of NMDARs appears to be expressed postsynaptically by a mechanism distinct from that previously described for metabotropic receptor-induced potentiation of NMDAR EPSCs in DA neurons (Borgland et al., 2006; Schilstrom et al., 2006; Ungless et al., 2003). For example, activation of orexin-1 receptors induces PKC-dependent translocation of NR2A-containing NMDARs to the synapse (Borgland et al., 2006). PKC-mediated recruitment of NMDARs has also been implicated in NMDAR LTP at hippocampal mossy fiber synapses (Kwon and Castillo, 2008). However, PKC blockade failed to affect NMDAR LTP in our study. Furthermore, the effects of NR2A- and NR2B-specific antagonists on NMDAR EPSCs were not altered after LTP expression. Although we cannot rule out potential changes in NR2C/2D subunits (Harney et al., 2008), these subunits make small contributions to NMDAR EPSCs in DA neurons (Borgland et al., 2006). Therefore, enhanced function of individual NMDAR channels and/or increased synaptic expression of existing NMDARs with no change in the subunit composition likely mediate the expression of LTP (Chen and Roche, 2007).
Burst-Timing Dependence and Reversibility of NMDAR Plasticity in DA Neurons: Potential Relevance to Reward Learning
In behaving animals, DA neurons “learn” to respond to inherently neutral environmental cues with synchronized bursts of activity after repeated cue-reward pairing (Pan et al., 2005; Schultz, 1998). Several modeling studies have addressed the neurobiological substrates underlying the conditioning of DA neuron responses (Brown et al., 1999; Contreras-Vidal and Schultz, 1999; Houk et al., 1995). One of these models postulates that plasticity of synapses onto DA neurons is involved in this learning process (Contreras-Vidal and Schultz, 1999). It has also been shown in awake rats that excitatory responses of pedunculopontine tegmental nucleus neurons to auditory cues, which play an important role in driving DA neuron burst responses to those cues, remain unaltered during cue-reward learning (Pan and Hyland, 2005). Since the pedunculopontine tegmental nucleus gives rise to direct glutamatergic (and cholinergic) inputs to DA neurons (Charara et al., 1996), this raises the possibility that plasticity of glutamatergic synapses onto DA neurons may play a role in the development of conditioned burst responses. Therefore, in light of the prominent role of NMDARs in the generation of DA neuron bursts (Chergui et al., 1994; Morikawa et al., 2003; Overton and Clark, 1997; Tong et al., 1996; Zweifel et al., 2009), the activity-dependent plasticity of NMDARs described in this study may contribute to the acquisition of cue responses. It should be noted that the synaptic stimulation-burst pairing protocol emulates the neural activity evoked during the cue-reward pairing paradigm. Here, sustained synaptic stimulation mimics the working memory-type persistent input activated by the presentation of the cue (Brown et al., 1999; Funahashi et al., 1989), whereas the postsynaptic burst corresponds to that triggered by the reward during conditioning. In this model, potentiated NMDARs at those synapses activated by the cue, accompanied by certain termination mechanism(s) (e.g., SK channel activation), mediate the transient burst response to the cue after conditioning.
Of particular interest is the burst-timing dependence of the induction of NMDAR plasticity, which appears analogous to the timing rule governing cue-reward learning in behaving animals. In the standard and most effective training paradigm, termed delay conditioning, there is a delay of hundreds of milliseconds to several seconds between the onset of the cue and that of the reward, with the two stimuli overlapping in time (Fiorillo et al., 2003; Schwartz et al., 2002). For NMDAR LTP in DA neurons, the requirement of the delay (~0.5–1 s) and the overlap between synaptic stimulation and burst firing during induction most likely reflects the involvement of PI-coupled receptors and NMDARs, respectively. Furthermore, induction of LTD when the burst precedes synaptic stimulation during the burst pairing protocol is congruent with the ineffectiveness of backward conditioning in which the reward is presented prior to the cue (Schwartz et al., 2002). The timing rule described here is distinct from that for the spike timing-dependent plasticity reported in a variety of neurons (Dan and Poo, 2004; Sjostrom and Nelson, 2002), including DA neurons (Liu et al., 2005; Luu and Malenka, 2008), in which the plasticity is sensitive to the timing of presynaptic and postsynaptic spikes on a timescale of tens of milliseconds, much shorter than the timescales encountered during behavioral conditioning (Drew and Abbott, 2006).
It is of note that the same induction protocol that caused NMDAR LTP resulted in LTD of AMPAR EPSCs in this study. Since AMPAR LTD did not require postsynaptic bursting, it presumably corresponds to the mGluR-dependent but postsynaptic activity-independent AMPAR LTD mediated by a shift in the AMPAR subunit composition in DA neurons (Mameli et al., 2007). This LTD has been shown to reverse the persistent and global potentiation of AMPARs produced by cocaine administration paired with environmental cues, and thus may act to reset AMPAR-mediated transmission to enable AMPAR plasticity required for future learning (Bellone and Luscher, 2006; Chen et al., 2008; Stuber et al., 2008). Therefore, simultaneous NMDAR LTP and AMPAR LTD may work in concert to promote the learning of new environmental cues in animals previously conditioned with powerful reinforcers such as addictive drugs. However, it is important to point out that the exact, and perhaps differential, roles of NMDAR plasticity vs. AMPAR plasticity in vivo remain to be determined.
The neural mechanisms underlying behavioral learning are thought to involve both reversible and irreversible components (Medina et al., 2002; Pan et al., 2008). Our results show that NMDAR LTP can be reversed, or depotentiated, by repeated delivery of synaptic stimulation alone, which is reminiscent of the extinction of learned responses when the conditioning cue is repeatedly presented without the expected reward. It is remarkable that the expression of LTP is maintained when synaptic stimulation is repeatedly paired with a single AP, suggesting that single AP-evoked Ca2+ transients, facilitated by IP3-dependent CICR, can serve to prevent depotentiation. Therefore, a pause in tonic single-spike activity of DA neurons, as observed at the time of the expected reward when the learned cue is presented alone, may be necessary to induce extinction of phasic burst responses to the cue (Pan et al., 2008; Tobler et al., 2003).
EXPERIMENTAL PROCEDURES
Electrophysiology
Horizontal midbrain slices were prepared from male Sprague-Dawley rats (4–7 weeks old). Recordings were made at 34–35°C in a chamber perfused at ~2.5 ml/min with recording solution containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 or 0.1 MgCl2, 2.4 CaCl2, 11 glucose, 21.4 NaHCO3, saturated with 95% O2/5% CO2 (pH 7.4, ~295 mOsm/kg). The pipette solution contained (in mM): 115 K-gluconate or K-methylsulfate, 20 KCl, 1.5 MgCl2, 10 HEPES, 0.025 EGTA, 2 Mg-ATP, 0.2 Na2-GTP, and 10 Na2-phosphocreatine (pH 7.25, 285 mOsm/kg).
Cells were visualized using an upright microscope with IR-DIC optics (Olympus). Whole-cell voltage-clamp recordings were made from electrophysiologically identified DA neurons at a holding potential of −62 mV, corrected for a liquid junction potential of −7 mV. Pipette resistance was 2.0–2.5 MΩ. Pipette capacitance was neutralized but series resistance was left uncompensated. Input resistance (typically ~250 MΩ) and holding current (typically 0 to −100 pA) were monitored continuously; experiments were discarded if they changed by more than 25% or 60 pA, respectively, or if series resistance increased above 16 MΩ. A Multiclamp 700B amplifier (Molecular Devices) was used to record the data, which were filtered at 2–10 kHz, digitized at 4–20 kHz, and collected using AxoGraph X (AxoGraph Scientific).
Synaptic Stimulation and LTP Induction
Synaptic stimuli were applied at 0.05 Hz using bipolar tungsten electrodes (100–120 μm tip separation) and pharmacologically isolated NMDAR EPSCs were monitored.
Immediately before LTP induction, the effect of sustained synaptic stimulation on IK(Ca), evoked by a single unclamped AP, was evaluated in each neuron. A 2-ms depolarizing pulse from −62 mV to −7 mV was used to elicit an unclamped AP. The integral of the outward tail current, i.e., IK(Ca), was calculated between 20 ms and 400–600 ms after the depolarizing pulse. We have shown previously that IK(Ca) thus measured is completely eliminated by TTX and also by apamin, a selective blocker of Ca2+-sensitive SK channels, and hence can be used as a readout of AP-induced Ca2+ transients (Cui et al., 2007). The magnitude of IK(Ca) facilitation by synaptic stimulation was calculated by comparing Ik(Ca) evoked 60 ms after a 1-s train of 50-Hz synaptic stimulation, after subtracting the trace elicited by synaptic stimulation alone, with Ik(Ca) evoked in isolation. A single AP, instead of a burst of APs, was used in evaluating synaptic facilitation of IK(Ca) in order to avoid potential influence on LTP induction.
The LTP induction protocol consisted of sustained synaptic stimulation (70 stimuli at 50 Hz) paired with a postsynaptic burst of 5 APs at 20 Hz, where the burst was delayed by 1 s from the onset of the synaptic stimulation. Synaptic stimulation was extended 200 ms beyond the end of the burst, i.e., until burst-evoked Ik(Ca) mostly decayed, to ensure that synapses were activated while cytosolic Ca2+ concentration was elevated. Synaptic stimulation-burst pairing was repeated 10 times every 20 s. The same stimulation intensity used for monitoring NMDAR EPSCs was used for synaptic stimulation during induction. The magnitude of LTP was calculated by comparing averaged EPSC amplitudes from 10-min windows (30 traces) immediately before and 30–40 min after LTP induction. These windows were also used to assess PPR and 1/CV2.
To test the independence of inputs in the two-pathway experiments (Figure 6), we used cross paired-pulse analysis. We first determined the PPR for each input. We then substituted the opposing input for the second pulse and confirmed the absence of interaction between the two inputs.
Flash Photolysis
Caged IP3 (100 μM) was loaded into the cytosol through the whole-cell pipette. A 1-ms UV pulse was applied using a xenon arc lamp (Cairn Research) to rapidly release IP3 and the resulting SK-mediated outward current (IIP3) was measured. The amount of photolysis is known to be proportional to the UV pulse intensity, which is proportional to the capacitance of the capacitor feeding current to the flash lamp. This capacitance was varied (50–4050 μF) to adjust the UV pulse intensity.
Ca2+ Imaging
Fluorescence imaging of intracellular Ca2+ was performed using fluo-5F (50 μM) loaded into the cytosol via the whole-cell pipette. Images were captured at 15 Hz with a spinning disk confocal imaging system (Olympus). Ca2+ signals from selected ROIs were expressed as ΔF/F = (F − Fbaseline)/(Fbaseline − Fbackground).
Additional methodological details are described in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Drs. Rishikesh Narayanan and Michael Roberts for comments on the manuscript. We also thank Dr. Michael Mauk for suggesting the single-spike depotentiation experiment. This work was supported by National Institutes of Health Grant DA015687. M.T.H. was supported by a National Science Foundation Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Atherton JF, Bevan MD. Synaptic Activation of Dendritic AMPA and NMDA Receptors Generates Transient High-Frequency Firing in Substantia Nigra Dopamine Neurons In Vitro. J Neurophysiol. 2007;97:2837–2850. doi: 10.1152/jn.01157.2006. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brown J, Bullock D, Grossberg S. How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci. 1999;19:10502–10511. doi: 10.1523/JNEUROSCI.19-23-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Akaoka H, Charlety PJ, Saunier CF, Buda M, Chouvet G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport. 1994;5:1185–1188. doi: 10.1097/00001756-199406020-00006. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Schultz W. A predictive reinforcement model of dopamine neurons for learning approach behavior. J Comput Neurosci. 1999;6:191–214. doi: 10.1023/a:1008862904946. [DOI] [PubMed] [Google Scholar]
- Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J Neurosci. 2007;27:4776–4785. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Abbott LF. Extending the effects of spike-timing-dependent plasticity to behavioral timescales. Proc Natl Acad Sci U S A. 2006;103:8876–8881. doi: 10.1073/pnas.0600676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci. 2008;28:10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Cholinergic inhibition of ventral midbrain dopamine neurons. J Neurosci. 2000;20:7855–7860. doi: 10.1523/JNEUROSCI.20-20-07855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Rowan M, Anwyl R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J Neurosci. 2006;26:1128–1132. doi: 10.1523/JNEUROSCI.2753-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Houk J, Adams J, Barto A. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Houk J, Davis J, Beiser D, editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 249–270. [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ. The return of the spike: postsynaptic action potentials and the induction of LTP and LTD. Neuron. 1999;22:661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Malenka RC. Spike timing-dependent long-term potentiation in ventral tegmental area dopamine cells requires PKC. J Neurophysiol. 2008;100:533–538. doi: 10.1152/jn.01384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Medina JF, Repa JC, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Tripartite mechanism of extinction suggested by dopamine neuron activity and temporal difference model. J Neurosci. 2008;28:9619–9631. doi: 10.1523/JNEUROSCI.0255-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ, Schrader LA, Anderson AE. Regulation of surface localization of the small conductance Ca2+-activated potassium channel, Sk2, through direct phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2006;281:11769–11779. doi: 10.1074/jbc.M513125200. [DOI] [PubMed] [Google Scholar]
- Sarkisov DV, Wang SS. Order-dependent coincidence detection in cerebellar Purkinje neurons at the inositol trisphosphate receptor. J Neurosci. 2008;28:133–142. doi: 10.1523/JNEUROSCI.1729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Wasserman EA, Robbins SJ. Psychology of learning and behavior. 5. New York, NY: W. W. Norton & Company; 2002. [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Shin N, Soh H, Chang S, Kim DH, Park CS. Sodium permeability of a cloned small-conductance calcium-activated potassium channel. Biophys J. 2005;89:3111–3119. doi: 10.1529/biophysj.105.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol. 2002;12:305–314. doi: 10.1016/s0959-4388(02)00325-2. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci. 2006;9:798–806. doi: 10.1038/nn1698. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase a and protein phosphatase 1alpha. J Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm. 1996;103:889–904. doi: 10.1007/BF01291780. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Joseph SK, Yule DI. Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J Physiol. 2008;586:3577–3596. doi: 10.1113/jphysiol.2008.152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci. 2004;7:518–524. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wilson CJ, Callaway JC. Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol. 2000;83:3084–3100. doi: 10.1152/jn.2000.83.5.3084. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.