Abstract
Charles Darwin laid the foundation for all modern work on sexual selection in his seminal book The Descent of Man, and Selection in Relation to Sex. In this work, Darwin fleshed out the mechanism of sexual selection, a hypothesis that he had proposed in The Origin of Species. He went well beyond a simple description of the phenomenon by providing extensive evidence and considering the far-reaching implications of the idea. Here we consider the contributions of Darwin to sexual selection with a particular eye on how far we have progressed in the last 150 years. We focus on 2 key questions in sexual selection. First, why does mate choice evolve at all? And second, what factors determine the strength of mate choice (or intensity of sexual selection) in each sex? Darwin provided partial answers to these questions, and the progress that has been made on both of these topics since his time should be seen as one of the great triumphs of modern evolutionary biology. However, a review of the literature shows that key aspects of sexual selection are still plagued by confusion and disagreement. Many of these areas are complex and will require new theory and empirical data for complete resolution. Overall, Darwin's contributions are still surprisingly relevant to the modern study of sexual selection, so students of evolutionary biology would be well advised to revisit his works. Although we have made significant progress in some areas of sexual selection research, we still have much to accomplish.
Keywords: Bateman gradient, direct benefits, female choice, indirect benefits, selection differential
Charles Darwin proposed the concept of sexual selection 150 years ago in On the Origin of Species by Means of Natural Selection (1), but his definitive work on sexual selection was undoubtedly The Descent of Man, and Selection in Relation to Sex (2), which was published in 1871. Now—200 years after Darwin's birth—is an excellent time to reflect on the modern relevance of his work and on progress that has been made in the study of sexual selection since his time. In typical Darwinian fashion, The Descent of Man, and Selection in Relation to Sex is a colossal tome (with a tongue-tripping title that we will henceforth abbreviate as The Descent of Man) that strolls through myriad topics relevant to evolutionary biology and ecology. Consequently, it is futile to attempt to characterize the full spectrum of topics addressed in this book, which touched upon such sundry issues as species concepts, taxonomy, correlated evolution, sex-limited inheritance, and group selection, to name a few. Rather, we focus on the contributions of Darwin in light of modern research in sexual selection, and in so doing we identify a few important topics for which a thread of reasoning can be traced from Darwin to the present.
One of the great strengths of Darwin was that he often constructed his literary works with a clear argument in mind and marshaled vast amounts of evidence to support his case. The Descent of Man is most famous for Darwin's contribution to the hypothesis of sexual selection, but the main goal of the book was to provide evidence that evolutionary principles applied to humans and that humans descended from some ape-like common ancestor. Darwin believed that sexual selection played a major role in the evolution of humans and the divergence among distinct human populations, so he felt a lengthy description of sexual selection was necessary. Indeed, the bulk of the book concerns sexual selection, but many of Darwin's insights regarding sexual selection appear in his chapters on humans.
Darwin's most lasting achievement with respect to sexual selection must be his definition of the term, as it is essentially the same as the one still in use today. It is difficult to find a quote from Darwin that captures the full essence of his concept of sexual selection, but he provides the following definition (ref. 2; Part I, pp 254–255):
“We are, however, here concerned only with that kind of selection, which I have called sexual selection. This depends on the advantage which certain individuals have over other individuals of the same sex and species, in exclusive relation to reproduction.”
However, Darwin makes it clear that not all selection related to reproduction constitutes sexual selection, as primary sexual traits—like ovaries and testes—can evolve as a consequence of natural selection. Even though he never spells it out in so many words, Darwin's working definition of sexual selection is essentially identical to the one used by Andersson (3) and most other scientists studying sexual selection. In particular, “sexual selection arises from differences in reproductive success caused by competition for access to mates” (ref. 3, p 3). This definition admittedly focuses primarily on precopulatory sexual selection, so a more complete definition should also include postcopulatory processes, which can be accomplished by tagging the phrase “or fertilization opportunities” onto the end of Andersson's definition.
Aside from the definition of sexual selection, what did Darwin accomplish in The Descent of Man? In a book as rich as Darwin's, every reader could potentially identify different sets of key conclusions, depending on the reader's background and research emphasis. Here we try to focus on those parts of the book that are still relevant to modern research, and from our perspective Darwin identified 2 major themes that set the stage for work on sexual selection. The first theme concerned the question of why sexual selection occurs in the first place. In the context of this question, Darwin identified the 2 major categories of sexual selection, namely intrasexual and intersexual selection, although he didn't use those terms (2). The second theme is related to the question of why sexual selection is strong in some lineages but not others. This question continues to be a major theme of modern research, but Darwin expressed an amazingly modern, intuitive understanding of some of the explanations for the patterns of sexual selection among diverse evolutionary lineages (2).
In this report, we summarize Darwin's contributions to these 2 major topics and consider how far we have progressed in our understanding of them. Rather than review all of the relevant literature, which for many of these topics has been done recently, we try to paint the current state of sexual selection with broad strokes. Like Darwin (2), we focus on precopulatory sexual selection, leaving the treatment of postcopulatory processes for a different paper in this volume (4). Our sojourn through sexual selection literature leads to the identification of at least 2 major triumphs of precopulatory sexual selection research since Darwin. It also identifies numerous areas ripe for additional work. We hope that this paper will inspire our fellow scientists by showing (i) that Darwin, despite having tremendous insight given the state of biology in the 19th century, did not get everything right, (ii) that we have made tremendous progress since Darwin's time, especially in the last several decades, and (iii) that many important questions regarding sexual selection remain to be answered.
The Mechanisms of Sexual Selection and the First Major Triumph
Darwin (2) correctly realized that sexual selection could be mediated by male–male combat or by a female's choice of attractive males. His original definition of sexual selection, which appeared in The Origin of Species, appears to emphasize male–male combat [i.e., “a struggle between the males for possession of the females” (ref. 1, p 88)], but even then he was clearly aware of female choice. Thus, Darwin identified the 2 main categories of sexual selection that persist to this day.
The Law of Battle.
Sexual selection would be relatively simple if there were nothing but what we call intrasexual selection. In fact, Darwin's understanding of intrasexual selection was essentially complete. In some species, males engage in fierce struggles among one another, and the victors in these contests tend to mate with the receptive females. Darwin provided numerous examples of species in which intrasexual combat for access to mates prevailed, some of the more interesting of which include male narwhals fencing with their tusks (which remains to be studied, but see ref. 5), male scarab beetles (family Scarabaeidae) battling with their elaborate horns (6), and female buttonquail (genus Turnix) fighting like gamecocks for reproductive access to the males (7). The latter example illustrates Darwin's appreciation of the fact that sexual selection, which normally acted most strongly on males, could sometimes operate on females, a point to which we will return later.
However, intrasexual selection is not a sufficient mechanism by which to explain all of the diversity wrought by sexual selection. Modern work shows that intersexual selection is a major aspect of sexual selection, a point that was deeply appreciated by Darwin. In fact, Darwin correctly realized that even in species characterized by the “law of battle,” mate choice often is important. As Darwin stated (ref. 2, Part II, p 269),
“The female could in most cases escape, if wooed by a male that did not please or excite her; and when pursued, as so incessantly occurs, by several males, she would often have the opportunity, whilst they were fighting together, of escaping with, or at least temporarily pairing with, some one male.”
Thus, any treatment of sexual selection will be incomplete without a treatment of mate choice.
Female (or Sometimes Male) Choice.
If the number of words devoted to each topic can serve as a guide, then Darwin felt that the topic of female choice required much more explanation than did male–male combat. Here we will treat female choice, while keeping in mind that Darwin knew that in some systems mirror-image processes could occur through male choice. Sexual selection as a consequence of female choice is easy to understand, provided we are willing to accept that female preferences exist. If females show a preference, then males with the preferred trait will leave greater numbers of offspring, and their trait values will tend to increase in frequency in the population.
But why would female preferences exist in the first place? The answer to this question is not entirely obvious. Darwin's approach was to contend that it was inconceivable that preferences did not exist and to provide evidence of female preferences in various animal species. Some of these examples, like the female mallard duck that experienced “love at first sight” upon encountering a male pintail (ref. 2, Part II, p 115), are rather humorous, but they do serve to show that females are not mating at random in most cases. Numerous passages in The Descent of Man address this issue, the following quote (ref. 2, Part I, p 421) among them:
“Nevertheless, when we see many males pursuing the same female, we can hardly believe that the pairing is left to blind chance—that the female exerts no choice, and is not influenced by the gorgeous colours or other ornaments with which the male alone is decorated.”
Here we see the essence of Darwin's argument: Given the variation among males with respect to their beautiful ornaments, it is difficult to believe that females have no preference whatsoever, and even a weak preference would be sufficient for sexual selection to operate. Hence, Darwin clearly understood that female preferences existed, but he never compellingly explained why such preferences would evolve.
The flavor of Darwin's argument for female choice may represent one of the largest shortcomings of his treatment of sexual selection because it gave the impression that animals would need a human-like sense of aesthetics for sexual selection to operate. Indeed, Darwin himself seemed to subscribe to this point of view, as he went to great lengths to argue that arthropod, insect, and vertebrate females possess sufficient intelligence to appreciate beauty. He further asserted that the “lowest classes” of animals, including echinoderms, annelids, mollusks, and so forth, “have too imperfect senses and too low mental powers to feel mutual rivalry, or to appreciate each other's beauty or other attractions” (ref. 2, Part I, p 321). However, Darwin also clearly appreciated that different species could possess different standards of beauty, explaining why not all sexually selected traits appear attractive to us. Regardless, in the midst of Darwin's tremendous insights pertaining to sexual selection, the suggestion that a sense of aesthetics is necessary for sexual selection to operate may have been his most significant shortcoming. It can be argued that it took almost 100 years for the study of sexual selection to overcome this erroneous view of mating preferences.
The First Major Triumph of Modern Sexual Selection Research.
The study of sexual selection entered its modern era during the latter half of the 20th century when scientists identified the evolution of female choice as a legitimate topic in its own right by expanding the ideas laid out much earlier by Fisher (8, 9). In other words, it was no longer sufficient to assume that females had preferences or even to provide empirical evidence of such preferences. Rather, we needed mechanisms that could explain the evolution of female preferences (10–15). Once the evolution of preferences could be explained, our understanding of selection of the preferred trait became entirely straightforward, and it remains today essentially as Darwin described it. Thus, the difficulty, which partly remains unsolved, is to understand the evolution of female (or male) preferences. We will briefly review the modern models, but we start with the one explicit model proposed by Darwin. Although his model is missing some elements and comes up short as a complete explanation for choice evolution, it does provide the core of a model with potential explanatory power.
Darwin's Model of Sexual Selection.
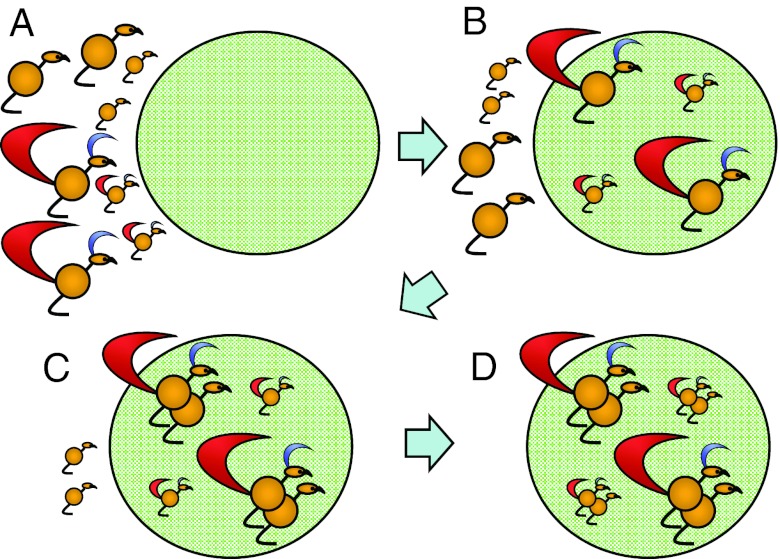
Darwin's model, which later came to be known as the Darwin–Fisher model (15, 16), has been invoked as a potential explanation for sexual selection in monogamous taxa. In Darwin's version of the model (ref. 2, Part I, pp 261–262), he assumes a population with 2 types of females: “the more vigorous and better-nourished individuals” and “the less vigorous and healthy” (Fig. 1A). Males also are variable in quality, and they arrive before females on the breeding grounds (Fig. 1B). The higher-quality females are ready to breed before the lower-quality females, and they choose to pair with the higher-quality males (Fig. 1C). Thus, the model predicts assortative mating by quality (Fig. 1D), and Darwin argues that the higher-quality pairs will produce more offspring than the lower-quality pairs. In Darwin's discussion of this model, we find the only occurrence of a clear explanation for the evolution of mate choice in his entire book (ref. 2, Part I, p 263):
“Such pairs would have an advantage in rearing offspring, more especially if the male had the power to defend the female during the pairing-season, as occurs with some of the higher animals, or aided in providing for the young.”
Fig. 1.
Darwin's model of sexual selection. (A) First, males and females are variable with respect to phenotypic quality. (B) Males, which have the ornaments, move to the breeding area (large circle) before the females. (C) The highest-quality females are ready to mate sooner, so they pair with the highest-quality males. (D) Finally, the lower-quality females pair with the lower-quality males. Sexual selection occurs because the higher-quality pairs produce more offspring than the lower-quality pairs.
Unfortunately, Darwin did not elaborate much on this line of reasoning, and he never provided a convincing explanation for the evolution of a preference for ornaments or other sexually selected characters not directly involved in territory defense or parental care. However, if we merge Darwin's model with a more modern understanding of sexual selection, as has been done by Fisher (8) and subsequent researchers (16, 17), we find a model that could explain female-choice evolution while retaining the main features of Darwin's original model. However, surprisingly little research has been directed at this model, and so far it has been used exclusively as an explanation for sexual selection in males of monogamous taxa. Additional theoretical and empirical work on this model is warranted, and attempts should be made to extend it to sexual selection on females and in nonmonogamous taxa.
Models of Direct Benefits.
Models for the evolution of mating preferences fall into 2 major categories: direct-benefits models and indirect-benefits models (Table 1). The direct-benefits models suppose that females (or males for sex-role-reversed species) choose mates that provide some immediate benefit to the chooser, such as parental care, a nuptial gift, or territory defense (18–21). We provide a list of some of the most commonly invoked direct benefits in Table 1, along with a few empirical examples. The evolution of choice for direct benefits is conceptually simple, as the selective advantage to choosing is entirely obvious (3). However, the situation is slightly more complex when the female prefers an ornament in the male that somehow indicates that the male will provide better-than-average parental care, resources, defense, and so forth (Table 1). Under these circumstances, there must be a mechanism that produces a correlation between the secondary sexual trait and the direct benefit provided by the male to the female. Our reading of the literature indicates that direct-benefits models enjoy excellent empirical support and are mostly not controversial, so we will treat them no further here.
Table 1.
Some empirical examples supporting various models of mate preference evolution
| Mechanism of preference evolution | Some sample organisms | References |
|---|---|---|
| Direct-benefits models | ||
| Resource acquisition | Bushcrickets | 80 |
| Protection | Elephant seals, dung flies | 81, 82 |
| Parental care | Blackbirds, sticklebacks | 83, 84 |
| Fertility | Lemon tetras, fruit flies | 85, 86 |
| Parasite avoidance | Grain beetles | 87 |
| Indirect-benefits models | ||
| Fisherian process | Guppies, sandflies | 88, 89 |
| Condition-dependent indicator | Ambush bugs, bank voles | 90, 91 |
| Condition-independent indicator | Possibly cockroaches | 92 |
| Other models | ||
| Sensory bias | Three-spined sticklebacks, Anolis lizards | 93, 94 |
| Sexual conflict | Cichlids, fireflies | 95, 96 |
| Genetic compatibility | Oldfield mice | 97 |
Models of Indirect Benefits.
In some species, the males appear to provide nothing to the females but sperm, yet they have elaborate ornaments for which females show preferences (19, 22–24). These systems are especially perplexing from a sexual selection standpoint because the benefits of choice are not at all obvious. Consequently, a tremendous amount of effort has been devoted to creating explanatory models of female-choice evolution in such systems (3, 14, 15, 25). These types of models have commonly been described as indirect-benefits models because the female's choice of males provides her with no immediate, measurable benefits. Rather, the female's fitness increases as a consequence of her offspring having higher fitness if she pairs with a preferred male. Several excellent reviews have addressed the dizzying array of such models (21, 24–26), so rather than review them, we attempt here to organize them into a few major categories. We would like to suggest that there are 3 main categories of indirect-benefits models. We have no doubt that such a categorization will be controversial. Indeed, we are at odds with at least one other perspective that suggests that all indirect-benefits models should be lumped into a single category of Fisher–Zahavi models (27, 28). We believe there is much heuristic value in keeping them separate, so to stimulate discussion we propose the following 3 classes of models.
Model 1: The Fisherian Model.
This model involves a single preference trait in females and a single ornament trait in males (ignoring multivariate preferences and signals for the moment). To understand this model, we must appreciate that females with a preference for a large ornament, for example, will have offspring with both the genes for the large ornament from the father and genes for the preference for large ornaments from the mother (8, 9, 14). Consequently, mate choice results in a genetic correlation between the ornament and preference (14, 25). The ornament evolves as a consequence of sexual selection imposed by female mate choice, and the preference is carried along as a consequence of a correlated response to selection (14). Almost any outcome is possible in the Fisherian model, depending on the strength of the genetic correlation between the ornament and the preference (14, 15, 25). However, under some circumstances, this model results in a self-reinforcing, open-ended process that produces never-ending trait elaboration. Eventually, the process is opposed by natural selection when the ornament becomes so large as to be a major impediment to survival, a point that was actually well appreciated by Darwin (2). This model has intuitive appeal because it seems like such a process can explain some of the most elaborate traits present in the animal world, such as the peacock's tail or the bowerbird's bower. It is also worth noting that some amount of a genetic correlation between ornament and preference will occur in any system in which mate choice operates, so a Fisherian process could act in concert with almost any other model of mate-choice evolution.
Model 2: The Condition-Dependent Indicator Model.
The condition-dependent indicator model is the most widely used of the “good genes” models (3, 25, 29). This model requires at least 3 traits (i.e., ornament, preference, and a viability trait), so it is clearly distinct from the Fisherian model (29). In the condition-dependent indicator model, the ornament is a costly condition-dependent trait. Thus, males closer to the optimum with respect to the viability trait will be in better condition and will be able to maintain a more elaborate version of the ornament (12, 30). Female choice evolves because females choosing males with more elaborate ornaments produce offspring with higher viability or that will be in good condition as adults. Of the indirect benefits models, the condition-dependent indicator model works most easily from a theoretical standpoint and enjoys the most empirical support (3). Because the ornament is condition dependent, it is always a reliable indicator of genetic quality, and for female preferences to evolve or be maintained, the condition-dependent indicator model does not require any variation in the genes determining the ornament (although the ornament may appear genetically variable due to genetic variation in condition). Of course, for the male trait to evolve it must be genetically variable. This model also requires genetic variation for fitness for the males in the population, so a complete understanding must address the mechanisms maintaining genetic variation for viability or vigor, an issue that has yet to be satisfactorily resolved (19, 24).
Model 3: The Condition-Independent Indicator Model.
Even though this model still involves 3 traits—ornament, preference, and viability—it differs from the condition-dependent indicator model in that the ability of a male to produce an elaborate ornament is no longer dependent on his condition (or nearness to the optimum for the viability trait). For the model to work, all 3 traits must exhibit genetic variation, and they may or may not have environmental variation. This model functions as a consequence of a genetic correlation between the ornament and the viability trait. Clearly, such a genetic correlation will allow a female to produce higher-fitness offspring by choosing males with better ornaments, and hence female choice will evolve (3, 29, 31). However, this model, if it works at all, requires more restrictive conditions than the condition-dependent indicator model. The major remaining challenges for this model are to explain what could maintain a genetic correlation between male viability and the ornament trait and to find additional empirical examples of this process.
Other Models of Mate-Choice Evolution.
In addition to the direct- and indirect-benefits models of mate choice, several other models have been proposed. For example, the sensory exploitation model suggests that males evolve sexually selected traits that take advantage of preexisting inclinations inherent to the female sensory systems (32–34). Thus, females may exhibit a preference simply because they are predisposed to do so, and the preference may have evolved as a consequence of evolutionary mechanisms, such as natural selection or drift, unrelated to sexual selection. Not only could sensory exploitation lead to the evolution of male secondary sexual traits, but it also could play a role in jump-starting other mechanisms of mate-choice evolution, such as the Fisherian process. Another class of models focuses on genetic compatibility by suggesting that females choose males who complement their own genome (35–37). Finally, sexual conflict, which occurs when the sexes have incompatible optima with respect to some aspects of reproduction (e.g., mating rate), has been suggested as a mechanism for female-choice evolution (38–40). All of these models enjoy some empirical and theoretical support, but all remain controversial. Of these 3 models, it seems that the sensory exploitation model is the most likely to explain the exaggerated traits that interested Darwin (2). Nevertheless, all 3 of these models present extremely fertile ground for future work.
Summary and Future Directions.
Although Darwin appreciated the importance of mating preferences in sexual selection, he did not cleanly identify the evolution of mate choice as a key topic in its own right. The progress in this area of research since Darwin has been nothing short of spectacular. It is now clear that the evolution of mate choice is one of the most important topics in sexual selection research, and we have several plausible models with which to work. We find that direct-benefits models and condition-dependent indicator models seem to be the most well-supported explanations for the evolution of elaborate traits via sexual selection. Nevertheless, definitive tests of these models are difficult to find, and the subject remains controversial. The Fisherian process is probably operating in some systems, but we do not know how ubiquitous it is. Maybe this process is operating in the background in all systems characterized by mate choice or maybe it comes into play episodically when conditions are especially favorable for its operation. On the other hand, depending on the evolution and maintenance of genetic correlations between traits and preferences, the possibility remains that the Fisherian process explains very little with respect to the evolution of female preferences. We will need more data before we can decide. Even more controversial are the models of condition-independent indicators and sensory exploitation, so they should be studied in more detail. Finally, genetic compatibility and sexual conflict models almost certainly describe real phenomena (41–43), but their role in the evolution of secondary sexual traits remains to be resolved.
One important success of these models is that we no longer need to invoke a human-like sense of aesthetics in animals as Darwin did (2). Rather, it is sufficient for the choosing sex to respond to a stimulus (e.g., an ornament) if the response to the stimulus increases the fitness of the chooser. Why the ornaments used by birds and other nonhuman animals usually appear so strikingly beautiful to humans is another question, but it's a mystery that does not have to be solved for us to understand sexual selection.
Despite our tremendous success so far, there remains much to be accomplished. Here, we suggest the following 5 areas in which we see a pressing need for additional research.
1. Costs of choice.
Every model of choice evolution is affected by assumptions regarding the costs of choosing, but insufficient empirical data exist from this challenging area of inquiry to properly parameterize the models. Perhaps we need a new concept called “the environmental potential for mate choice” in which costs of choice are considered in light of reproductive ecology.
2. The evolution of genetic correlations between ornaments and preferences.
The genetic correlations between ornaments and preferences affect the strength of the Fisherian process, which could be operating in tandem with any other model for preference evolution. In addition, we know that genetic correlations can be evolutionarily unstable (25, 44, 45), so how does the evolution of genetic correlations (and genetic variation) affect the Fisherian process and other processes of mate-choice evolution?
3. Mutual mate choice.
This topic is gaining momentum, but we need to understand the circumstances under which both sexes will be choosy and how easily sexual selection can simultaneously operate on both sexes. Should we expect sexual selection on both sexes to be the norm, or will it only occur under very special circumstances?
4. The evolution of multiple sexually selected traits and preferences.
The choosing sex may be integrating information from multiple traits assessed using several sensory modalities. Some theoretical work has been done regarding models of sexual selection when multiple traits and preferences are involved (46, 47), but we are far from a complete understanding.
5. The relative contributions of the various models of mate-choice evolution within and between taxa.
In many cases, several of the models may be working in concert to produce selection on mating preferences, and it would be of interest to assess empirically and theoretically the relative contributions of the different models. Such studies, applied within taxa and in a comparative phylogenetic framework, would help us to understand the relative contributions of the various models to the evolution of preferences.
The Intensity of Sexual Selection and the Second Triumph
Why Do Lineages Differ in Sexual Dimorphism?
One important question in morphological and behavioral evolution, especially from a human perspective, concerns why the sexes differ from one another more dramatically in some lineages than in others. Darwin (ref. 2, Part II, p 388) identified this topic as a key question, and he presented an example from butterflies to illustrate the issue. We are more familiar with fishes of the family Syngnathidae, which includes seahorses, sea dragons, and pipefish, so we will use examples from this family to illustrate the same problem. One interesting aspect of this group of fishes is that the entire family is characterized by male brooding of offspring, with the embryos either glued to the surface of the male or residing in an enclosed pouch. Despite the ubiquity of this “male pregnancy” in syngnathids, however, different species differ from one another with respect to their degrees of sexual dimorphism. For example, seahorses (genus Hippocampus) tend to be sexually monomorphic (48), but a related pipefish, Syngnathus scovelli, is extremely sexually dimorphic with secondary sexual traits appearing only in females (49). Several other species in Syngnathus, such as S. typhle and S. floridae, are sexually dimorphic but not nearly as much so as S. scovelli (50, 51). There are other species of pipefish, like Doryrhamphus excisus, in which both sexes are brightly colored with flag-like caudal fins (50), and in the sexually monomorphic leafy sea dragons, Phycodurus eques, both sexes are characterized by extremely elaborate leafy appendages (50), resulting in one of the most beautiful and bizarre examples of fish morphology.
Why Do Some Lineages Display Striking Colorations, Sexual Dimorphism, and Pronounced Morphological Traits While Others Do Not?
Of course, there are many potential answers to this question. Some of the differences among species are almost certainly due to natural selection on the traits or differences in sex-limited inheritance, but in some cases, especially in the case of secondary sexual characteristics, the differences among lineages are due to dissimilar intensities of sexual selection. In other words, while keeping in mind that observable sexual dimorphism is not an entirely reliable guide to the strength of sexual selection, a complete explanation for morphological differences among taxa requires consideration of factors affecting sexual selection in populations of organisms. Thus, one important question, which Darwin (2) originally posed and which is still valid today, is why the intensity of sexual selection varies in different populations or evolutionary lineages.
Darwin's Perspective.
Perhaps Darwin's thinking regarding factors affecting the intensity of sexual selection is most obvious when he considers sex-role-reversed taxa. In these species, sexual selection acts more strongly on females than on males, so there has been a reduction in the strength of sexual selection on males and an increase in the strength of selection on females relative to most other sexually selected taxa. One example of a sex-role-reversed species is the barred buttonquail (Turnix suscitator) mentioned by Darwin or the Gulf pipefish (S. scovelli), a species Darwin did not bring up. How did Darwin explain sex-role reversal? The following quote provides the flavor of his perspective (ref. 2, Part II, p 207):
“Now if we might assume that the males in the present class have lost some of that ardour which is usual for their sex, so that they no longer search eagerly for the females; or, if we might assume that females have become much more numerous than the males—and in the case of the Indian Turnix the females are said to be ‘much more commonly met with than the males’—then it is not improbable that females would have been led to court the males, instead of being courted by them.”
This quote illustrates 2 of the main causes that Darwin pointed out for variation in the intensity of sexual selection. The first, a loss of “ardour” by the males, is not a very satisfying explanation in the context of our modern understanding of sexual selection. However, the second explanation, a skewed sex ratio, does fit well with our current view of behavioral ecology. In addition, Darwin clearly had a good intuitive understanding of the concept of the operational sex ratio (which is the ratio of males ready to mate to receptive females in the population) and the implications of mating patterns. This understanding appears throughout the book, but is demonstrated clearly when he states that “the practice of polygamy leads to the same results as would follow from an actual inequality in the number of the sexes” (ref. 2, Part I, p 266). Thus, Darwin provided a glimmer of insight that has expanded over the ages into our current understanding of factors affecting sex roles and sexual selection.
The Second Major Triumph.
In our view, another major triumph in sexual selection research came from advances in quantitative genetics and formal selection theory, which resulted in quantitative techniques for the measurement of selection in natural populations (52–54). These approaches lead to the stark realization that what we are really talking about are selection coefficients on sexually selected phenotypic traits, which leads to the seemingly simple question of what determines the magnitude of a selection coefficient in a natural population.
Selection Coefficient Thinking.
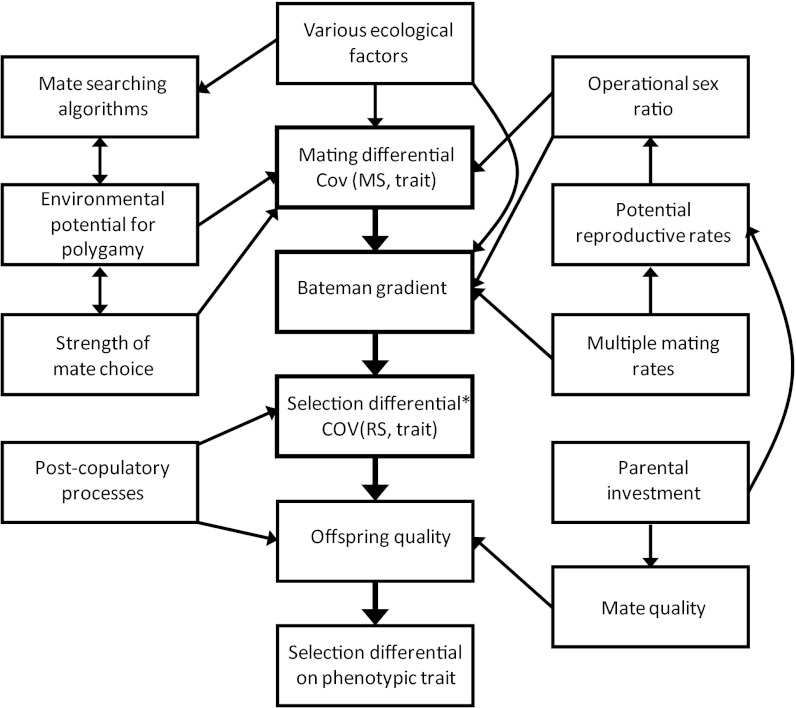
For the purposes of this discussion, we are going to focus mainly on premating sexual selection of the type Darwin (2) discussed. Assuming that a population is characterized by female choice or male–male combat, males with traits favorable for success in this mating competition will leave more offspring than males with unfavorable traits. Consequently, there will be a covariance between trait values and mating success (i.e., number of mates), which we would like to call a “mating differential” (55). Mathematically, how do we get from a mating differential to a selection differential? Recall that a selection differential can be measured as the covariance between trait values and relative fitness (52–54). For a positive mating differential to result in a positive selection differential, then, there must be a mechanism to convert mating success into fitness. This conversion is partially measured by a quantity known as the “Bateman gradient”, which is the relationship between number of mates and number of offspring (55–58). If the Bateman gradient is positive, success at mating will result in an increase in number of offspring (56). Hence, a nonzero mating differential will result in a nonzero selection differential only if the Bateman gradient is positive. The central path in Fig. 2 illustrates this important relationship.
Fig. 2.
One possible depiction of a “reproductive ecology web” showing the relationships between important ecological factors and variables that ultimately result in a selection gradient on a sexually selected trait. This figure was inspired especially by Andersson's figure 7 (3) and Arnold and Duvall's path diagram (56). Note that our figure only deals with a single selective episode, such as a breeding season. The selection differential marked with an asterisk is the covariance between number of offspring and trait values, so the final selection differential (lowest box) takes into account the number of offspring and offspring quality. The exact locations and sizes of the effects remain subjects for future research.
If all offspring are equal with respect to fitness, then numerical counts of offspring are sufficient for the measurement of sexual selection. However, we know that mate choice and postcopulatory sexual selection (4) can influence offspring quality (3), so ideally each offspring would be weighted somehow by its quality in the calculation of fitness. The precise way in which such a weighting could be accomplished in the context of sexual selection is a subject for future work, and the solution will no doubt be complex (59–61). Nevertheless, as Fig. 2 shows, mate quality and postcopulatory sexual selection (4) are 2 factors that must also be considered for a complete characterization of the intensity of sexual selection. We find the topic complicated enough without these factors, so we will not treat them further here.
If we accept this central path in Fig. 2, then we have 2 major questions to address in precopulatory sexual selection (55). First, what factors determine the magnitude of mating differentials? And second, how is the slope of the Bateman gradient established? In Fig. 2, we provide one tentative hypothesis for the relationships among important mating-system variables that interact to produce a selection differential on a sexually selected trait. This figure is not meant to be the final word on the subject, as we believe there is a great need for additional work.
Factors Affecting Mating Differentials.
The question here is how ecological factors can either enhance or decrease the likelihood of a correlation between sexually selected trait values and mating success. This topic has been a central one in sexual selection work for quite some time. We will give only a partial list of some of the most important factors, and these are shown in Fig. 2. Perhaps the most obvious cause is strong mate choice, so if a mechanism of female-preference evolution is especially effective in a population, we might expect a strong covariance between an ornament and male mating success. In addition, mating differentials can be caused by a high “environmental potential for polygamy,” which in turn could be affected by the spatial distribution of resources or temporal synchrony of female receptivity (62, 63). Another factor, which has been somewhat neglected, concerns mate-searching algorithms (64, 65). Environmental constraints, such as predation, may affect how mates find one another. If mate searching is efficient, very strong mating differentials may result, whereas constrained searching may impose limitations. In addition, the operational sex ratio (62) is almost certainly important, because as more individuals are excluded from reproduction, the magnitude of the mating differential will change. Finally, there may be numerous other ecological factors, such as population density (66) and others that have yet to be appreciated, that affect mating differentials.
Factors Affecting Bateman Gradients.
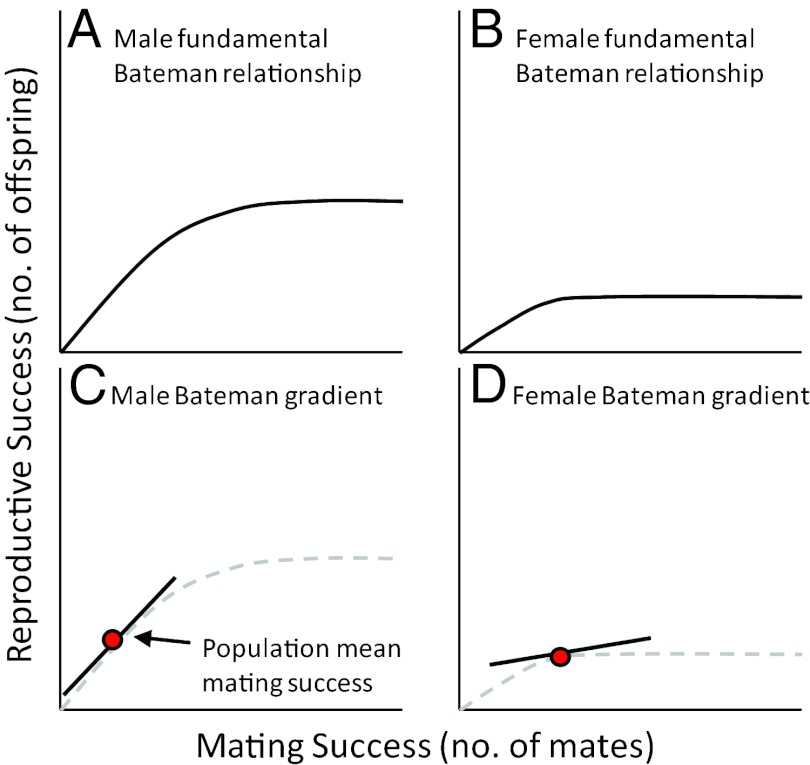
As noted above, a mating differential will result in a selection differential on a trait only if high mating success results in high relative fitness, and the Bateman gradient describes this conversion (55–58). To stimulate thought about Bateman gradients, we would like to propose a heuristic way of thinking about them (Fig. 3). Regardless of sex or species, there will be some point at which an individual runs out of reproductive potential, either by running out of gametes, time, or other resources required for reproduction. When the individual reaches this upper limit of reproductive potential, access to additional mates will no longer provide any increase in reproductive success. This curve, describing the potential relationship between number of mates and number of offspring can be thought of as the “fundamental Bateman relationship.” In Fig. 3, we show 2 such curves for males and females of a species like Drosophila melanogaster, in which males have much greater reproductive potential than females. After only a few mating events, a female will achieve her full reproductive output, whereas a male can go on mating for quite some time before he runs out of sperm (67). Note that in this treatment we are ignoring factors like sexual conflict that could cause a downward trajectory as the number of mates increased beyond some optimum. Given the fundamental Bateman relationship for a sex, the Bateman gradient is the slope of this curve at the population-mean mating success (Fig. 3 C and D). Thus, the Bateman gradient tells us whether, in the population under consideration, a sex is on the increasing part of the curve (and thus limited primarily by access to mates) or on the plateau (limited by intrinsic reproductive capacity).
Fig. 3.
One way to think about the Bateman gradient. (A and B) The “fundamental Bateman relationship” for males (A) and females (B) in a species like Drosophila melanogaster. For either sex, reproductive success will initially increase with an increasing number of mates, but both sexes will eventually reach a point at which they are limited by factors other than access to mates. This plateau could be caused by limitations imposed by time, parental care demands, gamete production, or other resources required for reproduction. (C and D) The Bateman gradient can then be conceptualized as the slope of the fundamental Bateman relationship evaluated at the population mean for mating success.
Given the heuristic way of thinking about Bateman gradients in Fig. 3, we can now consider the factors that might affect the magnitude of a Bateman gradient. Anything that could lower the point at which the relationship plateaus could potentially change the Bateman gradient, for example. A factor such as parental investment (68), through its effect on potential reproductive rates of the sexes (69), certainly could change the location of the plateau. Factors such as nuptial gifts or rates of multiple mating could in principle change the slope of the increasing part of the fundamental Bateman relationship (56, 70, 71), resulting in a change in the slope of the Bateman gradient. Finally, factors that change the position of the population-mean mating success on the x axis of the fundamental Bateman relationship graph certainly could affect the Bateman gradient. The most obvious such factor is the operational sex ratio (62), as a skewed sex ratio can easily change which sex is limiting for reproduction. When a sex becomes limiting, it is by definition on the plateau of the curve, so its Bateman gradient should be near zero. This view of mating systems based on the Bateman gradient remains controversial and is the subject of continuing debate (72–74), so much work remains to be done. Nevertheless, we hope our hypothesis in Fig. 2 will stimulate additional research.
Future Directions.
In some ways, our understanding of the causes of differences in sexual selection intensity among lineages is less well developed than our understanding of models of female mate-choice evolution, so there are many potential avenues for future work. We suggest the following 5 areas as the most pressing targets.
1. Comparative studies of mating systems among populations within species.
Such studies will be difficult, because in most cases they will require genetic studies of parentage to completely characterize mating systems (75). However, the benefits would be worth the effort, because such studies could facilitate the identification of putative ecological factors affecting mate choice and the intensity of sexual selection on traits.
2. Better integration of the ideas surrounding the mating differential and the Bateman gradient with the ecology of sexual selection.
This goal could be accomplished by focusing on diagrams like our Fig. 2 and attempting to establish the locations and magnitudes of the various arrows through empirical work in natural systems.
3. Explicit consideration of age structure in sexual selection.
Studies of sexual selection often focus on a single breeding season, and the results can be difficult to interpret in terms of lifetime fitness. Thus, a complete understanding of the intensity of sexual selection would seem to call for explicit consideration of age structure in the populations under study. For a complete picture, lifetime fitness should be conceptualized in terms of selection episodes so that effects of sexual selection can be separated from natural selection and stochastic effects.
4. Integration of models for the evolution of female choice with theory related to the intensity of sexual selection and mating-system evolution.
These areas of thought are often dealt with separately from theoretical and empirical standpoints, but the merging of these 2 areas of inquiry could result in interesting insights.
5. Integration of precopulatory and postcopulatory sexual selection.
To completely understand the entire selective history of any sexually selected trait, we will need to resolve the entire set of paths depicted in Fig. 2, including those that affect fitness after mating.
Summary and Conclusions.
Darwin presented an incredibly detailed and clear description of sexual selection in The Descent of Man. Even though Darwin's account of sexual selection was by no means complete and he had a garbled understanding of inheritance, Darwin was correct about almost every topic related to sexual selection that he discussed. For instance, he laid out essentially the modern version of intrasexual selection, and he correctly realized that female choice was an important mechanism in sexual selection. He also recognized that sexual selection could sometimes act on both sexes or more strongly on females than on males, and he demonstrated a good intuitive understanding of the effects of the operational sex ratio and mating systems on the intensity of sexual selection. However, Darwin did not clearly identify the evolution of female choice as a key topic for study in its own right. Rather, he tended to invoke a human-like sense of aesthetics in animals to explain their preferences for ornaments. He also never produced a clear picture of why some lineages seem to be experiencing stronger sexual selection than others. Regardless, The Descent of Man is an impressive scientific work, and well worth a read for anyone interested in sexual selection. Not only does it provide a clear intuitive explanation of the process, but the vast array of empirical examples could serve as the launching point for countless new studies.
Since Darwin, progress in the study of sexual selection has been astounding. Two of the greatest triumphs included the proliferation of models explaining the evolution of female preferences and quantitative approaches to the measurement of selection differentials. These advances provide clear, plausible mechanisms for the evolution of female choice and allow us to begin to address why sexual selection varies among species. A third major triumph in the study of sexual selection, which we did not have space to discuss, was the introduction of molecular markers into behavioral ecology (76–79). Molecular studies of parentage provide unprecedented opportunities to study patterns of mating in natural populations, so they have become a cornerstone of sexual selection research.
Despite the triumphs of modern sexual selection research, there are still many topics that need to be addressed. For example, some models of the evolution of mate choice enjoy only limited empirical support, and for the most part we are not sure which model explains the majority of choice evolution within or between systems. With respect to factors determining the intensity of sexual selection, there may be even more confusion. We are still in the process of building connections between reproductive ecology and selection differentials. Finally, there seems to be a lack of connections between theory related to mate-choice evolution and theory related to sexual selection intensity. There are many other unanswered questions, many of which will require new theory and empirical work.
Overall, the study of sexual selection has been a rich and exciting endeavor, especially in the last several decades. We owe a lot to Darwin for establishing a framework for all modern work in this area. However, we are far from complete resolution on many topics, so the next several decades should be at least as exciting as the recent past.
Acknowledgments.
We thank John Avise and Francisco Ayala for inviting us to participate in the Arthur M. Sackler Colloquium of the National Academy of Sciences, In the Light of Evolution III: Two Centuries of Darwin. We also are grateful to John Avise, Steve Shuster, and an anonymous referee for helpful comments on this manuscript.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution III: Two Centuries of Darwin,” held January 16–17, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Darwin.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection. London: Murray; 1859. [Google Scholar]
- 2.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: Murray; 1871. [Google Scholar]
- 3.Andersson M. Sexual Selection. Princeton, NJ: Princeton Univ Press; 1994. [Google Scholar]
- 4.Eberhard WG. Postcopulatory sexual selection: Darwin's omission and its consequences. Proc Natl Acad Sci USA. 2009;106(Suppl):10025–10032. doi: 10.1073/pnas.0901217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerson HB, Hickie JP. Head scarring on male narwhals (Monodon monoceros): Evidence for aggressive tusk use. Can J Zool. 1985;63:2083–2087. [Google Scholar]
- 6.Emlen DJ, Marangelo J, Ball B, Cunningham CW. Diversity in the weapons of sexual selection: Horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae) Evolution. 2005;59:1060–1084. [PubMed] [Google Scholar]
- 7.Starck JM. Biogeography and life-history of Turnix suscitator Gmelin, 1979: Small adult body size as a consequence of selection for rapid growth. Z Zool Syst Evol Forsch. 1991;29:213–237. [Google Scholar]
- 8.Fisher RA. The evolution of sexual preference. Eugenics Review. 1915;7:184–192. [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher RA. The Genetical Theory of Natural Selection. Oxford: Clarendon Press; 1930. [Google Scholar]
- 10.O'Donald P. The theory of sexual selection. Heredity. 1962;22:499–518. doi: 10.1038/hdy.1967.66. [DOI] [PubMed] [Google Scholar]
- 11.O'Donald P. Genetic Models of Sexual Selection. Cambridge, UK: Cambridge Univ Press; 1980. [Google Scholar]
- 12.Zahavi A. Mate selection: A selection for a handicap. J Theor Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- 13.Williams GC. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought. Princeton, NJ: Princeton Univ Press; 1966. [Google Scholar]
- 14.Lande R. Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick M. Sexual selection and the evolution of female choice. Evolution. 1982;36:1–12. doi: 10.1111/j.1558-5646.1982.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick M, Price T, Arnold SJ. The Darwin–Fisher theory of sexual selection in monogamous birds. Evolution. 1990;44:180–193. doi: 10.1111/j.1558-5646.1990.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 17.Dearborn DC, Ryan MJ. A test of the Darwin–Fisher theory for the evolution of male secondary sexual traits in monogamous birds. J Evol Biol. 2002;15:307–313. [Google Scholar]
- 18.Williams GC. Sex and Evolution. NJ: Princeton Univ Press Princeton; 1975. [Google Scholar]
- 19.Kirkpatrick M, Ryan MJ. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. [Google Scholar]
- 20.Møller AP, Jennions MD. How important are direct fitness benefits to sexual selection? Naturwissenschaften. 2001;88:401–415. doi: 10.1007/s001140100255. [DOI] [PubMed] [Google Scholar]
- 21.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Taylor PD, Williams GC. The lek paradox is not resolved. Theor Pop Biol. 1982;22:392–409. [Google Scholar]
- 23.Reynolds JD, Gross MR. Costs and benefits of female mate choice: Is there a lek paradox? Am Nat. 1990;136:230–243. [Google Scholar]
- 24.Kokko H, Brooks R, Jennions MD, Morley J. The evolution of mate choice and mating biases. Proc R Soc London Ser B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mead LS, Arnold SJ. Quantitative genetic models of sexual selection. Trends Ecol Evol. 2004;19:264–271. doi: 10.1016/j.tree.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Kotiaho JS, Puurtinen M. Mate choice for indirect genetic benefits: Scrutiny of the current paradigm. Funct Ecol. 2007;21:638–644. [Google Scholar]
- 27.Eschel I, Volovik I, Sansone E. On Fisher–Zahavi's handicapped sexy son. Evol Ecol Res. 2000;2:509–523. [Google Scholar]
- 28.Kokko H, Jennions MD, Brooks R. Unifying and testing models of sexual selection. Annu Rev Ecol Evol Syst. 2006;37:43–66. [Google Scholar]
- 29.Maynard Smith J. Theories of sexual selection. Trends Ecol Evol. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. [DOI] [PubMed] [Google Scholar]
- 30.Zahavi A. The cost of honesty (Further remarks on the handicap principle) J Theor Biol. 1977;53:205–214. doi: 10.1016/0022-5193(77)90061-3. [DOI] [PubMed] [Google Scholar]
- 31.Pomiankowski A. The evolution of female mate preferences for male genetic quality. Oxford Surv Evol Biol. 1988;5:136–184. [Google Scholar]
- 32.West-Eberhard MJ. Sexual selection, competitive communication and species-specific signals in insects. In: Lewis T, editor. Insect Communication. New York: Academic; 1984. pp. 283–324. [Google Scholar]
- 33.Ryan MJ. Signals, species, and sexual selection. Oxford Surv Evol Biol. 1990;7:157–195. [Google Scholar]
- 34.Fuller RC, Houle D, Travis J. Sensory bias as an explanation for the evolution of mate preferences. Am Nat. 2005;166:437–446. doi: 10.1086/444443. [DOI] [PubMed] [Google Scholar]
- 35.Zeh JA, Zeh DW. The evolution of polyandry I: Intragenomic conflict and genetic incompatibility. Proc R Soc London Ser B. 1996;263:1711–1717. [Google Scholar]
- 36.Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage. Mol Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- 37.Neff BD, Pitcher TE. Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Mol Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. [DOI] [PubMed] [Google Scholar]
- 38.Holland B, Rice WR. Perspective: Chase-away sexual selection: Antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- 39.Cameron E, Day T, Rowe L. Sexual conflict and indirect benefits. J Evol Biol. 2003;16:1055–1060. doi: 10.1046/j.1420-9101.2003.00584.x. [DOI] [PubMed] [Google Scholar]
- 40.Parker GA. Sexual conflict over mating and fertilization: An overview. Philos Trans R Soc London Ser B. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillingham MAF, et al. Cryptic preference for MHC-dissimilar females in male red jungle fowl, Gallus gallus. Proc R Soc London Ser B. 2009;276:1083–1092. doi: 10.1098/rspb.2008.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tregenza T, Wedell N. Polyandrous females avoid costs of inbreeding. Nature. 2002;415:71–73. doi: 10.1038/415071a. [DOI] [PubMed] [Google Scholar]
- 43.Holland B, Rice WR. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc Natl Acad Sci USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones AG, Arnold SJ, Bürger R. Stability of the G-matrix in a population experiencing pleiotropic mutation, stabilizing selection, and genetic drift. Evolution. 2003;57:1747–1760. doi: 10.1111/j.0014-3820.2003.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 45.Jones AG, Arnold SJ, Bürger R. Evolution and stability of the G-matrix on a landscape with a moving optimum. Evolution. 2004;58:1639–1654. doi: 10.1111/j.0014-3820.2004.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 46.Pomiankowski A, Iwasa Y. Evolution of multiple sexual preferences by Fisher runaway process of sexual selection. Proc R Soc London Ser B. 1993;253:173–181. [Google Scholar]
- 47.Iwasa Y, Pomiankowski A. The evolution of mate preferences for multiple sexual ornaments. Evolution. 1994;48:853–867. doi: 10.1111/j.1558-5646.1994.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 48.Vincent A, Ahnesjö I, Berglund A, Rosenqvist G. Pipefishes and seahorses: Are they all sex role reversed? Trends Ecol Evol. 1992;7:237–241. doi: 10.1016/0169-5347(92)90052-D. [DOI] [PubMed] [Google Scholar]
- 49.Jones AG, Avise JC. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: Insights from microsatellite-based studies of maternity. J Hered. 2001;92:150–158. doi: 10.1093/jhered/92.2.150. [DOI] [PubMed] [Google Scholar]
- 50.Dawson CE. Indo-Pacific Pipefishes. Ocean Springs, MS: Gulf Coast Research Laboratory; 1985. [Google Scholar]
- 51.Jones AG, Avise JC. Microsatellite of maternity and the mating system in the Gulf pipefish Syngnathus scovelli, a species with male pregnancy and sex-role reversal. Mol Ecol. 1997;6:203–213. doi: 10.1046/j.1365-294x.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 52.Robertson A. A mathematical model of the culling process in dairy cattle. Anim Prod. 1966;8:93–108. [Google Scholar]
- 53.Price GR. Selection and covariance. Nature. 1970;227:520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- 54.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 55.Jones AG. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution. 2009 doi: 10.1111/j.1558-5646.2009.00664.x. in press. [DOI] [PubMed] [Google Scholar]
- 56.Arnold SJ, Duvall D. Animal mating systems: A synthesis based on selection theory. Am Nat. 1994;143:317–348. [Google Scholar]
- 57.Andersson M, Iwasa Y. Sexual selection. Trends Ecol Evol. 1996;11:53–58. doi: 10.1016/0169-5347(96)81042-1. [DOI] [PubMed] [Google Scholar]
- 58.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc R Soc London Ser B. 2000;267:677–680. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore AJ, Brodie ED, Jr, Wolf JB. Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects of social interactions. Evolution. 1997;51:1352–1362. doi: 10.1111/j.1558-5646.1997.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 60.Wolf JB, Brodie ED, III, Moore AJ. Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. Am Nat. 1999;153:254–266. doi: 10.1086/303168. [DOI] [PubMed] [Google Scholar]
- 61.Wolf JB, Wade MJ. On the assignment of fitness to parents and offspring: Whose fitness is it and when does it matter? J Evol Biol. 2001;14:347–356. [Google Scholar]
- 62.Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 63.Shuster SM, Wade MJ. Mating Systems and Strategies. Princeton, NJ: Princeton Univ Press; 2003. [Google Scholar]
- 64.Parker GA. Evolution of competitive mate searching. Annu Rev Entomol. 1978;23:173–196. [Google Scholar]
- 65.Real LA. Search theory and mate choice. I. Models of single-sex discrimination. Am Nat. 1990;136:376–404. [Google Scholar]
- 66.Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc London Ser B. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 68.Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man, 1871–1971. London: Heinemann; 1972. pp. 136–179. [Google Scholar]
- 69.Clutton-Brock TH, Parker GA. Potential reproductive rates and the operation of sexual selection. Q Rev Biol. 1992;67:437–456. [Google Scholar]
- 70.Lorch PD. Understanding reversals in the relative strength of sexual selection on males and females: A role for sperm competition? Am Nat. 2002;159:645–657. doi: 10.1086/339992. [DOI] [PubMed] [Google Scholar]
- 71.Lorch PD, Bussiere LF, Gwynne DT. Quantifying the potential for sexual dimorphism using upper limits on Bateman gradients. Behaviour. 2008;145:1–24. [Google Scholar]
- 72.Tang-Martinez Z, Ryder TB. The problem with paradigms: Bateman's worldview as a case study. Integr Comp Biol. 2005;45:821–830. doi: 10.1093/icb/45.5.821. [DOI] [PubMed] [Google Scholar]
- 73.Parker PG, Tang-Martinez Z. Bateman gradients in field and laboratory studies: A cautionary tale. Integr Comp Biol. 2005;45:895–902. doi: 10.1093/icb/45.5.895. [DOI] [PubMed] [Google Scholar]
- 74.Snyder BF, Gowaty PA. A reappraisal of Bateman's classic study of intrasexual selection. Evolution. 2007;61:2457–2468. doi: 10.1111/j.1558-5646.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 75.Mobley KB, Jones AG. Environmental, demographic, and genetic mating system variation among five geographically distinct dusky pipefish (Syngnathus floridae) populations. Mol Ecol. 2009;18:1476–1490. doi: 10.1111/j.1365-294X.2009.04104.x. [DOI] [PubMed] [Google Scholar]
- 76.Gowaty PA, Karlin AA. Multiple maternity and paternity in single broods of apparently monogamous eastern bluebirds (Sialia sialis) Behav Ecol Sociobiol. 1984;143:91–95. [Google Scholar]
- 77.Burke T, Bruford MW. DNA fingerprinting in birds. Nature. 1987;327:149–152. doi: 10.1038/327149a0. [DOI] [PubMed] [Google Scholar]
- 78.Hughes C. Integrating molecular techniques with field methods in studies of social behavior: A revolution results. Ecology. 1998;79:383–399. [Google Scholar]
- 79.Jones AG, Ardren WR. Methods of parentage analysis in natural populations. Mol Ecol. 2003;12:2511–2523. doi: 10.1046/j.1365-294x.2003.01928.x. [DOI] [PubMed] [Google Scholar]
- 80.Gwynne DT. Courtship feeding increases female reproductive success in bushcrickets. Nature. 1984;307:361–363. [Google Scholar]
- 81.Galimberti F, Boitani L, Marzetti I. Female strategies of harassment reduction in southern elephant seals. Ethol Ecol Evol. 2000;12:367–388. [Google Scholar]
- 82.Borgia G. Mate selection in the fly Scatophaga stercoraria: Female choice in a male-controlled system. Anim Behav. 1981;29:71–80. [Google Scholar]
- 83.Préault M, Chastel O, Cézilly F, Faivre B. Male bill colour and age are associated with parental abilities and breeding performance in blackbirds. Behav Ecol Sociobiol. 2005;58:497–505. [Google Scholar]
- 84.Östlund S, Ahnesjö I. Female fifteen-spined sticklebacks prefer better fathers. Anim Behav. 1998;56:1177–1183. doi: 10.1006/anbe.1998.0878. [DOI] [PubMed] [Google Scholar]
- 85.Nakatsuru K, Kramer DL. Is sperm cheap? Limited male fertility and female choice in the Lemon Tetra (Pices, Characidae) Science. 1982;216:753–755. doi: 10.1126/science.216.4547.753. [DOI] [PubMed] [Google Scholar]
- 86.Markow TA, Quaid M, Kerr S. Male mating experience and competitive courtship success in Drosophila melanogaster. Nature. 1978;276:821–822. [Google Scholar]
- 87.Worden BD, Parker PG. Females prefer noninfected males as mates in the grain beetle Tenebrio molitor: Evidence in pre- and postcopulatory behaviours. Anim Behav. 2005;70:1047–1053. [Google Scholar]
- 88.Brooks R. Negative genetic correlation between male sexual attractiveness and survival. Nature. 2000;406:67–70. doi: 10.1038/35017552. [DOI] [PubMed] [Google Scholar]
- 89.Jones TM, Quinnell RJ, Balmford A. Fisherian flies: Benefits of female choice in a lekking sandfly. Proc R Soc London Ser B. 1998;265:1651–1657. [Google Scholar]
- 90.Punzalan D, Cooray M, Helen Rodd F, Rowe L. Condition dependence of sexually dimorphic colouration and longevity in the ambush bug Phymata americana. J Evol Biol. 2008;21:1297–1306. doi: 10.1111/j.1420-9101.2008.01571.x. [DOI] [PubMed] [Google Scholar]
- 91.Lopuch S, Radwan J. Condition dependence of sexual attractiveness in the bank vole. Behav Ecol Sociobiol. 2009;63:339–344. [Google Scholar]
- 92.Moore AJ. Genetic evidence for the good genes process of sexual selection. Behav Ecol Sociobiol. 1994;35:235–241. [Google Scholar]
- 93.Smith C, Barber I, Wootton RJ, Chittka L. A receiver bias in the origin of three-spined stickleback mate choice. Proc R Soc London Ser B. 2004;271:949–955. doi: 10.1098/rspb.2004.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fleishman LJ. The influence of the sensory system and the environment on motion patterns in the visual displays of Anoline lizards and other vertebrates. Am Nat. 1992;139:S36–S61. [Google Scholar]
- 95.Maan ME, Taborsky M. Sexual conflict over breeding substrate causes female expulsion and offspring loss in a cichlid fish. Behav Ecol. 2008;19:302–308. [Google Scholar]
- 96.Demary KC, Lewis SM. Male courtship attractiveness and paternity success in Photinus greeni fireflies. Evolution. 2007;61:431–439. doi: 10.1111/j.1558-5646.2007.00033.x. [DOI] [PubMed] [Google Scholar]
- 97.Ryan KK, Altmann J. Selection for male choice based primarily on mate compatibility in the oldfield mouse, Peromyscus polionotus rhoadsi. Behav Ecol Sociobiol. 2001;50:436–440. [Google Scholar]