Abstract
In one of his few major oversights, Darwin failed to appreciate that male–male competition and sexual selection can continue even after copulation has begun. The postcopulatory equivalents of both direct male–male battles (sperm competition) and female choice (cryptic female choice) occur within the female's body. Recognition of this hidden, but intense, sexual competition provides new insights into a variety of fields. These include the hyperdiverse and paradoxically elaborate morphology of both sperm and male genitalia, the equally puzzling and elaborate morphology of nongenitalic male structures that are specialized to grasp and stimulate females, powerful manipulative effects of substances in male semen on female reproductive physiology, paradoxical male courtship behavior that occurs after copulation has already begun, variability in parental investments, and the puzzlingly complex and diverse interactions between sperm and female products that surround animal eggs and between male gametophytes and female tissues in flowering plants. Many bizarre traits are involved, including male genitalia that are designed to explode or fall apart during copulation leaving behind parts within the female, male genitalia that “sing” during copulation, potent seminal products that invade the female's body cavity and her nervous system to influence her behavior, and a virtual Kama Sutra of courtship behavior performed after rather than before genital coupling, including male–female dialogues during copulation.
Keywords: cryptic female choice, sexually antagonistic coevolution
Picture a pile of freshly-cut weeds at the sunny edge of a tropical forest. Metallic green flies dart and circle over it, chasing one another in short dashes. Your eye is caught when a chase ends as one fly grasps another in midair and the pair immediately lands on the pile of weeds. Their genitalia are already coupled, and the male immediately turns to face away from the female. After a few seconds, paradoxically (because he is already securely attached), he begins to court, rhythmically waving his colorful hind legs and tapping the female's abdomen. The courtship continues for a few minutes as the pair remains coupled, and then the flies separate. The female walks down into the pile where she lays eggs (her larvae will feed on the rotting vegetation), while the male rejoins the frenetic chases above the pile.
Why would a male fly wait to court a female until after he has already achieved his evolutionary objective of copulating with her? The answer (recently worked out by a Brazilian graduate student, F. Barbosa, personal communication) had to wait for >100 years after Darwin's great book on sexual selection (1) that explained so many other aspects of male–female sexual interactions.
The Puzzle of Darwin's Omissions
Darwin was uncannily on target about most of the topics he discussed, and he seldom missed general phenomena that had important consequences for his ideas. Strangely, however, there is a major missing piece in Darwin's thinking on sexual selection. He discussed at length how competition between males for sexual access to females leads to sexual selection (1), but failed to realize that sexual selection [sperm competition and cryptic female choice (CFC) in Table 1] can also occur even after males have initiated copulation. Simply stated, Darwin missed the fact that not all copulations result in insemination, and that not all inseminations result in fertilization of the female's eggs. Any male ability to improve the chances that his copulations will lead to fertilizations of eggs will give him an advantage in competition with other males who mate with the same female.
Table 1.
Different types of competition among males for access to conspecific females and their gametes (types of sexual selection) that occur before and after copulation
| Time | Intrasexual selection | Intersexual selection |
|---|---|---|
| Before copulation | Male–male battles | Classic female choice |
| During and after copulation | Sperm competition | Cryptic female choice |
It was not until 99 years after Darwin's 1871 book that Geoff Parker (2) awakened evolutionary biologists to the evolutionary importance of processes that occur after the male has already achieved genital coupling (conventionally called, somewhat imprecisely, postcopulatory processes; they include processes during copulation). Parker saw that they, too, like the precopulatory events emphasized by Darwin, can result in sexual selection on a male by affecting his success in competition with other males. There are postcopulatory equivalents inside the female of Darwin's precopulatory male–male struggles and female choice (Table 1). If a female copulates with >1 male, and if one of these males is better than others at, for instance, removing sperm stored from previous males (3), this male will stand to sire more offspring and win out over the others. Appreciation that female biases can also have postcopulatory effects on male reproductive success, and thus exercise the postcopulatory equivalent of female choice among males, lagged behind (4, 5), and was not presented as a general theory, however, until 1996 (6). After an initial period of negative reactions (7–9), CFC is now routinely included as a possible factor in studies of possible postcopulatory sexual selection (10–12). Because important postcopulatory events are played out inside the female's body, where she is largely in control of what happens, female choice seems a priori more likely to be important after copulation than it is leading up to copulation. There is a surprisingly long list of female-controlled processes that must be executed if insertion of the male's genitalia into the female is to result in siring her offspring (Table 2). Other things being equal, any male better able to induce the female to carry out one of these processes more completely than she does when mating with other males stands to produce more offspring.
Table 2.
Female-controlled processes that occur in different species and are known to increase the chances that a given male will sire her offspring
|
Consequences of the Historical Isolation of Related Fields from Evolutionary Biology
Whatever the reasons for Darwin's original oversights, they correlate with a general failure by subsequent workers to link sexual selection to a variety of postcopulatory phenomena. Recently this isolation has been eroded. In this article I will explore several newly-established connections with previously-isolated fields and their consequences for evolutionary ideas. The phenomena I will discuss include the often elaborate structure of male genitalia; the diverse morphology of sperm and the chemical constitution of male seminal products and their striking physiological effects on female reproductive processes; the common, but paradoxical, male courtship behavior that occurs after copulation has already begun; and the otherwise puzzling complexity and diversity of interactions between sperm and eggs and between the pollen tube and the female tissue through which it grows in plants.
Mechanisms of Postcopulatory Sexual Selection
Direct Male–Male Interactions (Sperm Competition).
Sperm competition was originally defined in a general sense, as “competition between the sperm from 2 or more males for the fertilization of a given set of ova” (ref. 2, p. 4). A more restrictive definition is now used, to distinguish male from female effects on this competition (Table 1). Sperm competition is presently restricted to cases in which there is a direct action by 1 male or his semen on the sperm of another male. Sperm competition was quickly accepted as a potentially important evolutionary force after Parker's pioneering article (2). In fact, some studies have claimed to demonstrate sperm competition without having eliminated alternative possibilities such as female choice (below).
Males use several mechanisms in sperm competition, and sperm competition explains a number of hitherto paradoxical observations. A male can dilute the sperm from previous males with his own voluminous ejaculate, engaging in what is called “raffle competition” (8). This tactic is apparently common in vertebrates, where testes size (and thus ejaculate size) correlates with the degree of female polyandry (13, 14). Behavioral traits of males to prevail in sperm competition include transferring larger ejaculates when more males are in the vicinity (15) and performing “retaliatory” copulations when the female with which a male is paired copulates with another male (14). Another tactic involves the behavior and morphology of the sperm themselves, with the sperm from a male linking together so that the group can swim more vigorously (16). The male can also use his own genitalia or a spermatophore to physically displace sperm from previous males that are present in the female (10, 17). Waage‘s classic study of sperm removal in a damselfly (3) showed that during the first portion of copulation the female's sperm storage organs (spermathecae) gradually become depleted of sperm as the male moves his genitalia in and out, snagging sperm on thick arrays of spines on his genitalia. Then after the spermathecae are nearly empty, the male ejaculates and fills them again with his own sperm.
The male can also increase his chances of winning out in sperm competition by using defensive strategies, such as reducing the danger of competition for his own sperm by guarding the female from copulation with additional males (by staying with her after copulation, physically plugging her genitalia, or inducing nonreceptive behavior). Several other competitive mechanisms have been proposed, including “kamikaze” sperm that kill or disable the sperm of other males (18), and douche-like flushing out stored sperm from the female with a jet of water (5), but they have not been convincingly documented (see ref. 19 for rejection of the douche hypothesis in a shark).
Female Effects on Male–Male Competition [CFC and Sexually Antagonistic Coevolution (SAC)].
There are many different ways in which a female can bias the likelihood that 1 male rather than another with which she has mated will father her offspring (Table 2). If such a female bias is associated with some particular male trait, then it can result in selection favoring that trait. This phenomenon has been called CFC. The word “cryptic” emphasizes that the female selection is invisible with respect to Darwinian criteria for reproductive success, which supposed that all copulations are equally effective in producing offspring. Male traits associated with such biases include morphology, behavior, and physiology (e.g., differences in ejaculate composition). The likelihood that natural selection will favor female mechanisms to trigger her reproductive processes on the basis of whether she has mated makes the subsequent evolution of sexual selection via female-imposed biases particularly likely to occur. Natural selection on females will favor repression of reproductive responses such as oviposition, sperm transport, resistance to further mating, etc. (Table 2) while she is a virgin and will also favor female mechanisms that trigger such processes by using stimuli associated with mating. These triggering mechanisms favored by natural selection produce results that are favorable to the male's reproductive interests (e.g., induce ovulation, sperm transport, inhibit further mating). Thus, any male ability to emphasize such stimuli and thereby to elicit more a complete female response would be favored in competition with other males that might copulate with the same female. Sexual selection is also expected to mold female responsiveness. A female that makes it somewhat more difficult for the male to elicit these responses can be favored, because her offspring will be fathered by males who are better than average in eliciting these responses.
A more recent alternative hypothesis involving postcopulatory biases in sperm use that are imposed by females proposes that males and females are in a coevolutionary arms race over control of reproductive events (12, 20) is SAC. Male adaptations to promote the use of their own sperm rather than that of other males are thought to damage the female's reproductive interests by reducing her direct reproductive output. For instance, a male ability to induce the female to lay more of her eggs soon after copulation (and before mating with another male) may result in some eggs being laid at suboptimal times or sites. A female evolutionary response that makes it more difficult for the male to induce a damaging response of this sort would be favored by natural selection on the female and would incidentally result in further sexual selection on males to improve their abilities to induce the response.
There are 2 extreme versions of SAC. One emphasizes physical coercion by the male (21–23), and has been tested by checking for physical or chemical coercion of the female by the male and coevolution of potential resistance mechanisms in the female. Several types of indirect data do not fit its predictions, at least with respect to genital evolution (24). A second version, which emphasizes male stimuli that act as sensory traps (25), is less easily evaluated: the male produces a stimulus that elicits a particular female response because previous natural selection on the female in another context favored such a response to the same (or a similar) stimulus. An example would be the possible disadvantage just mentioned to the female from responding to male stimuli eliciting oviposition, when such stimuli mimic the triggering stimuli that females originally evolved to use under natural selection.
It is not clear whether the sensory trap version is likely to be a general phenomenon, because it depends on the female not being able to evolve an effective defense against this damaging manipulation by the male (25). But a simple female defense, such as modifying her tendency to respond to the stimulus depending on the context in which she receives it, would free her from this “trap.” Even in the absence of such decoupling, if the female responds to the evolution of a new male trap stimulus by simply raising her response threshold to this stimulus, she will gain from both a decrease in the maladaptiveness of her response in the sexual context and an increase in the fitness because of the expected increase in the quality of her sons (as in the CFC argument). Still another simple female defense would be to avoid multiple mating altogether.
Whether CFC or SAC has been more important in the evolution of female postcopulatory influence on sperm use is currently hotly debated. The 2 theories are difficult to distinguish in practice and are not mutually exclusive; both types of selection could act sequentially or even simultaneously in a given trait (12, 26). The difference between the theories revolves around the benefits that females are thought to derive from influencing sperm use patterns: the payoff from increased offspring quality from biasing paternity (an indirect benefit) is thought to be larger under the CFC hypothesis; that from increased numbers of the female's own offspring from avoiding male manipulations (a direct benefit) is thought to be larger under SAC. The heat in the debate is partly because of the lack of data that convincingly address directly the question of which female benefit is larger. This unfortunate situation promises to be protracted, because it will be devilishly hard to obtain such data.
The logical, direct way to test the crucial difference between CFC and SAC theories would be to measure the direct and indirect benefits and losses that a female derives from cooperating or failing to cooperate with males. Such data have been obtained in the laboratory with respect to the effects of male seminal products on female reproductive physiology in Drosophila melanogaster (27, 28), and these results have been widely cited (12, 29, 30). But unfortunately the gains and losses measured under laboratory conditions may be quite different from those experienced by flies in the field (31). For instance, even the selective significance of such a radical male effect as reducing the female's life span and thus her total reproductive output is uncertain, because it is possible that flies in the field (where they are subject to predation, parasites, bad weather, greater need for dispersal to find oviposition sites, etc.) may never live to the advanced ages they achieve in captivity; the reproductive output of wild females is almost certainly unlikely to suffer as much from reduced longevity as that of females in the laboratory. Male and female traits evolved in the field, so laboratory data cannot be expected to be reliable indicators of the balance in gains and losses. An attempt to get around this problem used a strain of D. melanogaster that had been in captivity for many generations (32). But (not surprisingly) this laboratory strain still has traits (probably hangovers from selection in the field) that are not favored in captivity (24). In fact, D. melanogaster is an especially poor species for discovering the selective significance of such traits, because its natural habitat is not even known for certain (33).
I believe that the best data available to judge the relative importance of CFC and SAC are indirect tests that involve the morphology of genitalia and nongenital male contact organs, and I will discuss them in the section on genitalia.
Evolutionary Consequences of Postcopulatory Selection
Genitalia (and General Lessons).
Genitalia offer a large and taxonomically diverse set of data that can be used to illustrate arguments regarding postcopulatory sexual selection in general. Male genitalia, like peacock trains and other sexually-selected traits, are often very complex in form and frequently differ sharply even among closely-related species. Taxonomists working on many different animal groups with internal insemination have found that the shapes of male genetalia are often more useful in distinguishing closely-related species than the forms of most other body parts. This is true for many groups, including flatworms, nematodes, annelids, insects, spiders, mites, fish with internal insemination, snakes, bats, primates, and rodents (5). In other words, the male genitalia of many animals tend to evolve divergent forms especially rapidly when compared with other structures. Many male genitalia also have complex and baroque designs that seem paradoxical in view of the relatively simple basic task of transferring sperm to the female. The widespread use of male genitalia in taxonomy means that there is more known about the genital morphology of many (perhaps most) animal species than about any other aspect of their morphology, behavior, or physiology.
This immense taxonomic literature grew in absence of insights linking genital morphology with sexual selection. Probably the earliest and historically most widely-cited hypothesis to explain genital divergence is the “lock and key” hypothesis proposed by Dufour in 1844 (34), which proposed that natural selection favored differences in genitalia to prevent cross-specific fertilizations; only the genital key of a conspecific male could fit into the lock of the female's genitalia. This idea explained both the baroque aspects of male genitalia and their rapid evolutionary divergence. And it fit well with ideas about speciation in the modern synthesis that emphasized the evolution of prezygotic reproductive isolation. Gradually, however, accumulating data contradicted several important predictions.
Most importantly, female locks are simply absent in many groups in which males have species-specific genitalia (summaries in refs. 5, 34, and 35). In addition, the hypothesis predicts that genitalia should not diverge rapidly in groups in which species have evolved in strict isolation from all close relatives and in which mistaken cross-specific matings have thus been impossible throughout the history of the species. Such “isolated” groups include species endemic to isolated islands or caves and parasitic species isolated from all relatives because they live in different species of hosts. Again, the data clearly fail to show this trend (5). In fact, in some groups of such isolated species, the male genitalia are the most important of all morphological traits for distinguishing species (36). Still another failed lock and key prediction was that there should be geographic “character displacement” in genital form. In regions in which a species coexisted with other closely-related species (and females were thus subject to the danger of cross-specific mating), the differences in both the female locks and the male keys of the 2 species were predicted to be greater than in regions in which no other closely species were present. Nevertheless, character displacement seems to be generally absent in genital morphology (5, 34, 37). Finally, lock-and-key theory does not explain the positive correlation found in some groups between the degree of interspecific genital divergence and the frequency with which females of different species mate with different males (5, 38–40).
As it gradually became clear that the species isolation hypothesis does not work as a general explanation, Mayr (41) proposed another alternative: that the diversity of genitalia was an incidental (pleiotropic) byproduct of selection on alleles that affected other, ecologically important traits. These alleles were proposed to have pleiotropic and selectively-neutral effects on the genitalia. Aside from the problem of hypothesizing neutral variation in such selectively crucial structures as those responsible for transferring gametes, this pleiotropism theory was incapable of explaining a large array of facts (5). Why do the genitalia of groups with external fertilization, in which males do not contact females directly, never show these incidental effects? Why are genitalia, rather than other body parts, so consistently subject to such pleiotropic effects, and only in the males? Why in groups in which males use organs other than their primary genitalia to introduce sperm into the female do the primary genitalia never show the incidental effects, whereas the other intromittent structures in these groups (“secondary genitalia”) consistently do display them? The pleiotropy hypothesis has been largely abandoned in recent discussions.
Both the CFC and the SAC hypotheses attempt to explain the frequency of rapid divergent evolution of male genitalia, and the abundant data on genitalia permit strong tests. One test contrasts genital evolution in groups in which SAC would seem a priori much more likely to occur with that in others in which it is less likely. The prediction is for more consistent divergence in male genitalia in groups more likely to show sexual conflict between males and females. In mating systems in which males frequently encounter and attempt to mate with females that are relatively unreceptive to mating (for instance, males defend resources such as food or oviposition sites, and oblige females to mate before allowing them access to the resources), conflict seems likely; in those in which conflict is much less likely because females only encounter males when they are receptive (for instance, species in which the male can only locate the female when she is receptive enough to emit a long-distance attractant pheromone or respond to a male calling song), male–female conflict over mating seems much less likely.
An extensive review of groups of insects and spiders in which SAC should be more or less common nevertheless showed no trend toward lack of rapid divergent genital evolution (to have less distinctive male genitalia) in groups in which male–female conflict over copulation is less likely (42). Several reanalyses of these data, in attempts to mitigate possible biases in the literature, failed to reveal any trend in the predicted direction; in fact, the only significant difference found in the reanalyses was in the opposite direction from that predicted by SAC. The very large sample (involving literally hundreds of thousands of species) and the lack of even a trace of a trend in the expected direction speaks strongly against SAC. A second survey of genital evolution groups in which male–female reproductive conflict is probably unusually intense (for instance, groups with hypodermic insemination) again failed to find the especially elaborate male genitalia predicted by SAC (43).
Another study (35) checked for the defensive morphological female coevolution that is predicted by SAC in species with rapidly-evolving male genitalia or other nongenital structures that are specialized to contact females in sexual contexts (see next section). More than half of the 84 different groups showed no female coevolution at all, and in only ≈20% was it even feasible that female morphology was coevolving defensively. A sample of literally thousands of species of spiders failed to reveal a single case of a particularly likely female SAC design, involving structures that can be deployed facultatively to defend against males.
Male Contact Organs.
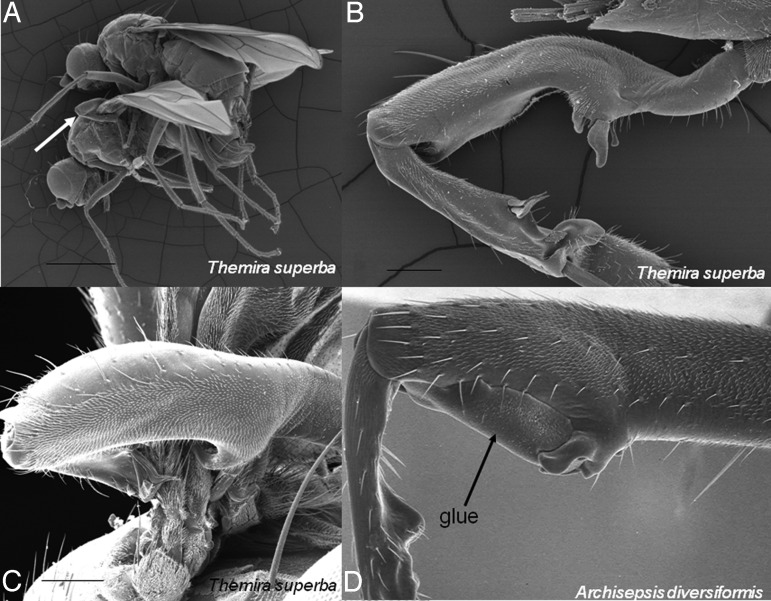
Males of many species have nongenitalic structures that are specialized to make contact with females during or immediately preceding copulation. These structures, which include Darwin's “prehensile organs” (1), include modifications of nearly all parts of the body. They are modified to clasp, press against, or otherwise contact the female directly during sexual interactions (Fig. 1). Comparisons of these contact organs among related species show that they diverge rapidly among closely related species, and have otherwise puzzling, “overly complex” designs to accomplish relatively simple mechanical tasks, just as in genitalia. The same hypotheses discussed for genitalia also apply here. Because in many cases the portion of the female's body that is contacted by these structures is easily studied, it is especially easy to test (and in many cases confidently reject) both lock-and-key and coercive SAC explanations for the male diversity: females often simply lack any sign of species-specific counterparts to these male structures (35, 44, 45). The relative ease with which the male and female structures can be modified (e.g., coating them with glue or paraffin) makes experimental studies of how they function possible. The data available to date indicate that male contact organs function to stimulate the female (46–48).
Fig. 1.
Male contact organs whose elaborate species-specific forms probably function to stimulate the female. (A)The front legs of male sepsid flies are specialized to grasp the female's wings (arrow) before and during copulation. (B) As in many such male contact organs, these legs are generally species-specific and sometimes quite complex in form. (C) Nevertheless, the portion of the female wing that they grasp is relatively uniform, giving little sign of the defensive coevolution predicted by the SAC hypothesis. (D) In 1 species, experimental modification of the male's femur (arrow) did not reduce his ability to grasp the female, but did result in decreased reproductive cooperation from the female; further experiments showed that the changes in female behavior were caused by changes in stimulation of her wing, as expected if the male legs have evolved under sexual selection by female choice. (Scale lines in A–C = 1 mm, and 0.1 mm, and 0.1 mm, respectively; width photo bottom right = 0.46 mm.) [Adapted with permission from ref. 44 (Copyright 2008, Biol J Linn Soc).] (D) [Adapted with permission from ref. 48 (Copyright 2002, J Ins Behav).]
Courtship Behavior During and After Copulation.
“Paradoxical” male courtship behavior that is performed after copulation has already begun, as in the fly described in the Introduction, may be common. A survey using 131 randomly-chosen species and conservative behavioral criteria showed that copulatory courtship occurred in just >80% of the species (49). Male insects and spiders use virtually all parts of their bodies as they tap, slam, squeeze, bite, lick, rub, shake, gently rock, or twist the female, coat her with liquid, cover her eyes with semitransparent colored plates, wrap her symbolically in weak silken lines, feed her, wave at her, and sing to her (49, 50). Similar behavior also occurs in other groups of animals (6). Unless one makes the unlikely supposition that this behavior, which is often energetic, persistent, and stereotyped, represents selectively neutral “mistakes” or incidental movements by the males (49, 51), courtship at this late stage would seem to function to induce favorable female responses. None of the male copulatory courtship behavior patterns observed to date involve direct manipulation of the female reproductive organs in a way that would suggest sperm competition, and in only a very few species is physical coercion of the female even feasible, so this courtship did not evolve under sperm competition or coercive SAC. As expected if it is under sexual selection, male copulatory courtship consistently differs among closely-related species (6, 49, 50). Also as expected, sex roles are inverted in 2 species in which males apparently donate resources to the female, and the female rather than the male courts during copulation (52, 53).
The discovery of copulatory courtship has opened the door on an entire new field of study, testing for the predicted effects of copulatory courtship on female reproductive processes, which is only beginning to be explored (e.g., refs. 54–58). The prejudice that courtship ends as soon as copulation begins probably caused earlier researchers (myself included) to overlook copulatory courtship behavior, because it did not seem to make adaptive sense. Research has already revealed a variety of copulatory courtship effects on female processes, including biased sperm use, sperm dumping, rapid oviposition, and resistance to remating. For instance, the copulatory courtship of the fly described in the Introduction has the effect of inducing the female to lay eggs immediately after copulation: if the male is prevented from courting during copulation, the female flies away without having laid any eggs (F. Barbosa, personal communication). Other effects likely await discovery as increasing numbers of taxa are studied in this expanding field. A recent study suggests the further possibility that male signals elicit female signals in response, and that male–female dialogues occur during copulation. Females of a spider “sing” periodically during copulation, apparently in attempts to induce the male to relax the squeezes that he performs with his powerful genitalia and rewarding cooperative males with greater paternity (58).
Sperm Morphology.
Sperm morphology is another important trait influenced by postcopulatory sexual selection. Sperm morphology differs widely and has been used to study phylogenetic relationships in many different groups (59, 60). Much of this diversity probably results from postcopulation sexual section, which can potentially act within the female in competition among sperm in a single ejaculate and competition between sperm from different males. The functions of sperm modifications are just beginning to be understood, however, and speculations are far ahead of the data. Discovering sperm function by direct observation is difficult, because both the morphology and the chemical mileu of the female reproductive tract can affect sperm behavior.
Perhaps the most general pattern is that sperm morphology tends to be simple and uniform in externally-fertilizing animals and more complex and diverse in those with internal fertilization (61). This pattern argues for the importance of sexual selection and the influence of female “playing fields.” Correlations have been found between the length and the form of sperm cells and the length of female storage organs or ducts in birds and insects (62–67); the functional effects of longer or shorter female structures are not clear, however, nor are the selective pressures that result in changes in these traits.
Swimming speed is one frequently-mentioned sperm competition mechanism that may exercise selection on sperm morphology. Greater flagellum length may correlate in some cases with greater swimming speed or greater force as the sperm nears the egg, but the functional significance of sperm length is often unclear. Sperm length is positively correlated with the probability of encountering sperm competition in some groups of animals, but not in others (62, 67, 68). Sperm length does not appear to be correlated with the thickness of the zona pellucida in mammals (69). The sperm of internally fertilizing species of fish and echinoids, which would seem to need less swimming ability, are nevertheless longer than those of external fertilizers in the same groups. In several vertebrate and invertebrate taxa sperm have traits such as hook-shaped heads that allow individual sperm cells to link up with each other at least temporarily; in several of these species the resulting collaborative groups may swim straighter or more rapidly (16). Groupings of this sort seem to correlate with competition between sperm from different males, rather than between sperm cells in the same ejaculate (16).
Males of some groups routinely produce both fertile sperm, and sperm that are designed to be infertile (parasperm); some even lack nuclei. Parasperm are widespread in Lepidoptera, have evolved repeatedly in other groups (66), and constitute more than half of the ejaculate in some species. Hypotheses for parasperm functions (66, 70) include provisioning the female with nutrients, displacing or killing rival sperm, blocking access for rival sperm, promoting movement of fertile sperm within the female, influencing CFC (for instance, by packing female storage organs to induce the female not to remate), influencing long-term vs. short-term survival in the female, and defense against female spermicides (71). The data needed to test these hypotheses are largely lacking (see critical discussions in ref. 66). Changes in the percentage of the ejaculate dedicated to parasperm under conditions of different intensities of sperm competition suggest that sperm competition is an important function of parasperm in some species (72) but not others (73).
Seminal Products.
A similar disconnect regarding sexual selection occurred in studies of semen and of its effects on the female. Insect physiologists developed a tradition of experimentally implanting glandular portions of the male reproductive tract or injecting extracts into the female and determining the effects of these treatments on female behavior and reproductive physiology. Their consistent finding was that these glandular products are diverse (74–76) and they induce females to oviposit or resist mating attempts from additional males; additional effects include inducing oogenesis, ovulation, or sperm storage (6, 74, 77). These kinds of data accumulated for many years in the absence of any theoretical expectations, but recognition of the importance of postcopulatory competition among males to trigger such female responses made immediate sense (under both CFC and SAC) of their diversity, their consistent effects on females (6, 78), and molecular signatures indicating that they evolved under selection (77, 79). The rapid divergence in Gryllus, in which females are thought to benefit (80) rather than suffer from repeated copulations, constitutes evidence against SAC being responsible for this divergence (77). Rapid divergence under selection has also occurred in seminal proteins of both primates and rodents (81, 82).
Interactions Between Egg and Sperm Molecules.
Recent summaries reveal that the genes coding for molecules involved in fertilization in mammals and marine invertebrates such as sea urchins and abalone show a general evolutionary pattern strikingly similar to the patterns seen in male genital morphology: in essentially all steps of animal fertilization where the molecular interactions between sperm and egg proteins have been studied, there is evidence for rapid divergence of the corresponding sperm and egg genes among closely-related species (83, 84), and changes in the genes coding for several of these divergent proteins indicates that many of these changes have resulted from positive selection. There are also informative exceptions to this rule (below). Strictly speaking, the term postcopulation is not appropriately applied to free-spawning species such as sea urchins, but they offer interesting comparisons. As in genitalia, species isolation hypotheses played a large role in early interpretations, but recent discoveries also suggest important roles for sexual selection.
Sperm proteins in free-spawning marine gastropods are among the most rapidly evolving proteins known (83). The abalone sperm molecule lysin, which digests a hole in the vitelline egg membrane, has evolved up to 15 times faster than introns (85), and there is a link between sites of positive selection and functional changes (83). Egg molecules with which lysin interacts have also undergone rapid divergence under selection (75, 86). Similarly, the bindin molecule of sea urchin sperm, which both attaches the acrosomal process of the sperm to the glycoprotein bindin receptor molecules in the egg's vitelline layer and promotes fusion of egg and sperm membranes, also shows rapid divergence (84). The section of the bindin molecule that is involved in attachment to the egg varies sharply among species in 3 genera, in each of which there is evidence that positive selection produced the changes; in 3 other genera, divergence in the attachment portion is low, and there is no evidence for positive selection (84). Egg molecules involved in both induction of the acrosome reaction and the docking process on the egg have diverged in 1 genus that has rapidly diverging bindin (87).
A SAC explanation of this divergence would posit a coevolutionary race between males and females over control of sperm entry into the egg. When multiple sperm enter the same egg (polyspermy), usually the embryo dies, so sperm competition favoring sperm cells that are especially quick to enter eggs might also result in loss of some eggs because of polyspermy. Females could respond by making it more difficult for sperm to enter, and the resulting coevolutionary race could result in rapid divergent evolution of both sperm and egg molecules. A CFC explanation is that females are under selection to favor sperm cells with particularly effective designs, to obtain sons with these same designs; increased female selectivity could result in competition among males to evolve even more effective designs, resulting in rapid coevolution between male and female molecules.
A species isolation function for the rapid divergence of the fertilization molecules of sea urchins and abalone is also attractive because their gametes meet in open water. Species that have evolved in the presence of close relatives would be expected to show greater divergence in their fertilization molecules than species that evolved in isolation from other congeneric species. Neither SAC nor CFC predicts this pattern. This predicted biogeographic pattern occurs in sea urchins: the 3 genera with rapidly diverging bindin molecules contain species that live in sympatry with congeners; in contrast, species in 3 other genera that do not show rapid divergence in bindin do not have overlapping geographic ranges (88). In addition, 1 species that overlaps with a congenor at some locations but not others shows the predicted accentuation of differences in areas of sympatry (84). Species isolation is a feasible (although not proven) explanation for the rapid divergence of their fertilization molecules of the abalone species, which are generally sympatric with congeners.
Nevertheless, further details suggest that sexual selection may have played a role, at least in bindin evolution. In the first place, cause and effect are not clear in the biogeographic correlation: perhaps the divergence in bindin was originally caused by other selective factors and only incidentally allowed congeneric species to subsequently coexist in sympatry without fusing or becoming extinct (89). In addition, there is rapid intraspecific divergence with signs of positive selection in 2 genera with sympatric congeners, contrary to species isolation predictions (89), and there is no evidence of selection having produced the substantial differences in the bindin (relative to mDNA) of 2 sympatric species of 1 genus (90). Furthermore, a possible explanation for intraspecific divergence in some but not other parts of a species' range that is based on the species isolation hypothesis is not supported in 1 group: the same bindin occurs in areas of overlap and nonoverlap with another congeneric species (H. Lessios, personal communication). Zigler (84) concluded that different types of selection may have acted on bindin, and that final answers are not yet available.
Both species isolation and sexual selection hypotheses predict that once an egg has responded to 1 sperm by erecting a barrier that excludes other sperm proteins that are involved in subsequent interactions should fail to show the rapid divergence typical of fertilization molecules. The data appear to support this prediction in sea urchin bindin. In sea urchins the raising of the egg's fertilization membrane, which prevents the entry of further sperm, is triggered by the by sperm–egg membrane fusion that follows attachment of sperm to the vitelline membrane. The core of the bindin molecule, which is involved in the fusion of egg and sperm membranes is, as predicted, uniform even among quite distantly-related sea urchins and other echinoids (84). Thus, 2 patterns occur in the same molecule: the portion involved in an early stage of fertilization is highly diverse in some lineages, whereas another portion that is involved in a later stage (after paternity has been decided) is very conservative.
The rapid diversification of fertilization molecules in mammals (83) is not likely to be explained by selection for species isolation, because the complex male–female interactions before copulation, and internal female barriers such as reduced sperm transport, probably make interactions between heterospecific eggs and sperm very rare (69). Both sperm proteins and egg coat zona pellucida glycoproteins show rapid divergence because of selection (91), and the ZP2 and ZP3 egg glycoproteins are among the 10% most different proteins between rodents and humans (83). Presumably SAC or CFC was involved in the divergence of these molecules.
Postcopulatory Sexual Selection in Plants.
Darwin did not apply his principle of sexual selection to plants, and this extension was a long time in coming (92–94). Competition between males leading to sexual selection can occur both before and after the plant equivalent of insemination (arrival of pollen on the stigma). There is ample opportunity for females to exercise postcopulatory choice in processes such as pollen germination, growth of the pollen tube to reach the ovule, and maturation (versus abortion) of the resulting seeds and their fruit. Some otherwise paradoxical traits of females, like the production of inhibitors of pollen grain germination at the site where pollen grains must germinate (the stigma) until it is loaded with pollen, and initiation of many more seeds and fruit than will eventually mature, may be female-imposed mechanisms that impose “rules of the game” for male–male competition (93). The consistent finding that a female's offspring show greater vigor when the competition among pollen grains from a single donor plant is more intense indicates a payoff for female selectivity that is compatible with CFC explanations (and contrary to those of SAC). Plant reproductive proteins are as yet only incompletely investigated, and evolutionary patterns of the genes and molecules involved cannot be checked (83).
Two widespread traits make postcopulatory selection in plants likely to differ from that in most animals. Many plants are hermaphroditic, and their largely passive roles in pollination can lead to a common postcopulatory problem that is largely absent in animals: avoidance of self-fertilization. Some of the diversity in postcopulatory traits in plants is probably related to selection favoring avoidance of fertilization with self-pollen (95). Species-specific diversity may also result from selection to avoid hybridization between species. For instance, specificity in the structural complexity of the outer cell wall of the pollen grain is apparently responsible for its ability to adhere to the conspecific stigma in species with “dry” stigmas (83, 96). But crucial data (see previous section) are generally not available to discriminate among the inbreeding avoidance, species isolation, CFC, and SAC explanations for generating these traits.
Second, female plants may be more likely than female animals to be able to reap indirect payoffs from screening among pollen grains. A large fraction of the genome of a pollen grain is expressed during the growth of the pollen tube (up to ≈⅔ of the expressed genome in a mature plant; ref. 97). In contrast, the genome of a sperm cell is largely silent (16). More vigorous pollen tube growth tends to correlate with more vigorous growth by the resulting offspring (93, 98), thus favoring female abilities to select among pollen tubes.
A further potentially intense filter of males in plants is the often substantial rate of abortion of zygotes before maturation of seed and fruit. One yet to be explored possibility is that sexual selection on males promoted genetic imprinting as a mechanism to reduce the chances that the male's offspring would be aborted.
Concluding Thoughts
The traits just reviewed share a strong trend: rapid divergent evolution. There are reasons to suppose that sexual selection has been important in many cases, but it is possible that no single explanation accounts for all cases or rapid divergence. Selection for species isolation mechanisms may have had an important role in the evolution of abalone lysins, but species isolation has probably been of little importance in producing the widespread rapid divergent evolution of male genitalia or mammal sperm and egg proteins.
It is impressive to see the long shadow that one of Darwin's few omissions had in the history of studies of sexual selection. Despite the resulting delays, Darwin's theory of sexual selection is now inspiring progress in explaining new findings and directing research in various fields that involve postcopulatory male–female interactions.
Acknowledgments.
I thank F. Ayala and J. Avise for inviting me to participate in the colloquium and M.-J. West-Eberhard, H. Lessios, and 2 referees for useful comments. This work was supported by the Sminthsonian Tropical Research Institute and the Universidad de Costa Rica.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution III: Two Centuries of Darwin,” held January 16–17, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Darwin.
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Darwin C. The Descent of Man and Selection in Relation to Sex. New York: Modern Library; 1871. reprinted (1959) [Google Scholar]
- 2.Parker GA. Sperm competition and its evolutionary consequences. Biol Rev. 1970;45:525–567. [Google Scholar]
- 3.Waage JK. Dual function of the damselfly penis: Sperm removal and transfer. Science. 1979;203:916–918. doi: 10.1126/science.203.4383.916. [DOI] [PubMed] [Google Scholar]
- 4.Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am Nat. 1983;122:765–788. [Google Scholar]
- 5.Eberhard WG. Sexual Selection and Animal Genitalia. Cambridge, MA: Harvard Univ Press; 1985. [Google Scholar]
- 6.Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton Univ Press; 1996. [Google Scholar]
- 7.Birkhead T, Møller AP. Sperm Competition and Sexual Selection. New York: Academic; 1997. [Google Scholar]
- 8.Parker GA. In: Sperm Competition and Sexual Selection. Birkhead T, Møller AP, editors. New York: Academic; 1997. pp. 3–54. [Google Scholar]
- 9.Birkhead T. Cryptic female choice: Criteria for establishing female sperm choice. Evolution (Lawrence, Kans) 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 10.Simmons LW. Sperm Competition and its Evolutionary Consequences in the Insects. Princeton: Princeton Univ Press; 2001. [Google Scholar]
- 11.Hosken DJ, Stockley P. Sexual selection and genital evolution. Trends Ecol Evol. 2004;19:87–93. doi: 10.1016/j.tree.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Arnqvist G, Rowe L. Sexual Conflict. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 13.Harcourt AH, Harvey PH, Larson SG, Short RV. Testis weight, body weight, and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- 14.Birkhead T, Møller AP. Sperm Competition in Birds: Evolutionary Causes and Consequences. New York: Academic; 1992. [Google Scholar]
- 15.Gage MJG, Baker RR. Ejaculate size varies with socio-sexual situation in an insect. Ecol Entomol. 1991;16:331–337. [Google Scholar]
- 16.Pizzari T, Foster KR. Sperm sociality: Cooperation, altruism, and spite. PLos Biol. 2008;130:925–931. doi: 10.1371/journal.pbio.0060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gack C, Peschke K. Spermathecal morphology, sperm transfer and a novel mechanism of sperm displacement in the rove beetle, Aleochara curtula (Coleoptera, Staphylinidae) Zoomorphology. 1994;114:227–237. [Google Scholar]
- 18.Baker RR, Bellis MA. Kamikaze sperm in mammals. Anim Behav. 1988;36:936–939. [Google Scholar]
- 19.Whitney NM, Pratt HL, Carrier JC. Group courtship, mating behavior, and siphon sac function in the whitetip reef shark, Triaenodon obesus. Anim Behav. 2004;68:1435–1442. [Google Scholar]
- 20.Holland B, Rice WR. Chase-away sexual selection: Antagonistic seduction versus resistance. Evolution (Lawrence, Kans) 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander RD, Marshall D, Cooley J. In: Social Competition and Cooperation in Insects and Arachnids. I. Evolution of Mating Systems. Choe J, Crespi B, editors. Cambridge, UK: Cambridge Univ Press; 1997. pp. 4–31. [Google Scholar]
- 22.Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- 23.Arnqvist G, Rowe L. Correlated evolution of male and female morphologies in water striders. Evolution (Lawrence, Kans) 2002;56:936–947. doi: 10.1111/j.0014-3820.2002.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 24.Eberhard WG. In: Evolution of Primary Sexual Characters in Animals. Leonard J, Cordoba-Aguilar A, editors. New York: Oxford Univ Press; 2009. in press. [Google Scholar]
- 25.Arnqvist G. Sensory exploitation and sexual conflict. Philos Trans R Soc London Ser B. 2006;361:375–386. doi: 10.1098/rstb.2005.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordero C, Eberhard WG. Interaction between sexually antagonistic selection and mate choice in the evolution of female responses to male traits. Evol Ecol. 2005;19:111–122. [Google Scholar]
- 27.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 28.Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 29.Pizarri T, Snook R. Sexual conflict and sexual selection: Chasing away paradigm shifts. Evolution (Lawrence, Kans) 2003;57:1223–1236. doi: 10.1111/j.0014-3820.2003.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 30.Arnqvist G. Sexual conflict and sexual selection: Lost in the chase. Evolution (Lawrence, Kans) 2004;58:1383–1388. doi: 10.1111/j.0014-3820.2004.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 31.Cordero C, Eberhard WG. Female choice of sexually antagonistic male adaptations: A critical review of some current research. J Evol Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 32.Orteiza N, Linder JE, Rice WR. Sexy sons from remating do not recoup the direct costs of harmful male interactions in the Drosophila melanogaster laboratory model system. J Evol Biol. 2005;18:1315–1323. doi: 10.1111/j.1420-9101.2005.00923.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith RL. In: Sperm Competition and Sexual Selection. Birkhead T, Møller AP, editors. New York: Academic; 1997. pp. xv–xxiii. [Google Scholar]
- 34.Shapiro AM, Porter AH. The lock-and-key hypothesis: Evolutionary and biosystematic interpretation of insect genitalia. Annu Rev Entomol. 1989;34:231–245. [Google Scholar]
- 35.Eberhard WG. Rapid divergent evolution of sexual morphology: Comparative tests of antagonistic coevolution and traditional female choice. Evolution (Lawrence, Kans) 2004;58:1947–1970. doi: 10.1554/04-143. [DOI] [PubMed] [Google Scholar]
- 36.Poinar GO, Jr, Herre EA. Speciation and adaptive radiation in the fig wasp nematode, Parasitodiplogaster (Diplogasteridae: Rhabditida) in Panama. Rev Nematol. 1991;14:361–374. [Google Scholar]
- 37.Ware AD, Opell BD. A test of the mechanical isolation hypothesis in two similar spider species. J Arachnol. 1989;17:149–162. [Google Scholar]
- 38.Roig-Alsina A. The evolution of the apid endophallus, its phylogenetic implications, and functional significance of the genital capsule (Hymenoptera, Apoidea) Bull Zool. 1993;60:169–183. [Google Scholar]
- 39.Dixson AF. Primate Sexuality. Oxford, UK: Oxford Univ Press; 1998. [Google Scholar]
- 40.Arnqvist G. Comparative evidence for the evolution of genitalia by sexual selection. Nature. 1998;393:784–786. [Google Scholar]
- 41.Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard Univ Press; 1963. [Google Scholar]
- 42.Eberhard WG. Male–female conflicts and genitalia: Failure to confirm predictions in insects and spiders. Biol Rev. 2004b;79:121–186. doi: 10.1017/s1464793103006237. [DOI] [PubMed] [Google Scholar]
- 43.Eberhard WG. Sexually antagonistic coevolution in insects is associated with only limited morphological diversity. J Evol Biol. 2006;19:657–681. doi: 10.1111/j.1420-9101.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 44.Ingram KK, Laamanen T, Puniamoorthy N, Meier R. Lack of morphological coevolution between male forelegs and female wings in Themira (Sepsidae: Diptera: Insecta) Biol J Linn Soc. 2008;93:227–238. [Google Scholar]
- 45.Robson GC, Richards OW. The Variation of Animals in Nature. Green & Co, London: Longmans; 1936. [Google Scholar]
- 46.Kreiger F, Krieger-Loibl E. Beiträge zum Verhalten von Ischnura elegans und Ischnura pumilio (Odonata) Z Tierpsychol. 1958;15:82–93. [Google Scholar]
- 47.Belk D. Antennal appendages and reproductive success in the Anostraca. J Crust Biol. 1984;4:66–71. [Google Scholar]
- 48.Eberhard Physical restraint or stimulation? The function(s) of the modified front legs of male Archisepsis diversiformis (Diptera, Sepsidae) J Ins Behav. 2002;15:831–850. [Google Scholar]
- 49.Eberhard WG. Evidence for widespread courtship during copulation in 131 species of insects and spiders, and implications for cryptic female choice. Evolution (Lawrence, Kans) 1994;48:711–733. doi: 10.1111/j.1558-5646.1994.tb01356.x. [DOI] [PubMed] [Google Scholar]
- 50.Eberhard WG. Copulatory courtship in insects. Biol Rev. 1991;66:1–31. [Google Scholar]
- 51.Shuker D, et al. Mating behavior, sexual selection, and copulatory courtship in a promiscuous beetle. J Ins Behav. 2002;15:617–631. [Google Scholar]
- 52.Ortiz P. San José: Universidad de Costa Rica; 2002. Historia natural, sitios de apareamiento, comportamiento sexual y posible función de la alimentación nupcial en Ptilosphen variolatus (Diptera: Micropezidae) Masters thesis. [Google Scholar]
- 53.Eberhard WG. Sexually reversed copulatory courtship roles and possible nuptial feeding in the soldier beetle Ditemnus acantholobus (Coleoptera, Cantharidae) J Kans Entomol Soc. 2006;79:13–22. [Google Scholar]
- 54.Arnqvist G, Danielsson I. Copulatory behavior, genital morphology, and male fertilization success in water striders. Evolution (Lawrence, Kans) 1999;53:147–156. doi: 10.1111/j.1558-5646.1999.tb05340.x. [DOI] [PubMed] [Google Scholar]
- 55.Edvardsson M Arnqvist G. Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc R Soc London Ser B. 2000;267:559–563. doi: 10.1098/rspb.2000.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tallamy DW, Darlington MB, Pesk JP, Powell BE. Copulatory courtship signals male genetic quality in cucumber beetles. Proc R Soc London Ser B. 2003;270:77–82. doi: 10.1098/rspb.2002.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuantianquiz C, Cordero C. Experimental manipulation of male behavior during copulation in Stenomacra marginella (Heteroptera: Largidae): Effect on copulation duration, female remating, and oviposition. Behav Proc. 2006;73:222–227. doi: 10.1016/j.beproc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Peretti A, Eberhard WG, Briceño RD. Copulatory dialogue: Female spiders sing during copulation to influence male genitalic movements. Anim Behav. 2006;72:413–421. [Google Scholar]
- 59.Jamieson BGM. The Ultrastructure and Phylogeny of Insect Spermatozoa. Cambridge, UK: Cambridge Univ Press; 1987. [Google Scholar]
- 60.Alberti G. In: The Acari: Reproduction, Development and Life-History Strategies. Schuster R, Murphy PW, editors. London: Chapman and Hall; 1991. pp. 77–105. [Google Scholar]
- 61.Baccetti B, Afzelius BA. The Biology of the Sperm Cell. New York: Karger; 1976. [PubMed] [Google Scholar]
- 62.Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- 63.Pitnick S, Miller GT, Schneider K, Markow TA. Ejaculate-female coevolution in Drosophila mojavensis. Proc R Socon London Ser B. 2002;270:1507–1512. doi: 10.1098/rspb.2003.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotrba M. The internal female genital organs of Chaetodiposis and Diasemopsis (Diptera: Diopsidae) and their stystematic relevance. Ann Natal Mus. 1995;36:147–159. [Google Scholar]
- 65.Kotrba M. The internal female reproductive tract of Campichoeta Macquart, 1835 and Diastata Meigen, 1830 (Diptera, Schizophora) Studia Dipt. 2006;13:309–315. [Google Scholar]
- 66.Swallow JG, Wilkinson GS. The long and short of sperm polymorphisms in insects. Biol Rev. 2002;77:153–182. doi: 10.1017/s1464793101005851. [DOI] [PubMed] [Google Scholar]
- 67.Minder AM, Hosken DJ Ward PI. Coevolution of male and female reproductive characters across the Scathophagidae (Diptera) J Evol Biol. 2005;18:60–69. doi: 10.1111/j.1420-9101.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 68.Oppliger A, Naciri-Graven Y, Ribi G, Hosken DJ. Sperm length influences fertilization success during sperm competition in the snail Viviparus ater. Mol Ecol. 2003;12:485–492. doi: 10.1046/j.1365-294x.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- 69.Gomendio M, Harcourt AH, Roldan ERS. In: Sperm Competition and Sexual Selection. Birkhead T, Møller AP, editors. New York: Academic; 1997. pp. 666–751. [Google Scholar]
- 70.Holman L, Snook RR. Spermicide, cryptic female choice, and the evolution of sperm form and function. J Evol Biol. 2006;19:1660–1670. doi: 10.1111/j.1420-9101.2006.01112.x. [DOI] [PubMed] [Google Scholar]
- 71.Holman R, Snook RR. A sterile sperm caste protects brother fertile sperm from female-mediated death in Drosophila pseudoobscura. Curr Biol. 2008;18:292–296. doi: 10.1016/j.cub.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 72.Oppliger A, Hosken DJ, Ribi G. Snail sperm production characteristics vary with sperm competition risk. Proc R Soc London Ser B. 1998;265:1527–1534. [Google Scholar]
- 73.Weddell N, Cook PA. Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc R Soc London Ser B. 1999;266:1033–1039. [Google Scholar]
- 74.Chen PS. The functional morphology and biochemistry of insect male accessory glands and their secretions. Annu Rev Entomol. 1984;29:233–255. [Google Scholar]
- 75.Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- 76.Kern AD, Jones CD, Begun DJ. Molecular genetics of male accessory gland in the Drosophila simulans complex. Genetics. 2004;167:725–735. doi: 10.1534/genetics.103.020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrés JA, Maroja LS, Bogdanowicz SM, Swanson WJ, Harrison RG. Molecular evolution of seminal proteins in field crickets. Mol Biol Evol. 2006;23:1574–1584. doi: 10.1093/molbev/msl020. [DOI] [PubMed] [Google Scholar]
- 78.Eberhard WG, Cordero C. Sexual selection by cryptic female choice on male seminal products: A new bridge between sexual selection and reproductive physiology. Trends Evol Ecol. 1995;10:493–496. doi: 10.1016/s0169-5347(00)89205-8. [DOI] [PubMed] [Google Scholar]
- 79.Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquando CF. Evolutionary EST analysis identifies rapidly evolving male reproductive products in Drosophila. Proc Natl Acad Sci USA. 2001;98:7375–7379. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner WE, Kelly RJ, Tucker KR, Harper CJ. Females receive a lifespan benefit from male ejaculates in a field cricket. Evolution (Lawrence, Kans) 2001;55:994–1001. doi: 10.1554/0014-3820(2001)055[0994:fralsb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 81.Dorus S, Evans PD, Wyckoff GJ, Choi SS, Lahn BT. Rate of molecular evolution of the seminal protein gene SEMG2 correlates with levels of female promiscuity. Nat Genet. 2004;36:1326–1329. doi: 10.1038/ng1471. [DOI] [PubMed] [Google Scholar]
- 82.Karn RC, Clark NL, Nguyen ED, Swanson WJ. Adaptive evolution in rodent seminal vesicle secretion proteins. Mol Biol Evol. 2008;25:2301–2310. doi: 10.1093/molbev/msn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clark NL, Aagaard JE, Swanson WJ. Evolution of reproductive proteins from animals and plants. Reproduction. 2006;131:11–22. doi: 10.1530/rep.1.00357. [DOI] [PubMed] [Google Scholar]
- 84.Zigler KS. The evolution of sea urchin sperm bindin. Int J Dev Biol. 2008;52:791–796. doi: 10.1387/ijdb.072521kz. [DOI] [PubMed] [Google Scholar]
- 85.Metz EC, Robles-Sikisaka R, Vacquiere VD. Nonsynonymous substitution in abalone sperm fertilization genes exceeds substitution in introns and mitochondrial DNA. Proc Natl Acad Sci USA. 1998;95:10676–10681. doi: 10.1073/pnas.95.18.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aagaard JE, Yi Z, MacCoss MJ, Swanson WJ. Rapidly evolving zona pellucida domain proteins are a major component of the vitelline envelope of abalone eggs. Proc Natl Acad Sci USA. 2006;103:17302–17307. doi: 10.1073/pnas.0603125103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamei N, Glabe CG. The species-specific egg receptor for sea urchin sperm adhesion is EBR1, a novel ADAMTS protein. Genes Dev. 2003;17:2502–2507. doi: 10.1101/gad.1133003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zigler KS, Lessios HA. 250 million years of bindin evolution. Biol Bull. 2003;205:8–15. doi: 10.2307/1543440. [DOI] [PubMed] [Google Scholar]
- 89.Zigler KS Lesios HA. Evolution of bindin in the pantropical sea urchin Tripneustes: Comparisons to bindin of other genera. Mol Biol Evol. 2003;20:220–231. doi: 10.1093/molbev/msg020. [DOI] [PubMed] [Google Scholar]
- 90.Zigler KS, Lessios HA. Speciation on the coasts of the New World: Phylogeography and the evolution of bindin in the sea urchin genus Lytechinus. Evolution (Lawrence, Kans) 2004;59:2399–2404. doi: 10.1111/j.0014-3820.2004.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 91.Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Mol Biol Evol. 2003;20:18–20. doi: 10.1093/oxfordjournals.molbev.a004233. [DOI] [PubMed] [Google Scholar]
- 92.Willson MF, Burley N. Mate Choice in Plants. Princeton: Princeton Univ Press; 1983. [Google Scholar]
- 93.Delph LF, Havens K. In: Sperm Competition and Sexual Selection. Birkhead T, Møller AP, editors. New York: Academic; 1997. pp. 149–174. [Google Scholar]
- 94.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 95.Kao T, Tsukamoto T. The molecular and genetic bases of S-RNase-based self-incompatibility. Plant Cell. 2004;16(Suppl):S72–S83. doi: 10.1105/tpc.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zinkl GM, Zwiebel BI, Grier DG, Preuss D. Pollen-stigma adhesion in Arabidopsis: A species-specific interaction mediated by lipophilic molecules in the pollen exine. Development. 1999;126:5431–5440. doi: 10.1242/dev.126.23.5431. [DOI] [PubMed] [Google Scholar]
- 97.Mascarenhas JP. Gene activity during pollen development. Annu Rev Plant Physiol Mol Biol. 1990;41:317–338. [Google Scholar]
- 98.Rocha O, Stephenson AG. Effect of ovule position on seed production, seed weight, and progeny performance in Phaseolus coccineus L. (Leguminosae) Am J Bot. 1990;77:1320–1329. [Google Scholar]