Abstract
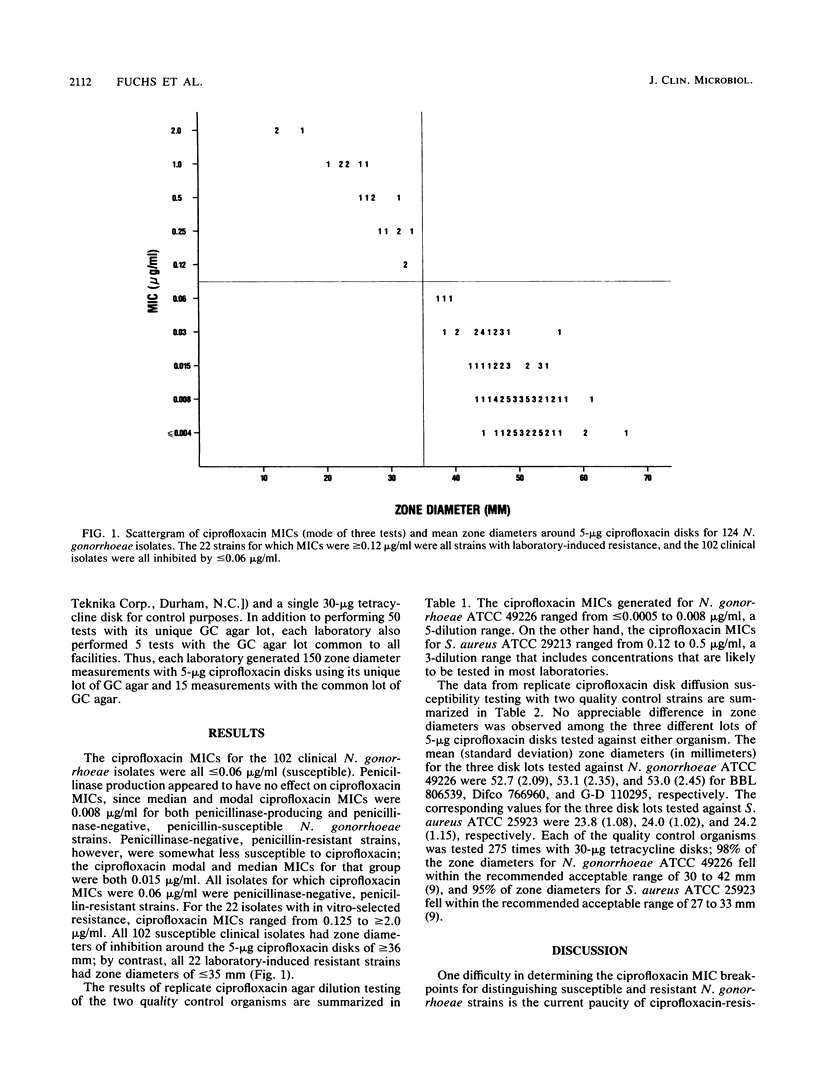
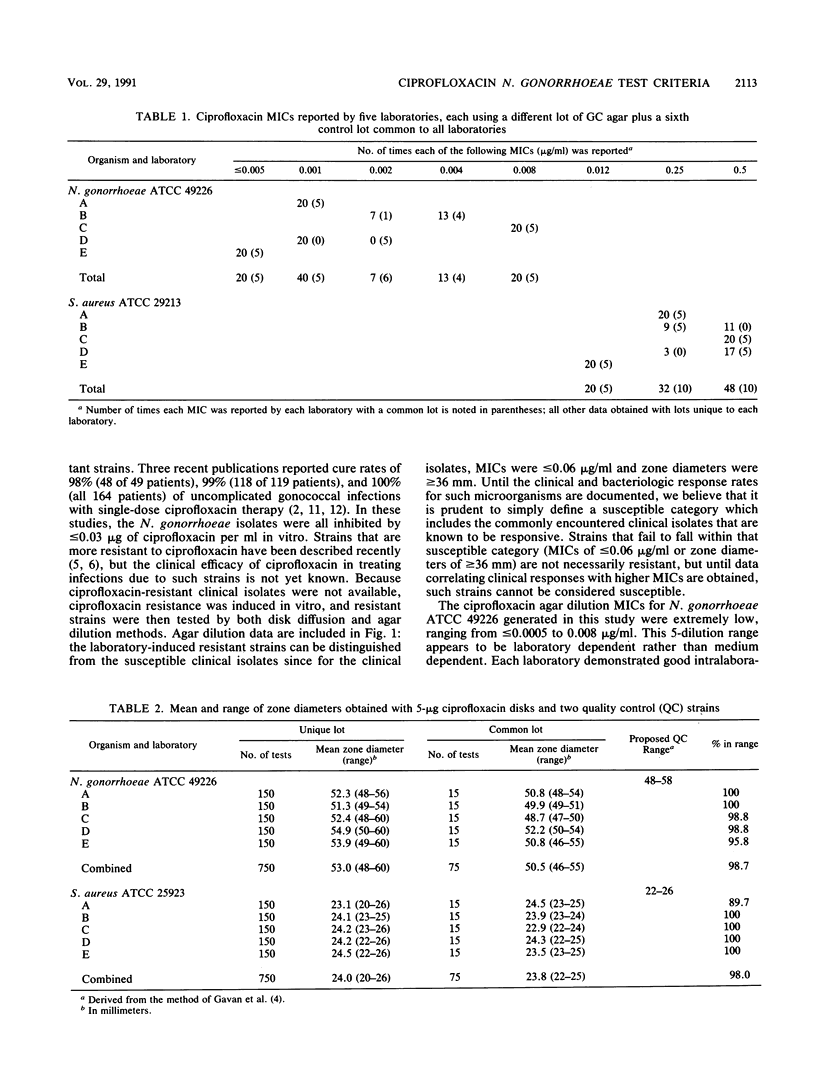
Ciprofloxacin was subjected to a multilaboratory study designed to determine its in vitro susceptibility criteria for Neisseria gonorrhoeae and its quality control parameters for both agar dilution and disk diffusion susceptibility testing for this species. All clinical isolates were susceptible, i.e., MICs were less than or equal to 0.06 microgram/ml and zones of inhibition were greater than or equal to 36 mm. A resistant category could not be defined, but in vitro-selected mutants gave zones of less than or equal to 35 mm, and MICs for these strains were greater than or equal to 0.12 microgram/ml. For quality control of ciprofloxacin agar dilution tests on supplemented GC agar, MICs for Staphylococcus aureus ATCC 29213 ranged from 0.12 to 0.5 microgram/ml. For quality control of 5-micrograms ciprofloxacin disk tests, N. gonorrhoeae ATCC 49226 and S. aureus ATCC 25923 produced acceptable zone diameter ranges of 48 to 58 mm and 22 to 26 mm, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N. Cross-resistance among cinoxacin, ciprofloxacin, DJ-6783, enoxacin, nalidixic acid, norfloxacin, and oxolinic acid after in vitro selection of resistant populations. Antimicrob Agents Chemother. 1984 Jun;25(6):775–777. doi: 10.1128/aac.25.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. P., Hira S. K., Brady W., Luo N., Mwale C., Mpoko G., Krieg R., Siwiwaliondo E., Reichart C., Waters C. Oral ciprofloxacin versus ceftriaxone for the treatment of urethritis from resistant Neisseria gonorrhoeae in Zambia. Antimicrob Agents Chemother. 1990 May;34(5):819–822. doi: 10.1128/aac.34.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L., Fuchs P. C., Gerlach E. H., Matsen J. M., Reller L. B., Thornsberry C., Thrupp L. D. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981 Jul;14(1):67–72. doi: 10.1128/jcm.14.1.67-72.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransden W. R., Warren C. A., Phillips I., Hodges M., Barlow D. Decreased susceptibility of Neisseria gonorrhoeae to ciprofloxacin. Lancet. 1990 Jan 6;335(8680):51–51. doi: 10.1016/0140-6736(90)90177-7. [DOI] [PubMed] [Google Scholar]
- Jephcott A. E., Turner A. Ciprofloxacin resistance in gonococci. Lancet. 1990 Jan 20;335(8682):165–165. doi: 10.1016/0140-6736(90)90035-4. [DOI] [PubMed] [Google Scholar]
- Moran J. S., Zenilman J. M. Therapy for gonococcal infections: options in 1989. Rev Infect Dis. 1990 Jul-Aug;12 (Suppl 6):S633–S644. doi: 10.1093/clinids/12.supplement_6.s633. [DOI] [PubMed] [Google Scholar]
- Roddy R. E., Handsfield H. H., Hook E. W., 3rd Comparative trial of single-dose ciprofloxacin and ampicillin plus probenecid for treatment of gonococcal urethritis in men. Antimicrob Agents Chemother. 1986 Aug;30(2):267–269. doi: 10.1128/aac.30.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelberg-Stassen M. J., van der Hoek J. C., Mooi L., Wagenvoort J. H., van Joost T., Michel M. F., Stolz E. Treatment of uncomplicated gonococcal urethritis in men with two dosages of ciprofloxacin. Eur J Clin Microbiol. 1986 Apr;5(2):244–246. doi: 10.1007/BF02013999. [DOI] [PubMed] [Google Scholar]



