Abstract
It has been established for a long time that brain glucose metabolism is impaired in Alzheimer's disease. Recent studies have demonstrated that impaired brain glucose metabolism precedes the appearance of clinical symptoms, implying its active role in the development of Alzheimer's disease. However, the molecular mechanism by which this impairment contributes to the disease is not known. In this study, we demonstrated that protein O-GlcNAcylation, a common post-translational modification of nucleocytoplasmic proteins with β-N-acetyl-glucosamine and a process regulated by glucose metabolism, was markedly decreased in Alzheimer's disease cerebrum. More importantly, the decrease in O-GlcNAc correlated negatively with phosphorylation at most phosphorylation sites of tau protein, which is known to play a crucial role in the neurofibrillary degeneration of Alzheimer's disease. We also found that hyperphosphorylated tau contained 4-fold less O-GlcNAc than non-hyperphosphorylated tau, demonstrating for the first time an inverse relationship between O-GlcNAcylation and phosphorylation of tau in the human brain. Downregulation of O-GlcNAcylation by knockdown of O-GlcNAc transferase with small hairpin RNA led to increased phosphorylation of tau in HEK-293 cells. Inhibition of the hexosamine biosynthesis pathway in rat brain resulted in decreased O-GlcNAcylation and increased phosphorylation of tau, which resembled changes of O-GlcNAcylation and phosphorylation of tau in rodent brains with decreased glucose metabolism induced by fasting, but not those in rat brains when protein phosphatase 2A was inhibited. Comparison of tau phosphorylation patterns under various conditions suggests that abnormal tau hyperphosphorylation in Alzheimer's disease brain may result from downregulation of both O-GlcNAcylation and protein phosphatase 2A. These findings suggest that impaired brain glucose metabolism leads to abnormal hyperphosphorylation of tau and neurofibrillary degeneration via downregulation of tau O-GlcNAcylation in Alzheimer's disease. Thus, restoration of brain tau O-GlcNAcylation and protein phosphatase 2A activity may offer promising therapeutic targets for treating Alzheimer's disease.
Keywords: tau phosphorylation, O-GlcNAcylation, glucose metabolism, protein phosphatase 2A, neurofibrillary degeneration
Introduction
The human brain constitutes only ∼2% of the body's mass, but its metabolism accounts for up to 50% of the total body glucose utilization under the basal condition (Fehm et al., 2006). The capacity of the brain to store energy and to use other fuels is very limited. Thus, a continuous supply of glucose is essential to maintain normal brain function. With increasing age, brain glucose utilization declines to regionally different degrees (Kuhl et al., 1982; Salmon et al., 1991; Loessner et al., 1995; Moeller et al., 1996; Ivancevic et al., 2000). This decline is accelerated in Alzheimer's disease, for which ageing is the most important risk factor. Extensive studies have established the impairment of glucose utilization and metabolism in the Alzheimer's disease brain (McGeer et al., 1989, 1990; Heiss et al., 1991; Smith et al., 1992; Minoshima et al., 1995). Brain glucose metabolism occurs prior to the appearance of clinical symptoms and in mild cognitive impairment, a likely precursor of Alzheimer's disease (Pietrini et al., 1993; Mielke et al., 1994; de Leon et al., 2001; Drzezga et al., 2003, 2005; Mosconi et al., 2004, 2007). These studies together suggest that impairment of brain glucose metabolism might be a cause, rather than a consequence, of Alzheimer's disease.
Most glucose in the brain is metabolized to produce ATP to maintain neuronal activity. Approximately 2–5% of total glucose feeds into the hexosamine biosynthesis pathway (HBP) to produce glucosamine-6-phosphate and, ultimately, UDP-N-acetylglucosamine (UDP-GlcNAc) (Love and Hanover, 2005). UDP-GlcNAc is the donor substrate for O-linked-β-N-acetylglucosamine (O-GlcNAc) transferase (OGT), which catalyses protein O-GlcNAcylation, a process transferring GlcNAc from UDP-GlcNAc to Ser/Thr residues of proteins. O-GlcNAcylation is a recently recognized post-translational modification of numerous cytoplasmic and nuclear proteins. The activity of OGT is sensitive to relatively small changes in UDP-GlcNAc availability over a wide range of concentrations (Kreppel and Hart, 1999). Thus, protein O-GlcNAcylation is regulated by intracellular glucose metabolism via alteration of UDP-GlcNAc production through the HBP.
It has been suggested that the impairment of brain glucose metabolism contributes to the pathogenesis of Alzheimer's disease (Perry et al., 2000, 2003; Iqbal and Grundke-Iqbal, 2005). However, the underlying molecular mechanism is not well understood. Arnold et al. (1996) found initially that bovine microtubule-associated protein tau is modified by O-GlcNAc. Tau is abnormally hyperphosphorylated and accumulated in the form of neurofibrillary tangles (NFTs) in Alzheimer's disease brain (Grundke-Iqbal et al., 1986). Many studies have demonstrated that abnormal hyperphosphorylation of tau is critical to neurodegeneration and promotes its aggregation into tangles (Iqbal et al., 1986; Alonso et al., 1994, 2001a, b, 2004; Lucas et al., 2001; Fath et al., 2002; Jackson et al., 2002; Perez et al., 2002). We recently found that tau phosphorylation is inversely regulated by O-GlcNAcylation and that decreased O-GlcNAcylation induces hyperphosphorylation of tau (Liu et al., 2004; Li et al., 2006). Therefore, we postulate that impaired brain glucose metabolism contributes to Alzheimer's disease pathogenesis via decreased HBP flux and, consequently, decreased tau O-GlcNAcylation, leading to abnormal hyperphosphorylation of tau.
To test the above hypothesis, we studied tau O-GlcNAcylation and the relationship between phosphorylation and O-GlcNAcylation of tau in Alzheimer's disease brain. We also investigated whether inhibition of the HBP induces tau hyperphosphorylation. We found that the protein O-GlcNAcylation level in Alzheimer's disease brain was decreased to nearly half of that in control brains and that hyperphosphorylated tau contained 4-fold less O-GlcNAc than did non-hyperphosphorylated tau. The decrease in O-GlcNAc correlated negatively to tau phosphorylation at most phosphorylation sites in Alzheimer's disease brains. Downregulation of O-GlcNAcylation by OGT knockdown with small hairpin RNA (shRNA) led to increased phosphorylation of tau in HEK-293 cells. We also found that inhibition of the HBP resulted in decreased O-GlcNAcylation and increased phosphorylation of tau, which resembled the changes of O-GlcNAcylation and phosphorylation of tau induced by fasting, but not by inhibition of protein phosphatase 2A (PP2A).
Materials and Methods
Human brain tissue
Frozen autopsied frontal cerebral cortices and cerebellar cortices from seven Alzheimer's disease cases and seven controls obtained from the Sun Health Research Institute Brain Donation Program (Sun City, AZ, USA) were used in this study. The ages, gender, post-mortem delays, Braak stages and tangle scores of these samples are listed in Table 1. Both Alzheimer's disease and control cases were confirmed histopathologically. The use of frozen human brain tissue was in accordance with the U.S. National Institutes of Health guidelines and was approved by the Institutional Review Board of the New York State Institute for Basic Research in Developmental Disabilities. The tissue was homogenized in cold buffer consisting of 50 mM Tri–HCl, pH 7.4, 8.5% sucrose, 2.0 mM EDTA, 10 mM β-mercaptoethanol, 100 mM GlcNAc, 1.0 mM orthovanadate, 50 mM NaF, 50 nM okadiac acid, 2.0 mM benzamidine, 1.0 mM phenyl-methylsulfonyl fluoride and 2.0 μg/ml each of aprotinin, leupeptin and pepstatin. After centrifugation of the homogenates at 16 000g at 4°C for 10 min, the supernatants were used to measure protein O-GlcNAcylation level and tau phosphorylation.
Table 1.
Human brain tissue of Alzheimer's disease and control cases used in this study
| Case | Age at death (year) | Gender | PMI (h) | Braak stagea | Tangle scoresb |
|---|---|---|---|---|---|
| AD 1 | 89 | F | 3 | V | 14.5 |
| AD 2 | 80 | F | 2.25 | VI | 14.5 |
| AD 3 | 85 | F | 1.66 | V | 12.0 |
| AD 4 | 78 | F | 1.83 | VI | 15.0 |
| AD 5 | 95 | F | 3.16 | VI | 10.0 |
| AD 6 | 86 | M | 2.25 | VI | 13.5 |
| AD 7 | 91 | F | 3 | V | 8.50 |
| Mean ± SD | 86.29 ± 5.99 | 2.45 ± 0.61 | 12.57 ± 2.51 | ||
| Con 1 | 85 | M | 25 | II | 4.25 |
| Con 2 | 86 | F | 2.5 | III | 5.00 |
| Con 3 | 81 | M | 2.75 | III | 6.41 |
| Con 4 | 88 | F | 3 | II | 2.00 |
| Con 5 | 90 | F | 3 | III | 4.50 |
| Con 6 | 88 | F | 3.5 | III | 2.50 |
| Con 7 | 88 | F | 3 | IV | 4.50 |
| Mean ± SD | 86.6 ± 2.9 | 2.89 ± 0.39 | 4.17 ± 1.50 |
PMI = post-mortem interval.
a Neurofibrillary pathology was staged according to Braak and Braak (1995).
b Tangle score was a density estimate and was designated none, sparse, moderate or frequent (0, 1, 2 or 3 for statistics), as defined according to CERAD Alzheimer's disease criteria (Mirra et al., 1991). Five areas (frontal, temporal, parietal, hippocampal and entorhinal) were examined, and the scores were totalled for a maximum of 15.
Analyses of levels of O-GlcNAcylation and phosphorylation of tau
Levels of O-GlcNAcylation and tau phosphorylation were determined by using quantitative immuno-dot-blot assay, as described (Liu et al., 2002). Levels of tau phosphorylation at each specific site were determined by using phosphorylation-dependent and site-specific tau antibodies from Biosource International (Camarillo, CA, USA). Total tau level was determined by using phosphorylation-independent tau antibodies R134d, 92e or 43D, which were produced in our laboratory. The protein O-GlcNAcylation level of human brains was measured by using RL2 as the primary antibody. The correlation between human brain O-GlcNAcylation and phosphorylation of tau was analysed by using linear regression method.
Immuno-affinity purification of tau
Non-hyperphosphorylated tau (AD-tau) was immunoaffinity-purified from Alzheimer's disease brain extracts in RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM NaF, 100 mM GlcNAc, 1.0 mM orthovanadate, 1.0 mM phenyl-methylsulfonyl fluoride and 2.0 μg/ml each of aprotinin, leupeptin and pepstatin) with Tau-1 antibody (from L. Binder of Northwestern University, Chicago, IL, USA) that was pre-linked covalently onto protein G-agarose beads using Seize™ X Protein G affinity kit (Pierce, Rockford, IL). The AD-tau-depleted extracts were further immunoprecipitated by using 43D (a phosphorylation-independent tau antibody generated in our laboratory) for immuno-purification of abnormally hyperphosphorylated tau (AD P-tau). Tau purified with this method was free of IgG and other proteins (Liu et al., 2004).
Double fluorescent immunohistochemistry
Frozen sections (40 μm thick) of the superior frontal gyri from Alzheimer's disease and control cases were blocked with 5% normal goat serum in TBS for 30 min, followed by incubation with primary antibodies overnight at 4°C in TBS containing 5% goat serum and 0.1% Triton. Primary antibodies used included polyclonal phospho-tau antibodies (1:250, Biosource International) and monoclonal antibody RL2 to O-GlcNAc (1:50, Affinity BioReagents, Rockford, IL, USA). After washing with TBS, the sections were incubated with Oregon green goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA) (1:1000) and Cy-3 goat anti-rabbit F(ab′)2 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) (1:1000) in TBS containing 5% goat serum and 0.1% Triton at room temperature for 1 h. The double immunostaining was analysed by using a laser scanning confocal microscope (PCM 200, Nikon).
HEK-293 cell culture and RNA interference
HEK-293 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum (Invitrogen, Carlsbad, CA, USA) in six-well plates. For expression of human tau and knockdown of OGT expression by using RNA interference technique, cells were co-transfected with pCI/tau441 (Liu et al., 2004) and SureSilencing shRNA plasmids for human OGT (SABioscience, Frederick, MD, USA) by using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturer's instruction. Three shRNA plasmids were used, and they were designed to target CCAAGGACGATACTGAAAGTT (shOGT1), AGATCTTCGAACAGCCAGAAT (shOGT3) and CCATCGCCAAGCTGATTAAAT (shOGT4), respectively, of the human OGT under the control of the U1 promoter and also included the green fluorescence protein (GFP) gene. The shRNA plasmid with the sequence CCATCGCCAAGCTGATTAAAT was used as a negative control. After transfection for 2 days, cells were lysed, and the cell lysates were analysed for OGT expression and the level and phosphorylation of tau by using Western blots, as described previously (Liu et al., 2004).
Intracerebroventricular injection
Rats were first anesthetized by intraperitoneal injection of chloral hydrate (30 mg/kg) and placed on a stereotactic instrument with the incisor bar set to 2 mm below the ear bars (i.e. flat skull). After the scalp was incised and retracted, a 10 µl syringe (Hamilton) was stereotactically placed into the lateral ventricle of the cerebrum according to stereotaxic coordinates (bregma and dura of anterior–posterior 0.8 mm, left lateral 1.2 mm and dorsal–ventral 3.5 mm). A total of 10 µl of 5 mM 6-diazo-5-oxonorleucine (DON) dissolved in artificial cerebrospinal fluid (aCSF) (140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2 and 1.2 mM Na2HPO4, pH 7.4) was injected into the left ventricle of the brain. The same volume of aCSF was injected into the left ventricle for control animals. Rats were killed 24 h after injection. The brains were immediately removed and either processed for double fluorescent immunohistochemistry, as described above, or homogenized at 4°C in 50 mM Tris–HCl, pH 7.4, 8.5% sucrose, 50 mM NaF, 1.0 mM Na3VO4, 10 mM β-mercaptoethanol, 2.0 mM EDTA, 2.0 mM benzamidine, 1.0 mM phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin, 5 µg/ml aprotinin and 2 µg/ml pepstatin. The homogenates were used for Western blot analysis.
Preparation of metabolically active rat brain slices and treatment with okadaic acid
Metabolically active brain slices (400 μm × 400 μm) were prepared from 6.5-month-old male Wistar rats and incubated in oxygenated aCSF at 33°C, as described (Gong et al., 2000). In some sets of slices, 0.1 μM okadaic acid was added into the aCSF during incubation. After incubation for 1–3 h, the slices were washed twice in homogenization buffer (50 mM Tris–HCl, pH 7.0, 10 mM β-mercaptoethanol, 1.0 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 2.0 mM benzamidine and 2.0 µg/ml each of aprotinin, leupeptin and pepstatin) and homogenized and analysed by Western blots.
Fasting of mice
Eight-week-old male C57BL/6NJCL mice (Charles River Laboratories, Wilmington, MA, USA) were housed singly in cages with grid floors to prevent coprophagy. After fasting for 48 h by removal of food, but not water, from the cages, the mice were sacrificed, and the brains were removed. The cerebral cortices were immediately dissected and homogenized for Western blot analysis.
Results
Decrease in O-GlcNAcylation correlates negatively to hyperphosphorylation of tau in Alzheimer's disease
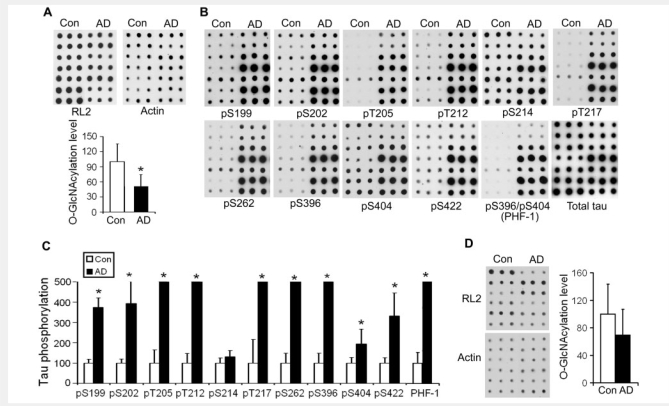
To determine the role of O-GlcNAcylation in abnormal hyperphosphorylation of tau, we first confirmed the decrease in protein O-GlcNAcylation in Alzheimer's disease brain by comparing O-GlcNAcylation levels in Alzheimer's disease and age-matched control brains with short post-mortem delay (<3 h). We found by immuno-dot-blot assays that the protein O-GlcNAcylation level in Alzheimer's disease brain was decreased to nearly half of that of control brains (Fig. 1A). The decrease in O-GlcNAcylation was accompanied by hyperphosphorylation of tau at all phosphorylation sites examined except Ser214 (Fig. 1B and C). We also determined O-GlcNAcylation level and tau phosphorylation in the cerebellum, which is not affected in Alzheimer's disease. We found that, unlike in the cerebral cortex, neither O-GlcNAcylation (Fig. 1D) nor phosphorylation of tau (data not shown) was changed significantly in the cerebellar cortex in Alzheimer's disease as compared to controls. These results suggest that downregulation of O-GlcNAcylation in Alzheimer's disease might relate to abnormal hyperphosphorylation of tau in the cerebrum.
Figure 1.
Decreased protein O-GlcNAcylation and increased phosphorylation of tau in Alzheimer's disease brain. (A) Protein O-GlcNAcylation levels in extracts of frontal cerebral cortices from seven Alzheimer's disease (AD) and seven control (Con) cases were determined by immuno-dot-blots with antibody RL2 to O-GlcNAc (upper panel). Each row shows blots in triplicate from an individual control case and an Alzheimer's disease case. Quantitation of the blots after being normalized with actin blots is shown as mean ± SD (lower panel). (B) The levels of total tau and tau phosphorylated at the indicated phosphorylation sites in the extracts of Alzheimer's disease and control brains were analysed by immuno-dot-blots developed with a mixture of total tau antibodies R134d and 92e and with several phosphorylation-dependent/site-specific tau antibodies. (C) Blots in panel B were quantified after normalization with the total tau level, and the relative levels of site-specific tau phosphorylation are shown as mean ± SD. Full scales and error bars are not shown for those sites where the increase in phosphorylation in Alzheimer's disease exceeded by 5-fold that of the controls. (D) Protein O-GlcNAcylation level in extracts of cerebellar cortices from six Alzheimer's disease (AD) and six control (Con) cases was determined by immuno-dot-blots with antibody RL2 to O-GlcNAc (upper panel). Each row shows blots in triplicates from an individual control case and an Alzheimer's disease case. Quantitation of the blots after being normalized with actin blots (lower panel) is shown as mean ± SD (right panel). *P < 0.05 versus control group.
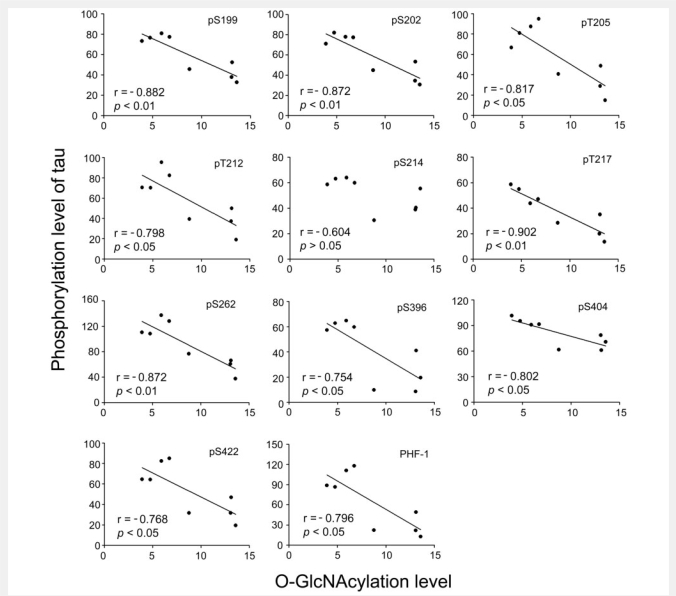
To further investigate whether the decrease in O-GlcNAcylation contributes to the site-specific hyperphosphorylation of tau, we analysed the relationship between the levels of O-GlcNAcylation and the levels of tau phosphorylation in the cerebral cortical extracts of seven Alzheimer's disease cases and one control case which had a detectable level of tau phosphorylation at most phosphorylation sites. We found that the levels of O-GlcNAcylation correlated negatively with the levels of tau phosphorylation at all the phosphorylation sites studied except Ser214 (Fig. 2). These results suggest that the decrease in O-GlcNAcylation might have contributed to hyperphosphorylation of tau in Alzheimer's disease brain.
Figure 2.
Correlation between O-GlcNAcylation and site-specific phosphorylation of tau. Levels of O-GlcNAcylation and tau phosphorylation at individual phosphorylation sites in extracts of frontal cortices from seven Alzheimer's disease and one control case (in six other controls, tau phosphorylation was not detectable at all phosphorylated sites determined) were determined by quantitative immuno-dot-blots. The levels of tau phosphorylation at individual phosphorylation sites were then plotted against the corresponding O-GlcNAcylation level. A linear regression line is shown where the correlation reached statistical significance.
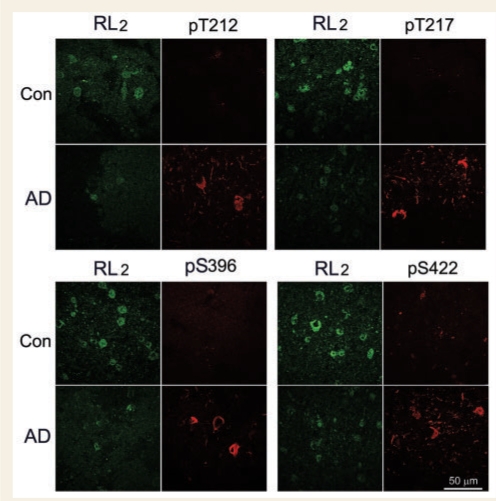
To confirm the contribution of decreased O-GlcNAcylation in hyperphosphorylation of tau, we performed a double-immunofluorescence study of the frontal cortices from Alzheimer's disease and control cases. We found a marked decrease in O-GlcNAcylation, as detected by monoclonal antibody RL2, in neurons in Alzheimer's disease brain as compared with controls (Fig. 3). In contrast, polyclonal antibodies against phosphorylated tau barely stained control brain tissue sections, but strongly stained neurons in Alzheimer's disease brain sections. In Alzheimer's disease brains, RL2-positive neurons were stained with antibodies to phosphorylated tau very weakly. There was no detectable co-labelling. These results are consistent with the above biochemical analyses and further support that the decrease in O-GlcNAcylation may have contributed to hyperphosphorylation of tau in Alzheimer's disease brain.
Figure 3.
Double-immunofluorescence staining of Alzheimer's disease and control brain sections. Frozen sections (40 μm) of the superior frontal gyrus of Alzheimer's disease (AD) and control cases were double-immunostained with monoclonal antibody RL2 against O-GlcNAc (green) and polyclonal antibodies against tau phosphorylated at the indicated phosphorylation sites (red).
Hyperphosphorylated tau is less O-GlcNAcylated than non-hyperphosphorylated tau in Alzheimer's disease brain
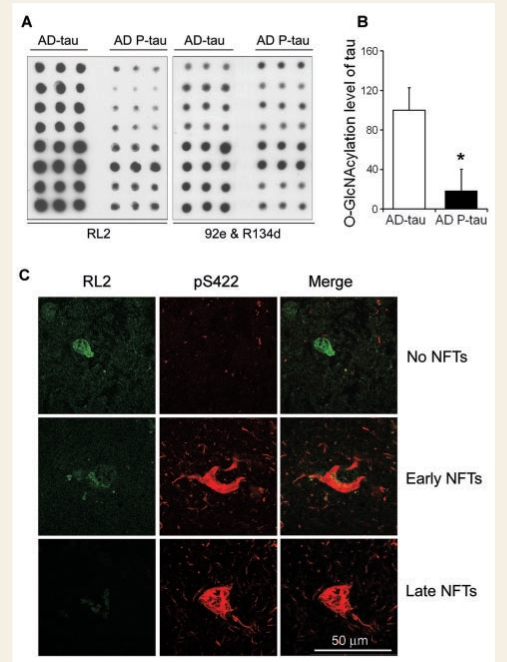
Tau in Alzheimer's disease brain can be isolated into three pools: (i) non-hyperphosphorylated tau that appears to be the same as normal tau and whose level is about the same as in normal brain (AD-tau); (ii) soluble, abnormally hyperphosphorylated tau (AD P-tau); and (iii) insoluble, hyperphosphorylated tau that is highly aggregated into NFTs (Khatoon et al., 1992; Kopke et al., 1993). To isolate AD-tau and AD P-tau from Alzheimer's disease brain, we first immuno-purified AD-tau from Alzheimer's disease brain extracts by using Tau-1 antibody that recognizes only non-phosphorylated tau between amino acids 192–204 and thus reacts with normal tau and AD-tau, but not AD P-tau (Grundke-Iqbal et al., 1986). AD P-tau was then purified from the Tau-1-immunodepleted extracts by using antibody 43D that reacts with human tau in a phosphorylation-independent manner. Then, we used immuno-dot-blots to measure the O-GlcNAcylation level of the isolated AD-tau and AD P-tau preparations and found that AD P-tau contained only ∼20% of the O-GlcNAc level of AD-tau (Fig. 4A and B). These results indicated that O-GlcNAcylation and phosphorylation both modify tau in a reciprocal manner in the human brain.
Figure 4.
O-GlcNAcylation of non-hyperphosphorylated tau (AD-tau) and hyperphosphorylated tau (AD P-tau). (A) Levels of O-GlcNAcylation and of tau in the AD-tau and AD P-tau preparations were quantified by immuno-dot-blots developed with RL2 to O-GlcNAc and antibodies R134/92e mixture to total tau, respectively. Each row includes triplicates of a tau preparation from an individual case. (B) Blots shown in panel A were quantified for the O-GlcNAcylation levels of AD-tau and AD P-tau after being normalized with the total tau level. Data are presented as mean ± SD. *P < 0.05 versus control group. (C) Double-immunofluorescence staining of Alzheimer's disease brain sections. Frozen sections (40 μm) of the superior frontal gyrus of Alzheimer's disease were double-immunostained with monoclonal RL2 against O-GlcNAc (green) and pS422 against tau phosphorylated at Ser422 (red). Representative neurons with no detectable (upper row), early stage (middle row) and late stage NFTs are shown.
We confirmed the reciprocal relationship between O-GlcNAcylation and phosphorylation of tau by double-immunofluorescence staining of Alzheimer's disease brain tissue sections with monoclonal antibody RL2 to O-GlcNAc and polyclonal anti-pS422 to hyperphosphorylated tau. We found that normal neurons with strong RL2 staining were not stained with anti-pS422, whereas neurons with strong anti-pS422 were negative or weakly stained with RL2 (Fig. 4C). These results are consistent with the reciprocal relationship between O-GlcNAcylation and phosphorylation of tau in Alzheimer's disease brain.
Inhibition of O-GlcNAcylation leads to increased phosphorylation of tau in cultured cells
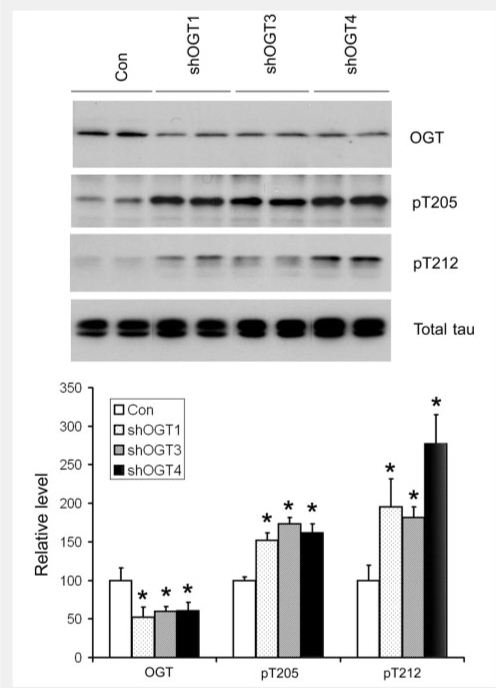
To investigate the effect of downregulation of O-GlcNAcylation on phosphorylation of tau, we co-transfected human tau and shRNA of human OGT into HEK-293 cells and studied the alterations of tau phosphorylation with OGT downregulation. We found that transfection with OGT shRNA knocked down OGT expression significantly, with reduction of ∼40–50% OGT expression (Fig. 5). The reduction of OGT expression was accompanied by a marked increase in tau phosphorylation at Thr205 and Thr212, whereas tau expression was not affected by OGT knockdown. These results indicate that downregulation of O-GlcNAcylation can lead to increased phosphorylation of tau in live cells.
Figure 5.
Increased phosphorylation of tau with OGT knockdown by shRNA. HEK-293 cells were co-transfected with SureSilencing shRNA of OGT (three different shRNAs were used) and pCI/tau441 for 2 days. The cells were then lysed, and the cell lysates were subjected to Western blots developed with anti-OGT, anti-pT205, anti-pT212 and anti-total tau (R134). Quantification of the blots (mean ± SD, n = 4) is shown in the lower panel. *P < 0.05 versus controls.
Inhibition of HBP decreases O-GlcNAcylation and increases phosphorylation of tau in mammalian brain
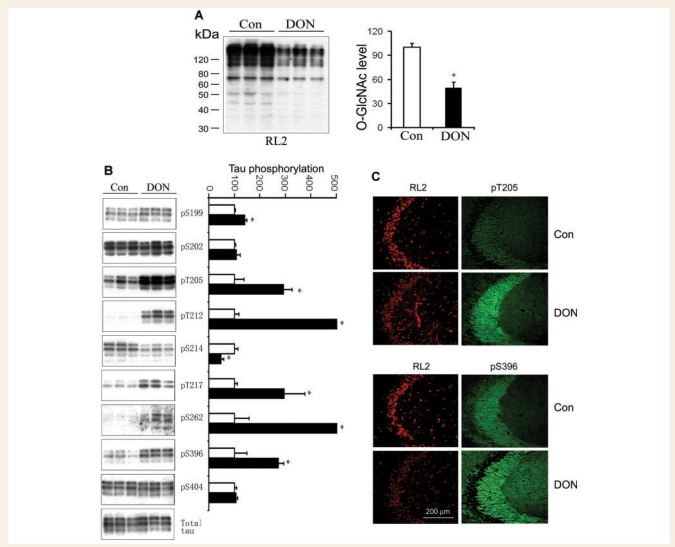
We had previously observed that decreasing glucose metabolism by fasting causes hyperphosphorylation of tau at multiple phosphorylation sites in the mouse brain (Liu et al., 2004; Li et al., 2006) and in the rat brain (data not shown). In addition to decreasing glucose supply for producing UDP-GlcNAc, fasting has been reported to downregulate PP2A activity in the brain, which, in turn, could induce hyperphosphorylation of tau (Planel et al., 2001, 2004). To elucidate the role of O-GlcNAcylation in the impaired glucose metabolism-induced hyperphosphorylation of tau, we injected DON, a specific inhibitor of the rate-limiting enzyme (glutamine:fructose-6-P amidotransferase, GFAT) of the HBP, into the lateral ventricle of rat brains. We found that inhibition of the HBP with DON reduced brain O-GlcNAcylation by 50% (Fig. 6A) and resulted in increased phosphorylation of tau at the majority of the phosphorylation sites studied (Fig. 6B). Double fluorescent immunohistochemical staining also showed decreased O-GlcNAc staining with monoclonal antibody RL2 and increased staining of phosphorylated tau, especially in the mossy fibres of the hippocampus (Fig. 6C). These results indicate that reduction of the HBP itself can cause hyperphosphorylation of tau via downregulation of O-GlcNAcylation.
Figure 6.
Changes of O-GlcNAcylation and tau phosphorylation after inhibition of the HBP pathway with DON in rat brain. (A) Rats were sacrificed 48 h after intracerebroventricular injection of DON, and the O-GlcNAcylation levels in the brain homogenates were analysed by Western blots developed with RL2. The right panel is the densitometric quantitation of all RL2-positive bands. (B) The total level and the levels of site-specific phosphorylation of tau in the rat brain homogenates were also analysed by Western blots developed with antibody 92e to total tau and with several phosphorylation-dependent/site-specific tau antibodies, respectively, as indicated. No significant immunostaining with antibody against pS422 was detected in the rat brain samples (data not shown). Quantitation of these blots after being normalized with the total tau is shown as mean ± SD (right panel). The tau phosphorylation levels of controls were defined as 100 for all sites. Full scales and error bars are not shown for those sites where the increase in phosphorylation in DON group exceeded by 5-fold that of the controls. *P < 0.05 versus control group. (C) Double-immunofluorescence staining of DON- and control-injected rat brain sections. Frozen sections were double-immunostained with monoclonal RL2 for O-GlcNAc (red) and polyclonal antibodies against tau phosphorylated at Thr205 or Ser396 (green).
Downregulation of both O-GlcNAcylation and PP2A activity may contribute to abnormal hyperphosphorylation of tau in Alzheimer's disease brain
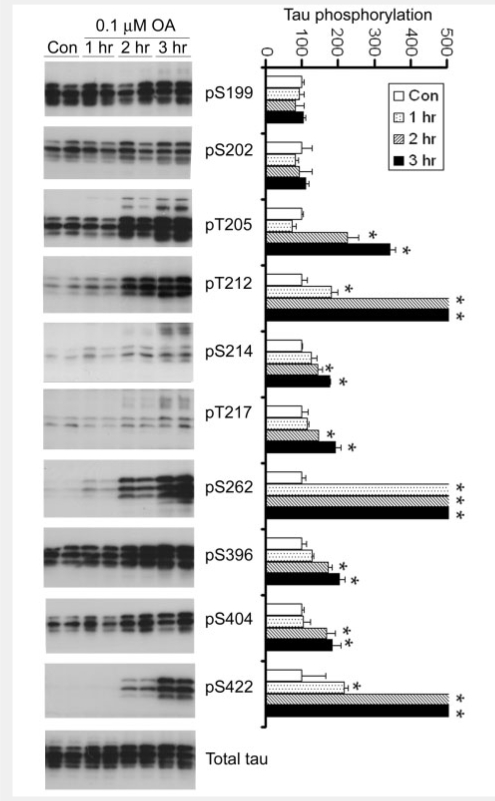
PP2A activity is decreased in Alzheimer's disease brain and this decrease has been suggested to contribute to abnormal hyperphosphorylation of tau (Gong et al., 1993, 1995; Loring et al., 2001; Vogelsberg-Ragaglia et al., 2001; Sontag et al., 2004; Liu et al., 2005, 2008a; Zhou et al., 2008). To study whether downregulation of PP2A or O-GlcNAcylation, or both, leads to abnormal hyperphosphorylation of tau in Alzheimer's disease brain, we compared the patterns of site-specific hyperphosphorylation induced by fasting, by DON and by PP2A inhibition with that in Alzheimer's disease brain. PP2A inhibition was induced by treatment of metabolically active rat brain slices with 0.1 μM okadaic acid, which selectively inhibits PP2A in rat brain slices (Gong et al., 2000). We found that PP2A inhibition resulted in tau hyperphosphorylation at multiple sites (Fig. 7). However, the overall pattern of tau hyperphosphorylation differed from that induced by HBP inhibition (compare Figs 7 with 6B, Table 2). These results suggest that PP2A activity and O-GlcNAcylation regulate tau phosphorylation with overlapping, but different, site specificities. When we compared these two patterns of tau hyperphosphorylation with that in Alzheimer's disease brain, we found that neither of these patterns was identical to that in Alzheimer's disease brain, despite the fact that tau hyperphosphorylation was seen under all these conditions at most of the sites. Comparing the overall patterns of tau hyperphosphorylation under these conditions, it appears that both PP2A downregulation and impaired brain glucose metabolism may contribute to abnormal hyperphosphorylation of tau in Alzheimer's disease.
Figure 7.
Site-specific tau hyperphosphorylation induced by PP2A inhibition with OA. Metabolically active rat brain slices were incubated with aCSF containing either 0 (control) or 0.1 μM OA for the indicated periods of time. The slices were then homogenized and analysed by Western blots developed with 92e (to detect total tau) and several phosphorylation-dependent and site-specific tau antibodies (to detect tau phosphorylation at the indicated phosphorylation sites). The blots were also quantified densitometrically after being normalized with the total tau levels, and the relative tau phosphorylation levels at individual sites are shown as mean ± SD (right panel). The tau phosphorylation levels of controls were defined as 100 for all sites. Full scales and error bars are not shown for those sites where the increase in phosphorylation exceeded by 5-fold that of the controls. *P < 0.05 versus control group.
Table 2.
Tau phosphorylation under different conditions and in Alzheimer's disease brain
| Phosphorylation site | OA treatment | DON treatment | Fasting | Alzheimer's disease |
|---|---|---|---|---|
| Ser199 | ↔ | ↑ | ↑ | ↑ |
| Ser202 | ↔ | ↔ | ↔ | ↑ |
| Thr205 | ↑ | ↑ | ↑ | ↑ |
| Thr212 | ↑ | ↑ | ↑ | ↑ |
| Ser214 | ↑ | ↓ | ↓ | ↔ |
| Thr217 | ↑ | ↑ | ↑ | ↑ |
| Ser262 | ↑ | ↑ | ND | ↑ |
| Ser396 | ↑ | ↑ | ↑ | ↑ |
| Ser404 | ↑ | ↔ | ↔ | ↑ |
| Ser422 | ↑ | ND | ND | ↑ |
↑ = increased; ↓ = decreased; ↔ = unchanged; ND = not detectable.
Fasting has been suggested to result in not only a decrease in brain glucose metabolism and O-GlcNAcylation (Liu et al., 2004; Li et al., 2006), but also hypothermia and decreased PP2A activity in the brain (Planel et al., 2001, 2004; Su et al., 2008). We, therefore, compared the pattern of site-specific hyperphosphorylation of tau induced by fasting with that induced by PP2A inhibition, induced by HBP inhibition and in Alzheimer's disease brain. We found that fasting-induced changes of tau phosphorylation resembled those induced by HBP inhibition with DON, but not with PP2A inhibition (Table 2). These results suggested that impaired brain glucose metabolism contributes to hyperphosphorylation of tau mainly via decreased HBP flux and thus downregulation of tau O-GlcNAcylation, rather than PP2A inhibition.
Discussion
In the present study, by using a cohort of seven Alzheimer's disease cases and seven controls with matched age, gender, post-mortem interval between the two groups and very short post-mortem delay (mean delay <3 h, range 1.7–3.5 h), we demonstrated that the protein O-GlcNAcylation level in the cerebrum, but not in the cerebellum, of Alzheimer's disease was reduced to approximately half of that in controls. The selective downregulation of O-GlcNAcylation in the cerebrum is consistent with the fact that tau pathology occurs only in the cerebrum in Alzheimer's disease, and not in the cerebellum. We also found that abnormally hyperphosphorylated tau contained four times less O-GlcNAc than did the non-hyperphosphorylated tau, although both species of tau were isolated from Alzheimer's disease brain. These findings demonstrated a reciprocal relationship between phosphorylation and O-GlcNAcylation in the brain. This reciprocal relationship was further confirmed by our double-immunofluorescence studies and correlation analyses, which showed an inverse relationship between O-GlcNAcylation and phosphorylation of tau at most of the phosphorylation sites examined. These findings are consistent with our previous observations showing such an inverse relationship between O-GlcNAcylation and phosphorylation of tau in cultured cells, rat brain slices and mouse brains (Liu et al., 2004; Li et al., 2006). Furthermore, we demonstrated that inhibition of the HBP decreased O-GlcNAcylation and increased phosphorylation of tau in rat brains. Because protein O-GlcNAcylation is regulated by intracellular glucose metabolism (Kreppel and Hart, 1999), which is impaired in Alzheimer's disease brain, our findings together reveal a mechanism by which impaired brain glucose metabolism could contribute to abnormal hyperphosphorylation of tau and, consequently, neurofibrillary degeneration via decreased HBP flux and the resultant decreased tau O-GlcNAcylation.
An animal model commonly used to mimic decreased brain glucose metabolism is fasting of animals (Liu et al., 2004; Li et al., 2006; Planel et al., 2004; Deng et al., 2008). Fasting of rodents has been reported to induce hyperphosphorylation of tau and neurofilaments in the brain (Planel et al., 2001, 2004; Liu et al., 2004; Li et al., 2006; Deng et al., 2008). Fasting results in both a decrease in PP2A activity (Planel et al., 2001, 2004) and a decrease in O-GlcNAcylation (Liu et al., 2004; Li et al., 2006), either of which could induce hyperphosphorylation of tau. Thus, it was not known which mechanism, fasting or decreased brain glucose metabolism, leads to hyperphosphorylation and neurofibrillary degeneration. To date, more than 40 phosphorylation sites have been identified in tau isolated from Alzheimer's disease brain (Gong et al., 2005; Hanger et al., 2007). Because not all phosphorylation sites of tau are hyperphosphorylated to the same extent, and O-GlcNAcylation and PP2A may regulate each of the phosphorylation sites differently, we compared the patterns of tau phosphorylation among tau from Alzheimer's disease brain and tau induced by inhibition of HBP (to reduce intracellular UDP-GlcNAc level) or PP2A. We found that although these tau species were all hyperphosphorylated at most of the phosphorylation sites studied, their phosphorylation patterns were different. Downregulation of O-GlcNAcylation via HSP inhibition led to a marked increase in tau phosphorylation at Ser199, Thr205, Thr212, Thr217, Ser262 and Ser396; a marked decrease at Ser214; and no change at Ser202 or Ser404. However, PP2A inhibition led to a marked increase in tau phosphorylation at Thr205, Thr212, Ser214, Thr217, Ser262, Ser396, Ser404 and Ser422, but it did not change tau phosphorylation at Ser199 or Ser202 (Table 2). The different patterns of tau hyperphosphorylation induced by these two pathways suggest that O-GlcNAcylation and PP2A regulate tau phosphorylation at overlapping yet different phosphorylation sites. This conclusion is consistent with our previous in vitro observation that Thr205, Thr212 and Ser262 of tau are the favourite sites for PP2A, followed by Ser214 and Ser396, whereas Ser202 and Ser199 are dephosphorylated by PP2A less efficiently (Liu et al., 2005). When we compared tau phosphorylation patterns induced by fasting with those induced by the two pharmacological means above, we found that fasting-induced change of tau phosphorylation resembled that induced by downregulation of O-GlcNAcylation via HBP inhibition with DON and differed from that induced by PP2A inhibition. These results suggest that decreased brain glucose metabolism due to fasting leads to hyperphosphorylation of tau mainly via downregulation of O-GlcNAcylation (through decreased HSP flux) instead of a decrease in PP2A activity due to hypothermia.
When we compared tau phosphorylation patterns in Alzheimer's disease brain with those in the rat models above, we found that the former do not resemble the tau phosphorylation patterns induced by fasting or DON or by PP2A inhibition. Detailed analysis of tau phosphorylation patterns suggests that both PP2A downregulation and impaired brain glucose metabolism may contribute to abnormal hyperphosphorylation of tau in Alzheimer's disease.
Alzheimer's disease is a chronic neurodegenerative disorder with multiple aetiologies (Perry et al., 2003; Iqbal and Grundke-Iqbal, 2005; Stozicka et al., 2007). The exact causes leading to abnormal hyperphosphorylation of tau in Alzheimer's disease remain elusive, although Aβ toxicity (Blurton-Jones and Laferla, 2006), deregulation of protein kinases and phosphatases (Gong and Iqbal, 2008; Pei et al., 2008), and mitochondrial oxidative stress (Lee et al., 2005; Melov et al., 2007) have been implicated. PP2A is the major phosphatase regulating tau phosphorylation level in the brain (Goedert et al., 1995; Sontag et al., 1996; Gong et al., 2000; Liu et al., 2005). Both the activity and the expression of PP2A are decreased in Alzheimer's disease brain (Gong et al., 1993, 1995; Loring et al., 2001; Vogelsberg-Ragaglia et al., 2001; Sontag et al., 2004; Liu et al., 2005), suggesting that the downregulation of PP2A might underlie, at least in part, abnormal hyperphosphorylation of tau in Alzheimer's disease. The present study demonstrated that impaired glucose metabolism, via decreased tau O-GlcNAcylation, is another cause of abnormal hyperphosphorylation of tau and neurofibrillary degeneration in Alzheimer's disease.
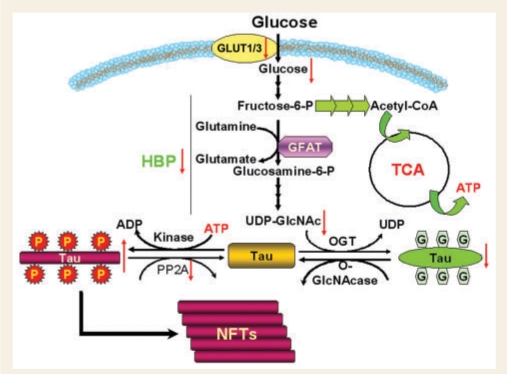
It is not well understood why brain glucose metabolism is impaired in Alzheimer's disease. Normally, the transport of glucose from the bloodstream into the brain is mediated by glucose transporters (GLUTs). Among the varieties of glucose transporters, GLUT1 and GLUT3 play the most important role in transporting glucose into the neuron. The levels of these two glucose transporters are decreased in the Alzheimer's disease brain (Simpson et al., 1994; Liu et al., 2008b). More importantly, we recently found that the decrease in GLUT1/3 correlates positively with the decrease in O-GlcNAcylation and negatively with the level of tau phosphorylation at multiple phosphorylation sites (Liu et al., 2008b). Taken together, we propose that impaired brain glucose metabolism due to deficient glucose transport contributes to abnormal hyperphosphorylation of tau and neurofibrillary degeneration via downregulation of the HBP and, consequently, O-GlcNAcylation of tau (Fig. 8).
Figure 8.
Proposed mechanism by which impaired brain glucose metabolism contributes to neurofibrillary degeneration in Alzheimer's disease. Decreased brain glucose metabolism due to deficient GLUT1/3 and possibly other causes leads to decreased HBP flux and thus decreased UDP-GlcNAc production in the neuron, which in turn results in decreased tau O-GlcNAcylation catalysed by OGT, whose activity is regulated by intracellular UDP-GlcNAc level. Because tau phosphorylation is inversely regulated by O-GlcNAcylation, decreased O-GlcNAcylation, together with downregulation of PP2A, leads to abnormal hyperphosphorylation of tau and, finally, neurodegeneration and formation of NFTs.
In conclusion, this study has demonstrated that: (i) protein O-GlcNAcylation was markedly decreased in the cerebrum, but not in the cerebellum, in Alzheimer's disease, and the decrease in O-GlcNAc correlated negatively with tau phosphorylation at most phosphorylation sites; (ii) that in the Alzheimer's disease brain, hyperphosphorylated tau contained 5-fold less O-GlcNAc than non-hyperphosphorylated tau; (iii) that inhibition of O-GlcNAcylation by OGT knockdown led to increased phosphorylation of tau in cultured cells; (iv) that inhibition of the HBP resulted in decreased O-GlcNAcylation and increased phosphorylation of tau, which resembled changes of O-GlcNAcylation and phosphorylation of tau induced by fasting, but not by inhibition of PP2A; and (v) that abnormal tau hyperphosphorylation in Alzheimer's disease brain may result from downregulation of both O-GlcNAcylation and PP2A. Together with our previous observations (Liu et al., 2004; Li et al., 2006; Deng et al., 2008), these findings allow us to propose a mechanism of tau pathology led by impaired brain glucose metabolism (Fig. 8). In Alzheimer's disease, deficient GLUT1/3 and possibly other causes lead to impaired brain glucose metabolism, which results in decreased HBP flux and thus decreased UDP-GlcNAc production in the neuron. Because OGT activity is regulated by intracellular UDP-GlcNAc level, the decreased intraneuronal UDP-GlcNAc level causes decreased tau O-GlcNAcylation. Since tau O-GlcNAcylation regulates its phosphorylation inversely, decreased O-GlcNAcylation, together with downregulation of PP2A, causes abnormal hyperphosphorylation of tau and, finally, formation of NFTs. Thus, increasing brain glucose uptake and PP2A activity offers a rational therapeutic target for preventing and treating Alzheimer's disease.
Funding
New York State Office of Mental Retardation and Developmental Disabilities, Nantong University, the National Institute of Health (grant R01 AG027 429 and R01 AG019 158, partial); the U.S. Alzheimer's Association (Grant IIRG-05-13 095 and NIRG-08-91126); National Natural Science Foundation of China (grants 30 572 076 and 30 770 468); Natural Science Foundation of Jiangsu Province (grant BK2 004 047); National Institute on Aging, The Brain Donation Program (grant P30 AG19 610, Arizona Alzheimer's Disease Core Center, partial).
Acknowledgements
We thank Ms. Janet Murphy for secretarial assistance and Ms. Maureen Marlow for editorial suggestions. We are also grateful to the Sun Health Research Institute Brain Donation Program of Sun City, Arizona, USA, for the provision of post-mortem human brain tissue.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AD P-tau
abnormally hyperphosphorylated tau isolated from Alzheimer's disease brain
- AD-tau
non-hyperphosphorylated tau isolated from Alzheimer's disease brain
- DON
6-diazo-5-oxonorleucine
- GFAT
glutamine:fructose-6-P amidotransferase
- GFP
green fluorescence protein
- GlcNAc
β-N-acetyl-glucosamine
- GLUT
glucose transporter
- HBP
hexosamine biosynthesis pathway
- NFTs
neurofibrillary tangles
- O-GlcNAc
O-linked-β-N-acetylglucosamine
- OGT
O-GlcNAc transferase
- PP2A
protein phosphatase 2A
- shRNA
small hairpin RNA
References
- Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–81. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer's disease. Proc Natl Acad Sci USA. 1994;91:5562–6. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. Interaction of tau isoforms with Alzheimer's disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem. 2001a;276:37967–73. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001b;98:6923–8. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–4. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–48. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 278–84. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–71. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, et al. Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer's disease. FASEB J. 2008;22:138–45. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Riemenschneider M, Lautenschlager N, Siebner H, Alexopoulus P, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–32. [PubMed] [Google Scholar]
- Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- Fath T, Eidenmuller J, Brandt R. Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer's disease. J Neurosci. 2002;22:9733–41. doi: 10.1523/JNEUROSCI.22-22-09733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm HL, Kern W, Peters A. The selfish brain: competition for energy resources. Prog Brain Res. 2006;153:129–40. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65:2804–7. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer's disease. Curr Med Chem. 2008;15:2321–8. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000;275:5535–44. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm. 2005;112:813–38. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer's disease brain. J Neurochem. 1995;65:732–8. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer's disease brain. J Neurochem. 1993;61:921–7. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, et al. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007;282:23645–54. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Szelies B, Kessler J, Herholz K. Abnormalities of energy metabolism in Alzheimer's disease studied with PET. Ann N Y Acad Sci. 1991;640:65–71. doi: 10.1111/j.1749-6632.1991.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Metabolic/signal transduction hypothesis of Alzheimer's disease and other tauopathies. Acta Neuropathol. 2005;109:25–31. doi: 10.1007/s00401-004-0951-y. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, et al. Defective brain microtubule assembly in Alzheimer's disease. Lancet. 1986;2:421–6. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- Ivancevic V, Alavi A, Souder E, Mozley PD, Gur RE, Benard F, et al. Regional cerebral glucose metabolism in healthy volunteers determined by fluordeoxyglucose positron emission tomography: appearance and variance in the transaxial, coronal, and sagittal planes. Clin Nucl Med. 2000;25:596–602. doi: 10.1097/00003072-200008000-00005. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, et al. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34:509–19. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer's disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem. 1992;59:750–3. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer's disease. J Biol Chem. 1993;268:24374–84. [PubMed] [Google Scholar]
- Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–22. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Metter EJ, Riege WH, Phelps ME. Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]fluorodeoxyglucose method. J Cereb Blood Flow Metab. 1982;2:163–71. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]
- Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, et al. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol Med. 2005;11:164–9. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Li X, Lu F, Wang JZ, Gong CX. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J Neurosci. 2006;23:2078–86. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:10804–9. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer's disease. FEBS Lett. 2008b;582:359–64. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Gong CX. Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience. 2002;115:829–37. doi: 10.1016/s0306-4522(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Liu R, Zhou XW, Tanila H, Bjorkdahl C, Wang JZ, Guan ZZ, et al. Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology. J Cell Mol Med. 2008a;12:241–57. doi: 10.1111/j.1582-4934.2008.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner A, Alavi A, Lewandrowski KU, Mozley D, Souder E, Gur RE. Regional cerebral function determined by FDG-PET in healthy volunteers: normal patterns and changes with age. J Nucl Med. 1995;36:1141–9. [PubMed] [Google Scholar]
- Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. A gene expression profile of Alzheimer's disease. DNA Cell Biol. 2001;20:683–95. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL, Akiyama H, Harrop R. Cortical glutaminase, beta-glucuronidase and glucose utilization in Alzheimer's disease. Can J Neurol Sci. 1989;16:511–5. doi: 10.1017/s0317167100029851. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Peppard RP, McGeer PL, Tuokko H, Crockett D, Parks R, et al. 18Fluorodeoxyglucose positron emission tomography studies in presumed Alzheimer cases, including 13 serial scans. Can J Neurol Sci. 1990;17:1–11. doi: 10.1017/s0317167100029930. [DOI] [PubMed] [Google Scholar]
- Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Clinical deterioration in probable Alzheimer's disease correlates with progressive metabolic impairment of association areas. Dementia. 1994;5:36–41. doi: 10.1159/000106692. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48. [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16:385–98. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–40. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, Rusinek H, De Santi S, Li Y, Wang GJ, et al. Quantitation, regional vulnerability, and kinetic modeling of brain glucose metabolism in mild Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2007;34:1467–79. doi: 10.1007/s00259-007-0406-5. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Sjogren M, Winblad B. Neurofibrillary degeneration in Alzheimer's disease: from molecular mechanisms to identification of drug targets. Curr Opin Psychiatry. 2008;21:555–61. doi: 10.1097/YCO.0b013e328314b78b. [DOI] [PubMed] [Google Scholar]
- Perez M, Hernandez F, Gomez-Ramos A, Smith M, Perry G, Avila J. Formation of aberrant phosphotau fibrillar polymers in neural cultured cells. Eur J Biochem. 2002;269:1484–9. doi: 10.1046/j.1432-1033.2002.02794.x. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Hirai K, Takeda A, Aliev G, Smith MA. Oxidative damage in Alzheimer's disease: the metabolic dimension. Int J Dev Neurosci. 2000;18:417–21. doi: 10.1016/s0736-5748(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Raina AK, Aliev G, Siedlak SL, Harris PL, et al. A metabolic basis for Alzheimer's disease. Neurochem Res. 2003;28:1549–52. doi: 10.1023/a:1025678510480. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Azari NP, Grady CL, Salerno JA, Gonzales-Aviles A, Heston LL, et al. Pattern of cerebral metabolic interactions in a subject with isolated amnesia at risk for Alzheimer's disease: a longitudinal evaluation. Dementia. 1993;4:94–101. doi: 10.1159/000107349. [DOI] [PubMed] [Google Scholar]
- Planel E, Miyasaka T, Launey T, Chui DH, Tanemura K, Sato S, et al. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease. J Neurosci. 2004;24:2401–11. doi: 10.1523/JNEUROSCI.5561-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Yasutake K, Fujita SC, Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001;276:34298–306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- Salmon E, Maquet P, Sadzot B, Degueldre C, Lemaire C, Franck G. Decrease of frontal metabolism demonstrated by positron emission tomography in a population of healthy elderly volunteers. Acta Neurol Belg. 1991;91:288–95. [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann Neurol. 1994;35:546–51. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Smith GS, de Leon MJ, George AE, Kluger A, Volkow ND, McRae T, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer's disease. Pathophysiologic implications. Arch Neurol. 1992;49:1142–50. doi: 10.1001/archneur.1992.00530350056020. [DOI] [PubMed] [Google Scholar]
- Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, et al. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer's disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–7. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Stozicka Z, Zilka N, Novak M. Risk and protective factors for sporadic Alzheimer's disease. Acta Virol. 2007;51:205–22. [PubMed] [Google Scholar]
- Su B, Wang X, Drew KL, Perry G, Smith MA, Zhu X. Physiological regulation of tau phosphorylation during hibernation. J Neurochem. 2008;105:2098–108. doi: 10.1111/j.1471-4159.2008.05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp Neurol. 2001;168:402–12. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- Zhou XW, Gustafsson JA, Tanila H, Bjorkdahl C, Liu R, Winblad B, et al. Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol Dis. 2008;31:386–94. doi: 10.1016/j.nbd.2008.05.013. [DOI] [PubMed] [Google Scholar]