Abstract
Retinal lesions caused by eye diseases such as glaucoma and age-related macular degeneration can, over time, eliminate stimulation of parts of the visual cortex. This could lead to degeneration of inactive cortical neuronal tissue, but this has not been established in humans. Here, we used magnetic resonance imaging to assess the effects of prolonged sensory deprivation in human visual cortex. High-resolution anatomical magnetic resonance images were obtained in subjects with foveal (age-related macular degeneration) and peripheral (glaucoma) retinal lesions as well as age-matched controls. Comparison of grey matter between patient and control groups revealed density reductions in the approximate retinal lesion projection zones in visual cortex. This indicates that long-term cortical deprivation, due to retinal lesions acquired later in life, is associated with retinotopic-specific neuronal degeneration of visual cortex. Such degeneration could interfere with therapeutic strategies such as the future application of artificial retinal implants to overcome lesion-induced visual impairment.
Keywords: macular degeneration, glaucoma, visual field, visual cortex, voxel-based morphometry, grey matter density
Introduction
Age-related macular degeneration (AMD) and glaucoma, eye diseases associated with the occurrence of visual field defects, are the two leading causes of visual impairment in the developed world (Resnikoff et al., 2004). AMD is caused by accumulated waste products in the tissues underneath the macula that interfere with retinal metabolism and lead to retinal atrophy (Holz et al., 2004; Zarbin, 2004). The disease causes field defects that are located in or near the central visual field. In glaucoma, progressive retinal ganglion cell loss and optic nerve damage occurs, in most cases induced by an elevated intra-ocular pressure (Fechtner and Weinreb, 1994; Nickells, 1996). Visual field deterioration typically starts peripherally and progresses towards the fovea. Because of the retinotopic organization of the visual cortex (Dougherty et al., 2003; Horton and Hoyt, 1991), when field defects occur in both eyes and overlap, the corresponding part of visual cortex is no longer stimulated. An absence of stimulation may result in changes in the cortical structure (Johansson, 2004; Merzenich et al., 1984). This makes it relevant to ask whether field defects that have their origin at the level of the retina can lead to deterioration in the structure of the occipital cortex.
Indeed, there is evidence that developmental visual disorders such as amblyopia (Mendola et al., 2005) and albinism (von dem Hagen et al., 2005) affect the structure of the human occipital cortex. However, surprisingly little is known about the consequence of visual deprivation later in life. A recent case study showed degenerative changes in the visual cortex of a glaucoma patient based on post-mortem examination (Gupta et al., 2006). Previously, using magnetic resonance imaging (MRI), Kitajima and colleagues had reported wider calcarine sulci in a small group of patients with a variety of retinal pathology indicating a possible link between visual field defects and cortical degeneration (Kitajima et al., 1997). In another study, compared with healthy participants, subjects with normal-tension glaucoma, but not those with primary open-angle glaucoma, showed more pathological findings (ischemia) in sulci, fissures, subarachnoid spaces and ventricles (Acaroglu et al., 2001). However, the status of the grey matter was not examined. Finally, using MRI, in glaucoma patients a reduced size of the lateral geniculate nuclei was reported (Gupta et al. 2009).
In the present article, we determined whether structural changes in human visual cortex occur once disease of the eye has resulted in an established homonymous visual field defect. This question is also relevant given conflicting reports on the presence of functional occipital reorganization following retinal lesions (Baker et al., 2005; Masuda et al., 2008; Smirnakis et al., 2005; Sunness et al., 2004).
In the present study, visual field measurements were used to chart changes in visual sensitivity associated with the retinal visual field defects from AMD and glaucoma. MRI and subsequent voxel-based morphometric analysis methods were used to selectively assess the presence of any associated changes in grey matter density in the two groups.
Materials and Methods
Subjects
Subjects with visual field defects were recruited among the patient population of the Department of Ophthalmology of the University Medical Centre Groningen (Groningen, The Netherlands) and through advertizements in magazines of patient associations. The group consisted of nine patients suffering from AMD (two females and seven males; mean age 73 years, range 51–82 years), and eight patients with primary open-angle glaucoma (one female and seven males; mean age 72 years, range 61–85 years).
AMD was defined as the presence of a decreased visual acuity in a patient with abnormalities in the macular area without any other explanation. Abnormalities might be either drusen, hyper- or de-pigmentation, geographic atrophy or signs of previous exudative changes. A glaucoma patient was defined as a patient with a reproducible visual field defect on conventional perimetry. The defects had to be compatible with glaucoma and without other explanation. Optic disc excavation had to be in line with glaucoma as well.
In addition, in both AMD and glaucoma groups, patients had to have a homonymous scotoma of at least 10 degrees diameter in at least one quadrant, for a minimum of 3 years. Patients with any other (neuro-) ophthalmic disease that might affect the visual field were excluded.
Homonymous visual fields defect in both groups were located centrally or paracentrally. In glaucoma, the visual field defect generally starts paracentrally, progresses peripherally and finally expands towards central parts in the last stage of the disease. This difference in location and severity of visual field losses is reflected in the visual acuity (logMAR; logarithmic minimum angle of resolution) and in the mean deviation (MD) scores of both groups. LogMAR is a logarithmic transformation of the more common clinically used decimal notation. A logMAR value of 0 corresponds to a visual acuity of 1.0 in decimal notation. More positive numbers indicate that the minimum angle of resolution is larger, and thus corresponds to a lower visual acuity. The MD is the weighted average decrease in visual field sensitivity relative to the norm for a particular age group. Negative values indicate a reduced sensitivity. Table 1 lists these characteristics.
Table 1.
Subject characteristics
| Subject | Group | Age | Visual acuity of the right eye (logMAR) | Visual acuity of the left eye (logMAR) | Visual field sensitivity of the right eye (MD; dB) | Visual field sensitivity of the left eye (MD; dB) |
|---|---|---|---|---|---|---|
| 1 | AMD | 74 | 1.8 | 1.3 | n/a | −7.57 |
| 2 | AMD | 67 | 0.5 | 0.1 | −5.5 | −4.50 |
| 3 | AMD | 62 | 1.3 | 0.7 | −11.48 | −2.69 |
| 4 | AMD | 82 | 1.1 | 1.0 | −6.28 | −4.96 |
| 5 | AMD | 78 | 1.0 | 1.8 | −3.51 | −7.04 |
| 6 | AMD | 81 | 1.1 | 0.7 | −6.06 | −3.64 |
| 7 | AMD | 76 | 0.5 | 0.8 | −5.79 | −2.64 |
| 8 | AMD | 51 | 1.0 | 1.0 | −12.01 | −14.08 |
| 9 | AMD | 82 | 0.5 | 0.4 | −3.43 | −2.20 |
| Mean | AMD | 72.6 | ||||
| 10 | POAG | 66 | 0.1 | 0.1 | −25.08 | −22.96 |
| 11 | POAG | 69 | 0.1 | 0.1 | −27.20 | −13.82 |
| 12 | POAG | 85 | 0.0 | 1.0 | −8.80 | −24.59 |
| 13 | POAG | 80 | 0.2 | 0.1 | −5.23 | −16.15 |
| 14 | POAG | 64 | l.p. | 0.7 | l.p. | −14.45 |
| 15 | POAG | 61 | 0.05 | 0.05 | −11.62 | −3.67 |
| 16 | POAG | 74 | 0.0 | 0.1 | −23.28 | −18.27 |
| 17 | POAG | 79 | 0.1 | 0.15 | −6.36 | −13.41 |
| Mean | POAG | 72.3 |
Characteristics of the two patient groups (AMD and glaucoma) were visual acuity for one or both eyes (expressed in logMAR), visual sensitivity (expressed as MD in dB) and age. logMAR = logarithm of minimum angle of resolution; MD = mean deviation; dB = decibel; AMD = age-related macular degeneration; POAG = primary open-angle glaucoma; l.p. = light perception; n/a = not available.
For the control group, 12 healthy age-matched subjects (three females and nine males; mean age 66 years, range 60–82 years) were recruited. They were recruited from among the partners of the visual field impaired participants or via advertisement in a local newspaper. Control subjects were required to have good (or corrected to good) visual acuity (logMAR ≤ 0), not to have any visual field defect and to be free of any ophthalmic, neurologic or general health problem.
This study conformed to the tenets of the Declaration of Helsinki and was approved by the medical review board of the University Medical Center Groningen (Groningen, The Netherlands). All participants gave their informed written consent prior to participation.
Visual field measurements
Visual fields were recorded using the Humphrey Field Analyser (Carl Zeiss Meditec, Dublin, CA, USA) running the 30-2 program SITA (Swedish Interactive Threshold Algorithm)-Fast, a standard method for the examination of the 30° central visual field. In this type of measurement, the subject is facing a white illuminated sphere, on which points of light with varying intensities are briefly flashed. The subject has to fixate a set of centrally positioned fixation points during the measurement. Stimuli are presented one by one on a grid in the central 30° of the visual field. Subjects respond when they perceive the flash. The sensitivity at each location in the visual field is determined by changing the intensity of the flash on subsequent presentations. Each eye is measured independently, one eye is covered while the other is tested.
To assess the relationship between the reduction in visual field sensitivity and changes in cortical grey matter density, for each group, mean sensitivity deviation maps were calculated. This was done in two steps. First, for each subject, a combined visual field map was created by taking, at each position, the smallest sensitivity deviation from the left and right eye's monocular measurements. Second, the combined maps from all subjects in a group were averaged to yield an average binocular sensitivity deviation map.
Magnetic resonance imaging
High-resolution MRI was performed on a 3.0 Tesla Philips Intera (Eindhoven, The Netherlands). A three-dimensional structural image was acquired on each subject using a T1-weighted magnetization sequence T1W/3D/TFE-2, 8° flip angle, repetition time 8.70 ms, matrix size 256 × 256, field of view 230 × 160 × 180, yielding 160 slices and voxel size 1 × 1 × 1 mm3.
Voxel-based morphometric analysis
We performed voxel-based morphometry analysis (Ashburner and Friston, 2000), which is part of the SPM5 (Statistical Parametric Mapping) software (Wellcome Department Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Voxel-based morphometry statistically assesses local changes in grey matter density between groups of anatomical scans.
The brain images were registered to the International Consortium for Brain Mapping space template. Using the standard segmentation protocol in SPM5, each voxel was classified into one of the three different tissues: grey matter, white matter and cerebrospinal fluid. Non-brain voxels were excluded from the statistical analysis by applying a brain mask. Finally, the images resulting from the segmentation were smoothed with a Gaussian kernel of 10 mm full-width at half-maximum.
For the statistical analysis, two contrasts were defined: the first comparing grey matter density in the AMD group and the control group and the second comparing grey matter density in the glaucoma group and the control group. Even though the participant groups were age-matched, there were some slight differences in the age distribution. The subjects’ ages were therefore added as a covariate to the analysis as an additional measure to control for any potential remaining effect of age.
Volume-of-interest-based analysis
In addition to the voxel-based morphometric analysis, an anatomical volume-of-interest (VOI) analysis was performed. For this analysis, in each individual participant, 21 mm diameter sphere VOIs were defined at the location that corresponded to the approximate expected anatomical position of the foveal and peripheral visual field representations. In each hemisphere, posterior (at the occipital pole; foveal projection zone) and anterior (∼10 mm posterior to the junction of parieto-occipital and calcarine sulci) VOIs were defined in both the superior and inferior banks of the calcarine sulcus. Mean grey matter density values were extracted from each subject's grey matter for each VOI and averaged over groups. For each VOI, relative grey matter densities were calculated for the AMD and glaucoma group by dividing the mean grey matter values by those of the control group. Analysis of variance was used to examine the interaction between the factors group (AMD and glaucoma) and cortical location (posterior and anterior).
Results
Binocular visual fields
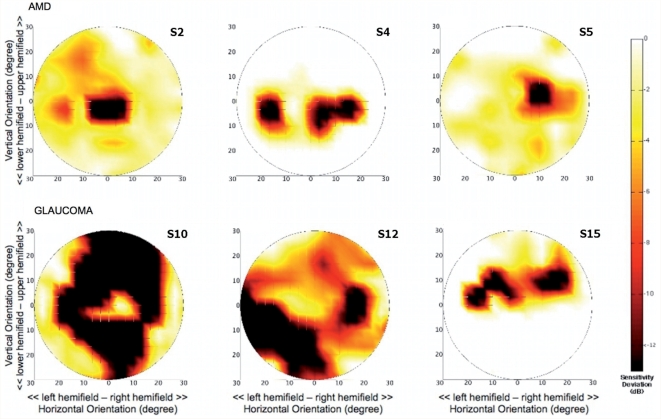
Figure 1 shows the binocular sensitivity deviaton maps for six representative subjects, three from each group. On the basis of the individual maps, for each group a mean binocular sensitivity deviaton map was calculated. These mean maps are shown in Figures 2D and 3D.
Figure 1.
Individual binocular sensitivity deviation maps. Upper row shows maps for three representative subjects from the AMD group, the lower row shows maps for three subjects with glaucoma. Subject numbers correspond to those in Table 1.
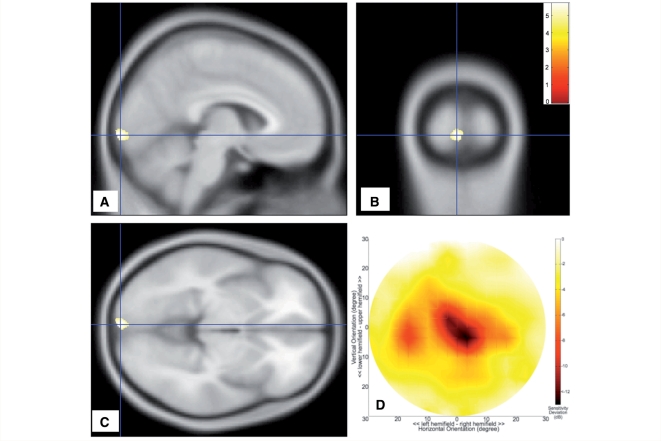
Figure 2.
Analysis of subjects with AMD. (A–C) Sections of the brain showing regions of grey matter density reduction. Colours indicate statistical significance (t-values), with red indicating more significant changes, and yellow/green indicating less significant changes (all changes are at least P < 0.001, uncorrected). (D) Mean visual field sensitivity deviation (in dB). The largest change in sensitivity is located centrally in the visual field. dB = decibel.
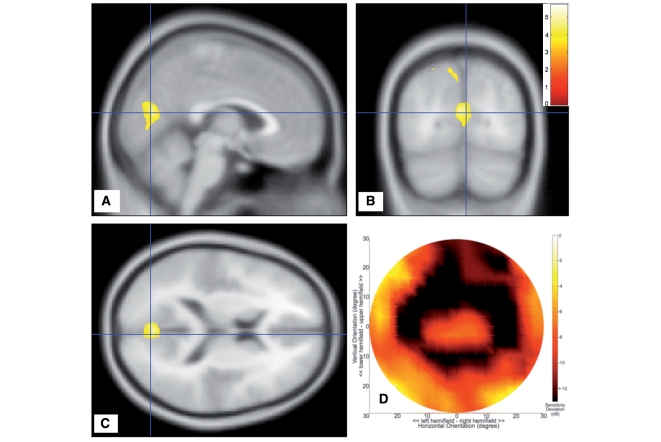
Figure 3.
Analysis of subjects with glaucoma. (A–C) Sections of the brain showing the regions of grey matter density reduction. Colours indicate statistical significance (t-values), with red indicating more significant changes, and yellow/green indicating less significant changes (all changes are at least P < 0.001, uncorrected). (D) Mean visual field sensitivity deviation (in dB). The largest changes in sensitivity are located peripherally in the visual field, leaving the macular region relatively unaffected. dB = decibel.
Age-related macular degeneration
Figure 2D shows the mean visual field sensitivity deviation map for the AMD group. A reduction in sensitivity is evident centrally on and near the location of the fovea (< −6 dB). Figure 2A–C shows the comparison of grey matter density in the AMD group compared with controls superimposed on the mean anatomical image of all subjects. In subjects with AMD, grey matter density is reduced compared with the control group. This main reduction in density is located near the occipital pole (primarily in the left hemisphere), particularly around the posterior part of the calcarine fissure (P < 0.001).
Glaucoma
Figure 3D indicates that in the glaucoma group mean visual field sensitivity is markedly reduced in the periphery of the visual field, in both the upper and lower hemifields (< −8 dB). Importantly, in contrast to the AMD group, the sensitivity is relatively spared in the macular region. Figure 3A–C shows the comparison of grey matter density between the glaucoma and control groups. The results indicate the presence of a bilateral reduction of grey matter density on the medial aspect of the occipital lobe, at the anterior half of the calcarine fissures (P < 0.001).
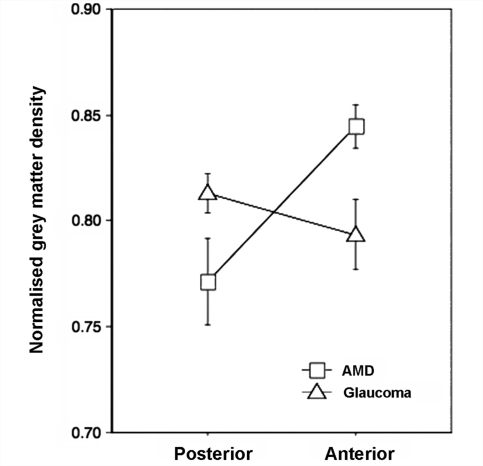
Figure 4 shows the results of an additional volume-of-interest-based analysis. For this analysis, on each participant's brain, small sphere VOIs were defined that correspond to the approximate anatomical projection zones of the fovea and peripheral visual field. For each participant group, average relative grey matter density in the anterior and posterior VOIs is plotted. This analysis confirms the results of the voxel-based morphometric analysis. In the AMD group, the relative grey matter density is more reduced in the posterior than in the anterior region. The glaucoma group shows exactly the opposite result. Relative grey matter density is more reduced in the anterior than in the posterior region. This interaction was statistically significant (P < 0.01).
Figure 4.
Results from a VOI-based analysis. Relative change in grey matter density for the AMD and glaucoma groups compared to the control group in anatomically defined volumes of interest in posterior (approximate foveal projection zone) and anterior (approximate peripheral visual field projection zone) visual cortex. Relative changes were calculated by dividing for each participant group the averaged grey matter density in each VOI by that of the control group.
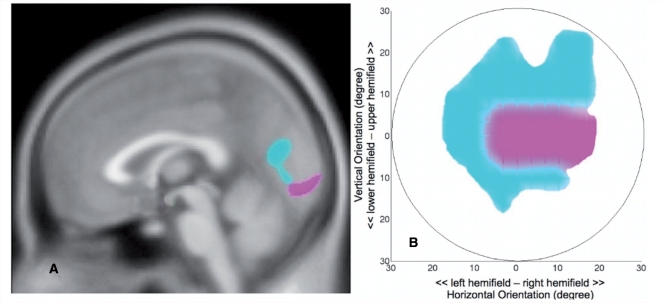
Figure 5 summarizes the main results of this study, and shows the pattern of decrements in grey matter density in the glaucoma and AMD groups, as well as their respective (thresholded) sensitivity deviation maps. Retinotopically, the pattern of grey matter reduction that we find in our study correlates well with the pattern of changes in visual field sensitivity in both types of pathology. In AMD, the reduction in visual field sensitivity is most pronounced in the foveal region (Fig. 5A). Corresponding with this, the focus of grey matter reduction in this group is located near the posterior pole of the occipital cortex (Fig. 5A). In glaucoma, the main reductions in visual field sensitivity are located more peripherally (Fig. 5B). Consistent with this, the focus of the grey matter reduction in this group is located more anterior in the occipital cortex (Fig. 5A).
Figure 5.
Summary of visual field sensitivity and grey matter reduction in AMD and glaucoma. (A) Cortical grey matter density reduction in glaucoma is found in the anterior half of the medial occipital cortex (cyan). Cortical grey matter density reduction in AMD (magenta) is found in the posterior part of the occipital cortex. (B) Thresholded mean visual field sensitivity deviation maps indicating central defects in AMD (magenta) and more peripheral defects in glaucoma (cyan). For this thresholded map, for the glaucoma group, sensitivity deviations below −12 dB are shown in cyan. For the AMD group, sensitivity deviations below −8 dB are shown in magenta.
Discussion
The main finding of this study is that visual field defects caused by long-standing retinal pathology due to glaucoma and AMD are associated with reductions in grey matter density in occipital cortex. Moreover, in both the AMD- and glaucoma-participant groups, the location of the grey matter density reduction corresponded with the approximate visual field defect projections in visual cortex. The more central scotoma of the AMD subjects correlated with a reduction located more posteriorly in occipital cortex, in correspondence with the location of the foveal representation in visual cortex. The difference was more pronounced in the left hemisphere. In agreement, the sensitivity deviation map shows a macular defect that was more pronounced in the right visual field. The more peripherally located scotoma in the glaucoma subjects correlated with a reduction of grey matter density located more anterior in occipital cortex. This is in correspondence with the location of the cortical representation of the peripheral visual field. Therefore, our results suggest that retinal visual field defects acquired later in life can lead to retinotopically specific grey matter density reduction in the visual cortex.
An earlier report on this study (Boucard, 2006) found no significant difference in grey matter density between the AMD and the control groups. The analysis carried out in this preliminary report used SPM99 rather than SPM5. The difference in results can be attributed to improvements in the segmentation process where SPM5 reaches a more detailed segmentation than SPM99. The segmentation process in SPM5 takes advantage of a spatial normalization that does not rely on the simple relationship between the intensities of two images. The segmentation is achieved by warping tissue probability maps in order to overlay them on to the image to segment. The obtained spatially normalized tissue class images of each subjects are averaged and used as prior probabilities for each voxel to belong to a particular tissue class (Ashburner and Friston 2005). Since this procedure is more refined and accurate, we report here the results obtained with SPM5. Nevertheless, we believe that discrepancies in results originating from differences in analyses should be considered serious issues.
Our present results indicate a good correspondence between the locations of the mean scotoma and the cortical regions showing a reduction in grey matter density. When examining the results at a more detailed level, however, a number of factors may contribute to uncertainty (and thus deviations) in the correspondence. First, we rely on normalization of the brains of the subjects. Normalization, particularly in occipital cortex, is delicate due to anatomical variability. Second, there will be differences in how the functional visual field maps onto the anatomy. Third, we show the grey matter density reductions on the mean anatomical image of all participants. Each participant's contribution to the average anatomy is equal. Yet, the contribution to the group's focus on the density reduction is not necessarily equal for each participant. Fourth, the extent of the cortical regions showing a reduction varies with the statistical threshold used. Finally, it is not a priori clear when (in terms of sensitivity deviation and diameter) a retinal scotoma will result in a reduction of grey matter density. We have here, for both the participant selection and analysis of the visual field, made the assumption that this is only in the case of relatively large, homonimous scotoma. For this reason, we computed the binocular maps for individual participants using the minimum deviation in sensitivity and averaged these. Yet, grey matter density reduction may occur for monocular defects as well. A more extensive analysis including patients with different (also monocular) field defects would be required to indicate precisely how the depth and extent of the retinal lesion is related to changes in grey matter density and functional anatomy of the visual cortex.
This study involved older participants, so the possibility that the reported changes are simply due to ageing effects should be considered. Salat et al. (2004) showed that cortical thinning due to ageing might involve the primary visual cortex among other cortical areas, although the correlation with age was only weak. Raz et al. (2005) confirmed that regional volume change due to ageing in the visual cortex is very weak. To control for possible ageing effects, we ensured that the groups were, on average, age-matched. Our volume-of-interest-based analysis indicates a slight overall reduction in grey matter density in both patient groups compared with the control group, in addition to the specific scotoma-related reductions. In the voxel-based comparisons of cortical grey matter density we included age as a covariate to remove any age-related residual influences. Hence, we are confident that the present results are not merely due to age. The generalized reduction in grey matter density observed in the VOI-based analysis could be due to variability in the field defects. The defects in both patient groups, although primarily centrally located for AMD and more peripherally for glaucoma patients, do show some spreading between individual subjects (Fig. 1). Grey matter density reduction related to field defects that vary between the individual members of a patient group could show up as a generalized effect. Nevertheless, for each patient group the location of the focus of the grey matter density reduction clearly corresponds to the expected lesion projection zone of mean visual field defect (Figs. 2, 3 and 5).
We believe this to be the first report that establishes a firm relationship between acquired retinal visual field loss and grey matter degeneration in human visual cortex. An earlier MRI study had suggested calcarine atrophy to be related to retinal degeneration (Kitajima et al., 1997). However, the participants in this study had not only widely varying retinal pathology but also relatively coarse measures of degeneration (width of the calcarine sulcus). Since no segmentation of grey and white matter was performed, the findings may not be due to grey matter degeneration. A post-mortem study reported to have found a reduction of the cortical ribbon thickness in the visual cortex in glaucoma. However, this study reported on anatomical and histological examination of a single subject only (Gupta et al., 2006).
In glaucoma, cortical degeneration can be interpreted as a result of the loss of retinal ganglion cells. The atrophy from damaged parts of the retina likely propagates by means of transneuronal degeneration through the optic nerve towards the cortex, thereby provoking its subsequent degeneration. Experimentally, this sequence of events has been shown to occur in cats and monkeys where the retina was experimentally injured by an induced elevated intra-ocular pressure (Yucel et al., 2003).
In AMD, the relationship between retinal pathology and cortical degeneration might be slightly more indirect, in that photoreceptor damage may first lead to retinal ganglion cell loss. It has been shown that the retinal ganglion cell count is significantly lower in wet AMD than in otherwise healthy eyes (Medeiros and Curcio, 2001). Eyes with geographic atrophy due to AMD also have a lower count of retinal ganglion cells (Kim et al., 2002). In agreement with the idea that cortical atrophy is linked to a reduction in retinal ganglion cell count and optic nerve damage, a voxel-based morphometry study reported abnormally reduced grey matter volume at the occipital poles of a group of human albinos (von dem Hagen et al., 2005). There is no direct evidence demonstrating that retinal ganglion cell loss occurred in our AMD group. However, a reduction in visual field sensitivity is linked to retinal ganglion cell loss and not to other components of the neuro-retina (Garway-Heath et al., 2000; Swanson et al., 2004). Moreover, foveal sensitivity is greatly reduced in AMD. Together, this suggests the possibility that retinal ganglion cell loss does occur in AMD-associated visual field defects. This loss could be responsible for the cortical degeneration we have observed.
A number of studies have examined whether functional reorganization accompanies retinal lesions. Thus far, reports have presented conflicting results with some groups reporting an absence of reorganization (Smirnakis et al., 2005; Sunness et al., 2004) and others reporting its presence (Baker et al., 2005). This difference in findings has recently been attributed to differences in stimuli and tasks used in the various studies (Masuda et al., 2008). More cognitively engaging tasks appear to result in feedback signals from later visual areas that also drive neuronal activity in the lesion projection zones. Such feedback signals appear absent in visually healthy subjects. While one could argue that this indicates a form of functional reorganization, it leaves open the question whether the lesion projection zone of primary visual cortex is still able to process retinal signals. This question is particularly relevant given the rapid developments in retinal prostheses. Our study indicates a reduction in grey matter in the approximate lesion projection zones of primary visual cortex. Given that feedback signals still appear to be processed, we speculate that the grey matter reduction reported on here could be selective to the input layers of primary visual cortex.
In summary, this study shows that long-standing visual field defects due to retinal pathology are associated with retinotopic specific grey matter reduction in early visual cortex. These findings contribute to the understanding of brain plasticity at a later age. From a clinical point of view, a better understanding of the relation between retinal visual field defects and structural changes in visual cortex may help understand disease symptoms as well as their progression. For instance, cortical degeneration may limit the efficacy of rehabilitation and training programmes (Safran and Landis, 1996), retinal prostheses (Hossain et al., 2005) and may require new therapeutic strategies (Gupta and Yucel, 2007; Taub et al., 2002) to prevent blindness. Prevention of cortical degeneration associated with eye diseases may also need to become a new therapeutic goal.
Acknowledgements
The first and the second author contributed equally to this study. A.T.H is supported by the ‘RuG Fellowship Program’ and C.C.B. is supported by an Ubbo Emmius grant, both from the University of Groningen, the Netherlands. This work was supported by Uitzicht Stichting Oogheelkundig Onderzoek Nederland and an equipment grant from the Prof. Mulder foundation. The authors thank the BCN Neuroimaging center for the use of their scanner, Hans Hoogduin and Anita Kuiper for assistance in magnetic resonance scan acquisition, Michiel Kunst for assistance in setting up the voxel-based morphometry analysis, Shriprakash Sinha for assistance with the region of interest analysis, Remco Renken for fruitful suggestions regarding data analysis and Martin Pavlovsky for contributing to the discussion.
Glossary
Abbreviations
- AMD
age-related macular degeneration
- dB
decibel
- POAG
primary open-angle glaucoma
- SPM99
Statistical Parametric Mapping analysis program version of 1999
- SPM5
Statistical Parametric Mapping analysis program version of 2005
- VBM
voxel-based morphometry
References
- Acaroglu G, Tali T, Batman A, Sinik B, Oskan S. Comparative study of brain magnetic resonance imagings in normal tension glaucoma, primary open-angle glaucoma, and normal subjects. Neuro-Ophthalmology. 2001;26:103–7. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25:614–8. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard CC. Neuro-imaging of visual field defects. PhD thesis, University of Groningen; 2006. [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3:586–98. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. 1994;39:23–42. doi: 10.1016/s0039-6257(05)80042-6. [DOI] [PubMed] [Google Scholar]
- Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41:1774–82. [PubMed] [Google Scholar]
- Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–8. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Greenberg G, de Tilly LN, Gray B, Polemidiotis M, Yücel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol. 2009;93:56–60. doi: 10.1136/bjo.2008.138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Yucel YH. Should we treat the brain in glaucoma? Can J Ophthalmol. 2007;42:409–13. [PubMed] [Google Scholar]
- Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137:504–10. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991;109:816–24. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Hossain P, Seetho IW, Browning AC, Amoaku WM. Artificial means for restoring vision. Bmj. 2005;330:30–3. doi: 10.1136/bmj.330.7481.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB. Brain plasticity in health and disease. Keio J Med. 2004;53:231–46. doi: 10.2302/kjm.53.231. [DOI] [PubMed] [Google Scholar]
- Kim SY, Sadda S, Humayun MS, de Juan E, Melia BM, Green WR. Morphometric analysis of the macula in eyes with geographic atrophy due to age-related macular degeneration. Retina. 2002;22:464–70. doi: 10.1097/00006982-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T, et al. MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol. 1997;18:1291–5. [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 Projection zone signals in human macular degeneration depend on task, not stimulus. Cereb Cortex. 2008;18:2483–93. doi: 10.1093/cercor/bhm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NE, Curcio CA. Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:795–803. [PubMed] [Google Scholar]
- Mendola JD, Conner IP, Roy A, Chan ST, Schwartz TL, Odom JV, et al. Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp. 2005;25:222–36. doi: 10.1002/hbm.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Nickells RW. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma. 1996;5:345–56. [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- Safran AB, Landis T. Plasticity in the adult visual cortex: implications for the diagnosis of visual field defects and visual rehabilitation. Curr Opin Ophthalmol. 1996;7:53–64. doi: 10.1097/00055735-199612000-00009. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Brewer AA, Schmid MC, Tolias AS, Schuz A, Augath M, et al. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–7. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–8. doi: 10.1016/j.ophtha.2003.12.050. [DOI] [PubMed] [Google Scholar]
- Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004;45:466–72. doi: 10.1167/iovs.03-0374. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–36. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Houston GC, Hoffmann MB, Jeffery G, Morland AB. Retinal abnormalities in human albinism translate into a reduction of grey matter in the occipital cortex. Eur J Neurosci. 2005;22:2475–80. doi: 10.1111/j.1460-9568.2005.04433.x. [DOI] [PubMed] [Google Scholar]
- Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–81. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]