Abstract
Hearing developmental dyslexics and profoundly deaf individuals both have difficulties processing the internal structure of words (phonological processing) and learning to read. In hearing non-impaired readers, the development of phonological representations depends on audition. In hearing dyslexics, many argue, auditory processes may be impaired. In congenitally profoundly deaf individuals, auditory speech processing is essentially absent. Two separate literatures have previously reported enhanced activation in the left inferior frontal gyrus in both deaf and dyslexic adults when contrasted with hearing non-dyslexics during reading or phonological tasks. Here, we used a rhyme judgement task to compare adults from these two special populations to a hearing non-dyslexic control group. All groups were matched on non-verbal intelligence quotient, reading age and rhyme performance. Picture stimuli were used since this requires participants to generate their own phonological representations, rather than have them partially provided via text. By testing well-matched groups of participants on the same task, we aimed to establish whether previous literatures reporting differences between individuals with and without phonological processing difficulties have identified the same regions of differential activation in these two distinct populations. The data indicate greater activation in the deaf and dyslexic groups than in the hearing non-dyslexic group across a large portion of the left inferior frontal gyrus. This includes the pars triangularis, extending superiorly into the middle frontal gyrus and posteriorly to include the pars opercularis, and the junction with the ventral precentral gyrus. Within the left inferior frontal gyrus, there was variability between the two groups with phonological processing difficulties. The superior posterior tip of the left pars opercularis, extending into the precentral gyrus, was activated to a greater extent by deaf than dyslexic participants, whereas the superior posterior portion of the pars triangularis extending into the ventral pars opercularis, was activated to a greater extent by dyslexic than deaf participants. Whether these regions play differing roles in compensating for poor phonological processing is not clear. However, we argue that our main finding of greater inferior frontal gyrus activation in both groups with phonological processing difficulties in contrast to controls suggests greater reliance on the articulatory component of speech during phonological processing when auditory processes are absent (deaf group) or impaired (dyslexic group). Thus, the brain appears to develop a similar solution to a processing problem that has different antecedents in these two populations.
Keywords: inferior frontal gyrus, deaf, dyslexia, rhyming, phonology
Introduction
It is well established that knowledge about the internal structure of a word (phonological awareness) is an important correlate of learning to read in hearing children (e.g. Goswami and Bryant, 1990). Moreover, it has been argued that auditory processing skills underpin these phonological skills since they are good longitudinal predictors of phonological development in pre-schoolers (Corriveau et al., 2009). Hearing children with developmental dyslexia, by definition, have difficulties in learning to read and there is consensus in the literature that they have specific problems with phonological representations and processing (e.g. Ziegler and Goswami, 2005). In addition, there is increasing evidence suggesting that their phonological processing difficulties arise from impaired auditory processing, particularly of suprasegmental cues such as amplitude envelope structure (Goswami et al., 2002; Rocheron et al., 2002; Richardson et al., 2004; Hämäläinen et al., 2005, 2009; Boets et al., 2008; Thomson and Goswami, 2008). These auditory processing and associated phonological difficulties do not ameliorate with age (Thomson et al., 2006; Pasquini et al., 2007). Thus, growing evidence highlights the importance of auditory processing to phonological development in hearing children, especially those with developmental dyslexia.
Children born profoundly deaf also have difficulty learning to read (e.g. Nielsen and Luetke-Stahlman, 2002). Inability to access spoken language makes reading very difficult. Due to the importance of phonological processing to reading development in hearing children, there has been a particular focus on phonological processing of spoken language in deaf children and adults in relation to reading skills (e.g. Perfetti and Sandak, 2000). Unsurprisingly, numerous studies have identified phonological processing deficits in those born profoundly deaf compared to hearing peers (e.g. Miller, 1997; James et al., 2005, 2008; Kyle and Harris, 2006). Nevertheless, many studies report above-chance performance by deaf participants on phonological tasks (e.g. Campbell and Wright, 1988; Miller, 1997; Sterne and Goswami, 2000; Dyer et al., 2003). Some of these studies have also reported a relationship between phonological skills and reading (e.g. Campbell and Wright, 1988; Harris and Beech, 1998; Dyer et al., 2003).
The fact that congenitally profoundly deaf readers can perform spoken language phonological tasks at an above-chance level suggests that they gain knowledge about phonological structure from modalities other than audition. Information may be derived from visual input in the form of orthography. For example, deaf children find it easier to decide that words rhyme when they are spelled the same (e.g. cat–mat) than when they are not (e.g. wine–sign), even when the stimuli are presented as pictures (e.g. Sterne and Goswami, 2000). This effect has also been shown for aurally presented words in hearing adults (Seidenberg and Tanenhaus, 1979; Ziegler et al., 2004) and children (Goswami et al., 2005). Visual information about the phonological structure of speech may also be derived from speech-reading. A number of studies have shown a positive correlation between speech-reading and reading skill in deaf children and adults (Campbell and Wright, 1988; Harris and Moreno, 2006; Kyle and Harris, 2006; Mohammed et al., 2006). This same pattern has also been reported in hearing dyslexics (Mohammed et al., 2006). Such studies suggest that phonological representations may best be thought of as supramodal or amodal (Hanson, 1989; Liberman and Shankweiler, 1991; Fowler, 2004; MacSweeney et al., 2008).
Of particular relevance to the study reported here, information about the phonological structure of spoken words may also be derived from articulation. ‘Chair’ and ‘bear’ not only sound the same; the motor representation to produce the rime of the word is the same. In hearing children and adults, articulatory suppression (silently repeating an irrelevant word) can interfere with phonological decisions such as rhyme judgement (Besner et al., 1981; Wilding and White, 1985; Johnston and McDermott, 1986; Arthur et al., 1994). Although not involving a phonological judgement task, short-term memory for pictures in deaf children is also disrupted by articulatory suppression (MacSweeney et al., 1996). Thus, studies involving articulatory suppression suggest that phonological processing is impaired, in both deaf and hearing individuals, when the neural networks that support articulatory processes are otherwise engaged. Neuroimaging data support the proposal that articulatory processes/representations may be especially important during phonological tasks in those born profoundly deaf and those with developmental dyslexia.
The left dorsal inferior frontal gyrus (IFG) is reliably recruited during phonological tasks in hearing readers (Sergent et al., 1992; Poldrack et al., 1999; Kareken et al., 2000; Lurito et al., 2000; Xu et al., 2001; Seghier et al., 2004; Burton et al., 2005; Gough et al., 2005; Booth et al., 2007). We found enhanced activation of left dorsal IFG in deaf, compared to hearing, adults during a picture rhyme judgement task (MacSweeney et al., 2008). Participants were presented with two pictures (e.g. chair–bear) and had to judge whether or not they rhymed. This was contrasted with a ‘same–different’ picture judgement task. Deaf participants recruited the left dorsal IFG to a greater extent than hearing participants even when the groups were matched on rhyme performance, reading level, non-verbal IQ, and numerous other behavioural characteristics. A similar pattern has been reported in response to written word stimuli (Aparicio et al., 2007). Given the involvement of the left dorsal IFG in articulation (e.g. Ojemann and Mateer, 1979; Fiez and Petersen, 1998), we argued that enhanced recruitment of this region was due to increased reliance on the articulatory component of speech when auditory input is absent (MacSweeney et al., 2008).
The left dorsal IFG has also been a focus of the literature exploring the neural basis of phonological processing in developmental dyslexics. Enhanced activation of the left IFG in dyslexic adults compared to controls has been reported during written non-word rhyming (Shaywitz et al., 1998) and reading aloud (Brunswick et al., 1999; see also Rumsey et al., 1997 for enhanced activation in left insular cortex). This enhanced activation is typically accounted for in terms of greater reliance on articulatory processes when phonological processing is somehow impaired (see Pugh et al., 2005).
The aim of the current study was to contrast the neural systems supporting a phonological judgement task in three groups of adults: congenitally profoundly deaf, hearing compensated dyslexics, and hearing non-dyslexics. Different literatures have identified the left IFG as showing enhanced activation in deaf and dyslexic readers in contrast to hearing non-dyslexics. By testing these three groups of participants on the same task, we addressed whether these literatures had identified altered activation levels in the same or different regions in both deaf and dyslexic groups. If similar regions are enhanced in both groups, this may suggest that similar compensatory strategies are used when phonological processing skills are poor. Critically, all three groups were good readers and were well matched on numerous behavioural characteristics, including reading level and rhyme performance. As validation that both dyslexic and deaf participants had residual phonological processing difficulties, both groups performed significantly worse than hearing controls on a test of initial phoneme judgement (e.g. gin–jet, see Methods section). Given that the data reported here from deaf and non-dyslexic participants are a subset of those reported previously (MacSweeney et al., 2008), we anticipated that the deaf subgroup would show greater activation in left dorsal IFG than the hearing non-dyslexic subgroup. Of interest, was whether activation in the dyslexic group would differ to that seen in non-dyslexics and, if so, whether the differential pattern was similar to that observed between deaf and hearing non-dyslexics.
Methods
Participants
Three groups of seven participants were contrasted. Participants were either deaf without dyslexia, hearing with dyslexia or hearing without dyslexia. These groups shall be referred to as ‘deaf’, ‘dyslexic’ and ‘hearing’, respectively. The small sample size is due to the difficulty in recruiting from the deaf and dyslexic populations such that groups are well matched.
Deaf and hearing participants were selected from a larger set of participants, whose data on this task we have reported previously (MacSweeney et al., 2008). All were right-handed and had normal or corrected-to-normal vision. All participants gave informed, written consent to participate in the study, which was approved by the Institute of Psychiatry/South London and Maudsley NHS Trust Research Ethics Committee.
All deaf participants reported being born profoundly deaf. Audiograms obtained at the time of testing confirmed that each deaf participant had a mean hearing loss greater than 92 dB in the better ear over four octaves, spanning 500–4000 Hz. Three of the seven deaf participants reported current daily use of hearing aids. All deaf participants encountered written English upon entering primary school, aged four or five. Four of the deaf participants had deaf, signing parents. The remaining three had hearing parents. All had attended ‘oral’ schools in which spoken English was the main form of communication. To enable close matching between the groups, the deaf participants included in this study were all good readers (mean reading age = 17 years 8 months), in comparison to the mean reading level for the deaf population (∼9/10 years; see Conrad, 1979; Allen, 1986; Holt, 1993).
All dyslexic participants had received a diagnosis of developmental dyslexia from either an educational psychologist or a speech and language therapist. The dyslexic participants in our study matched the profile of ‘compensated’ dyslexics in that all attained good reading levels when tested in adulthood. The mean reading age of the group was 17 years 11 months. The dyslexic participants had attained good levels of education and performed at near-ceiling on a test of picture rhyme judgment performed outside the scanner (Table 2). However, evidence of their dyslexia was apparent in their performance on a phoneme awareness task performed in a session prior to the scan. In that task, participants were required to judge whether two pictures shared the same initial phoneme (e.g. gin–jug); clustered onsets were also tested (e.g. clown–kick). Dyslexic participants were poorer on this task than the rhyme task and were significantly poorer than hearing controls (see Table 1 and below for group contrasts).
Table 2.
Mean (SD) accuracy (max = 30) and reaction times on ‘rhyme’ and ‘same picture’ tasks for each group
| Same? |
Rhyme? |
|||
|---|---|---|---|---|
| Acc. | RT (s) | Acc. | RT (s) | |
| Deaf | 29.6 (0.54) | 1.29 (0.23) | 26.6 (2.64) | 2.64 (0.42) |
| Dyslexic | 29.4 (0.54) | 1.30 (0.25) | 28.0 (1.53) | 2.41 (0.35) |
| Hearing | 29.0 (1.53) | 1.30 (0.42) | 27.3 (1.50) | 2.45 (0.52) |
There were no group differences in task performance (see text).
Table 1.
Participant characteristics—mean (SD) and range of age, reading age, non-verbal IQ (scaled scores and percentiles), English productive vocabulary score, rhyme and initial phoneme judgement tasks performed out of the scanner
| Age | Reading age | NVIQ (scaled-score) (mean = 10) | NVIQ (percentiles) | Vocabulary (max = 30) | Rhyme (% accurate) | Phoneme (% accurate) | |
|---|---|---|---|---|---|---|---|
| Deaf (n = 7) (male = 3) | 38:04 years (12:04 years) 26:08–54:08 years | 17:08 years (22 months) 15–19:06 years | 12.86 (1.77) 11–15 | 79.6 (15.6) 63–95 | 27.3 (1.98) 24–29 | 90.1 (4.2) 82–94 | 75.2 (13.3) 55–86 |
| Dyslexic (n = 7) (male = 6) | 24:08 years (7:02 years) 18:05–39:08 years | 17:07 years (23 months) 15:04–21:00 years | 13 (2.31) 11–17 | 78.7 (15.7) 63–99 | 28.6 (1.27) 27–30 | 88.9 (5.67) 78–93 | 78.6 (9.9) 61–91 |
| Hearing (n = 7) (male = 5) | 32:07 (8:07 years) 22:01–48:06 years | 17:11 years (21 months) 16–21 years | 12.86 (2.19) 11–16 | 78.1 (14.6) 63–98 | 28.4 (1.51) 26–30 | 91.9 (4.04) 86–99 | 90.4 (6.2) 80–100 |
There were no significant differences between the groups on reading age (Hedderly, 1996), non-verbal IQ (Block Design, WAIS-R), English productive vocabulary (shortened version of the Boston Naming Test, Kaplan and Goodglass, 1983) or a test of picture rhyme judgement performed outside the scanner (all P-values > 0.1; Table 1). The groups did however differ in gender (Table 1) and the difference in age just reached significance [F(2,18) = 3.57, P = 0.049]. The hearing non-dyslexic group and deaf group did not differ in age, but both groups were older than the dyslexic group [t(12) = −2.5, P < 0.05; t(12) = −2.53, P < 0.05, respectively]. There were also group differences on a task of initial phoneme identification, using picture stimuli, performed outside the scanner [e.g., gin–jug; F(2,18) = 4.25, P < 0.05]. There was no significant difference in initial phoneme judgement by deaf and dyslexic participants (P > 0.1). However, both groups were significantly poorer than the hearing participants [t(12) = −2.73, P < 0.025 and t(12) = −2.68, P < 0.025, respectively; Table 1]. These data provide evidence of residual phonological processing difficulties in both the deaf and dyslexic participants, despite their relatively high levels of reading attainment.
Stimuli
Sixty pictures were presented. All depicted highly familiar, high-frequency, monosyllabic words in spoken English. Thirty were from the Snodgrass and Vanderwart (1980) normed picture set. The remaining 30 were selected from a range of standardized language assessments. Fifty-eight of the pictures were black and white line drawings; two colour pictures were included to represent the colours ‘red’ and ‘blue’ (see Appendix 1 in MacSweeney et al., 2008 for stimuli).
Thirty pictures were combined to form 15 rhyming pairs. Although the spoken English labels for the pictures rhymed, orthography was inconsistent in all cases (e.g. tail–whale; chair–bear). This constraint was imposed to ensure that the rhyme decision could not be made on the basis of orthography alone. The ‘no’ trials were established by pairing the remaining 30 items such that there was no phonological overlap (e.g. hat–pig). Items in the rhyming and non-rhyming trials were matched on familiarity, concreteness and frequency (P > 0.1).
The stimuli used in the rhyme condition were also used in the ‘same picture?’ control task. Fifteen of the pictures were presented as identical pairs (e.g. chair–chair). Another 30 pictures were re-paired to form the ‘different picture?’ trials (hat–whale). Thus, of the 60 pictures seen in the rhyme condition, 45 were also presented in the ‘same picture?’ control condition. Adaptation to repeated stimuli may cause a decrease in haemodynamic response (e.g. Henson and Rugg, 2003). To address this, whether an item was first seen in the rhyme or control condition, was counterbalanced such that any repetition effects were balanced across conditions. All participants performed a picture naming pre-test outside the scanner to ensure that they named the pictures with the desired English labels.
Design
In a session prior to the scan, all participants were given a number of different verbal and non-verbal assessments to enable group matching. Participants were assessed on non-verbal IQ (block design, WAIS-R); reading [Kirklees Reading Assessment Schedule (Hedderly, 1996)]; and English vocabulary. English vocabulary was tested using a shortened version of the Boston picture naming test (Kaplan and Goodglass, 1983) during which spoken or finger-spelled responses were accepted from deaf participants. Participants were also tested on rhyme judgement (e.g. suit–boot) and initial phoneme judgement (e.g. king–cat). Both phonological judgement tasks used picture stimuli.
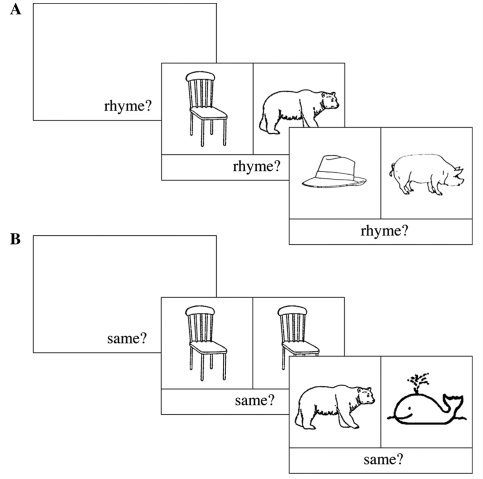
The fMRI run lasted 6 min and consisted of six 30-s blocks of the rhyme task, alternating with six 30-s blocks of the ‘same picture?’ control task (Fig. 1). In the control task, participants were required to decide whether two pictures were the same. In the rhyme task, they were required to decide whether the spoken English labels for two pictures rhymed. Deaf participants had already completed a behavioural study of rhyme awareness as part of a wider project. They were reminded of the concept of rhyme (introduced in the previous session) and were given examples and practice trials prior to the experiment in the scanner. Half the trials in each condition were ‘yes’ trials and half were ‘no’ trials. Subjects indicated their response using a two-choice button box held in their right hand.
Figure 1.
Schematic representation of order of events during the: (A) rhyme; (B) same picture judgement tasks.
A one-syllable task prompt appeared at the bottom of the screen, without a pair of pictures, for 2000 ms at the beginning of each block (‘Rhyme?’–rhyme task; ‘Same?’–picture matching task). This prompt remained on the screen throughout the block to keep participants on-task. Each pair of pictures was presented for 5 s. The inter-stimulus interval was 500 ms. Thus, in each 30 s block, five trials were presented.
fMRI data acquisition and analysis
Imaging parameters
Gradient echo echoplanar MRI data were acquired with a General Electric (Milwaukee, WI, USA) 1.5T Neuro-optimized MR system using a standard quadrature head coil. 120 T2* weighted images depicting BOLD contrast were acquired at each of 38 near-axial 3 mm thick planes parallel to the inter-commissural (AC–PC) line (0.3 mm interslice gap; TR = 3 s, TE = 40 ms). An inversion recovery EPI dataset was also acquired to facilitate registration of individual fMRI datasets to Talairach space (Talairach and Tournoux, 1988). This comprised 43 near-axial 3 mm slices (0.3 mm gap) which were acquired parallel to the AC–PC line (TE = 80 ms, TI = 180 ms, TR = 16 s).
Data analysis
The fMRI data were analysed using an in-house software package (XBAM_v3.2), which uses standard preprocessing steps: realignment, normalization, baseline correction, spatial smoothing, and GLM parameter estimation using a combination of gamma variate basis functions (for details see Brammer et al., 1997; Bullmore et al., 1999, 2001; Suckling and Bullmore, 2004). This analysis method is based on permutation testing and therefore does not assume normality of the fMRI data. Furthermore, first level (within subject) variance is taken into account by using a standardized statistic (SSQ ratio) rather than assuming this to be equal, as is typical in random effects analyses using non-permutation approaches. This approach is especially appropriate for analyses of small groups. For more information about the validity of this approach, see Thirion et al. (2007). The significance level used for each analysis reported here is that necessary to result in less than one false-positive cluster. Therefore, the appropriate significance level to establish this level of control can differ between analyses.
Following motion correction, fMRI data were smoothed using a Gaussian filter (FWHM 7.2 mm) and the least-squares fit computed between the observed time series at each voxel and the convolutions of two one-parameter gamma variate functions (peak responses 4 and 8 s) with the experimental design (Friston et al., 1998). In order to limit the range of fits to those known to reflect the physiological features of BOLD responses, the constraints described by Friman et al. (2003) were applied during the fitting process. The relative weighting of the fits to these two convolutions permits the peak time of BOLD response to adapt to local variations within the time range 4–8 s. Following fitting, a statistic describing the standardized power of response was derived by calculating the ratio between the sum of squares due to the model fit and the residual sum of squares (SSQ ratio). Significant values of this statistic were identified by comparison with its null distribution computed by repeating the fitting procedure twenty times at each voxel after wavelet-based permutation of the time series (Bullmore et al., 2001). This procedure preserves the noise structure of the time-series during the permutation process and gives good control of Type-I error rates. The voxelwise SSQ ratios were calculated for each subject from the observed data and following time-series permutation were transformed into standard space (Talairach and Tournoux, 1988) as described previously (Brammer et al., 1997).
Group analysis
Further analysis was carried out to identify 3D clusters of voxels showing significant responses to the paradigm (Table 3). This was achieved by first thresholding the median voxel-level SSQ ratio maps at a voxelwise false positive probability of 0.01. These ‘activated’ voxels were then assembled into 3D connected clusters and the sum of the SSQ ratios (statistical cluster mass) determined for each cluster. The same procedure was repeated for the median SSQ ratio maps obtained from the wavelet-permuted data to compute the null distribution of statistical cluster masses under the null hypothesis. This distribution could then be used to determine the critical threshold for the cluster mass statistic under the null hypothesis at any required Type-I error level and applied to the observed cluster mass data to determine significantly activated clusters (for details see Bullmore et al., 1999).
Table 3.
Regions activated during rhyme judgement task by each group relative to ‘same picture?’ control task
| L/R | Volume (cm3) | Rhyme task > baseline |
||||
|---|---|---|---|---|---|---|
| X | Y | Z | BA | |||
| Deaf group | ||||||
| Inferior frontal gyrus | L | 12.36 | −40 | 4 | 33 | 6/44 |
| Precuneus/superior parietal lobule | L | 7.01 | −22 | −63 | 50 | 7 |
| Medial superior frontal gyrus/anterior cingulate | – | 5.82 | 4 | 7 | 56 | 6/32 |
| Hearing group | ||||||
| Inferior frontal gyrus | L | 7.87 | −40 | 4 | 33 | 6/44 |
| L | 2.73 | −47 | 22 | 3 | 45 | |
| Precuneus/superior parietal lobule | L | 9.09 | −18 | −78 | 36 | 7 |
| Medial superior frontal gyrus/anterior cingulate | – | 3.23 | 0 | 15 | 50 | 6/32 |
| Fusiform gyrus | R | 2.55 | 36 | −70 | −13 | 19 |
| Inferior temporal gyrus | L | 3.31 | −58 | −37 | −13 | 20 |
| Dyslexic group | ||||||
| Inferior frontal gyrus | L | 11.28 | −40 | 7 | 30 | 6/44 |
| Cuneus/superior parietal lobule | L | 4.67 | −25 | −74 | 23 | 18/7 |
| Medial superior frontal gyrus/anterior cingulated | – | 6.61 | 0 | 11 | 50 | 6/32 |
| Cerebellum (extending into Fusiform gyrus) | L | 6.15 | −33 | −59 | −20 | – |
| Fusiform gyrus | R | 4.53 | 36 | −70 | −7 | 19 |
Foci represent the most strongly activated voxel in each 3D cluster. All group analyses were conducted at the following threshold: voxelwise P-value < 0.05; clusterwise P-value < 0.005.
Group contrasts
A permutation-based analysis of variance test was first undertaken to examine the main effect of group. This was done by first calculating an F-statistic based on the between and within groups sums of squares of deviations from mean values. This calculation was then repeated 50 times at each voxel after randomly permuting the group labels to achieve the null hypothesis of no main effect of group. The permuted statistics were then pooled over all intracerebral voxels to give the final data-driven null distribution. The significance of any observed F-statistic (from the non-permuted data) could then be assessed by directly determining its probability of occurrence in the null distribution. Subsequent pairwise group tests were then performed by computing the difference in median BOLD responses between the groups. Medians are used to reduce the effects of outliers, a potentially serious issue in small groups of subjects. The probability under the null hypothesis of the difference in BOLD response was then derived from the null distribution of median differences. This was derived by re-computation of these differences following random permutation of subjects between groups.
Results
Behavioural data
Mean accuracy and reaction time data for the rhyme and control tasks for each group are shown in Table 2. A mixed-model ANOVA was conducted on the accuracy data with Task (rhyme/control) as the within subjects factor and Group (deaf/dyslexic/hearing) as the between subjects factor. A main effect of Task indicated that the control task was performed better than the rhyme task [F = 19.1, (1,18), P < 0.005]. There was no significant main effect of Group and no interaction. The same mixed-model ANOVA was applied to the reaction time data. There was a main effect of Task indicating faster reaction times to the control task than the rhyme task [F = 615.7 (1,18), P < 0.005]. There was no main effect of Group and no interaction. These data suggest that all three groups performed similarly on each of the two tasks.
fMRI data
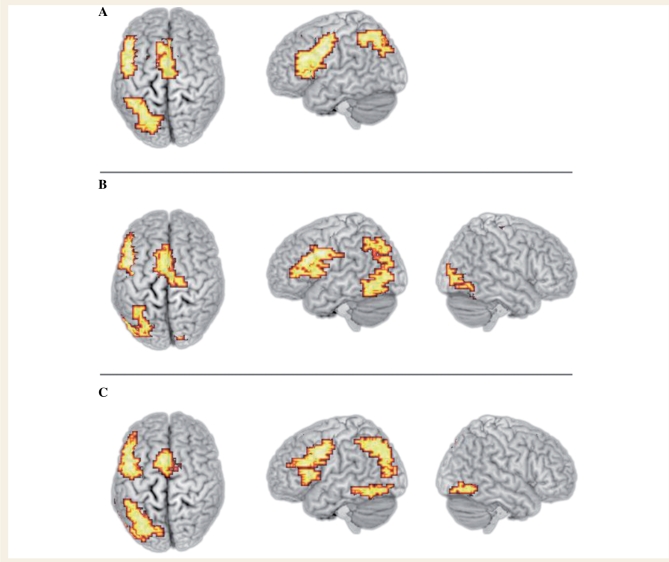
All three groups activated the core network that we have previously identified for this task (MacSweeney et al., 2008). This network consists of the medial portion of the superior frontal gyrus at the border with the anterior cingulate, the left superior parietal lobule extending medially to the precuneus and, most extensively, the left lateral frontal cortex, focused in the left dorsal inferior frontal gyrus (Table 3 and Fig. 2). Additionally, the hearing dyslexics and non-dyslexics, but not the deaf participants, activated the fusiform and inferior temporal gyri bilaterally (Table 3 and Fig. 2).
Figure 2.
Activation during the rhyme task relative to the ‘same picture?’ control task in (A) deaf participants; (B) hearing dyslexic participants; (C) hearing non-dyslexic participants. Voxelwise P < 0.05; clusterwise P < 0.005. Activated voxels up to 25 mm beneath the cortical surface are displayed.
Group differences
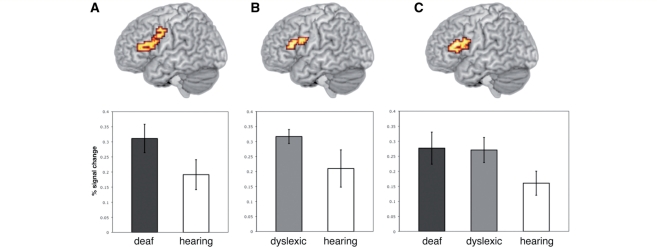
The main question of interest was whether there were differences between the three groups in their patterns of activation during the task. To address this we carried out a one-way ANOVA involving the three groups (clusterwise P = 0.05, voxelwise P = 0.01). The only region of significant difference between the three groups was a large area of frontal cortex with a focus at the junction of the left dorsal IFG and the ventral precentral gyrus (13.19 cm3 volume; X = −40, Y = 4, Z = 33). This extended from ventral IFG (pars orbitalis) into pars triangularis and pars opercularis and into ventral precentral gyrus. The activation also extended superiorly to include ventral parts of the middle frontal gryus.
The area identified as showing significantly different activation across groups in the one-way ANOVA was used as a mask to constrain the brain volume included in follow-up pairwise group comparisons (clusterwise P = 0.05, voxelwise P = 0.01). Deaf participants showed greater activation than hearing participants in a large portion of the left lateral frontal cortex extending from the pars triangularis in the IFG (BA 45; Z = 13) posteriorly and superiorly to include the pars opercularis and a large portion of the pre-central gyrus (BA 6; Z = 46; Fig. 3). This activation had two local peaks: one in the ventral precentral gyrus (BA 6) at the junction with the pars opercularis (BA 44; 0.79 cm3 volume; X = −40, Y = 4, Z = 33) and the other in a more anterior and inferior region, pars triangularis (BA 45; 2.26 cm3 volume; X = −40, Y = 30, Z = 20). Dyslexic participants showed greater activation than hearing participants in very similar, though more constrained, areas of the left lateral frontal cortex. This extended from the pars triangularis in the IFG (BA 45: Z = 16) to the pars opercularis (BA 44), at the junction with the precentral gyrus (BA 6; Z = 30). Again this activation had two local peaks: pars opercularis (BA 44), at the junction with the precentral gyrus (BA 6; 0.90 cm3 volume; X = −40, Y = 7, Z = 30) and pars triangularis (BA 45; 0.97 cm3 volume; X = −43, Y = 22, Z = 23). The regions activated more by deaf and dyslexic participants than hearing participants are shown in Fig. 3.
Figure 3.
(A) Regions showing greater activation in deaf compared with hearing participants (voxelwise P < 0.05; clusterwise P < 0.01). (B) Regions showing greater activation in dyslexic compared with hearing participants (voxelwise P < 0.05; clusterwise P < 0.01). (C) Regions showing greater activation in deaf and dyslexic participants combined compared with in hearing participants (voxelwise P < 0.05; clusterwise P < 0.005). Activated voxels up to 20 mm beneath the cortical surface are displayed. Plots represent the mean per cent signal change across all voxels in the activated cluster across all participants. Error bars represent the standard error of the mean.
Given the apparent overlap in the regions activated to a greater extent by deaf and dyslexic participants than hearing participants, a further whole-brain analysis was carried out. Deaf and dyslexic participants were combined and contrasted with hearing participants (voxelwise P = 0.05; clusterwise P = 0.005). This analysis was motivated by the pairwise contrasts reported above and by the fact that both deaf and dyslexic participants have phonological processing difficulties. A large portion of the left IFG was activated to a greater extent in the deaf and dyslexic than hearing participants. The focus of this activation was in the pars triangularis (BA 45; 2.55 cm3 volume; X = −43, Y = 26, Z = 20) extending superiorly into the middle frontal gyrus and posteriorly to include the pars opercularis (BA 44; Fig. 3).
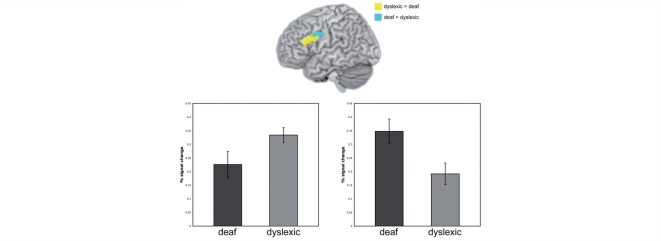
Small regions of difference were also identified between deaf and dyslexic participants in a pairwise contrast using the mask from the one-way ANOVA. These differences only partially overlapped with regions identified in the contrast between the combined dyslexic and deaf groups and the hearing group. Specifically, the dyslexic versus deaf group differences were situated in more superior portions of the IFG and precentral gyrus. There was greater activation in dyslexic than deaf participants in the superior posterior portion of the pars triangularis, extending into the superior inferior pars opercularis and the ventral middle frontal gyrus (BA 46; 1.11 cm3 volume; X = −40, Y = 19, Z = 26; Fig. 4). The region superior and posterior to this, the most superior tip of the pars opercularis extending into the ventral precentral gyrus, was activated more by deaf than dyslexic participants (0.75 cm3 volume; X = −40, Y = 4, Z = 33; Fig. 4). In both of these regions, activation in the hearing non-dyslexics fell between that of the dyslexic and deaf groups and did not differ significantly from either.
Figure 4.
Blue area shows region of dorsal IFG, with a focus in pars opercularis, showing greater activation in deaf compared with dyslexic participants. Yellow area shows region of IFG, with a focus in pars triangularis, showing greater activation in dyslexic compared with deaf participants (voxelwise P < 0.05; clusterwise P < 0.01). A mask from the one-way ANOVA contrasting the three groups was used to constrain this analysis. Activated voxels up to 20 mm beneath the cortical surface are displayed.
Discussion
We tested deaf, hearing dyslexic and hearing non-dyslexic good readers on a picture-based rhyme judgement task. All three groups recruited a core phonological network involving the medial portion of the superior frontal gyrus at the border with the anterior cingulate, the left superior parietal lobule extending medially to the precuneus and, most extensively, the left lateral frontal cortex, focused in the left dorsal IFG. This pattern replicates that seen in our previous study with larger numbers of deaf and hearing non-dyslexic participants (MacSweeney et al., 2008).
Of primary interest was whether the two groups of adults that we established had difficulties in phonological processing—adults born profoundly deaf and hearing developmental dyslexics—differed to hearing non-impaired readers in similar ways. In contrast to the hearing non-dyslexic controls, when deaf and dyslexic participants were combined they showed enhanced recruitment of the pars triangularis, extending superiorly into the ventral middle frontal gyrus and posteriorly to include the dorsal IFG, pars opercularis. In the direct pairwise contrasts between the hearing participants and each of the dyslexic and deaf groups separately, group differences also extended into the most dorsal portion of the pars opercularis and into the ventral precentral gyrus. These group differences were observed even though the three groups of participants were all good readers and were matched on a number of behavioural characteristics including task performance in the scanner, non-verbal IQ and reading level. The current study was necessarily conducted with small sample sizes due to our strict group-matching criteria. However, our findings seem unlikely to be unduly influenced by this since the statistical approach used is non-parametric, which is particularly robust when testing small groups (Nichols and Hayasaka, 2003).
Enhanced IFG activation in deaf and dyslexic participants
Our data suggest that poor phonological processing of spoken language is related to enhanced activation of the left dorsal IFG and the ventral pre-central gyrus, the regions that make up Broca's area. We argue that a ‘greater cognitive effort’ account of our data is unlikely since the three groups were well matched on behavioural characteristics and did not differ in errors or reaction times on the rhyme judgment task. Rather, we argue that compensatory articulatory processes are used to support phonological processing when auditory processes are either absent (deaf participants) or somehow impaired (dyslexic participants).
Numerous studies of hearing non-impaired readers report a developmental increase from childhood to adulthood in the recruitment of the left IFG during word reading (Simos et al., 2001; Turkeltaub et al., 2003), pseudoword reading (Simos et al., 2001), and auditory and visual rhyming and spelling tasks (Booth et al., 2004). When the left IFG is activated in young children, it is engaged significantly later following stimulus presentation in children than adults (Simos et al., 2001). The left IFG is especially strongly activated during difficult rhyme decisions, such as in the present study, in which phonology and orthography conflict (e.g. chair–bear; see Bitan et al. 2007). Using functional connectivity analyses, Bitan et al. (2007) also showed that the coupling of the dorsal IFG with the ventral IFG and the lateral temporal cortex increased with age. A complementary developmental reduction in activation in superior temporal regions led the authors to suggest a developmental progression from reliance on auditory-based phonological representations/processing to abstract phonological representations, associated with lateral temporal cortex, and on phonological segmentation and covert articulation associated with dorsal IFG (Bitan et al., 2007). These studies with non-impaired readers suggest that a greater reliance on the IFG during phonological and language-related tasks reflects the typical developmental progression (see Bitan et al., 2007 for review).
This developmental progression is also evident in developmental dyslexics (Shaywitz et al., 2002, 2007), however, studies suggest it may start later in dyslexic than non-dyslexic children and be more pronounced in adulthood. The data from dyslexic children (aged 8–14 years) overwhelmingly suggest reduced IFG activation in contrast to controls. This pattern has been reported during non-word reading (Georgiewa et al., 1999; Shaywitz et al., 2002); auditory rhyme judgment (Corina and McBurney, 2001); visual rhyme judgement of conflicting spellings (Cao et al., 2006; e.g., has–jazz/pint–mint); phonological manipulation (Georgiewa, et al., 1999) and letter to sound mapping (Aylward et al., 2003; for the reverse pattern, however, see Richards et al., 1999; Georgiewa et al., 2002). This contrasts with data from adults, reported here and in previous studies, showing enhanced IFG activation in dyslexic compared to non-dyslexic participants during reading and phonological tasks (Shaywitz et al., 1998; Brunswick et al., 1999; see also Rumsey et al., 1997). In contrast to this increased reliance on the left IFG, the left temporo-parietal cortex is reliably reported to show reduced activation in contrast to controls in both dyslexic children (Simos et al., 2000; Temple et al., 2001) and adults (Rumsey et al., 1992, 1997; Paulesu et al., 1996; Shaywitz et al., 1998, 2002, 2003; Brunswick et al., 1999).
The previous literature therefore suggests that the normal developmental progression of increased reliance on the left IFG during reading-related tasks is delayed in dyslexic children. However, by the time they reach adulthood, those with developmental dyslexia have engaged the left IFG and may need to rely more on this region than controls to compensate for reduced activation in temporo-parietal cortex. In terms of cognitive processing, it is likely that reduced temporo-parietal activation reflects impoverished ‘auditory-based’ processing, while enhanced IFG activation reflects greater reliance on fine-grained articulatory recoding (covert pronunciation) during phonological and reading tasks (e.g. Pugh et al., 2000; Shaywitz et al., 2002).
This developmental progression may not be driven by age alone but also by extent and type of remedial training received. Increases in IFG activation following remedial training have been reported in a number of studies of dyslexic children (Aylward et al., 2003; Temple et al., 2003; Shaywitz et al., 2004). In dyslexic adults, Eden et al. (2004) found enhanced activation following phonological training involving explicit instruction in articulatory awareness and phonics training in the left ventral middle frontal gyrus, very close to the dorsal pars triangularis activation identified in the current study.
The proposal that, in developmental dyslexics, the involvement of left IFG in phonological tasks reflects a delayed, but in adulthood enhanced, version of the typical developmental pattern needs to be explored longitudinally. Such studies would permit the relationship between changes in left IFG activation and behavioural performance to be examined throughout development. Whether this developmental pattern also applies to deaf people is not clear. Only two neuroimaging studies have examined phonological processing in deaf people and these have been in adults (Aparicio et al., 2007; MacSweeney et al., 2008). Both showed enhanced IFG activation in deaf compared to hearing readers.
In summary, we argue that a large portion of the left inferior frontal cortex is recruited to a greater extent during phonological processing by people with phonological difficulties than in those without and that this is due to compensatory recruitment of articulatory processes. However, we acknowledge the possibility of alternative interpretations. Activation in the IFG during rhyming may be driven by the resolution of the conflict between orthography and phonology (e.g. Bitan et al., 2007). If so, observed group differences could be due to differential mechanisms for resolving this conflict. However, since our task was picture-based we propose this explanation is unlikely to provide a complete account of the differences observed. A further alternative explanation for enhanced IFG activation in the deaf group is that they imagined the British Sign Language (BSL) labels for the pictures, since imagined finger and hand movements can activate this region (Iacoboni et al., 1999; Binofski et al., 2000). Indeed, in MacSweeney et al., (2008) we reported that deaf participants activated the pars opercularis during a British Sign Language phonological judgement task in response to pictures. Thus, this region is engaged in phonological judgement tasks based on both speech and sign. However, in the same study we also reported greater activation in this region in deaf participants for phonological decisions about speech (rhyme) than sign (location). Therefore, the pars opercularis activation reported for the deaf group in the current study is unlikely to reflect motor imagery for sign language alone. The most parsimonious interpretation, given that both the dyslexic and deaf groups showed enhanced IFG activation, involves a compensatory reliance on articulatory phonology.
Differences between deaf and dyslexic participants
Deaf participants recruited the superior portion of the left pars opercularis extending into the ventral precentral gyrus to a greater extent than dyslexic participants. In contrast, dyslexic participants recruited the superior posterior portion of the pars triangularis, extending into the superior inferior pars opercularis and the ventral middle frontal gyrus to a greater extent than deaf participants. The regions identified in this analysis only partially overlapped with the regions identified in the deaf plus dyslexic versus hearing contrast. Rather these regions were located in the most superior portions of the IFG extending into the pre-central gyrus. Furthermore, in both cases, activation in the hearing group fell between that of the deaf and dyslexic groups. Whether these small sub-regions play different functional roles in phonological processing cannot be determined on the basis of the present data.
It has been argued that different parts of the left IFG show preferential engagement in different aspects of language processing: the more posterior/dorsal region being involved in phonological processes (pars opercularis; BA 44/6), the more anterior region (pars triangularis; BA 45) being involved in syntactic processes and the ventral portion (pars orbitalis; BA 47) being especially involved in semantic processing (e.g. Bookheimer et al., 2002; see also Fiez, 1997; Price et al., 1997; Poldrack et al., 1999; Devlin et al., 2003). Thus, the enhanced involvement of the pars opercularis during phonological processing in both the deaf and dyslexic groups in comparison to the hearing group, and in the deaf group compared to the dyslexic group is readily accounted for by this functional characterization of the IFG.
In contrast, patterns of activation in dorsal pars triangularis and the ventral middle frontal gryus (BA 46; X = −40, Y = 19, Z = 26) fit less well with the linguistic functional specificity account of the left IFG outlined above. These regions were activated more by deaf and dyslexic participants than by hearing participants, and by dyslexic more than deaf participants. Whether or not the IFG should be parcelated in this fashion is currently a matter of debate (e.g. Thompson-Schill et al., 2005). Our current data cannot inform this debate since we did not test these participants on different levels of linguistic processing. However, it is clear that a strict parcelation of the IFG into separate processing regions is inappropriate. In addition to our data showing the involvement of the pars triangularis and ventral middle fontal gyrus in a phonological task, in dyslexic participants in particular, Shaywitz et al. (1998) identified the entire left IFG (BA 44/45/47), and also BAs 46 and 11, as being more activated in dyslexic than in hearing adults during a written non-word rhyme task. Furthermore, Eden et al. (2004) found enhanced activation in dyslexic adults following phonological training in left middle frontal gryus (BA 46; X = −34, Y = 27, Z = 26), very close to the region identified here as showing greater activation in dyslexic than in deaf adults. Future studies, using different task manipulations, are necessary to further explore the differences observed between deaf and dyslexic participants.
Absence of temporo-parietal hypo-activation
The main finding from the current study is that a large portion of the left inferior frontal cortex is more actively involved in phonological processing in those with phonological difficulties than in those without. We propose that this is due to compensatory recruitment of articulatory processes. However, when one argues for compensatory activity within a neural system, it is reasonable to ask: compensation for what? That is, were there any areas of under-activation in the deaf and dyslexic groups? In the current study, and in our related study involving larger deaf and hearing groups, we did not find any regions of hypo-activation in dyslexic or deaf participants in contrast to controls. This was the case even though we used whole brain analyses in both ANOVAs involving all three groups. This is surprising, since many previous studies have found reduced activation, in contrast to controls, in the left temporo-parietal junction in dyslexic adults (Rumsey et al., 1992, 1997; Paulesu et al., 1996, 2001; Shaywitz et al., 1998, 2002, 2003; Brunswick et al., 1999) and children (Simos et al., 2000; Temple et al., 2001). Disrupted connectivity between this region and the rest of the reading network in dyslexics has also been reported during a range of reading and phonological tasks (Paulesu et al., 1996; Horwitz et al., 1998; Pugh et al., 2000).
One possible explanation for this discrepancy is that it is due to the stimuli used. The vast majority of studies reporting under-activation in dyslexics compared to controls have used written word stimuli (Paulesu et al., 1996, 2001; Shaywitz et al., 1998; Brunswick et al., 1999; Simos et al., 2000; Temple et al., 2001). Indeed, it appears as though only one early PET study has reported this pattern using auditory stimuli (Rumsey et al., 1992). In the current study picture stimuli were used. To our knowledge, this is the first neuroimaging study to explore rhyme judgment in dyslexic participants using picture, as opposed to written or spoken, stimuli. Picture stimuli necessitate that participants generate their own phonological representations, rather than receive them auditorily or (partially) via text. Pictures, therefore, provide a more reliable test of phonological awareness skills than either spoken or printed stimuli (Katzir et al., 2005).
It has been argued that the left temporo-parietal region is involved in multi-modal integration: mapping between orthographic and phonemic representations (Booth et al., 2002; Eden et al., 2004). Reduced activation in this region might therefore be predicted when picture stimuli are presented. Indeed, in the only other published study to use pictures in a phonological judgement task (initial phoneme judgement), Katzir et al. (2005) reported no activation in temporo-parietal regions in hearing non-dyslexic readers. Although we argue that orthographic representations are likely to be activated during phonological judgements based on pictures (MacSweeney et al., 2008), and indeed all three groups activated this region during the current task (Table 3), it is likely that this region is engaged to a lesser extent when pictures, rather than text, are presented. A direct contrast between picture and written word stimuli in a phonological judgment task would further illuminate the role of the left temporo-parietal junction within the network involved in phonological processing.
The role of the left fusiform gyrus
Although not a significant group difference, it is worth noting that both dyslexic and non-dyslexic hearing, but not deaf, participants activated the fusiform and inferior temporal gyri bilaterally. In a larger group of deaf and hearing participants tested on the same task we reported activation in right fusiform gyrus in the hearing group, but not the deaf group (MacSweeney et al., 2008). At a less conservative threshold than that reported by MacSweeney et al. (2008; voxelwise P < 0.05; clusterwise P < 0.005), there was also significant activation in the left fusiform gyrus in the hearing group (2.41 cm3 volume; X = −40, Y = −63, Z = −7).
The left mid-fusiform gyrus has been referred to as the ‘visual word form area’ (Cohen et al., 2000, 2002) and is reliably activated by rhyme judgements in response to written words (Kareken et al., 2000; Booth et al., 2002; Burton et al., 2005). There was no orthographic input in the current rhyme task, nevertheless activation in hearing participants was located within the range of the proposed VWFA (as defined by Cohen et al., 2002). Activation in this region has also been reported during auditory phonological tasks (Booth et al., 2002; Burton et al., 2005). Thus, these data support the proposal that in literate individuals, phonology and orthography are intimately intertwined (Ziegler and Goswami, 2005). Hearing participants may automatically access orthographic representations of words when making phonological decisions. The lack of activation in this region in the deaf group may reflect less robust connections between orthography and spoken language phonology in these individuals.
Conclusion
We have shown that hearing dyslexic and profoundly deaf adults, who are good readers but have ongoing phonological processing difficulties, engage the left IFG to a greater extent than hearing non-impaired readers during a picture rhyme judgement task. This enhancement occurred even though the groups were well matched on both reading level and rhyme performance. We argue that in both groups this reflects compensation in terms of greater reliance on the articulatory component of spoken language phonology when the auditory component is compromised. However, as outlined in the Introduction, alternative sources of information about the phonological structure of speech also exist. Deaf and hearing readers, especially those that are dyslexic, are likely to extract information from orthography, speech-reading and a number of other sources. Establishing the relative contribution of these inputs to phonological representations and processing in these two populations and between individuals may provide vital insights into the most appropriate educational strategies for individuals for whom skilled reading poses a great challenge.
Funding
Wellcome Trust (GR062441AIA to M.MacS., U.G., M.J.B.; GR075214MA to M.MacS.).
Acknowledgements
We are grateful to Tara Mohammed for assistance with behavioural testing and to all of the volunteers who took part in this research.
References
- Allen TE. Patterns of academic achievement among hearing impaired students: 1974 and 1983. In: Schildroth AN, Karchmer MA, editors. Deaf children in America. San Diego, CA: College-Hill Press; 1986. pp. 161–206. [Google Scholar]
- Aparicio M, Gounot D, Demont E, Metz-Lutz MN. Phonological processing in relation to reading: an fMRI study in deaf readers. Neuroimage. 2007;35:1303–16. doi: 10.1016/j.neuroimage.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Arthur TAA, Hitch GJ, Halliday MS. Articulatory loop and children. Br J Psychol. 1994;85:283–300. [Google Scholar]
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, et al. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–19. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Besner D, Davies J, Daniels S. Reading for meaning:the effects of concurrent articulation. Q J Exp Psychol A. 1981;33:415–37. [Google Scholar]
- Binofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, et al. Broca's region subserves imagery of motion:a combined cytoarchitectonic and fMRI study. Hum Brain Mapp. 2000;11:273–85. doi: 10.1002/1097-0193(200012)11:4<273::AID-HBM40>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, et al. Developmental changes in activation and effective connectivity in phonological processing. Neuroimage. 2007;38:564–75. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Wouters J, van Wieringen A, De Smedt B, Ghesquiere P. Modelling relations between sensory processing, speech perception, orthographic and phonological ability, and literacy achievement. Brain Lang. 2008;106:29–40. doi: 10.1016/j.bandl.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TD, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. J Cogn Neurosci. 2004;16:1234–49. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Cho S, Burman DD, Bitan T. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Dev Sci. 2007;10:441–51. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, et al. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging. 1997;15:763–70. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain. 1999;122:1901–17. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, et al. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Burton MW, LoCasto PC, Krebs-Noble D, Gullapalli RP. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage. 2005;26:647–61. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Campbell R, Wright H. Deafness, spelling and rhyme: how spelling supports written word and picture rhyming skills in deaf subjects. Q J Exp Psychol A. 1988;40:771–88. doi: 10.1080/14640748808402298. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 2006;47:1041–50. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Conrad R. The deaf schoolchild: language and cognitive function. London: Harper and Row; 1979. [Google Scholar]
- Corina DP, McBurney SL. The neural representation of language in users of American Sign Language. J Commun Disord. 2001;34:455–71. doi: 10.1016/s0021-9924(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Corriveau K, Thomson JM, Goswami U. Auditory processing and early literacy skills in a preschool and kindergarten population. J Learn Disabil. 2009 doi: 10.1177/0022219410369071. (in press) [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Dyer A, MacSweeney M, Szczerbinski M, Green L, Campbell R. Predictors of reading delay in deaf adolescents: the relative contributions of rapid automatized naming speed and phonological awareness and decoding. J Deaf Stud Deaf Educ. 2003;8:215–29. doi: 10.1093/deafed/eng012. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, et al. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–22. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci USA. 1998;95:914–21. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CA. Speech as a supramodal or amodal phenomenon. In: Calvert G, Spence C, Stein BE, editors. The handbook of multisensory processes. Cambridge, MA: MIT Press; 2004. pp. 189–201. [Google Scholar]
- Friman O, Borga M, Lundberg P, Knutsson H. Adaptive analysis of fMRI data. Neuroimage. 2003;19:837–45. doi: 10.1016/s1053-8119(03)00077-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Gaser C, Gerhard UJ, Vieweg U, Freesmeyer D, et al. Phonological processing in dyslexic children: a study combining functional imaging and event related potentials. Neurosci Lett. 2002;318:5–8. doi: 10.1016/s0304-3940(01)02236-4. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, et al. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–65. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Goswami U, Bryant P. Phonological skills and learning to read. Hove: Lawrence Erlbaum; 1990. [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S, et al. Amplitude envelope onsets and developmental dyslexia: a new hypothesis. Proc Natl Acad Sci USA. 2002;99:10911–16. doi: 10.1073/pnas.122368599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U, Ziegler JC, Richardson U. The effects of spelling consistency on phonological awareness: a comparison of English and German. J Exp Child Psychol. 2005;92:345–65. doi: 10.1016/j.jecp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–16. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen JA, Leppänen PHT, Torppa M, Muller K, Lyytinen H. Detection of sound rise time by adults with dyslexia. Brain Lang. 2005;94:32–42. doi: 10.1016/j.bandl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Leppänen PHT, Eklund K, Thomson J, Richardson U, Guttorm TK, et al. Common variance in amplitude envelope processing tasks and their impact on phoneme duration processing and reading and spelling in children with reading disabilities. Applied Psycholinguistics. 2009;30:9–25. [Google Scholar]
- Hanson VL. Phonology and reading: evidence from profoundly deaf readers. In: Shankweiler D, Liberman IY, editors. Phonology and reading disability: solving the reading puzzle. Ann Arbor: University of Michigan Press; 1989. pp. 69–89. [Google Scholar]
- Harris M, Beech J. Implicit phonological awareness and early reading development in prelingually deaf children. J Deaf Stud Deaf Educ. 1998;3:205–16. doi: 10.1093/oxfordjournals.deafed.a014351. [DOI] [PubMed] [Google Scholar]
- Harris M, Moreno C. Speech reading and learning to read: a comparison of 8-year-old profoundly deaf children with good and poor reading ability. J Deaf Stud Deaf Educ. 2006;11:189–201. doi: 10.1093/deafed/enj021. [DOI] [PubMed] [Google Scholar]
- Hedderly R. Vernon-Warden Reading Test, Restandardised 1993 and 1994. Dyslexia Rev. 1996;7:11–16. [Google Scholar]
- Henson RNA, Rugg M. Neural response suppression, haemodynamic repetition effects and behavioural priming. Neuropsychologia. 2003;41:263–70. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Holt JA. Stanford achievement test, 8th edition. Reading comprehension subgroup results. Am Ann Deaf. 1993;138:172–75. [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–44. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–28. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- James D, Rajput K, Brinton J, Goswami U. Phonological awareness, vocabulary, and word reading in children who use cochlear implants: does age of implantation explain individual variability in performance outcomes and growth? J Deaf Stud Deaf Educ. 2008;13:117–37. doi: 10.1093/deafed/enm042. [DOI] [PubMed] [Google Scholar]
- James D, Rajput K, Brown T, Sirimanna T, Brinton J, Goswami U. Phonological awareness in deaf children who use cochlear implants. J Speech Lang Hear Res. 2005;48:1511–28. doi: 10.1044/1092-4388(2005/105). [DOI] [PubMed] [Google Scholar]
- Johnston RS, McDermott EA. Suppression effects in rhyme judgement tasks. Q J Exp Psychol A. 1986;38:111–24. [Google Scholar]
- Kaplan E, Goodglass HWS. Boston Naming Test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- Kareken DA, Lowe M, Chen SH, Lurito J, Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:264–70. [PubMed] [Google Scholar]
- Katzir T, Misra M, Poldrack RA. Imaging phonology without print: assessing the neural correlates of phonemic awareness using fMRI. Neuroimage. 2005;27:106–15. doi: 10.1016/j.neuroimage.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Kyle FE, Harris M. Concurrent correlates and predictors of reading and spelling achievement in deaf and hearing school children. J Deaf Stud Deaf Educ. 2006;11:273–88. doi: 10.1093/deafed/enj037. [DOI] [PubMed] [Google Scholar]
- Liberman IY, Shankweiler D. Phonology and beginning reading: a tutorial. In: Rieben L, Perfetti CA, editors. Learning to read: basic research and its implications. Hillsdale, NJ; London: Lawrence Erlbaum Associates; 1991. pp. 3–17. [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SH, Mathews VP. Comparison of rhyming and word generation with FMRI. Hum Brain Mapp. 2000;10:99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSweeney M, Campbell R, Donlan C. Varieties of short-term memory coding in deaf teenagers. J Deaf Stud Deaf Educ. 1996;1:249–62. doi: 10.1093/oxfordjournals.deafed.a014300. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Waters D, Brammer MJ, Woll B, Goswami U. Phonological processing in deaf signers and the impact of age of first language acquisition. Neuroimage. 2008;40:1369–79. doi: 10.1016/j.neuroimage.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. The effect of communication mode on the development of phonemic awareness in prelingually deaf students. J Speech Lang Hear Res. 1997;40:1151–63. doi: 10.1044/jslhr.4005.1151. [DOI] [PubMed] [Google Scholar]
- Mohammed T, Campbell R, MacSweeney M, Barry F, Coleman M. Speechreading and its association with reading among deaf, hearing and dyslexic individuals. Clin Linguist Phon. 2006;20:621–30. doi: 10.1080/02699200500266745. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12:419–46. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Nielsen DC, Luetke-Stahlman B. Phonological awareness: one key to the reading proficiency of deaf children. Am Ann Deaf. 2002;147:11–9. doi: 10.1353/aad.2012.0213. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205:1401–3. doi: 10.1126/science.472757. [DOI] [PubMed] [Google Scholar]
- Pasquini ES, Corriveau KH, Goswami U. Auditory processing of amplitude envelope rise time in adults diagnosed with developmental dyslexia. Sci Stud Read. 2007;11:259–86. [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, et al. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165–67. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, et al. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–57. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Sandak R. Reading optimally builds on spoken language: implications for deaf readers. J Deaf Stud Deaf Educ. 2000;5:32–50. doi: 10.1093/deafed/5.1.32. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. J Cogn Neurosci. 1997;9:727–33. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fullbright RK, Constable RT, et al. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11:51–6. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Sandak R, Frost SJ, Moore D, Mencl WE. Examining reading development and reading disability in English language learners: potential contributions from functional neuroimaging. Learn Disabi Res Pract. 2005;20:24–30. [Google Scholar]
- Richards TL, Dager SR, Corina D, Serafini S, Heide AC, Steury K, et al. Dyslexic children have abnormal brain lactate response to reading-related language tasks. Am J Neuroradiol. 1999;20:1393–98. [PMC free article] [PubMed] [Google Scholar]
- Richardson U, Thomson JM, Scott SK, Goswami U. Auditory processing skills and phonological representation in dyslexic children. Dyslexia. 2004;10:215–33. doi: 10.1002/dys.276. [DOI] [PubMed] [Google Scholar]
- Rocheron I, Lorenzi C, Fullgrabe C, Dumont A. Temporal envelope perception in dyslexic children. Neuroreport. 2002;13:1683–87. doi: 10.1097/00001756-200209160-00023. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, et al. Failure to activate the left temporoparietal cortex in dyslexia. An oxygen 15 positron emission tomographic study. Arch Neurol. 1992;49:527–34. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch Neurol. 1997;54:562–73. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23:140–55. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, Tanenhaus MK. Orthographic effects on rhyme monitoring. J Exp Psychol [Hum Learn] 1979;5:546–54. [PubMed] [Google Scholar]
- Sergent J, Zuck E, Levesque M, MacDonald B. Positron emission tomography study of letter and object processing: empirical findings and methodological considerations. Cereb Cortex. 1992;2:68–80. doi: 10.1093/cercor/2.1.68. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fullbright RK, Skudlarski P, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 2004;55:926–33. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Menci WE, Fullbright RK, Skudlarski P, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–10. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fullbright RK, et al. Age-related changes in reading systems of dyslexic children. Ann Neurol. 2007;61:363–70. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Menci WE, Constable RT, et al. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fullbright RK, Constable RT, Menci WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–41. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: a magnetic source imaging approach. Cereb Cortex. 2000;10:809–16. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Mouzaki A, Papanicolaou AC. Age-related changes in regional brain activation during phonological decoding and printed word recognition. Dev Neuropsychol. 2001;19:191–210. doi: 10.1207/S15326942DN1902_4. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sterne A, Goswami U. Phonological awareness of syllables, rhymes, and phonemes in deaf children. J Child Psychol Psychiatry. 2000;41:609–25. doi: 10.1111/1469-7610.00648. [DOI] [PubMed] [Google Scholar]
- Suckling J, Bullmore E. Permutation tests for factorially designed neuroimaging experiments. Hum Brain Mapp. 2004;22:193–205. doi: 10.1002/hbm.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci USA. 2003;100:2860–65. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, et al. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Deheane S, Poline J-B. Analysis of a large fMRI cohort: statistical and methodlogical issues for group analysis. Neuroimage. 2007;35:105–20. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Thomson JM, Fryer B, Maltby J, Goswami U. Auditory and motor rhythm awareness in adults with dyslexia. J Res Read. 2006;29:334–48. [Google Scholar]
- Thomson JM, Goswami U. Rhythmic processing in children with developmental dyslexia: auditory and motor rhythms link to reading and spelling. J Physiol Paris. 2008;102:120–29. doi: 10.1016/j.jphysparis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–73. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Wilding J, White W. Impairment of rhyme judgments by silent and overt articulatory suppression. Q J Exp Psychol A. 1985;37:95–107. [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb Cortex. 2001;11:267–77. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Ferrand L, Montant M. Visual phonology: the effects of orthographic consistency on different auditory word recognition tasks. Mem Cognit. 2004;32:732–41. doi: 10.3758/bf03195863. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol Bull. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]