Abstract
Acquired sensory neuronopathies encompass a group of paraneoplastic, dysimmune, toxic or idiopathic disorders characterized by degeneration of peripheral sensory neurons in dorsal root ganglia. As dorsal root ganglia cannot easily be explored, the clinical diagnosis of these disorders may be difficult. The question as to whether there exists a common clinical pattern of sensory neuronopathies, allowing the establishment of validated and easy-to-use diagnostic criteria, has not yet been addressed. In this study, logistic regression was used to construct diagnostic criteria on a retrospective study population of 78 patients with sensory neuronopathies and 56 with other sensory neuropathies. For this, sensory neuronopathy was provisionally considered as unambiguous in 44 patients with paraneoplastic disorder or cisplatin treatment and likely in 34 with a dysimmune or idiopathic setting who may theoretically have another form of neuropathy. To test the homogeneity of the sensory neuronopathy population, likely candidates were compared with unambiguous cases and then the whole population was compared with the other sensory neuropathies population. Criteria accuracy was checked on 37 prospective patients referred for diagnosis of sensory neuropathy. In the study population, sensory neuronopathy showed a common clinical and electrophysiological pattern that was independent of the underlying cause, including unusual forms with only patchy sensory loss, mild electrical motor nerve abnormalities and predominant small fibre or isolated lower limb involvement. Logistic regression allowed the construction of a set of criteria that gave fair results with the following combination: ataxia in the lower or upper limbs + asymmetrical distribution + sensory loss not restricted to the lower limbs + at least one sensory action potential absent or three sensory action potentials <30% of the lower limit of normal in the upper limbs + less than two nerves with abnormal motor nerve conduction study in the lower limbs.
Keywords: sensory neuronopathy, sensory ganglionopathy, sensory neuropathy, paraneoplastic neurological syndrome, cisplatin
Introduction
Acquired sensory neuronopathies (SNN) or ganglionopathies encompass different disorders characterized by a primary degeneration of sensory neurons in dorsal root ganglia (Kuntzer et al., 2004; Sghirlanzoni et al., 2005). This has been pathologically demonstrated with paraneoplastic SNN (Graus et al., 1990; Dalmau et al., 1991; Wanschitz et al., 1997), HIV infection (Scaravilli et al., 1992; Esiri et al., 1993), Sjögren's syndrome, unclassified connective diseases and rare idiopathic cases (Okajima et al., 1983; Sobue et al., 1988; Griffin et al., 1990; Hainfellner et al., 1996; Kurokawa et al., 1998; Colli et al., 2008). Interestingly, in all of these circumstances, dorsal root ganglia degeneration was associated with an inflammatory T-cell reaction suggesting that the disorder is mainly driven by a cell-mediated immune response. That sensory neuron cell body is the target of cisplatin toxicity is also recognized (Gill and Windebank, 1998; Krarup-Hansen et al., 2007). Conversely, with vitamin B6 toxicity (Windebank, 1985; Xu et al., 1989) or anti-disialosyl antibodies (Kusunoki et al., 1996), the demonstration of dorsal root ganglia involvement relies on animal models only. Finally, recent reports suggest that a variety of pure small fibre neuropathy may depend on a ganglionopathy, but this has not yet been demonstrated by dorsal root ganglia examination (Mori et al., 2003; Brannagan et al., 2005; Gibbons et al., 2008).
Differentiating SNN from other sensory neuropathies is important owing to the possibility of detecting disorders that may benefit from specific investigations and treatments. However, this is difficult in the absence of methods that allow easy and non-traumatic exploration of dorsal root ganglia. In addition, several conditions associated with SNN such as Sjögren's syndrome are not specifically connected with a ganglionopathy, as they also occur with other forms of neuropathy. Lastly, there is evidence that both dorsal root ganglia and peripheral nerves can simultaneously be affected in the same patient. This explains why some authors maintain descriptive terminologies such as ataxic sensory neuropathy or even sensory neuropathy (Dalakas, 1986; Windebank et al., 1990). Hence, there is a need for diagnostic criteria for SNN that can easily be used in general practice. Several years ago, Asbury (Asbury, 1987; Asbury and Brown, 1990) proposed that a non-length-dependent distribution of sensory loss and an almost pure and severe electrophysiological sensory involvement are distinctive of SNN. Specific criteria have also been proposed for paraneoplastic cases (Graus et al., 2004). Recently, skin biopsy has been used to demonstrate non length-dependence of small fibre loss (Lauria et al., 2001) and spinal cord MRI to show degeneration of the central process of large sensory neurons (Lauria et al., 2000). Although universally used, Asbury's criteria have not been validated and do not take into account several questions: is there a uniform pattern of acquired SNN? What is the sensitivity and specificity of the proposed criteria? Can different easy-to-use criteria differentiate SNN from other sensory neuropathies? We addressed these questions in a case–control study.
Materials and Methods
Patient selection
Patients consisted of two populations, the study and the test population.
The study population was used for the construction of diagnostic criteria. For this, we retrospectively reviewed the files of 85 patients with paraneoplastic neurological disorders and 511 with sensory non-paraneoplastic neuropathy referred between January 1993 and January 2007 to the Rhône-Alpes Reference Centre for Rare Neuromuscular Diseases. To be selected for the study, patients had to present a clinically pure sensory neuropathy even though electrophysiological investigations may have shown motor nerve conduction study abnormalities, and a complete and detailed record of the clinical and electrophysiological investigations had to be available. All of the patients had to have been examined by one of us and to have received a biological check-up with a search for at least diabetes mellitus, renal failure, abnormal white blood cell count, plasma ion abnormalities, monoclonal (M) gammopathy, liver perturbations, B12 deficiency, thyroid hormone abnormalities, well-characterized onconeural antibodies and organ- and non-organ-specific antibodies. Patients with multiple causes of neuropathy, clinical radiculopathy, entrapment neuropathy or hereditary neuropathy were excluded.
The study population consisted of two groups:
-
–
SNN patients were provisionally classified as having unambiguous or likely SNN. Unambiguous SNN included definite paraneoplastic SNN according to the PNS Euronetwork criteria (Graus et al., 2004) and acute or subacute sensory neuropathy due to cisplatin toxicity. SNN was considered likely by the clinician of the reference centre in the absence of gold standard criteria in those patients presenting with a clinically pure sensory neuropathy with a non-length dependent distribution and pure or predominant sensory abnormalities on the nerve conduction study independently of the associated context according to Asbury's criteria. The rationale for this classification relies on the fact that in the unambiguous group there was no alternative diagnosis for the neuropathy, and a fair demonstration of dorsal root ganglia involvement exists in the literature while in the likely group several mechanisms of neuropathy were theoretically possible for a given aetiology as it is the case with Sjögren's syndrome, unspecific dysimmune or idiopathic disorders.
-
–
Controls were used as a reference population for the elaboration of diagnostic criteria by comparison with patients with unambiguous or likely SNN. They consisted of patients with a clinically pure sensory neuropathy and either a length-dependent distribution, or an etiological context or electrophysiological pattern that clearly excluded SNN. They were a priori selected among our population of patients with sensory neuropathy to represent the largest possible panel of neuropathies of different origins and patterns, whatever the actual relative frequency of each of these neuropathies in the population.
The test population was an external group used for the validation of diagnostic criteria established on the study population. It consisted of 37 unselected consecutive patients prospectively investigated in our centre for the diagnosis of a pure sensory neuropathy between January 2007 and June 2008. In the test population, the selected models were compared with the final diagnosis of the clinician taken as an expert centre diagnosis.
Data recorded for the study
The following data were recorded and analysed for the study: sex and age; clinical information including: at disease onset, modalities of onset (acute ≤1 month; subacute >1 month and ≤6 months; progressive >6 months), presence of paresthesia/dysesthesia, ataxia, pain, first involvement in the lower, upper or four limbs; at maximum development of the neuropathy: topography of sensory loss in the four limbs (proximal or distal), face or trunk, presence of pain, dysesthesia/paresthesia, ataxia in the upper or lower limbs, small (thermal and pin-prink sensation) or large (vibration and joint position sense) fibre involvement, number of elicited tendon reflexes, symmetry or asymmetry of the sensory loss, modified Rankin score, autonomic system abnormalities including orthostatic hypotension, constipation or diarrhoea, sexual impotence, bladder disturbances, abnormal sweating and pupil abnormalities. In addition, the distribution of sensory involvement was classified as consistent or not with a length-dependent pattern. For this, limbs were segmented into six sections from distal to proximal and the trunk into two vertical anterior and posterior sections. Criteria for a length-dependent distribution were as reported (Thomas and Ochoa, 1993). Cerebrospinal fluid (CSF) analysis abnormalities included protein concentration >0.5 g/l, white cell count > 1/mm3 or oligoclonal pattern.
For the electrophysiological study, conduction velocities were recorded at full development of the neuropathy with classical procedure in median, ulnar and radial nerves in the forearm and peroneal, tibial, superficial peroneal and sural nerves in the leg. Sensory action potentials (SAP) were recorded with an orthodromic procedure for median, ulnar and radial nerves, antidromically in the superficial peroneal and sural nerves and expressed as a percentage of the lower limit of the laboratory normal value. Motor distal latencies, compound muscle action potential and minimal F-wave latencies were recorded for median, ulnar, tibial and peroneal nerves. The pattern of each motor nerve was classified as normal, axonal/neuronal, demyelinating or intermediate according to published criteria (Camdessanche et al., 2002). To compare action potentials, conduction velocities and distal and F wave latencies, the worst value of the right or left recorded nerve was kept. Several dichotomized electrophysiological criteria were tested including the presence of at least one, two or three abolished SAP or SAP <30% of lower limit of normal of the laboratory and one, two or three motor nerve with abnormal nerve conduction study in the upper or lower limbs. MRIs were analysed for the presence of spinal cord T2 high signal and somatosensory evoked potentials (SEPs) were evaluated for the presence of an involvement of the peripheral and central pathway. Superficial peroneal or radial nerve biopsy were analysed on semithin sections for the estimation of myelinated fibre density, presence of demyelination, remyelination, wallerian degeneration or regenerating clusters and on paraffin embedded sections stained with H&S and by immunohistochemistry with an anti-T3 antibody (Dako, Glostrup, Denmark) for the detection of inflammatory T lymphocytes.
Statistical analysis
First, to test the homogeneity of SNN patients, we compared the unambiguous and likely SNN groups with respect to demographic, clinical, electrophysiological and biological data. Differences were determined using the Fisher exact test for qualitative data and Students t-test or Wilcoxon Mann–Whitney test for quantitative data.
Second, SNN patients (unambiguous and likely) were compared with controls as previously. Areas under receiver operator characteristic (ROC) curves were used to determine thresholds of sensory nerve conduction studies differentiating SNN from controls. Sensitivity and specificity were estimated for each of 85 items analysed using controls as the reference group. When related to a test procedure, sensitivity is defined as the probability of correctly detecting a condition which is present while specificity is the probability of failing to detect the condition when it is indeed absent. Likelihood ratios for positive (sensitivity divided by 1—specificity) and negative (1—sensitivity divided by specificity) test results were computed to evaluate the relative clinical utility of each item. Likelihood ratios above 10 and below 0.1 were considered to provide strong evidence to rule in or rule out diagnosis, respectively.
Third, logistic regression was used to construct multivariable models to identify the association of variables that may discriminate the SNN group from controls with the best accuracy in which each selected variable was weighted according to the logistic regression coefficient. Goodness-of-fit was evaluated using the Hosmer–Lemeshow test. Areas under ROC curves (AURC) + 95% confidence interval were computed as a measure of the overall discrimination and to determine the cut-off value separating patients as having or not having SNN. We first tested the Asbury's and PNSEuronet group criteria and then tried several a priori constructed models by introducing in block in the logistic regression either the items included in the aforesaid criteria or different combinations of items having the best likelihood ratios. Finally, to select the best discriminative model, we performed a stepwise logistic regression with all the clinical and electrophysiological items recorded at onset or maximum development of the neuropathy and having the best likelihood ratio+ or likelihood ratio–.
The validity of the models selected on the study population was then evaluated in two ways. First, the ‘jackknife’ method was performed on the study population and the results were presented as correct classification defined as the fraction of patients and controls, who were correctly classified. The jackknife method is a statistical cross-validation technique in which one patient is removed and the rule is rederived and used to classify the excluded patient (Efron, 1982; Wasson et al., 1985). The patient's predicted state is then compared with the reference state. This process is repeated systematically for each patient to determine the frequency with which the excluded patient is correctly or incorrectly classified.
Second, models were tested on the external prospective test population. In this population, the clinician diagnosis was made blindly to this model approach. The number and percentage of patients well classified using the diagnostic final model compared with the clinician diagnosis were computed, and sensitivity, specificity and likelihood ratio+ were derived. Statistics were performed using SPSS TM 14.0 software.
Results
Patients and controls
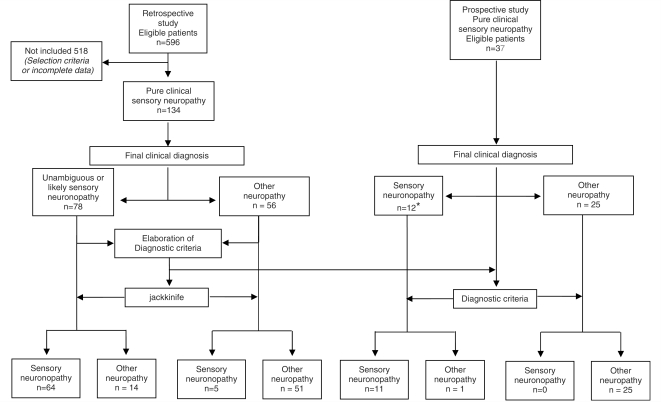
In the retrospective study population, 78 patients were in the SNN group (mean age 58 years, male 59%). Forty-four had an unambiguous diagnosis of SNN with an acute or subacute sensory neuropathy due to cisplatin toxicity in 11 cases and a definite paraneoplastic SNN in 33 cases (30 anti-Hu and one anti-amphiphysin antibody) (Fig. 1). SNN was considered as likely in 34 patients. A dysimmune context occurred in 11 patients including detection of anti-nuclear, SSA or mitochondria antibodies, lupus anticoagulant, positive salivary gland biopsy or M component without known antibody activity. Sjögren's syndrome was diagnosed in three of them. One patient had HIV infection. In 22 patients with a negative diagnosis workup, SNN was considered as idiopathic.
Figure 1.
Flow chart of the study design. Asterisk represents nine patients with SNN and three with suspected SNN. The diagnostic criteria applied to both the study and test populations is Model 6 selected by the logistic regression.
The control group consisted of 56 patients with clinical sensory neuropathy (mean age 62 years, male gender 57%). The neuropathy was dysimmune in 16: three had a chronic inflammatory demyelinating polyneuropathy, one the Lewis and Sumner syndrome, one an acute inflammatory demyelinating polyneuropathy, seven a distal demyelinating sensory neuropathy with anti-MAG M IgM, one a chronic ataxic neuropathy with anti-disialosyl M IgM, two mononeuritis multiplex with vasculitis and one Sjögren's syndrome. A metabolic neuropathy occurred in 10 patients including diabetes mellitus (4), B12 deficiency (4), amyloidosis (1) and hypothyroidism (1). The neuropathy was toxic in six patients: two alcoholic and four treatment-induced toxicity other than cisplatin. Finally, 24 patients had an axonal neuropathy of unknown origin. The diagnosis of Lewis and Sumner syndrome was retained in patients with a clinically pure multifocal sensory presentation on the presence on the electroneuromyographic study (ENMG) of signs of multifocal motor nerve demyelination and that of mononeuritis multiplex on ENMG signs of motor axonal degeneration and nerve biopsy findings of vasculitis.
The prospective test population consisted of 37 patients with clinically pure sensory neuropathy (mean age 60.7 years, male gender 70%). The final diagnosis before evaluation of the criteria was unambiguous SNN in one patient with onconeural antibody and likely SNN in eight patients. Three additional patients were suspected to have SNN but this diagnosis could not be definitively retained because of borderline clinical or ENMG data. The other patients had chronic inflammatory demyelinating polyneuropathy (4), Lewis-Sumner syndrome (2), amyloidosis (1), diabetes mellitus (1), B12 deficiency (1), alcoholic neuropathy (2), neuropathy with M gammopathy (1), small fibre neuropathy (1), neuropathy associated with heart graft (1) or neuropathy of unknown origin (11).
Comparison of unambiguous versus likely SNN
Results are summarized in Table 1. At full development, there was no significant difference in term of distribution, topography and quality of sensory involvement between the two groups. Characteristics that were shared by >80% of patients were the topography of sensory abnormalities involving the distal part of the four limbs, a non-length-dependent distribution, and presence of paraesthesia. Ataxia in the upper or lower limbs occurred in 71% of cases, small fibre involvement in 62% and pain in 50%. Asymmetrical distribution was present in 42% of patients. Rare particular clinical patterns that were not specific of one subgroup of SNN included: pure upper limb or lower limb involvement, sensory loss restricted to small fibres and multifocal distribution of patchy sensory loss that may be suggestive of mononeuritis multiplex.
Table 1.
Items differentiating unambiguous and likely SNN in the study population
| Unambiguous SNN (%) | Likely SNN (%) | P-value | |
|---|---|---|---|
| Sex (Male/total patients) | 31/44 (70.5) | 15/34 (44.1) | 0.019 |
| Acute–subacute–progressive onset | 15–24–5/44 (34.1–54.5–11.4) | 4–10–20/34 (11.7–29.4–58.8) | <0.0001 |
| Lower limb involvement only at onset | 8/44 (18.2) | 17/34 (50.0) | 0.0036 |
| Four limb involvement at onset | 17/44 (38.6) | 4/34 (11.8) | 0.01 |
| Pain at full development | 27/44 (61.4) | 12/34 (35.3) | 0.0224 |
| Raised CSF protein | 25/27 (92.6) | 8/21 (38.1) | <0.0001 |
| Oligoclonal CSF pattern | 7/18 (38.9) | 2/20 (10.0) | 0.0365 |
| Electroneuromyography with all motor nerves normal | 13/42 (30.9) | 20/34 (58.8) | 0.0148 |
P-value was determined with the Fisher's exact test.
The total number of unambiguous and likely SNN on which the statistics was performed for each item corresponds to the denominator. The numerator indicates the number of cases fulfilling the item and the percentage is given into brackets.
Significant clinical differences only occurred during the neuropathy onset. Usually, in possible SNN, onset was progressive and lower limbs were affected at first while in definite SNN, onset was acute/subacute and more frequently affected the upper limbs or the four limbs.
The electrophysiological study also demonstrated a similar pattern in the two subgroups. There were no significant differences in term of sensory abnormalities. The number of abolished SAP and the mean SAP amplitudes were similar in the four limbs. Concerning motor nerve conduction studies, abnormalities were significantly more frequent in the lower limbs in patients with paraneoplastic SNN consisting mostly of an axonal or intermediate pattern.
CSF examination was performed in 48 patients (3 cisplatin, 24 paraneoplastic and 21 likely SNN). It was significantly abnormal in patients with paraneoplastic SNN with a more frequent raised protein level and oligoclonal pattern. Twenty-two patients underwent spinal cord MRI (4 cisplatin, 2 paraneoplastic and 16 likely SNN). It showed abnormal high signal of the posterior column in only one of them. SEPs were studied in 25 patients (5 paraneoplastic, 2 cisplatin and 18 likely). They were not recordable in five and showed a clear involvement of both the peripheral and central process in seven. The last 13 patients had a severe peripheral involvement preventing any valuable recording of the central pathway.
Nerve biopsy was obtained in 16 patients, eight paraneoplastic and eight non-paraneoplastic (dysimmune or idiopathic) SNN. There were no differences concerning myelinated fibre density and morphological fibre changes between paraneoplastic and non-paraneoplastic SNN. Fibre loss was universal with a proportion of fibre undergoing axonal degeneration varying from 0% to 4%. Occasional regenerating clusters were encountered in one case of each group. One patient with dysimmune SNN had T-cell inflammatory infiltrates around epineurial blood vessels.
Comparison of SNN versus control sensory neuropathies
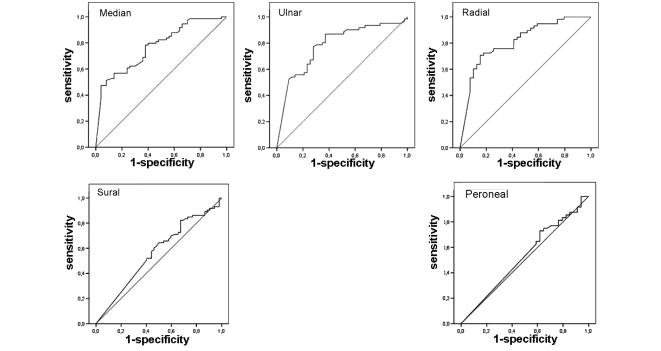
As unambiguous and likely SNN showed only few differences concerning clinical and electrophysiological presentation, data of the whole SNN population and controls were compared. Results are summarized in Table 2. Comparatively to SNN, controls had a neuropathy significantly restricted to the lower limbs, especially the distal part, with a symmetrical distribution. If the trunk was equally involved in both groups, controls did not show facial involvement. Ataxia and large sensory fibre involvement or autonomic perturbations were rarer. From the electrophysiological point of view, in the SNN group, motor nerve conduction was less severely altered in the four limbs. SAP amplitude was more severely reduced in the median, ulnar and radial nerves, and abolished SAP were more frequent in the upper limbs, but there was no difference in the lower limbs. ROC curves analysis of SAP amplitude expressed as a percentage of the lower limit of normal showed that it was not possible to determine a threshold value distinguishing SNN from controls in the lower limbs (superficial peroneal and sural nerves). In the upper limbs, the best compromise was a threshold of SAP amplitude <30% of the lower limit of normal that gave 70% sensitivity and specificity for the ulnar and median nerves, and 70% sensitivity and 85% specificity for the radial nerve in favour of SNN (Fig. 2).
Table 2.
Items differentiating SNN and controls in the study population
| Total cases = 134 | P-value | SNN (%) | Controls (%) | Sensitivity | Specificity | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Onconeural antibodies | <0.0001 | 31/78 (39.7) | 0/56 (0) | 0.4 | 1 | ∞ | 0.6 |
| Onseta | |||||||
| Acute | 0.0236 | 19/78 (24.3) | 5/56 (8.9) | 0.24 | 0.91 | 2.73 | 0.83 |
| Subacute | 0.0148 | 34/78 (43.6) | 13/56 (23.2) | 0.44 | 0.77 | 1.88 | 0.73 |
| Progressive | <0.0001 | 25/78 (32.0) | 37/56 (66.0) | 0.32 | 0.34 | 0.49 | 2 |
| Ataxia | <0.0001 | 33/78 (42.3) | 5/56 (8.9) | 0.42 | 0.91 | 4.74 | 0.63 |
| Asymmetry of sensory loss | 0.0067 | 31/78 (39.7) | 10/56 (17.8) | 0.4 | 0.82 | 2.23 | 0.73 |
| Including upper limb | <0.0001 | 54/78 (69.2) | 15/56 (26.8) | 0.69 | 0.73 | 2.58 | 0.42 |
| Including LL | 0.001 | 46/78 (58.9) | 48/56 (85.7) | 0.59 | 0.14 | 0.69 | 2.87 |
| Clinical manifestations—at full development | |||||||
| Distal upper limb | <0.0001 | 69/78 (88.5) | 28/56 (50.0) | 0.88 | 0.5 | 1.77 | 0.23 |
| Proximal upper limb | 0.0103 | 9/78 (11.5) | 0/56 (0) | 0.12 | 1 | ∞ | 0.88 |
| Including upper limb | <0.0001 | 73/78 (93.6) | 28/56 (50.0) | 0.94 | 0.5 | 1.87 | 0.13 |
| Lower limb only | <0.0001 | 5/78 (6.4) | 27/56 (48.2) | 0.06 | 0.52 | 0.13 | 1.81 |
| Four limbs | <0.0001 | 70/78 (89.7) | 28/56 (50.0) | 0.9 | 0.5 | 1.79 | 0.21 |
| Face involvement | 0.0206 | 8/78 (10.2) | 0/56 (0) | 0.1 | 1 | ∞ | 0.9 |
| Asymmetry of sensory loss | <0.0001 | 36/78 (46.1) | 7/56 (12.5) | 0.46 | 0.88 | 3.69 | 0.62 |
| Non length dependent distribution | <0.0002 | 64/78 (82.0) | 29/56 (51.8) | 0.82 | 0.48 | 1.58 | 0.37 |
| Superficial and deep sensation | 0.0023 | 73/78 (93.6) | 42/56 (75) | 0.94 | 0.25 | 1.25 | 0.26 |
| Ataxia (upper limb or lower limb) | <0.0001 | 55/78 (70.5) | 14/56 (25) | 0.71 | 0.75 | 2.82 | 0.39 |
| Dysautonomia | 0.0124 | 17/78 (21.8) | 3/56 (5.4) | 0.22 | 0.95 | 4.07 | 0.83 |
| CSFb | |||||||
| Raised protein | 0.0052 | 33/48 (68.8) | 10/20 (50.0) | 0.69 | 0.5 | 1.38 | 0.63 |
| Raised cell number | 0.0002 | 18/48 (37.5) | 0/20 (0) | 0.38 | 1 | ∞ | 0.63 |
| Oligoclonal pattern | 0.023 | 9/29 (31.0) | 0/20 (0) | 0.24 | 1 | ∞ | 0.76 |
| Nerve conduction study: sensory nerves | |||||||
| Sensory action potential median (μV) | <0.0001 | 2.53 ± 3.34 | 7.06 ± 5.91 | – | – | – | – |
| Sensory action potential ulnar (μV) | 0.0054 | 1.92 ± 4.78 | 4.44 ± 3.92 | – | – | – | – |
| Sensory action potential radial (μV) | <0.0001 | 3.69 ± 4.72 | 12.21 ± 9.04 | – | – | – | – |
| ≥1 Sensory action potential abolished in upper limb | <0.0001 | 48/78 (61.5) | 7/56 (12.5) | 0.62 | 0.88 | 4.92 | 0.44 |
| ≥2 Sensory action potential abolished in upper limb | <0.0001 | 28/78 (35.9) | 3/56 (5.4) | 0.36 | 0.95 | 6.7 | 0.68 |
| ≥3 Sensory action potential abolished in upper limb | 0.0078 | 15/78 (19.2) | 2/56 (3.6) | 0.19 | 0.96 | 5.38 | 0.84 |
| ≥2 Sensory action potential <30% lower limit of normal upper limb | <0.0001 | 49/78 (62.8) | 12/56 (21.4) | 0.63 | 0.79 | 2.93 | 0.47 |
| ≥3 sensory action potential <30% lower limit of normal upper limb | <0.0001 | 29/49 (37.2) | 4/56 (7.1) | 0.37 | 0.96 | 9.25 | 0.11 |
| ≥1 sensory action potential = 0 or 3 sensory action potential <30% lower limit of normal upper limb | <0.0001 | 53/78 (67.9) | 8/56 (17.8) | 0.68 | 0.86 | 4.76 | 0.20 |
| Nerve conduction study: motor nerves | |||||||
| Motor conduction velocities median (ms–1) | 0.0046 | 50.19 ± 5.69 | 46.5 ± 8.23 | – | – | – | – |
| Motor conduction velocities ulnar (ms−1) | 0.0128 | 51.39 ± 6.19 | 47.43 ± 10.4 | – | – | – | – |
| Compound muscle action potentials Peroneal (mV) | <0.0001 | 3.48 ± 1.9 | 1.84 ± 1.84 | – | – | – | – |
| Motor conduction velocities Peroneal ms–1) | <0.0001 | 43.7±4.97 | 33.44 ± 12.9 | – | – | – | – |
| Compound muscle action potentials Tibial (mV) | <0.0001 | 6.19 ± 3.49 | 2.76 ± 2.99 | – | – | – | – |
| Motor conduction velocities Tibial (ms–1) | <0.0001 | 40.64 ± 4.28 | 30.44 ± 14.2 | – | – | – | – |
| All motor nerves normal lower limb | 0.0001 | 34/55 (61.2) | 8/55 (14.5) | 0.62 | 0.85 | 4.25 | 0.45 |
| All motor nerves normal | 0.0005 | 33/76 (43.4) | 8/56 (14.3) | 0.43 | 0.86 | 3.04 | 0.66 |
| ≥2 abnormal motor nerve lower limb | <0.0001 | 29/76 (38.1) | 42/54 (77.8) | 0.38 | 0.22 | 0.49 | 2.78 |
| No/minor motor abnormalities | <0.0001 | 49/76 (64.5) | 12/56 (21.4) | 0.64 | 0.79 | 3.01 | 0.45 |
Compares the clinical manifestations at onset and at the maximum development of the neuropathy, cerebrospinal fluid and nerve conduction study abnormalities.
P-value was determined with the Fisher exact test for frequency comparison and with the Student t-test for numerical continuous variables. The number of SNN and controls on which the statistics was performed for each item corresponds to the denominator. The numerator indicates the number of case fulfilling the item and the percentage is given into brackets. For compound muscle action potentials, sensory action potential and motor conduction velocities the mean value_standard deviation is indicated.
LR = likelihood ratio.
Figure 2.
ROC curves for the determination of threshold differentiating SNN from other neuropathies for sensory action potentials expressed as a percentage of the lower limit of normal for the median, ulnar, radial, sural and superficial peroneal nerves.
Similar results were observed when comparing independently unambiguous SNN and likely SNN with controls.
Model testing for diagnostic criteria for SNN
Among the 85 items tested on the study population for the elaboration of diagnostic criteria, those with the best positive discriminative value (highest likelihood ratio+) were ataxia at onset or main development of the neuropathy, clinical asymmetry of sensory loss, at least one or two abolished SAP in the upper limb or the combination of at least one abolished SAP or at least three SAPs <30% of the lower limit of normal in the upper limbs, and no or less than one motor nerve with abnormal nerve conduction studies in the lower limbs. The best negative discriminative items (lowest likelihood ratio–) were the absence of clinical upper limb involvement or no large sensory fibre perturbation and a clinical involvement restricted to lower limbs (Table 2). However, as none of them reached a discriminative level (likelihood ratio+ > 10 or likelihood ratio– <0.1), several models of combined criteria were constructed with the study population. Each model was elaborated by entering into a logistic regression the variables included in these models (Table 3). For scoring the different models, variables in the model were weighted by their respective logistic regression coefficient. Models 1 and 5 were obtained by entering in block the items of the Asbury's and PNSEuronet group criteria, respectively. Models 2–4 are examples of models built arbitrarily by testing in block several combinations of variables with the best likelihood ratio+ or likelihood ratio–. Finally, Model 6 was provided by the stepwise logistic regression after entering all the items having the best likelihood ratio+ or likelihood ratio−. Model 7 corresponded to Model 6 with the adjunction of two further variables corresponding to items obtained after the initial workup and (i) making the diagnosis of SNN unambiguous (detection of onconeural antibodies or presence of a context of cisplatin treatment) or (ii) excluding SNN (presence of anti-MAG or anti-disialosyl antibodies, nerve conduction studies indicating demyelination, a context of diabetes mellitus, treatment-induced toxicity other than cisplatin or B12 deficiency).
Table 3.
Models of diagnostic criteria
| Model | Study population |
Study population Percentage of correct diagnosis with the jackknife method |
Test population |
|||||
|---|---|---|---|---|---|---|---|---|
| Area under ROC curve (95% CI) | All SNN | Unambiguous SNN | Likely SNN | Control | Sensitivity | Specificity | Likelihood ratio | |
| 1 | 0.78 (0.70–0.86) | 51.3 | 42.8 | 61.8 | 85.7 | 0.92 | 0.96 | 23.00 |
| 2 | 0.87 (0.80–0.93) | 39.5 | 33.3 | 47.0 | 91.1 | 0.75 | 0.84 | 4.69 |
| 3 | 0.92 (0.85–0.95) | 63.8 | 67.5 | 59.4 | 94.5 | 0.75 | 0.88 | 6.25 |
| 4 | 0.89 (0.83–0.94) | 86.8 | 83.3 | 91.2 | 79.6 | 0.92 | 0.84 | 5.75 |
| 5 | 0.88 (0.83–0.94) | 66.7 | 77.3 | 52.9 | 78.6 | 0.92 | 0.84 | 5.75 |
| 6 | 0.94 (0.90–0.98) | 81.6 | 76.2 | 88.2 | 90.7 | 0.92 | 1.00 | ∞ |
| 7 | 0.99 (0.98–1.00) | 94.7 | 100.0 | 88.2 | 94.4 | 0.92 | 1.00 | ∞ |
| Model | Model formulation | Threshold | ||||||
| 1 | 1.35 × NLD distribution + 1.95 × no or minor motor NCS abnormalities (Asbury's criteria) | 2.6 | ||||||
| 2 | 1.23 × NLD distribution + 2.5 × ataxia + 2.35 × no or minor motor NCS abnormalities | 4 | ||||||
| 3 | 2.12 × upper limb involvement + 2.34 × ataxia + 3.06 × normal motor NCS in LL + 2.12 × > 1 SAP = 0 in UL | 6 | ||||||
| 4 | 2.35 × UL involvement + 2.62 × > 1 SAP = 0 in UL + 2.22 × <2 motor nerves with abnormal NCS in LL | 4.5 | ||||||
| 5 | 1.09 × subacute onset – 0.41 × paresthesia + 0.14 × pain + 1.16 × asymmetry + 2.51 × Rankin >3 + 1.64 × UL involvement + 1.44 × deep sensation involvement – 1.37 > 1 SAP = 0 (PNSEuronet group criteria) | 3 | ||||||
| 6 | 3.1 × ataxia + 2.04 × sensory loss not limited to LL + 1.74 × asymmetrical distribution of sensory loss + 2.82 × > 1 SAP = 0 or 3 SAP < 30% lower limit of normal in UL + 3.08 × < 2 motor nerves with abnormal NCS in LL | 6.5 | ||||||
| 7 | Model 6 + 22.26 × onconeural antibody/cisp – 22.13 × biology or electroneuromyography excluding SNN | 6.5 | ||||||
Different tested models are showed with their area under the ROC curve + 95% CI and the percentage of correct diagnosis after jackknife on the study population and their sensitivity, specificity and likelihood ratio on the test population.
The different models are described as a mathematical formula where each clinical or electrophysiological item must be coded as 1 or 0 according as to whether the condition is fulfilled or not and multiplied by the logistic regression coefficient. The threshold differentiating patients as having or not having SNN is determined by the ROC curve.
NLD = non-length-dependent distribution, UL = upper limb, LL = lower limb, NCS = nerve conduction study, Cisp = cisplatin treatment.
NLD or asymmetrical distribution and UL or LL involvement correspond to the clinical distribution of sensory loss.
In the study population, the jackknifed Asbury's criteria gave wholly poor results while the PNSEuronet group criteria correctly identified 77.3% of unambiguous SNN (84.8% in the paraneoplastic group), but only 52.9% of patients in the likely group. The best results were obtained with Model 6 which correctly identified 81.6% of the SNN patients and 90.7% of controls. Adjunction of contextual items improved the correctness of the diagnosis in the unambiguous and control groups.
In the small external prospective test population, Model 1 had good accuracy when compared with the clinician diagnosis (likelihood ratio+ = 23), but Model 6 was superior as the Asbury's criteria detected as a positive case a patient initially diagnosed as having SNN, a diagnosis ruled out later by a complementary ENMG and biological investigations and excluded by Model 6.
Finally, considering Model 6 as the best one in both the jackknifed study and test population, and taking into account the interest of the initial biological and electrophysiological workup, we propose the following easy-to-use score form for the diagnosis of SNN in which the coefficient of each variable is the logistic regression coefficient:
| A In a patient with a clinically pure sensory neuropathy a diagnosis of SNN is considered as possible if score >6.5 | ||
| Yes | Points | |
| a—Ataxia in the lower or upper limbs at onset or full development | □ | +3.1 |
| b—Asymmetrical distribution of sensory loss at onset or full development | □ | +1.7 |
| c—Sensory loss not restricted to the lower limbs at full development | □ | +2.0 |
| d—At least 1 SAP absent or 3 SAP <30% of the lower limit of normal in the upper limbs, not explained by entrapment neuropathy | □ | +2.8 |
| e—Less than two nerves with abnormal motor nerve conduction studies in the lower limbs | □ | +3.1 |
| If >6.5, a diagnosis of SNN is possible | Total | |
| B A diagnosis of SNN is probable if the patient's score is >6.5 and if: | ||
| 1. The initial workup does not show biological perturbations or ENMG findings excluding SNN and | ||
| 2. The patient has one of the following disorders: onconeural antibodies or a cancer within 5 years (Graus et al., 2004), cisplatin treatment, Sjögren's syndrome (Vitali et al., 2002). | ||
| 3. Or MRI shows high signal in the posterior column of the spinal cord | ||
| C A diagnosis of SNN is definite if dorsal root ganglia degeneration is pathologically demonstrated although dorsal root ganglia biopsy is not recommended. | ||
Discussion
Because of the frequent absence of reference standard, diagnostic criteria of peripheral neuropathies have often been established on expert consensus raising the question of whether methodologies independent of subjective appreciations would be more pertinent. However, this remains a difficult challenge because none of these methods is free of potential bias especially in the selection of the reference and control populations. Here, to limit bias due to selection of diagnostic criteria on a preconceived representation of what a SNN should be, we used logistic regression to identify clinical and electrophysiological items that may accurately identify SNN from other sensory neuropathies. In the absence of definite proof of dorsal root ganglia involvement or other gold standard, we provisionally classified the SNN patients in the study population according to two levels of certitude for the diagnosis of SNN. The first was based on the fair demonstration in the scientific literature that the underlying disorder results from a primary degeneration of sensory neurons in dorsal root ganglia (e.g. paraneoplastic cases) and the second, on the absence of ambiguity concerning the main lesion site when several forms of neuropathy may be associated with the said disorder (Sjögren's syndrome or HIV infection for example). Despite this distinction, the general clinical and electrophysiological pattern was similar in unambiguous and likely SNN. This is an important point since it first shows that a general pattern of SNN can be established independently of the underlying disorder and second that patients here provisionally classified as likely SNN and who mostly conformed to the Asbury's criteria have a great chance to actually have developed a lesion in the dorsal root ganglia and hence that the whole study population could be used to establish diagnostic criteria.
Selection of the control population is another important methodological issue since it determines the negative reference against which the SNN population was compared. Here, we selected patients with neuropathies that can be without doubt (e.g. demyelinating neuropathy or mononeuritis multiplex) or likely (e.g. distal axonal sensory neuropathy) classified as non-SNN. These cases were selected not for the relative frequency of their neuropathy in our general population of neuropathy but to represent an as large as possible panel of the different patterns of sensory neuropathies.
By comparing SNN to other sensory neuropathies several statistically significant distinctive features appeared. Although we cannot rule out that some of them may have been selected by chance, the whole pattern of a predominantly distal sensory neuropathy with almost universal upper limb involvement extending sometimes to the face or trunk and frequent alteration of deep sensation conforms to what has been previously reported (Dalakas, 1986; Griffin et al., 1990; Windebank et al., 1990; Sobue et al., 1993; Mori et al., 2005). However, behind this general presentation we observed variants that were not specific of a given aetiology, but need to be identified as SNN by any proposed set of diagnostic criteria. These include forms with only patchy sensory loss, mild electrical motor nerve abnormalities and predominant small fibre or isolated lower limb involvement. The existence of SNN restricted to small sensory neurons is still debated. It has been reported with Sjögren's syndrome (Mori et al., 2003; Chai et al., 2005), coeliac disease (Brannagan et al., 2005) or idiopathic cases (Gorson et al., 2008), but has not been demonstrated yet by an examination of dorsal root ganglia. In this study, all the patients with a clinical small fibre neuropathy had abnormal SAP indicating an associated involvement of large neurons.
An electrophysiological pattern common to acquired SNN could also be established. Diffuse abnormal SAP recording was a universal feature but with important individual variations. Abolition of SAP in the upper limbs was frequent but not universal and a reduction of SAP <30% of the lower limit of normal in at least three nerves in the upper limbs, especially in the radial nerve was more discriminative. Mild motor nerve abnormalities were not uncommon particularly with paraneoplastic disorders (Camdessanche et al., 2002). These results contrast with studies that stressed the severity of sensory nerve abnormalities and the almost absence of motor nerve perturbations in SNN possibly because patients have been selected with too restrictive criteria (Dalakas, 1986; Windebank et al., 1990; Chalk et al., 1992; Lauria et al., 2003). Extension of electrophysiological abnormalities to motor fibres without any clinical counterpart probably results from a diffusion of the pathological process beyond sensory ganglia into the peripheral nerves. This is in keeping with the finding of inflammatory changes in peripheral nerves of patients with demonstrated SNN (Antoine et al., 1998; Mori et al., 2005). Finally, the only distinctive features between unambiguous and likely SNN concerned onset and CSF. Both were linked to the nature of the process underlying the neuropathy and could not be used for the elaboration of diagnostic criteria.
As peripheral neuropathy is a frequent cause of referral, there is a need for easy-to-use criteria that can differentiate SNN from other neuropathies and be sensitive enough to pick up the unusual variants. To be utilized in non-specialized centres, these criteria must also draw on materials available on first-hand investigation such as clinical examination and electrophysiological study, and so the Asbury's criteria was built (Asbury, 1987; Asbury and Brown, 1990). However, we found that although patients with likely SNN mostly conform to the Asbury's criteria, these criteria had low accuracy because first, a non-length-dependent distribution is not specific enough as it also occurs with other neuropathies such as B12 deficiency, sensory variants of, or neuropathies due to vasculitis or leprosy; and second, a significant but limited electrophysiological motor nerve involvement does not rule out a diagnosis of SNN. The PNSEuronet group criteria were accurate in patients with paraneoplastic SNN but were not reliable with other forms of SNN. Among the different models tested in this study, we selected the one built by the stepwise logistic regression that only used first-hand clinical data and ENMG recording of motor and sensory nerve conduction in the four limbs with at least a study of three sensory nerves in the upper limbs. This model was sensitive with both definite and possible SNN in the jackknifed study population where it identified the unusual forms of SNN and gave fairly good results in the external test population. In particular, it excluded cases for which the clinician diagnosis was initially hesitant. In addition it was effective in excluding non SNN sensory neuropathies. However, the perfect specificity of this model in the test population probably results from the relative small size of the sample that likely did not include all the possible presentations. Validation on a larger population of patients originating from different centres will be needed.
The patients detected by these criteria should be investigated for the usual aetiologies of SNN. This set of criteria does not take into account items that can guide to specific underlying causes. However, an acute or subacute onset and an inflammatory CSF strongly argues for a paraneoplastic origin while chronic evolution or normal CSF favours the hypothesis of a dysimmune or idiopathic SNN (Chalk et al., 1992; Dalakas, 1986; Graus et al., 2001). The diagnostic criteria specifically built for paraneoplastic disorders (Graus et al., 2004) or Sjögren's syndrome (Vitali et al., 2002) can be used for the aetiological diagnosis of SNN. Thus, in a patient with possible SNN according to clinical and electrophysiological data, a context of cisplatin treatment, detection of onconeural AB, occurrence of cancer in a short delay within the evolution of the neuropathy, diagnosis of an HIV infection or Sjögren's syndrome can be considered as evidences of probable SNN. The absence of diagnosis items that rule out SNN such as demyelinating features on the ENMG, or biological perturbations may also contribute to a probable diagnosis of SNN. However, this may be modulated since it cannot be excluded that some conditions to date considered as not associated with SNN may in the future prove to be a cause of ganglionopathy as it has been for example suggested with diabetes mellitus (Kishi et al., 2002). Here, we restricted the definite level of diagnosis to cases for which there is a pathological examination of dorsal root ganglia which is at present the only way to demonstrate neuron degeneration (Griffin et al., 1990; Colli et al., 2008). However, in want of a still unavailable method of non-traumatic exploration of dorsal root ganglia in living people, this level will seldom be reached as biopsy of these cells cannot be recommended as a routine examination.
Other markers have been proposed for the diagnosis of SNN. Spinal cord MRI may be used as a proxy of large sensory neuron degeneration. In our series, it was disappointing, possibly for technical reasons. Although it should be underlined that T2-high signal in the dorsal column is not specific of SNN as it occurs in B12 deficiency (Lauria et al., 2000), its presence may be a supportive argument and was considered as such in the proposed criteria. SEPs may also help to demonstrate an involvement of both the central and peripheral sensory pathway but the rapid degeneration of the peripheral process is a strong limitation to their interest.
Finally, skin biopsy has been used to demonstrate a non-length-dependant distribution of small fibre loss for the diagnosis of SNN (Lauria et al., 2001) and has recently been proposed for the investigation of patients with pure small fibre neuropathy (Sommer and Lauria, 2007). However, skin biopsy cannot presently be considered as a routine exam for the investigation of patients with sensory neuropathy, since it needs to be performed in specialized centres. This is the reason why we did not introduce this method in our criteria. When applied to the patients reported by Gorson et al. (2008) as having a painful neuropathy possibly due to a SNN restricted to small sensory neurons and diagnosed on skin biopsy the selected model gave positive results in only 3/23 of these patients. Skin biopsy can be helpful in difficult cases. Whether it may be used as an equivalent of the ENMG when the latter is normal needs specific investigations.
In conclusion, the method used here was for the first time applied to the elaboration of diagnostic criteria in a group of peripheral neuropathy for which there is yet no validated gold standard. Our study confirms that despite their different origins, acquired SNN have a common clinical and electrophysiological pattern distinctively different from other sensory neuropathies despite the existence of variants. With statistical methods powered for determination of diagnostic accuracy, we evaluated several sets of diagnostic criteria applicable in routine clinical care by any clinician as they rely only on clinical examination and ENMG. We found that one set had a good discriminative value and may be useful for the diagnosis of patients with sensory neuropathy.
Funding
Projet de Recherche Clinique Région Rhône-Alpes 2006.
Acknowledgement
The authors express their grateful thanks to Silvy Laporte and Emilie Presles for methodological statistical counselling and Tam Quash for English correction.
Glossary
Abbreviations
- CSF
cerebrospinal fluid
- ENMG
electroneuromyographic study
- ROC
receiver operator characteristic
- SAP
sensory action potentials
- SNN
sensory neuronopathies
References
- Antoine JC, Mosnier JF, Honnorat J, Convers P, Absi L, Lapras J, et al. Paraneoplastic demyelinating neuropathy, subacute sensory neuropathy, and anti-Hu antibodies: clinicopathological study of an autopsy case. Muscle Nerve. 1998;21:850–7. doi: 10.1002/(sici)1097-4598(199807)21:7<850::aid-mus2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Asbury AK. Sensory neuronopathy. Semin Neurol. 1987;7:58–66. doi: 10.1055/s-2008-1041406. [DOI] [PubMed] [Google Scholar]
- Asbury AK, Brown MJ. Sensory neuronopathy and pure sensory neuropathy. Curr Opin Neurol Neurosurg. 1990;3:708–11. [Google Scholar]
- Brannagan TH 3rd, Hays AP, Chin SS, Sander HW, Chin RL, Magda P, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Arch Neurol. 2005;62:1574–8. doi: 10.1001/archneur.62.10.1574. [DOI] [PubMed] [Google Scholar]
- Camdessanche JP, Antoine JC, Honnorat J, Vial C, Petiot P, Convers P, et al. Paraneoplastic peripheral neuropathy associated with anti-Hu antibodies. A clinical and electrophysiological study of 20 patients. Brain. 2002;125:166–75. doi: 10.1093/brain/awf006. [DOI] [PubMed] [Google Scholar]
- Chai J, Herrmann DN, Stanton M, Barbano RL, Logigian EL. Painful small-fiber neuropathy in Sjogren syndrome. Neurology. 2005;65:925–7. doi: 10.1212/01.wnl.0000176034.38198.f9. [DOI] [PubMed] [Google Scholar]
- Chalk CH, Windebank AJ, Kimmel DW, McManis PG. The distinctive clinical features of paraneoplastic sensory neuronopathy. Can J Neurol Sci. 1992;19:346–51. [PubMed] [Google Scholar]
- Colli BO, Carlotti CG, Jr, Assirati JA, Jr, Lopes Lda S, Marques W, Jr, Chimelli L, et al. Dorsal root ganglionectomy for the diagnosis of sensory neuropathies. Surgical technique and results. Surg Neurol. 2008;69:266–73. doi: 10.1016/j.surneu.2007.01.057. dicussion 273. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Chronic idiopathic ataxic neuropathy. Ann Neurol. 1986;19:545–54. doi: 10.1002/ana.410190605. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Furneaux HM, Rosenblum MK, Graus F, Posner JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology. 1991;41:1757–64. doi: 10.1212/wnl.41.11.1757. [DOI] [PubMed] [Google Scholar]
- Efron B. The jackknife, the bootstrap and other resampling plans. Philadelphia: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- Esiri MM, Morris CS, Millard PR. Sensory and sympathetic ganglia in HIV-1 infection: immunocytochemical demonstration of HIV-1 viral antigens, increased MHC class II antigen expression and mild reactive inflammation. J Neurol Sci. 1993;114:178–87. doi: 10.1016/0022-510x(93)90295-a. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Vernino SA, Freeman R. Combined immunomodulatory therapy in autoimmune autonomic ganglionopathy. Arch Neurol. 2008;65:213–7. doi: 10.1001/archneurol.2007.60. [DOI] [PubMed] [Google Scholar]
- Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998;101:2842–50. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorson KC, Herrmann DN, Thiagarajan R, Brannagan TH, Chin RL, Kinsella LJ, et al. Non-length dependent small fibre neuropathy/ganglionopathy. J Neurol Neurosurg Psychiatry. 2008;79:163–9. doi: 10.1136/jnnp.2007.128801. [DOI] [PubMed] [Google Scholar]
- Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Keime-Guibert F, Rene R, Benyahia B, Ribalta T, Ascaso C, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–48. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- Graus F, Ribalta T, Campo E, Monforte R, Urbano A, Rozman C. Immunohistochemical analysis of the immune reaction in the nervous system in paraneoplastic encephalomyelitis. Neurology. 1990;40:219–22. doi: 10.1212/wnl.40.2.219. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Cornblath DR, Alexander E, Campbell J, Low PA, Bird S, et al. Ataxic sensory neuropathy and dorsal root ganglionitis associated with Sjogren's syndrome. Ann Neurol. 1990;27:304–15. doi: 10.1002/ana.410270313. [DOI] [PubMed] [Google Scholar]
- Hainfellner JA, Kristoferitsch W, Lassmann H, Bernheimer H, Neisser A, Drlicek M, et al. T-cell-mediated ganglionitis associated with acute sensory neuronopathy. Ann Neurol. 1996;39:543–7. doi: 10.1002/ana.410390418. [DOI] [PubMed] [Google Scholar]
- Kishi M, Tanabe J, Schmelzer JD, Low PA. Morphometry of dorsal root ganglion in chronic experimental diabetic neuropathy. Diabetes. 2002;51:819–24. doi: 10.2337/diabetes.51.3.819. [DOI] [PubMed] [Google Scholar]
- Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rorth M, Krarup C. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain. 2007;130:1076–88. doi: 10.1093/brain/awl356. [DOI] [PubMed] [Google Scholar]
- Kuntzer T, Antoine JC, Steck AJ. Clinical features and pathophysiological basis of sensory neuronopathies (ganglionopathies) Muscle Nerve. 2004;30:255–68. doi: 10.1002/mus.20100. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Noda K, Mimori Y, Watanabe C, Katayama S, Nakamura S, et al. A case of pandysautonomia with associated sensory ganglionopathy. J Neurol Neurosurg Psychiatry. 1998;65:278–9. doi: 10.1136/jnnp.65.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki S, Shimizu J, Chiba A, Ugawa Y, Hitoshi S, Kanazawa I. Experimental sensory neuropathy induced by sensitization with ganglioside GD1b. Ann Neurol. 1996;39:424–31. doi: 10.1002/ana.410390404. [DOI] [PubMed] [Google Scholar]
- Lauria G, Pareyson D, Grisoli M, Sghirlanzoni A. Clinical and magnetic resonance imaging findings in chronic sensory ganglionopathies. Ann Neurol. 2000;47:104–9. [PubMed] [Google Scholar]
- Lauria G, Pareyson D, Sghirlanzoni A. Neurophysiological diagnosis of acquired sensory ganglionopathies. Eur Neurol. 2003;50:146–52. doi: 10.1159/000073055. [DOI] [PubMed] [Google Scholar]
- Lauria G, Sghirlanzoni A, Lombardi R, Pareyson D. Epidermal nerve fiber density in sensory ganglionopathies: clinical and neurophysiologic correlations. Muscle Nerve. 2001;24:1034–9. doi: 10.1002/mus.1107. [DOI] [PubMed] [Google Scholar]
- Mori K, Iijima M, Koike H, Hattori N, Tanaka F, Watanabe H, et al. The wide spectrum of clinical manifestations in Sjogren. Brain. 2005;128:2518–34. doi: 10.1093/brain/awh605. [DOI] [PubMed] [Google Scholar]
- Mori K, Iijima M, Sugiura M, Koike H, Hattori N, Ito H, et al. Sjogren. J Neurol Neurosurg Psychiatry. 2003;74:1320–2. doi: 10.1136/jnnp.74.9.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima T, Yamamura S, Hamada K, Kawasaki S, Ideta T, Ueno H, et al. Chronic sensory and autonomic neuropathy. Neurology. 1983;33:1061–4. doi: 10.1212/wnl.33.8.1061. [DOI] [PubMed] [Google Scholar]
- Scaravilli F, Sinclair E, Arango JC, Manji H, Lucas S, Harrison MJ. The pathology of the posterior root ganglia in AIDS and its relationship to the pallor of the gracile tract. Acta Neuropathol. 1992;84:163–70. doi: 10.1007/BF00311390. [DOI] [PubMed] [Google Scholar]
- Sghirlanzoni A, Pareyson D, Lauria G. Sensory neuron diseases. Lancet Neurol. 2005;4:349–61. doi: 10.1016/S1474-4422(05)70096-X. [DOI] [PubMed] [Google Scholar]
- Sobue G, Yanagi T, Hashizume Y. Chronic progressive sensory ataxic neuropathy with polyclonal gammopathy and disseminated focal perivascular cellular infiltrations. Neurology. 1988;38:463–7. doi: 10.1212/wnl.38.3.463. [DOI] [PubMed] [Google Scholar]
- Sobue G, Yasuda T, Kachi T, Sakakibara T, Mitsuma T. Chronic progressive sensory ataxic neuropathy: clinicopathological features of idiopathic and Sjogren's syndrome-associated cases. J Neurol. 1993;240:1–7. doi: 10.1007/BF00838437. [DOI] [PubMed] [Google Scholar]
- Sommer C, Lauria G. Skin biopsy in the management of peripheral neuropathy. Lancet Neurol. 2007;6:632–42. doi: 10.1016/S1474-4422(07)70172-2. [DOI] [PubMed] [Google Scholar]
- Thomas PK, Ochoa J. Symptomatology and differential diagnosis of peripheral neuropathy. In: PJ Dyck, PK Thomas., editors. Peripheral neuropathy. II. Philadelphia: Saunders, W.B. Company; 1993. pp. 749–74. [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanschitz J, Hainfellner JA, Kristoferitsch W, Drlicek M, Budka H. Ganglionitis in paraneoplastic subacute sensory neuronopathy: a morphologic study. Neurology. 1997;49:1156–9. doi: 10.1212/wnl.49.4.1156. [DOI] [PubMed] [Google Scholar]
- Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313:793–9. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- Windebank AJ. Neurotoxicity of pyridoxine analogs is related to coenzyme structure. Neurochem Pathol. 1985;3:159–67. doi: 10.1007/BF02834268. [DOI] [PubMed] [Google Scholar]
- Windebank AJ, Blexrud MD, Dyck PJ, Daube JR, Karnes JL. The syndrome of acute sensory neuropathy: clinical features and electrophysiologic and pathologic changes. Neurology. 1990;40:584–91. doi: 10.1212/wnl.40.4.584. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–83. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]