Summary
The Leishmania major aquaglyceroporin, LmAQP1, is responsible for the transport of antimonite [Sb(III)], an activated form of Pentostam or Glucantime. Downregulation of LmAQP1 provides resistance to trivalent antimony compounds and increased expression of LmAQP1 in drug resistant parasites can reverse the resistance. Besides metalloid transport, LmAQP1 is also permeable to water, glycerol, methylglyoxal, dihydroxyacetone, and sugar alcohols. LmAQP1 also plays a physiological role in volume regulation and osmotaxis. In this study we examined the role of extracellular C-loop glutamates (Glu143, Glu145, and Glu152) in LmAQP1 activity. Alteration of both Glu143 and Glu145 to alanines did not affect either the biochemical or physiological properties of the protein suggesting that neither residue is critical for LmAQP1 activity. Alteration of Glu152 to alanine, aspartate and glutamine affected metalloid transport in the order, wild-type > E152Q > E152D > E152A. In fact, axenic amastigotes expressing E152A LmAQP1 accumulated negligible levels of either arsenite [As(III)] or Sb(III). Alteration of Glu152 significantly affected volume regulation and osmotaxis suggesting that Glu152 is critical for the physiological activity of the parasite. More importantly, alteration of Glu152 to alanine did not affect glycerol permeability. Although the metalloids, As(III) and Sb(III), are believed to be transported through aquaglyceroporin channels as they behave as inorganic molecular mimic of glycerol, this is the first report where metalloid and glycerol transport can be dissected by a single mutation at the extracellular pore entry of LmAQP1 channel.
Keywords: Antimonite, arsenite, aquaglyceroporin, C-loop, glutamates, LmAQP1, Leishmania, NIPs, osmotaxis, volume regulation
Introduction
Leishmania are parasitic protozoa that are transmitted by the bite of a sand fly and cause leishmaniasis in humans and other mammals. The disease ranges from self-healing cutaneous lesions to nonhealing mucocutaneous and visceral ailments that affect over 12 million people worldwide. The Leishmania parasite exists in two morphologically distinct forms. The promastigote form of the parasite resides in the intestinal tract of the sand fly vector and appears as a slender, spindle shaped structure with an anterior flagellum. The amastigote forms of the parasite are small, oval shaped structures that reside in macrophages and other mononuclear phagocytes in the mammalian host. The first line choice of treatment against all forms of leishmaniasis uses drugs containing pentavalent antimony [Sb(V)] such as sodium stibogluconate (Pentostam) and meglumine antimonate (Glucantime).
The Leishmania major aquaglyceroporin (LmAQP1) is responsible for the transport of antimonite [Sb(III)], an activated form of Pentostam or Glucantime (Gourbal et al., 2004). Disruption of one of the two LmAQP1 alleles in L. major confers a 10-fold increase in resistance to Sb(III) (Gourbal et al., 2004). We have previously shown that loss of LmAQP1 can produce resistance and conversely increased expression of LmAQP1 in drug resistant parasites can reverse resistance (Gourbal et al., 2004, Marquis et al., 2005). LmAQP1 mRNA levels are significantly less in either the Sb(III) or arsenite [As(III)] resistant L. major and L. tarentolae cells, indicating that downregulation of LmAQP1 leads to drug resistance (Marquis et al., 2005).
Besides being an adventitious metalloid transporter, LmAQP1 is also permeant to water; its water conduction capacity is 65% that of human AQP1, which is a classical water channel. In contrast to Plasmodium and Trypanosome AQPs that are inhibited by mercurials, LmAQP1 water movement is not inhibited by mercuric chloride and is therefore a mercurial independent water channel. LmAQP1 also conducts glycerol, glyceraldehyde, dihydroxyacetone, and sugar alcohols. In contrast, there is negligible urea conduction by LmAQP1, and this property probably helps the parasite to survive the hostile environment of liver cells (Figarella et al., 2007). Expression of the protein is limited exclusively to the flagellum of promastigotes and in amastigotes it is found in the flagellar pocket, rudimentary flagellum, and contractile vacuoles. LmAQP1 plays an important physiological role in water and solute transport, volume regulation and osmotaxis. These properties help the parasite to face the osmotic challenges during its swim towards the proboscis of the sandfly and transmission to the vertebrate host (Figarella et al., 2007). It is therefore clearly evident that LmAQP1 plays a major role in Leishmania cellular physiology and drug resistance.
LmAQP1 shares 32% identity and 50% similarity with the Plasmodium falciparum aquaglyceroporin PfAQP. In comparison to the Escherichia coli aquaglyceroporin, GlpF, which is a glycerol channel with low water permeability, both LmAQP1 and PfAQP conduct glycerol and water at high rates (Figarella et al., 2007, Hansen et al., 2002). Beitz et al (Beitz et al., 2004) have shown that Glu125 in the extracellular C-loop is critically responsible for the high water permeability of PfAQP. Alteration of Glu125 to serine greatly reduces water but not glycerol permeability. The crystal structure of PfAQP indicates that Glu125 anchors the C-loop in place by hydrogen bonding with Ser200 and Thr212, and consequently the neighboring Trp124 is hydrogen bonded with Arg196 in the selectivity filter. Alteration of Glu125 to serine eliminates the stabilization of the C-loop, which in turn disrupts the hydrogen bonding between Arg196 and Trp124, resulting in increased solvent interaction of Arg196 and a higher barrier for passage of water. In contrast, the hydroxyl groups of glycerol have lower polarity than in water, and are not held back similarly (Newby et al., 2008).
A topology prediction of LmAQP1 based on the crystal structure of PfAQP (Newby et al., 2008) is shown in Fig. 1. The model indicates that LmAQP1 consists of six membrane spanning helices, and two membrane spanning half-helices containing the canonical Asn-Pro-Ala (NPA) motifs. These helices are connected by five loops (AE). The C-loop of LmAQP1 is slightly longer than PfAQP and has three glutamates at positions 143, 145 and 152; Glu152 being at the homologous position to Glu125 of PfAQP. Arg 230 (homologous to Arg196 of PfAQP) forms part of the selectivity filter and alteration of this residue severely affects the transport property of LmAQP1 (Figarella et al., 2007). The other residues that are hydrogen bonded to Glu125 and Arg196 of PfAQP are not conserved in LmAQP1. The objective of this work was to examine the role of C-loop glutamates (Glu143, Glu145 and Glu152) of LmAQP1 in solute permeability and physiological activity of the parasite. The results indicate that while Glu143 and Glu145 have no role in LmAQP1 activity, alteration of Glu152 to alanine selectively abrogates metalloid permeability but does not affect glycerol transport. We also demonstrate that Glu152 is critical for the physiological response of Leishmania to osmotic stress conditions.
Figure 1.
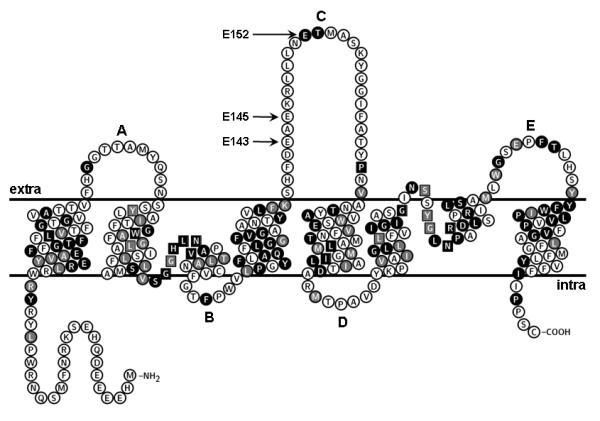
Predicted topology of LmAQP1. Amino acids labeled as white letters in black circles are conserved between LmAQP1 and PfAQP; residues marked as white letters in gray circles are similar to PfAQP; black and gray squares indicate conserved and similar residues, respectively, which directly interact with glycerol in PfAQP. This topology was plotted using the TeXtopo package (Beitz, 2000).
Results
Expression and localization of the C-loop glutamate mutants
LmAQP1 C-loop glutamates, E143 and E145, were altered by site directed mutagenesis to create E143A, E145A, and E143A/E145A derivatives. Similarly, Glu152 was individually changed to alanine, aspartate, and glutamine to produce LmAQP1 derivatives E152A, E152D, and E152Q, respectively. Xenopus laevis oocytes were injected with the mutant LmAQP1 cRNA and expression of the altered proteins were monitored by Western blotting with a rabbit polyclonal antibody raised against a synthetic peptide corresponding to amino acids 139-152 of LmAQP1. Each of the point mutants of Glu143, Glu145, and Glu152 were expressed on oocyte membranes at levels similar to that of wild-type LmAQP1 (data not shown). Immunofluorescence experiments were also performed to reveal the localization of the altered proteins in L. donovani promastigotes. Promastigotes expressing either the wild-type or altered LmAQP1 showed selective immunostaining of the flagella (data not shown).
Effect of alteration of C-loop glutamates on water and solute permeability
The water permeability of both wild-type and altered LmAQP1 was evaluated by expression in Xenopus oocytes. After 3 days of incubation at 16°C, oocytes expressing either the wild-type or altered LmAQP1 were subjected to hypotonic shock in 1:3 diluted ND96 medium, which established an outwardly directed osmotic gradient of 140 mOsm. Oocytes injected with wild-type LmAQP1 cRNA exhibited a ∼20-fold (Pf ≈ 182 μm s-1) increase in swelling rate compared with the water injected control (Fig. 2). The water permeability of the doubly altered E143A/E145A mutant was similar to that of the wild-type. However, the water permeability of the Glu152 mutants varied considerably in the order, wild-type > E152Q > E152D > E152A; E152A exhibiting a 50% reduction in water permeability than the wild-type.
Figure 2.
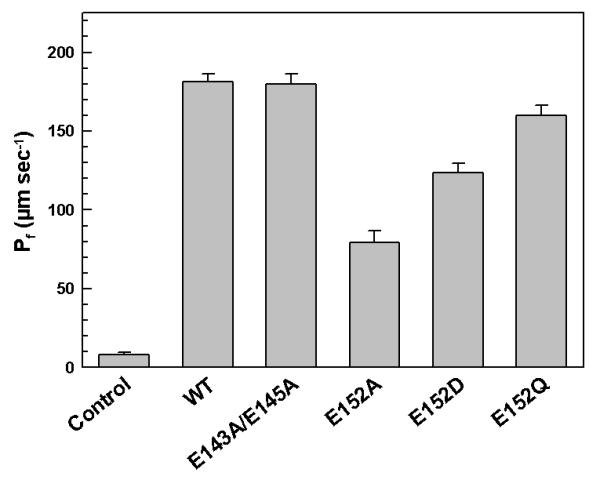
Water transport in Xenopus oocytes expressing altered LmAQP1. Oocytes were injected with water (control), wild-type, E143A/E145A, E152A, E152D, and E152Q LmAQP1-cRNA.
We have previously shown that LmAQP1-expressing oocytes also transport glycerol, methylglyoxal, dihydroxyacetone, glyceraldehyde and adonitol (Figarella et al., 2007). The glycerol permeability of the LmAQP1 mutants was determined by an isosmotic swelling assay with a 130 mM inwardly directed gradient of the solutes. This concentration gradient leads to an influx of solutes, resulting in a secondary influx of water, and consequently swelling of the oocytes. Injection of oocytes with either the wild-type or C-loop glutamate mutants showed similar transport activity for glycerol, methylglyoxal, and dihydroxyacetone (Fig. 3). In contrast, both E152D and E152Q LmAQP1 showed a marginal reduction in glyceraldehyde transport, but ∼50% reduction in adonitol permeability. The E152A mutant showed 2-fold and 6-fold reduction in glyceraldehyde and adonitol permeability, respectively (Fig. 3).
Figure 3.
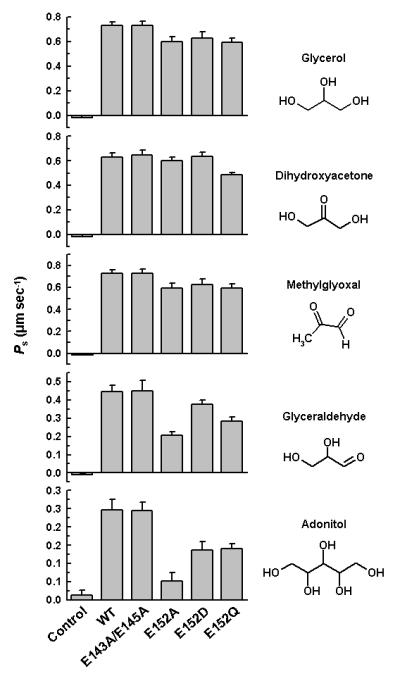
Solute permeability in Xenopus oocytes expressing altered LmAQP1. Oocytes were injected with water (control), wild-type, E143A/E145A, E152A, E152D, and E152Q LmAQP1-cRNA.
Effect of alteration of C-loop glutamates on metalloid sensitivity and transport
Both wild-type and altered LmAQP1 were cloned in the Leishmania expression vector pSP72αhygroα, and the resulting plasmids were transfected into L. donovani strain LdBob, as described in the Experimental Procedures section. As(III) and Sb(III) sensitivity was examined in Leishmania promastigotes while Sb(V) sensitivity was studied in intracellular amastigotes. Sb(V) is taken up by macrophages, and is reduced to Sb(III), which is then transported into Leishmania amastigote by LmAQP1. Each of the transfected strains showed varying levels of sensitivity to As(III), Sb(III), and Sb(V), when compared to vector alone control (Table I). Promastigotes or amastigotes overexpressing either wild-type or E143A/E145A LmAQP1 were hypersensitive to metalloids and both showed similar EC50 values. Although, cells expressing E152Q LmAQP1 showed slightly lower sensitivity than the wild-type, E152D mutant showed an intermediate level of resistance, whereas cells expressing E152A LmAQP1 were even more resistant to metalloids but less than cells expressing the vector control.
Table I.
Metalloid sensitivity of L. donovani expressing altered LmAQP1
| LmAQP1 | EC50 |
||
|---|---|---|---|
| [As(III)] μM | [Sb(III)] μM | [Sb(V)] μg/ml | |
| vector only | 18 ± 1 | 145 ± 45 | 245 ± 40 |
| wild type | 0.3 ± 0.02 | 0.4 ± 0.05 | 48 ± 4 |
| E143A/E145A | 0.3 ± 0.04 | 0.4 ± 0.1 | 50 ± 5 |
| E152A | 1.6 ± 0.2 | 70 ± 8 | 220 ± 50 |
| E152D | 0.7 ± 0.2 | 8 ± 3 | 90 ± 6 |
| E152Q | 0.3 ± 0.04 | 2.2 ± 0.7 | 65 ± 8 |
EC50 values for As(III) and Sb(III) were determined in L. donovani promastigotes transfected with wild type, E143A/E145A, E152A, E152D, E152Q LmAQP1 or vector control. Values for Sb(V) were determined for intracellular amastigotes.
The ability of LmAQP1 C-loop glutamate mutants to transport As(III) and Sb(III) was also investigated (Fig. 4). Promastigotes and amastigotes expressing E143A/E145A LmAQP1 showed similar metalloid uptake as the wild-type. Cells expressing E152Q LmAQP1 accumulated slightly lower levels of As(III) or Sb(III) than wild-type. Interestingly, although axenic amastigotes expressing E152Q LmAQP1 showed similar sensitivity to Sb(III) as the wild-type (Table I), they transported much less Sb(III) (Fig. 4D). Promastigotes and amastigotes expressing E152D LmAQP1 showed an intermediate level of metalloid uptake. Axenic amastigotes expressing E152A LmAQP1 accumulated negligible levels of As(III) or Sb(III) (Fig. 4B, D) whereas the promastigotes showed slightly higher levels of uptake than the vector alone control (Fig. 4A, C).
Figure 4.
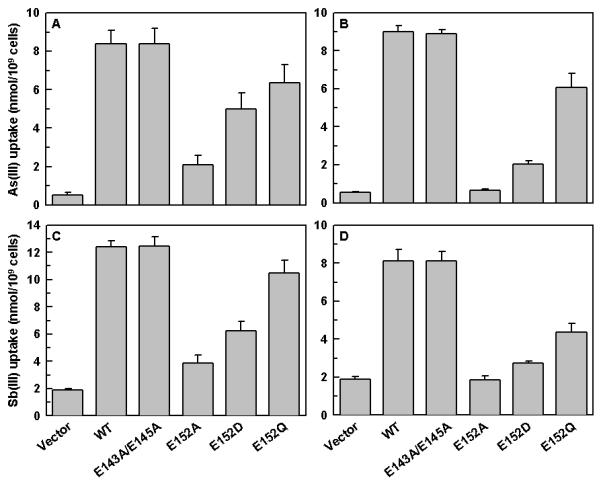
Metalloid uptake of L. donovani cells expressing wild-type or altered LmAQP1. Panels A and B depict As(III) uptake in L. donovani promastigotes and amastigotes, respectively; Panels C and D represent Sb(III) uptake in L. donovani promastigotes and amastigotes, respectively. Cells were transfected with wild-type, E143A/E145A, E152A, E152D, E152Q LmAQP1 or vector control.
Effect of alteration of C-loop glutamates on volume regulation
Upon exposure to hypoosmotic stress, several mammalian cell types as well as a number of protozoa, swell initially but later return to their normal volume. This phenomenon, dubbed as the regulatory volume decrease (RVD), is accomplished by the efflux of various osmolytes to the extracellular environment followed by passive water flow (Rohloff et al., 2003). We have previously shown that LmAQP1 plays a major role in volume regulation in both the promastigote and amastigote forms of the parasite (Figarella et al., 2007). The volume regulation ability of both promastigotes and axenic amastigotes expressing either wild-type or altered LmAQP1 was examined. L. donovani promastigotes and amastigotes transfected either with wild-type or altered LmAQP1 were subjected to a 50% reduction in osmolarity (from 300 to 150 mOsm). As cell swelling leads to a decrease in the absorbance, the process of volume recovery was monitored by following the absorbance of the cell suspension at 550 nm. Subsequent to a hypoosmotic shock, L. donovani promastigotes expressing wild-type LmAQP1 showed a drop in absorbance, indicating cell swelling, followed by a steady rise in absorbance to near original levels, signifying volume recovery (Fig. 5A). The volume recovery process was essentially complete over a 3 min period. Promastigotes expressing E143A/E145A LmAQP1 showed identical volume recovery as the wild-type. In contrast, promastigotes transfected with the vector control exhibited increased cell swelling, as reflected by a higher drop in absorbance, and lower volume recovery than cells expressing the wild-type protein (Fig. 5A). Promastigotes expressing the Glu152 mutants showed varying levels of volume recovery. Cells expressing E152A LmAQP1 behaved similarly as the vector alone control; cells expressing the altered E152D protein showed slight, but statistically significant, volume recovery; while cells expressing E152Q LmAQP1 showed an intermediate level of recovery between wild-type and vector control. Similar results were observed with axenic amastigotes. Fig. 5B shows that after a hypoosmotic stress, amastigotes overexpressing either the wild-type or E143A/E145A LmAQP1 swelled up as reflected by a decrease in absorbance, and recovered their volumes much faster than the Glu152 mutants or the vector alone control. This observation is interesting in the light of the fact that, although the Glu152 mutants showed water transport in Xenopus oocyte experiments (Fig. 2), their water permeability is not sufficient enough to perform volume regulation in vivo (Fig. 5).
Figure 5.
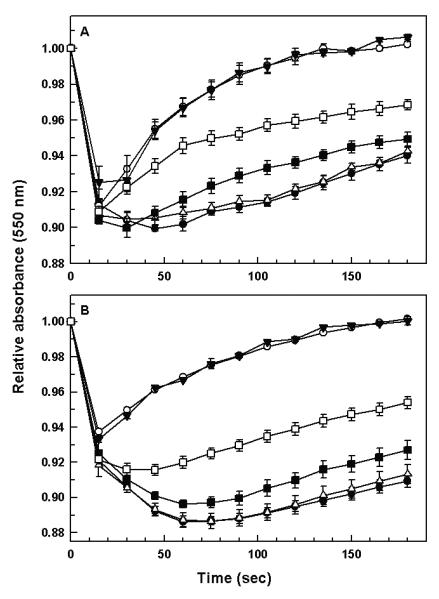
Volume regulation in the two life-cycle stage of Leishmania expressing either wild-type or altered LmAQP1. (A) L. donovani promastigotes, and (B) L. donovani amastigotes expressing the vector, (●); wild-type (○); E143A/E145A (▼); E152A (△); E152D (■); and E152Q (□) LmAQP1 were subjected to a hypoosmotic shock, and the relative changes in cell volume were followed by monitoring the absorbance at 550 nm, as described in Experimental procedures.
Effect of alteration of C-loop glutamates on osmotaxis
Leishmania promastigotes travel towards concentrations of sugar substrates and this movement requires the presence of an osmotic gradient (Leslie et al., 2002). We have earlier shown that LmAQP1 plays a significant role in osmotaxis in Leishmania promastigotes (Figarella et al., 2007). The effect of alteration of C-loop glutamates on the osmotaxis of Leishmania promastigotes was investigated. Leishmania promastigotes transfected with vector, wild-type and altered LmAQP1 were subjected to osmotaxis assay as described in Experimental procedures. In this assay, a capillary tube is filled with agarose gel containing 100 mM glucose, while leaving 1 cm of the end of the tube filled with buffer only. The diffusion of glucose from the agarose-filled end to the open end of the capillary tube establishes a concentration gradient that simulates the solute gradient within the insect gut. The number of promastigotes that move over a certain interval from a buffered cell suspension to the open end of a capillary tube was determined. Promastigotes overexpressing either wild-type or E143A/E145A LmAQP1 showed a higher osmotaxis response, as significantly more promastigotes were isolated from the capillary, compared to vector alone control (Fig. 6). In contrast, promastigotes expressing E152Q LmAQP1 showed a 3-fold loss in osmotaxis response compared to wild-type, E152D showed a 9-fold decline in activity, while E152A mutants showed near similar osmotaxis as the vector control. The background level of osmotaxis observed in Leishmania promastigotes transfected either with the vector or altered LmAQP1 is most likely due to expression of the chromosomal copy of wild-type LmAQP1. These data indicate that of the three C-loop glutamates, only Glu152 is critical for osmotaxis.
Figure 6.
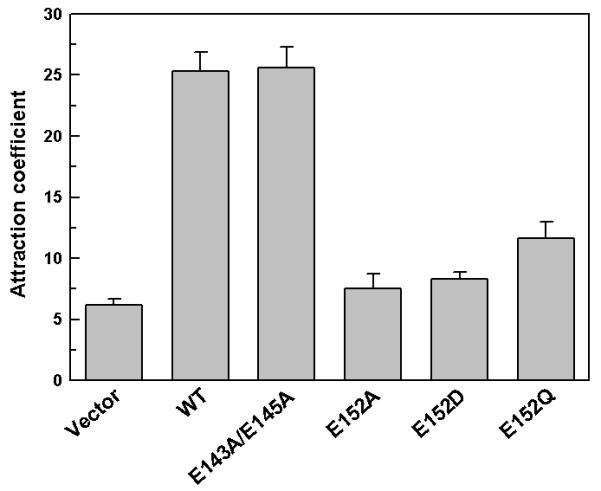
Movement of L. donovani promastigotes in response to an osmotic gradient. Cells transfected with wild-type, E143A/E145A, E152A, E152D, and E152Q LmAQP1 or vector control were counted for their ability to move into capillary tubes containing ±100 mM glucose.
Discussion
The crystal structure indicates that the C-loop Glu125 contributes to PfAQP selectivity and mutation of this residue to serine abolishes water transport (Newby et al., 2008, Beitz et al., 2004). The C-loop of LmAQP1 is 12 residues longer than PfAQP and has only three conserved residues: Glu152, Thr153, and Pro166 (Fig. 1). However, Trp124 that forms hydrogen bonding with Glu125 and crucial for maintaining the water permeability of PfAQP channel is not conserved in LmAQP1, but is replaced by an asparagine. Additionally, the C-loop of LmAQP1 has two extra glutamates, Glu 143 and Glu145. The objective of this study was to examine the role of the three C-loop glutamates (Glu143, Glu145, and Glu152) in solute permeability of LmAQP1.
Alteration of Glu143, Glu145, and Glu152 with Ala, Asp or Gln residues did not affect the overall conformation. Each of the altered proteins is produced in normal amounts and is not degraded in vivo (data not shown). Also, analogous to wild type LmAQP1, each of the altered proteins localized exclusively to the flagellum of promastigotes (data not shown). When expressed in Leishmania promastigotes, E143A/E145A LmAQP1 exhibited similar water and solute transport activity as the wild-type protein (Fig. 2 and 3). The E143A/E145A derivative showed no difference in metalloid sensitivity as reflected by near identical EC50 values as wild-type LmAQP1 (Table I), and consequently, both proteins showed no differences in metalloid transport (Fig. 4). These data indicate that Glu143 and Glu145 are not critical for LmAQP1 activity. However, any perturbation in Glu152 markedly affected the physiological properties of the protein. Alteration of Glu152 to alanine significantly affected both water and metalloid transport (Fig. 2 and 4). In fact, amastigotes expressing E152A LmAQP1 are unable to support metalloid transport; leading to higher EC50 values that are comparable to parasites harboring the vector alone control (Fig. 4 and Table I). On the other hand, transport of glycerol, dihydroxyacetone, and methylglyoxal are negligibly affected upon alteration of Glu152 to Ala, Asp, and Gln (Fig. 3). Alteration of Glu152 to Asp or Gln did not significantly affect oocyte water permeability (Fig. 2). Both E152D and E152Q LmAQP1 showed considerable metalloid transport in Leishmania (Fig. 4). However, only an intermediate level of volume recovery and osmotaxis was observed in L. donovani cells expressing E152Q but not E152D and E152A mutants (Fig. 5 and 6). Therefore, any alteration of Glu152 significantly affects the ability of the parasite to deal with osmotic stress or migrate in response to an osmotic gradient. These data indicate that Glu152 in the extracellular C-loop of LmAQP1 is critical for the physiological performance of the parasite. Since Glu152 mutants have lower osmotaxis ability, Leishmania promastigotes expressing such an altered protein will be less likely to move from the midgut to the proboscis of the sandfly, during the insect phase of the parasite life cycle, and consequently might be less infective to the vertebrate host.
It has been proposed that the metalloids As(III) and Sb(III) are transported by aquaglyceroporins, as both compounds exhibit similar conformation and charge distribution at physiological pH, and behave as inorganic molecular mimic of glycerol (Ramirez-Solis et al., 2004, Porquet & Filella, 2007). Our experiments indicate that the transport chemistry of metalloid and glycerol might follow distinct mechanisms as Glu152 in LmAQP1 is critical for metalloid but not glycerol transport. The crystal structure of PfAQP indicates that alteration of Glu125 (homologous to Glu152 of LmAQP1) disrupts the hydrogen bonding network around Arg196 in the selectivity filter, which results in a higher desolvation penalty for the passage of water (Newby et al., 2008). Very likely, alteration of Glu152 of LmAQP1 to alanine interferes with the hydrogen bonding of Arg230 (homologous to Arg196 of PfAQP1), which presents a higher energy barrier to the passage of metalloid. To the best of our knowledge, this is the first report on the molecular dissection of metalloid and glycerol permeability by a single mutation in an aquaglyceroporin. It is tempting to speculate that this might form the basis of a novel pathway of drug resistance, as Leishmania may develop suitable mutations that selectively block the transport of metalloids, but allow the uptake of physiological substrates. This hypothesis needs to be tested on drug-resistant field isolates.
Our observation that a single mutation affects metalloid but not glycerol transport in an aquaglyceroporin may have wider ramifications, especially in the development of arsenic resistant crops. In several regions of the globe, cultivation in arsenic-rich soil and irrigation with arsenic-contaminated water has led to accumulation of high levels of arsenic in rice, wheat, fruits and vegetables (Tripathi et al., 2007). Since plant aquaglyceroporins (nodulin-26 like intrinsic proteins (NIPs)) appear to be the major routes of As(OH)3 uptake into plants (Bienert et al., 2008, Isayenkov & Maathuis, 2008, Ma et al., 2008), genetically engineering NIPs that are permeable to essential metalloids such as boron (Takano et al., 2006) and silicon (Ma et al., 2006), but not to As(III) is a plausible approach towards the creation of low arsenic crops.
Experimental procedures
Strains and media
L. donovani strain LdBob was a kind gift from Professor Stephen M. Beverley, Washington University School of Medicine. LdBob was cycled between the promastigote and axenic amastigote forms using an established protocol (Goyard et al., 2003). L. donovani promastigotes and amastigotes were grown in culture media as described earlier (Goyard et al., 2003). Promastigotes were grown at 25°C while the axenic amastigotes were cultivated at 37°C with 5% CO2. Human leukemia monocyte cell line THP1 (ATCC) was maintained in RPMI-1640 medium with 10% fetal bovine serum (Invitrogen) and 50 μM β-mercaptoethanol (Sigma, cell culture grade) at 37°C with 5% CO2. Xenopus laevis were maintained in our animal facility and oocytes were harvested periodically.
Oligonucleotide-directed mutagenesis
The cloning of wild-type LmAQP1 into pGEM-T Easy vector (LmAQP1/pGEMEasy) has been described previously (Gourbal et al., 2004). Mutations in LmAQP1 and hAQP7 were introduced by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis Procedure (Stratagene), as described previously (Mukhopadhyay et al., 2003). The mutagenic oligonucleotides used for both strands and the respective changes introduced (underlined) are as follows: E143A/145A, 5′-G CTC TTC AAA TCG CAC TTT GAT GCA GCC GCA AAG AGG TTG CTT CTG-3′ (sense) and 5′-CAG AAG CAA CCT CTT TGC GGC TGC ATC AAA GTG CGA TTT GAA GAG C-3′ (antisense); E152A, 5′-G TTG CTT CTG AAT GCA ACG ATG GCG TCC AAG-3′ (sense) and 5′-CTT GGA CGC CAT CGT TGC ATT CAG AAG CAA C-3′ (antisense); E152D, 5′-GTTG CTT CTG AAT GAT ACG ATG GCG TCC AAG-3′ (sense) and 5′-CTT GGA CGC CAT CGT ATC ATT CAG AAG CAA C-3′ (antisense); E152Q, 5′-G TTG CTT CTG AAT CAA ACG ATG GCG TCC AAG-3′ (sense) and 5′-CTT GGA CGC CAT CGT TTG ATT CAG AAG CAA C-3′ (antisense). Each mutation was confirmed by sequencing the entire gene using a CEQ2000 DNA sequencer (Beckman Coulter).
Expression of LmAQP1 in oocytes
For expression in Xenopus oocytes, wild-type and mutant LmAQP1 in pGEM-T Easy vector were subcloned into the EcoRI site of the pL2-5 vector (Seyfang et al., 1997) (a generous gift from Professor Scott M. Landfear, Oregon Health Sciences University). Following linearization with SmaI, cRNAs were transcribed in vitro using the mMessage mMachine® kit (Ambion). Stage V and VI defolliculated X. laevis oocytes were injected either with 50 nl of water (control) or 50 nl of water containing 10 ng of cRNA (wild-type, E143A/E145A, E152A, E152D or E152Q LmAQP1). The oocytes were maintained at 16°C for 3 days in ND96 buffer containing 5 mM HEPES, pH 7.4, 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 2.5 mM sodium pyruvate, 0.5 mM theophylline, and 2 μg ml-1 of gentamicin sulphate.
Cloning of LmAQP1 in Leishmania expression vector pSP72αhygroα
pSP72αhygroα was created on the backbone of pSP72 (Promega) and pGEM7αhygroα vectors. pGEM7αhygroα was created as described previously (Gourbal et al., 2004, Papadopoulou et al., 1992). Either the wild-type or mutant LmAQP1/pGEMEasy was digested with XbaI and HindIII and cloned into the same sites of pSP72αhygroα.
Transfection of LmAQP1 in Leishmania
Transfection of pSP72αhygroα bearing either the wild-type or mutant LmAQP1 gene into LdBob promastigotes was accomplished as described previously (Ouellette et al., 1990). All transfectants were maintained in the presence of 0.3 mg ml-1 hygromycin (Invitrogen).
Western blot and immunofluorescence analysis
Flagella from LdBob promastigotes transfected with vector alone or altered LmAQP1 were isolated as described previously (Figarella et al., 2007). Total protein content of the flagellar fraction was estimated using BCA protein assay kit (Pierce). For oocytes expressing either wild-type or altered LmAQP1, fifteen oocytes from each category, were lysed at room temperature in 0.15 ml of lysis buffer (7.5 mM Na2HPO4, 1 mM EDTA pH 7.4). The lysates were centrifuged at 500 g for 5 minutes. Thirty microgram of the flagellar protein samples and 10 μl of the supernatant from oocyte lysates were analyzed by 12% SDS-PAGE (Sambrook & Russell, 2001). The proteins were transferred to a nitrocellulose membrane (Whatman) and probed with the LmAQP1 antipeptide antibody at 1:300 dilution, performed as described previously (Figarella et al., 2007). Immunofluorescence analyses of late log phase promastigotes expressing either wild-type or altered LmAQP1 were performed as described earlier (Figarella et al., 2007).
Standard oocyte swelling assays
Oocyte swelling assays were performed as described by Hansen et al (Hansen et al., 2002). Water permeability was measured by transferring oocytes into 1:3 diluted ND96 medium. Solute permeability was measured in an isoosmotic ND96 in which 65 mM NaCl was substituted with 130 mM of a non-ionic test solute. While determining the permeability of methylglyoxal, NaCl was entirely replaced with 115 mM of methylglyoxal to maintain buffer isoosmoticity. The swelling assays were performed at room temperature and video-monitored. The relative oocyte volume was calculated from the covered area. Osmotic water permeability (Pf, μm s-1) and solute permeability (Ps) were calculated using the following equation:
Pf = V0 × d(V/V0)/dt [S × Vw × (osmin − osmout)] (Hansen et al., 2002)
Ps = [osmtotal × V0 × d(V/V0)/dt]/[S × (solout − solin)] (Carbrey et al., 2003)
where, S is the oocyte surface area (= 0.045 cm2), Vo is the initial oocyte volume (= 9 × 10-4 cm3), Vw represents the molecular water volume (= 18 cm3 mol-1), osmin − osmout is the osmotic gradient, osmtotal is the total osmolarity of the system (200 mOsm), solout − solin is the osmotic solute gradient, and d(V/V0)/dt in s-1 is measured from the initial slope of the relative volume increase.
Determination of EC50 of metalloids in promastigotes
Metalloid sensitivity of L. donovani promastigote transfectants were determined as described previously (Gourbal et al., 2004). Briefly, log phase promastigote cultures were diluted to 106 cells ml-1 in a culture medium containing various concentrations of As(III) in the form of sodium arsenite, or Sb(III) in the form of potassium antimonyl tartrate. Growth was monitored from the absorbance at 600 nm after 5 days using a microplate reader (Spectramax 340, Molecular Devices). EC50 was calculated from the sigmoidal analysis of percentage live cells versus metalloid concentrations using the Origin Graphing Software. Each assay was performed at least three times and the results are represented as mean ± SE.
Determination of EC50 of antimonate in amastigotes
L. donovani promastigotes were used to infect the human leukemia cell line THP-1, as described previously (Zhou et al., 2004), with some minor modifications. THP-1 cells were differentiated with phorbol myristate acetate and infected with L. donovani promastigotes at a ratio of 20:1 for 4 h. Noninternalized parasites were washed away and the infected macrophages were treated with varying concentrations of potassium hexahydroxoantimonate (Sb(V)) (Sigma). After 4 days of culture, wells containing adherent differentiated THP-1 cells were washed, and luciferase activity was determined essentially as described (Zhou et al., 2004), using a microtiter plate luminometer (LMaxII, Molecular Devices). EC50 was calculated from the sigmoidal analysis of percentage light emitted (compared to untreated macrophages) versus antimonate concentrations using the Origin Graphing Software. Each assay was performed at least three times and the results are represented as mean ± SE.
Uptake assays
Log phase Leishmania promastigotes or amastigotes were washed twice with phosphate-buffered saline (PBS), pH 7.4 (Invitrogen) and suspended in PBS at a density of 108 cells ml-1. The promastigotes were then incubated with 10 μM As(III) or Sb(III) for 10 min at room temperature. A 0.2 ml portion was filtered through a 0.22-μm nitrocellulose filter and the filter washed once with 5 ml of ice-cold PBS. The filters were digested with 0.5 ml of concentrated HNO3 (69-70%) (EM Science) for 1 h at 70°C, allowed to cool to room temperature, and diluted with high pressure liquid chromatography grade water (Sigma) to produce a final concentration of HNO3 of approximately 3%, and then analyzed by inductively coupled plasma mass spectrometry (ICP-MS) with a PerkinElmer ELAN 9000. Standard solutions were made in the range of 0.5-10 ppb in 3% HNO3 using arsenic and antimony standards (Ultra Scientific). Each transport experiment was repeated at least three times with duplicate samples. Error bars were calculated from the mean ± SE.
For measurement of metalloid transport in oocytes, five oocytes were incubated with either 1 mM As(III) or Sb(III) in ND96 buffer, for 90 sec, at room temperature. The oocytes were washed four times with 1 ml of ice-cold ND96 buffer. Each oocyte was then transferred to a separate tube and digested with 0.1 ml of concentrated HNO3 as described above. Each sample was diluted with high pressure liquid chromatography grade water (Sigma) to produce a final concentration of HNO3 of approximately 3%, and the metalloid concentration was determined by ICP-MS. Each transport experiment was repeated at least three times. Error bars were calculated from the mean ± SE.
Cell volume measurements
Relative changes in cell volume following the induction of hypoosmotic shock were followed as described earlier (Figarella et al., 2007). Briefly, log phase promastigotes or amastigotes were washed twice in PBS and resuspended at a density of 109 cells ml-1. One hundred microlitre portions of the cell suspension were transferred to a microtiter plate. Hypoosmotic shock was induced by dilution of the isotonic cell suspension with an equal volume of deionized water and the absorbance at 550 nm was recorded every 15 s for 3 min in a microplate reader (Spectramax 340, Molecular Devices). A decrease in absorbance corresponds to an increase in cell volume. Isosmotic control experiments consisted of dilution of cell suspensions with appropriate volumes of isosmotic buffer. Unless otherwise noted, all hypoosmotic shock experiments were conducted at a final osmolarity of 150 mOsm (1:1 dilution of isosmotic buffer and water). Each experiment was repeated at least three times. Error bars were calculated from the mean ± SE.
Osmotaxis assay
Migration of promastigotes through osmotic gradients was measured as described earlier (Figarella et al., 2007). Glass capillary tubes (75 mm long × 1 mm internal diameter) were loaded with a 1% agarose solution, prepared either with PBS or PBS containing 100 mM D-glucose, such that exactly 1 cm at the end of each tube remained unoccupied by the gel. Late log phase LdBob promastigotes, overexpressing wild-type LmAQP1 or altered LmAQP1 or vector controls, were washed once with PBS, resuspended in the same buffer (5 ml) at a density of 2.5 × 107 cells ml-1, and placed in a microcentrifuge tube. The open end of each capillary tube was filled with PBS, and the capillary tubes were suspended vertically into the microcentrifuge tube, so that the open end of each capillary was immersed in the promastigote suspension. The capillary tubes were incubated at room temperature for 1 h. The capillary tubes were removed carefully from the cell suspension and the buffer (approximately 6 μl) was collected from the open end of each tube using a fine pipette-tip and mixed with 5 μl of PBS. The density of the promastigotes in each sample was determined using a haemocytometer and the mean density and standard error was calculated for both control and test samples. Interexperimental variability was eliminated by expressing the mean number of cells moving into the capillary tube containing 100 mM glucose relative to the mean number of cells moving towards the capillary tube in absence of glucose (attraction coefficient). An attraction coefficient of 1 thus indicates no specific movement towards glucose. Each experiment was repeated at least three times. Error bars were calculated from the mean ± SE.
Acknowledgements
This work was supported by National Institutes of Health Grant GM55425 and AI58170.
References
- Beitz E. TEXtopo: shaded membrane protein topology plots in LATEX2e. Bioinformatics. 2000;16:1050–1051. doi: 10.1093/bioinformatics/16.11.1050. [DOI] [PubMed] [Google Scholar]
- Beitz E, Pavlovic-Djuranovic S, Yasui M, Agre P, Schultz JE. Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis. Proc Natl Acad Sci USA. 2004;101:1153–1158. doi: 10.1073/pnas.0307295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Thorsen M, Schussler MD, Nilsson HR, Wagner A, Tamas MJ, Jahn TP. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella K, Uzcategui NL, Zhou Y, Lefurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, Turco SJ, Beverley SM. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kun JF, Schultz JE, Beitz E. A single, bi-functional aquaglyceroporin in blood-stage Plasmodium falciparum malaria parasites. J Biol Chem. 2002;277:4874–4882. doi: 10.1074/jbc.M110683200. [DOI] [PubMed] [Google Scholar]
- Isayenkov SV, Maathuis FJ. The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett. 2008;582:1625–1628. doi: 10.1016/j.febslet.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Leslie G, Barrett M, Burchmore R. Leishmania mexicana: promastigotes migrate through osmotic gradients. Exp Parasitol. 2002;102:117–120. doi: 10.1016/s0014-4894(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol Microbiol. 2005;57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Zhou Y, Rosen BP. Directed evolution of a yeast arsenate reductase into a protein-tyrosine phosphatase. J Biol Chem. 2003;278:24476–24480. doi: 10.1074/jbc.M302610200. [DOI] [PubMed] [Google Scholar]
- Newby ZE, O’Connell J, III, Robles-Colmenares Y, Khademi S, Miercke LJ, Stroud RM. Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol. 2008;15:619–625. doi: 10.1038/nsmb.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M, Fase-Fowler F, Borst P. The amplified H circle of methotrexate-resistant Leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990;9:1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou B, Roy G, Ouellette M. A novel antifolate resistance gene on the amplified H circle of Leishmania. Embo J. 1992;11:3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porquet A, Filella M. Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake. Chem Res Toxicol. 2007;20:1269–1276. doi: 10.1021/tx700110m. [DOI] [PubMed] [Google Scholar]
- Ramirez-Solis A, Mukopadhyay R, Rosen BP, Stemmler TL. Experimental and theoretical characterization of arsenite in water: insights into the coordination environment of As-O. Inorg Chem. 2004;43:2954–2959. doi: 10.1021/ic0351592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- Seyfang A, Kavanaugh MP, Landfear SM. Aspartate 19 and glutamate 121 are critical for transport function of the myo-inositol/H+ symporter from Leishmania donovani. J Biol Chem. 1997;272:24210–24215. doi: 10.1074/jbc.272.39.24210. [DOI] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wiren N, Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ. Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol. 2007;25:158–165. doi: 10.1016/j.tibtech.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Messier N, Ouellette M, Rosen BP, Mukhopadhyay R. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug pentostam. J Biol Chem. 2004;279:37445–37451. doi: 10.1074/jbc.M404383200. [DOI] [PubMed] [Google Scholar]


