Abstract
The field of neurotoxicology needs to satisfy two opposing demands: the testing of a growing list of chemicals, and resource limitations and ethical concerns associated with testing using traditional mammalian species. National and international government agencies have defined a need to reduce, refine or replace mammalian species in toxicological testing with alternative testing methods and non-mammalian models. Toxicological assays using alternative animal models may relieve some of this pressure by allowing testing of more compounds while reducing expense and using fewer mammals. Recent advances in genetic technologies and the strong conservation between human and non-mammalian genomes allows for the dissection of the molecular pathways involved in neurotoxicological responses and neurological diseases using genetically tractable organisms. In this review, applications of four non-mammalian species, Zebrafish, cockroach, Drosophila, and Caenorhabditis elegans, in the investigation of neurotoxicology and neurological diseases are presented.
Introduction
There was a time when non-mammals were thought to be far from ideal materials for the study of biomedical sciences because they are phylogenically too distant from humans. However, it has now become abundantly clear that some non-mammals are not only convenient materials but also are endowed with physiological and pharmacological properties common to humans. Thus, several such species have become very popular alternative organisms and are being used extensively as models. Here we would like to present a few such examples: Drosophila, Caenorhabditis elegans, cockroach, and zebrafish. Each of them is now being used not only for genetics, biochemistry, physiology and pharmacology of the nervous system, but also for neurotoxicology. This article summarizes the symposium on “Use of Non-Mammals for Neurotoxicological Study” which was held as part of the 11th Meeting of the International Neurotoxicological Association in 2007.
Zebrafish are amenable to high-throughput screening in small molecule discovery and cardiac toxicology. Zebrafish small molecule screening takes advantage of the small size, chemical permeability, and optical transparency of the zebrafish embryo. Transgenic lines expressing fluorescent proteins in specific neuronal subpopulations have also been developed, which could facilitate screening. Cardiotoxicity is perhaps the most thoroughly tested zebrafish toxicity to date. Zebrafish screens have also been used to discover novel compounds that suppress the effects of genetic vascular defects.
The nematode C. elegans, another useful neurotoxicological model, has been used to study of Parkinson’s disease and manganism. The nematode’s nervous system is highly conserved with mammals, and contains almost all of the known signaling and neurotransmitter systems found in vertebrates. In addition, the means to screen potential neurological and developmental toxicants using C. elegans have been developed in a medium-throughput setting. Assays are designed to assess chemical sensitivity to specific endpoints including growth, reproduction, movement, and feeding. Several additional toxicological assays are currently under development including green fluorescent protein-based, stress-responsive transgenic C. elegans.
Mammalian Na+ channels consist of a large pore-forming α-subunit and several small auxiliary β-subunits in various tissues and cell types. In Drosophila melanogaster, however, the para appears to be the only gene that encodes a functional sodium channel. Insects employ extensive alternative splicing and RNA editing to generate many functionally diverse sodium channel variants from a single sodium channel gene. Most of these alternative splice sites are conserved in D. virilis, the house fly Vssc1, and the cockroach BgNav. The cockroach sodium channel gene BgNav undergoes extensive alternative splicing and RNA editing to produce functionally distinct sodium channel variants. Interestingly, variants BgNav1-1 and BgNav2-1 showed different sensitivities to pyrethroids. BgNav2-1 channel variant is 100-fold less sensitive to deltamethrin than the BgNav1-1 variant. This is the first example of involvement of alternative splicing of a sodium channel gene in differential sensitivity to neurotoxins. In many cases, insects and mammals have the same type of target site for an insecticide, but with differential sensitivity. It has become increasingly clear that invertebrates including insects and C. elegans have inhibitory glutamate-gated chloride channels (GluCls) which are not present in mammals and which are highly sensitive to insecticides. Because of the presence only in invertebrates, GluCls are a potentially important target of insecticides. At least three types of currents were recorded in response to 100 μM glutamate: a fast-desensitizing current, a slow-desensitizing current, and a mixed type of current. Methods have recently been developed for recording them differentially. Slow-desensitizing currents could be inhibited selectively by trypsin, whereas fast-desensitizing currents were blocked selectively by soybean trypsin inhibitor or polyvinylpyrrolidone. The slow-desensitizing GluCls were much more sensitive to the blocking action of fipronil than the fast-desensitizing GluCls with IC50s of 10 nM and 800 nM, respectively. Fipronil is known to be degraded to fipronil sulfone via biotic/abiotic oxidation and to a desulfinyl photoproduct via photolysis. Fipronil sulfone blocked both slow- and fast-desensitizing GluCls, the former being slightly more sensitive than the latter.
Potential applications for zebrafish in neurotoxicology (R.T.P.)
The zebrafish, a favorite model organism for developmental geneticists, has been shown to be amenable to high-throughput screening in applications including small molecule discovery and cardiac toxicology (Zon and Peterson, 2005) (Fig. 1). Recent developments in zebrafish small molecule screening were reviewed to explore the possibility that the approach could be applied to neurotoxicity testing.
Figure 1.
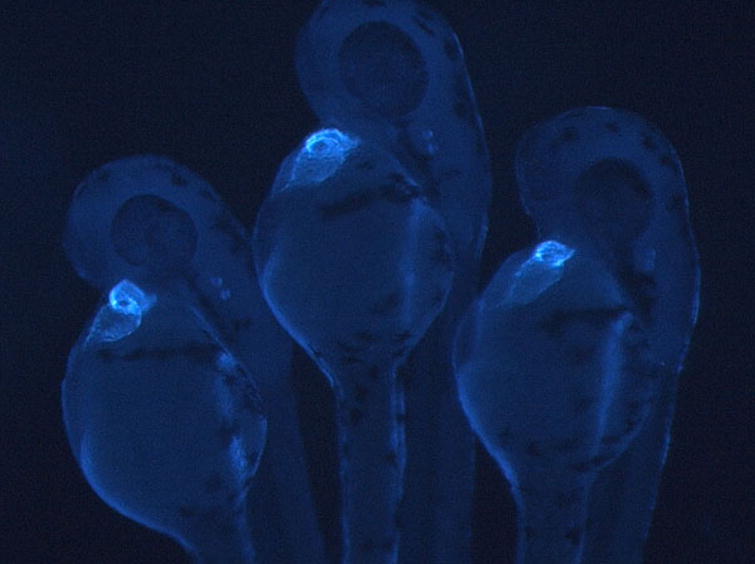
Transgenic zebrafish expressing a blue fluorescent protein from the cardiac myosin light chain 2 promotor. Image courtesy of Peter Schlueter.
Zebrafish small molecule screening takes advantage of the small size, chemical permeability, and optical transparency of the zebrafish. Embryonic and larval stages of the zebrafish can be grown in 96- or 384-well assay plates, exposed to small molecules by adding the compounds to the water in the wells, and the effects can be observed in the transparent embryos using microscopy. The first small molecule screens performed employed wild-type zebrafish and visual screening to identify obvious morphological defects (Peterson et al., 2000; Sternson et al., 2001;Spring et al., 2002; Shafizadeh et al., 2004; Moon et al., 2002; Khersonsky et al., 2003). These screens identified defects in numerous organ systems including the central nervous system (CNS). Phenotypes identified in this way were generally severe, and for the CNS ranged from loss or expansion of brain ventricles to truncation of the telencephalon to severe neuronal necrosis. While these studies demonstrate the ability of this approach to identify small molecules that cause severe developmental neurotoxicities, it is doubtful that such screens could reliably identify subtle neurotoxicities that aren’t manifest in obvious morphological changes. More sophisticated assays will likely be necessary. Vital dyes like acridine orange have been reported to stain apoptotic cells in zebrafish and may help detect subtle neurotoxicities (Parng et al., 2004). Transgenic lines expressing fluorescent proteins in specific neuronal subpopulations have also been developed, which could facilitate screening. Numerous functional and behavioral assays, including assays of vision, hearing, touch responsiveness, memory, anxiety, and startle habituation have been developed and could also be useful for identifying neurotoxicants that do not cause obvious developmental phenotypes (Brockerhoff et al., 1995; Bang et al., 2002; Fetcho et al., 1998; Peitsaro et al., 2003). It is possible that a panel of several high-throughput morphological and functional assays could be used to screen broadly for neurotoxicants.
Increasing the number and sophistication of high-throughput neuronal assays for zebrafish will be of little value if zebrafish and human neurotoxicities do not correlate. Much work remains to be done to determine the extent to which zebrafish toxicities are predictive, but initial data from other organ systems are encouraging. Cardiotoxicity is perhaps the most thoroughly tested zebrafish toxicity to date. In an assay for drug-induced bradycardia, 22 of 23 compounds known to cause human QT prolongation were detected among 100 tested compounds, suggesting a high degree of correlation between zebrafish and human cardiotoxicity (Milan et al., 2003). Similar types of studies focused on neurotoxicity would be very useful but have not been reported. However, some individual compounds have been reported to have predictable neurotoxicities in zebrafish, including ethanol, 6-hydroxydopamine, acrylamide, MPTP, and pentylenetetrazole (Parng et al., 2007; McKinley et al., 2005; Baraban et al., 2005).
Beyond screening for neurotoxicants, might the zebrafish high-throughput platform be useful for identifying neuroprotectants? Zebrafish screens have been used to discover novel compounds that suppress the effects of a genetic vascular defect (Peterson et al., 2004; Hong et al., 2006). Similar screens have discovered a small molecule that suppresses the effects of a mutation that causes a cell cycle defect in zebrafish (Stern et al., 2005). This approach could be applied to neuroprotection by exposing thousands of zebrafish en masse to a neurotoxicant, then screening in high-throughput for novel small molecules that block the neurotoxic effects of the toxicant. As preliminary evidence that such an approach may be feasible, several known neuroprotectants have been shown to protect zebrafish from L-hydroxyglutaric acid neurotoxicity (Parng et al., 2006), and in a separate study, l-deprenyl and nomifensine were shown to protect zebrafish from MPTP-induced neurotoxicity (McKinley et al., 2005).
Conclusions
In the search for alternative models for neurotoxicity testing, the zebrafish offers much: low cost, high throughput, an almost limitless range of morphological and functional assays, and an apparently high degree of correlation with mammalian systems. What is missing is history. Unlike many mammalian models that have been used for decades, the zebrafish cannot benefit from a large reservoir of historical data establishing the system’s validity and limitations. If the zebrafish is to become useful, it will require a commitment to accumulating and sharing that reservoir, a process that hopefully could be accelerated by the ability to acquire data rapidly in zebrafish. Although such an effort would be formidable, the competing pressures for additional testing and reduced use of mammals may indicate that an investment in zebrafish is a sound one.
Toxicogenetic analysis in a novel C. elegans model of Parkinson’s disease and manganism (R.N.)
The identification of the molecular components and mechanisms of neurodegenerative diseases have often been inhibited by the complexities of the vertebrate brain and the difficulties of modeling the diseases in cell cultures. The recent advances in genetic technologies and the high sequence similarity between human and invertebrate genomes allows for the dissection of the molecular pathways involved in neurological diseases using model organisms. Here we briefly summarize how we are using the nematode C. elegans to model Parkinson’s disease and manganism, and describe how this worm has the potential for identifying novel proteins and compounds that may be involved in, or protect against, these neurodegenerative disorders.
C. elegans is a powerful genetic model for exploring the molecular mechanisms of neuron function and human disease (Riddle et al., 1997; Nass et al., 2001). The worm’s nervous system contains almost all of the known signaling and neurotransmitter systems found in the vertebrates (Bargmann, 1998; Nass and Blakely, 2003). Its genome has been sequenced and contains approximately 20,000 genes, over 90% of the human genome. Its small size (1 mm long), large brood size, quick generation time of 3.5 days, and ease of maintenance in the laboratory allows for inexpensive production and rapid (Riddle et al., 1997). The animals can also easily be propagated in liquid medium in standard 96-well microtiter plates, allowing for high throughput screening (HTS) of animals with particular behavioral phenotypes or optical properties (Link et al., 2000; Kaletta and Hengartner, 2006). The worms are anatomically simple and well-characterized with approximately one-third of the over 1000 cells identified as neurons. The nematode is transparent and the ease of making transgenic animals containing reporter constructs greatly facilitates examination of neuron protein expression and localization (Mello and Fire, 1995; Thomas and Lockery, 1999). Primary cultures, first developed by Laird Bloom in 1993 during his MIT Ph.D thesis, differentiate and appear to retain many of their in vivo cellular properties (Bloom, 1993; Buechner et al. 1999).
Significant advantages of using C. elegans for genetic analysis is the opportunity to incorporate forward and reverse genetic screens to relatively quickly identify proteins and molecular pathways that are involved in particular cellular processes. Forward genetic screens using chemical mutagens have the advantage that no a priori knowledge is needed to determine a gene’s function in order to determine whether it plays a role in the particular phenotype. Mutations can be identified within as little as a week (Wicks et al., 2001), and since the worms can easily be mated with each other, strains containing several mutations can be generated within days (Wicks et al., 2001) C. elegans is also amendable to reverse genetics that allows for the identification of molecular pathways in which a particular gene acts upon. RNA interference, or RNAi, where the introduction of dsRNA induces sequence specific degradation of homologous mRNAs and subsequent protein expression, is one example of a reverse genetic approach. The 2006 Nobel Prize in Physiology or Medicine was awarded to Andrew Fire and Craig Mello for their RNAi efforts.
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease and results from the loss of at least 80% of the DA neurons within the substantia nigra pars compacta (SNpc). A hallmark of the disorder is the formation of protein aggregates termed Lewy bodies (LBs) in a significant number of the surviving neurons. Although the molecular basis of this disorder has not been identified, etiological and pathological data suggest that there is both a genetic and environmental component that causes and contributes to the DA neuron cell death (Jenner, 1998). Environmental exposure to manganese, as well as several other metals, has been associated with the development of PD. Mn-induced Parkinsonism, also called manganism, has been associated with Mn mining and welding (Calne et al., 1994; Chia et al., 1993). Acute Mn2+ toxicity results in symptoms similar to those seen in patients with PD, including rigidity, tremors, and bradykinesia (Stredrick et al., 2004).
One of the most common mechanisms to model Parkinson’s disease in vertebrates is through exposure of the animals to a DA neuron neurotoxin. Within weeks following toxin exposure, most of the animals display a Parkinsonian-like syndrome. The most common neurotoxins used are 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 1-methyl-4-phenylpyridinium ion (MPP+ [the active metabolite of MPTP]). 6-OHDA and MPP+ are transported into the cell by the high affinity dopamine transporter, DAT, which is also the target of drugs of abuse such as cocaine and amphetamine (Reading, 1994).
We generated the first C. elegans PD model by developing a C. elegans transgenic line that expresses GFP in all eight DA neurons within the hermaphrodite (Nass et al., 2002). When we briefly exposed the animals to 6-OHDA (0.5–1 hr), we find a time and concentration-dependent loss of DA neuron integrity. Also, this affect can be blocked by co-incubation with DAT agonist (e.g. amphetamine) or antagonist (e.g. cocaine), consistent with these vertebrate models of PD that DAT is required (Nass et al., 2002). These exciting results mimic several significant aspects of the vertebrate PD model, and should allow us to utilize the power of invertebrate genetics to explore in vivo the function of genes previously identified to play a role in PD, as well as identify potential environmental components such as Mn2+ exposure and novel endogenous molecules that could contribute to the DA neuron cell death.
For example, epidemiological studies indicate that the etiology of PD likely involves specific molecular pathways involved in protein aggregation and degradation. To date, at least seven genes have been independently identified that are associated with rare, familial forms of PD, including α-synuclein, parkin, DJ-1, UCH-1, NURR1, PINK-1, and LRRK2. Most of these proteins have strong homologues in the worm. α-synuclein, the first gene identified, is a presynaptic protein that appears to interact with synaptic vesicles and could be involved in the regulation of both dopamine biosynthesis and dopamine transporter function, and is also a major component of the PD associated protein aggregates LBs. α-synuclein aggregation is also dramatically accelerated in vitro in the presence of the transition metal Mn2+ (Uversky et al., 2001). Mn2+ exposure to DA and α-synuclein expressing mammalian cells also cause greater cell death than those not exposed to Mn+2 (Pifl et al., 2004). These examples suggest that Mn2+ exposure could be an aggravating factor for triggering DA neuron cell death in PD.
We have also generated transgenic animals overexpressing either human wild-type (WT) or mutant A53T α-synuclein and gfp in the DA neurons within the worm. We have found that both WT and A53T expression confer DA neuron cell death in the worm even in the absence 6-OHDA (Lakso et al., 2003). Motor deficits were also observed when α-synuclein was expressed behind the pan-neuronal promotor, and α-synuclein containing inclusion bodies are seen in some of the DA neurons. Our preliminary results also indicate that the DA neurons are sensitive to a number of heavy metals, and that some of these metals amplify the affects of 6-OHDA and α-synuclein in vivo. These observations suggest that C.elegans could be a useful model for α-synuclein-induced pathologies, and help to identify the relationship between α-synuclein, heavy metals, and PD-associated proteins.
The establishment and initial characterization of our C. elegans PD model also provides an opportunity to utilize genetic screens to identify novel genes involved in DA neuron cell death. For example, in one forward genetic screen we could utilize our DAT-1::GFP reporter line to identify molecules involved in Mn2+-induced neuronal death. We would first mutagenize the genome of the parental animals and then isolate the second generation hermaphrodites (to homozygose the mutation; Nass et al., 2003) in which GFP is still retained in the DA neurons following exposure to Mn2+. Animals that have DA neurons that are insensitive to Mn2+ could have mutations within Mn2+ transport proteins or proteins involved in DA neuron viability or cell death. We have implemented a similar genetic screen with 6-OHDA and have identified a number of mutants that have varying degrees of DA neuron 6-OHDA insensitivity (Nass et al., 2005). Three of these mutants contain mutations within DAT that render them completely resistant to 6-OHDA. The identification of these mutants provides proof-of-concept that we should be able to isolate genes involved in toxin-induced cell death.
With our worm PD model we should also be able to identify novel genes involved in Mn2+-induced DA neuron sensitivity by utilizing the worm friendly methods of RNA interference (RNAi). The Medical Research Council (Cambridge) has generated a remarkable library of bacteria that express RNAi molecules against roughly 90% of the known worm genes (~18,000 genes). Since feeding the bacteria expressing the dsRNA to the worms is an efficient way to knockdown the expression of a gene (Timmons et al., 2001), we could envision feeding our gfp reporter line to the bacteria also on medium containing Mn2+. We would then select for animals in which the DA neurons are not affected by the Mn2+, and therefore could quickly map and identify genes are involved in Mn2+-induced DA neuron sensitivity.
Conclusions
C. elegans provides remarkable opportunities to identify and characterize genes and proteins involved in Parkinson’s disease and manganism in vivo. The high similarities on the molecular level between the worm and humans suggest that the paradigms discovered using this system are highly relevant to these devastating diseases. C.elegans can also be utilized to screen and identify environmental agents such as heavy metals that could cause or contribute to susceptibility to disease.
Toxicological studies of environmental agents using C. elegans (W.A.B. and J.H.F.)
The National Toxicology Program (NTP) is responsible for the development of sound scientific tests designed to estimate the effects of chemicals on human health. National and international government agencies, such as the NTP and the Environmental Protection Agency (EPA), have defined a need to reduce, refine or replace vertebrate animals in toxicological testing with alternative testing methods and models (Becker et al. 2006). Toxicological assays using invertebrate species are more rapid and less expensive than traditional mammalian-based tests due in part to shorter life spans and the ability to assay in multi-well plate formats. Invertebrate species are also ideal model organisms because of the lack of animal welfare concerns.
C. elegans has been recognized as an attractive biological and genetic model organism for some time (Brenner 1974). Recently, these advantages have led to a rise in the use of C. elegans as a toxicity test organism. Short life cycles, easy and inexpensive maintenance, and detailed biological knowledge allow for the development of rapid, low-cost tests that readily lend themselves to mechanistic studies of toxicant action. There is also a high degree of conservation in the molecular toxicological responses between C. elegans and mammals. For example, many signal transduction pathways involved in general stress responses are well conserved (National Research Council, 2000). Several studies have also demonstrated the predictive potential of C. elegans lethality and changes in locomotion for mammalian toxicity (Cole et al. 2004; Tatara et al. 1998). With advances in technology, the assessment of phenotypes of thousands of nematodes can now be quantified in a high-throughput fashion, rather than by direct observation of only a few organisms.
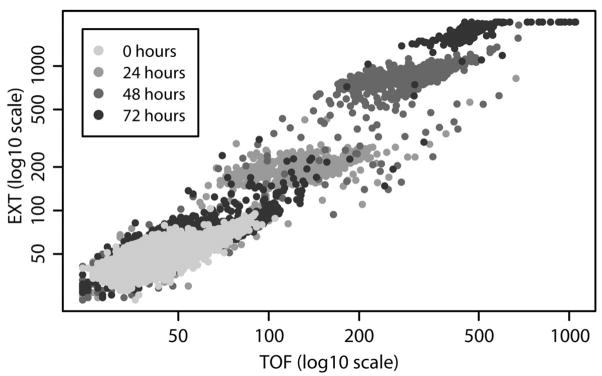
Our group has developed the means to screen potential neurological and developmental toxicants using C. elegans. To this point, sublethal toxicological assays have been automated using liquid handling robotic workstations, a Complex Object Parametric Analyzer and Sorter (COPAS) Biosort (Pulak 2006), which is used to dispense and analyze nematode length and fluorescence, and an imaging workstation for motion tracking and multidimensional image analyses. To optimize the toxicological assays, a 96-well plate format is used for sample preparation, dispensing of nematodes, and quantification of specific toxicological endpoints. As nematodes are dispensed, the COPAS Biosort measures the time of flight (TOF) or length, extinction (EXT) or optical density, and green and red fluorescence of each nematode.
C. elegans matures from fertilized egg to adult through four distinct larval stages, termed L1-L4, in approximately 3.5 days and has an average life span of 10 days. Fig. 2 illustrates the size distributions of C. elegans as they developed from the first larval stage (L1) to adults over 72 h, as measured by the COPAS Biosort. After 48 h, the nematode population was observed by microscopy to be mostly L4s. After 72 h, nematodes were either gravid adults, with the highest TOF and EXT, or the second generation of L1s and eggs, with the lowest TOF and EXT. As the nematodes grow and develop, they increase in length and optical density.
Figure 2.
Development of C. elegans from larvae to adult over 72 h. Extinction (optical density) versus time of flight (length) for nematodes incubated at 20°C for 0, 24, 48, or 72 h. Each point corresponds to a single nematode.
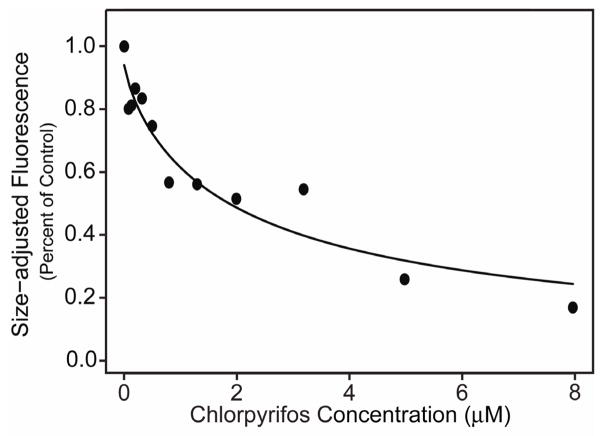
Three toxicological assays have been developed in order to assess chemical sensitivity at specific developmental stages: L1s for growth, L4s for reproduction, and adults for movement and feeding. In experiments similar to the one shown in Fig. 2, the C. elegans growth assay was used to measure the TOF and EXT from L1s at the beginning of the assay and then after a 72 h exposure to the test toxicant. The C. elegans reproduction assay was developed to assess the effects of chemicals at the L4 stage, just before nematodes are reproductive. After 48 h exposures, the COPAS Biosort was used to count the number of offspring. The C. elegans feeding assay was developed to monitor neurotoxicity in adult nematodes exposed to toxicants for 24 h (Boyd et al., 2007). After exposures, adult nematodes are allowed to feed on 0.5 μm red fluorescent microspheres and the level of red fluorescence in individual nematodes is measured using the COPAS Biosort. Feeding rates of exposed and non-exposed control C. elegans can then be calculated. Fig. 3 presents a typical dose-response curve showing the effects on feeding after exposing C. elegans to increasing concentrations of toxicant. In this study, nematodes were exposed to the organophosphate pesticide chlorpyrifos. In all assays, dose-response curves are calculated to estimate toxicological endpoints such as EC50s and benchmark doses. Our group has validated the growth, reproduction, and feeding assays by screening almost 60 chemicals, which include metals, pesticides, mutagens, and non-toxic agents (Table 1).
Figure 3.
Neurotoxic effects of chlorpyrifos on adult C. elegans feeding. Fitted concentration-response curve of a representative experiment based on observed mean size-adjusted fluorescence measurements as a percent of the control for chlorpyrifos. Each point represents approximately 120 nematodes on average, with counts ranging from 104 to 141 (For details, see Boyd et al., 2007).
Table 1.
Chemicals tested in C. elegans medium-throughput growth, reproduction and feeding assays.
| Metals | Organics | ||
|---|---|---|---|
| Aluminum Chloride | Acetaminophen | EtOH | Pyridine |
| Cadmium Chloride | α-Cyclodextrin | Fumonisin | Sodium Metam |
| Chromium Oxide | All-trans Retinoic Acid | Glyphosate | Tamoxifen |
| Cobalt Chloride | Ascorbic acid | Lindane | Tebuconazol |
| Copper Sulfate | β-Cyclodextrin | Methadone | Valproic acid |
| Lead Acetate | β-Cyclodextrin hydrate | Methanol | |
| Lead Nitrate | Caffeine | Methyl cellulose | |
| Manganese Oxide | Carbaryl | Methyl Parathion | |
| Mercuric Chloride | Chlorpyrifos | Methylisothiocyanate | |
| Methyl Mercury | Demeton-S-methylsulfone | MMS | |
| Nickel Sulfate | Dichlorvos | MNNG | |
| Silver Nitrate | Diphenylhydantoin | Monocrotophos | |
| Sodium Arsenite | Diquat | Nicotine | |
| Sodium Selenite | DMSO | Paraquat | |
| Thimerosal | EMS | Parathion | |
| Vanadium Oxide | ENU | PCB mixture | |
| Zinc Sulfate | Ethephon | PEG-60 | |
In order to increase the throughput of the assays, the reproduction assay has been modified and used to screen a library of 1408 chemicals selected by the NTP. Results from the C. elegans reproduction assay will then be compared to those obtained by the NIH Chemical Genomics Center, which performs cell-based high-throughput screens (Feng et al. 2007). All of the chemicals that cause a significant effect on reproduction will be entered into a second tier of testing. In the second tier, detailed concentration responses will be assayed.
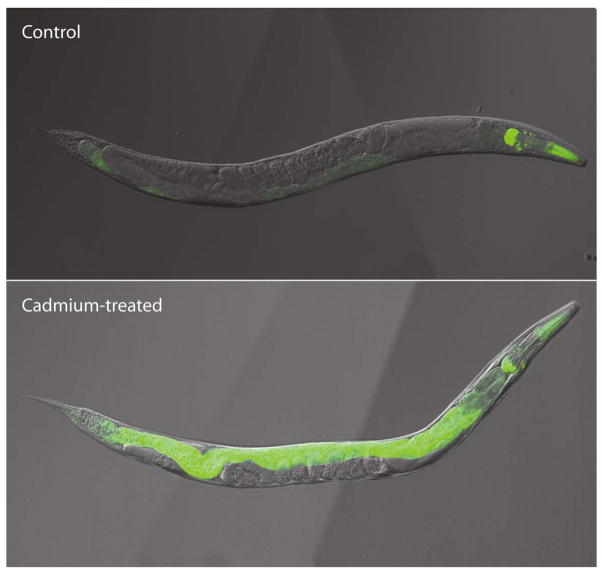
Several additional toxicological assays are currently under development. These include the development of green fluorescent protein-based, stress-responsive transgenic C. elegans, which will be used to improve the sensitivity and specificity of toxicity screens and semi-automated motion tracking (Fig. 4). Transgenic strains are being constructed that will be used to monitor several toxicological processes including metal response, biotransformation, apoptosis, and DNA damage response. After creating the transgenic strains, the effects of toxicant exposure on GFP expression will be measured using a COPAS Biosort or a plate-reading fluoremeter. Semiautomated motion tracking is being developed to monitor the effects of potential neurotoxicants on C. elegans locomotion. Many parameters are simultaneously calculated from individual nematode tracks including rate of movement, number of reversals, and sinuosity.
Figure 4.
Effect of cadmium-exposure on mtl-1::GFP transgenic C. elegans. Transgenic nematode expressing GFP under the control of the C. elegans metallothionein promoter (mtl-1) were grown in the absence (upper panel) or presence (lower panel) of 100 μM cadmium for 24 h. Constitutive mtl-1 transcription is observed in the pharynx of the nematodes, whole metal-inducible transcription occurs in the nematode intestine.
In addition to the technological deployment of medium and high throughput C. elegans toxicological assays, our group is continuing to develop statistical tools that will allow us to properly analyze the large volume of data that is produced in each of these assays. Several of the tools currently under development include multi-dimensional (e.g. TOF, EXT, GFP, etc.) modeling to assess changes in C. elegans populations after exposure to chemicals. Statistical algorithms that can be used to classify nematodes into discrete growth stages and to characterize the statistical properties of nematode populations at different developmental stages are also being developed. These new statistical tools will be used to quantify changes in C. elegans biology in response to toxicant exposure.
Conclusions
In response to the need to develop inexpensive and rapid toxicological assays, and to decrease the numbers of vertebrate animals used in the current assays, our group has explored the use of C. elegans as a test organism and developed several toxicological assays. We have found that C. elegans will make a valid alternative test organism for the screening of toxic agents.
Differential sensitivity of insect sodium channel variants to pyrethroid insecticides (K.D.)
Sodium channels are responsible for the initiation and propagation of action potentials in almost all excitable cells. Because of their crucial role in membrane excitability, sodium channels are targeted by a great variety of naturally occurring neurotoxins, such as tetrodotoxin, scorpion toxins, and batrachotoxin, which are produced by plants and animals for defense or predation (Cestele and Catterall, 2000; Wang and Wang, 2003). Sodium channels are also the primary target of the synthetic insecticides pyrethroids, which are structural derivatives of pyrethrins found in extracts of the flowers of Chrysanthemum species (Narahashi, 1988). Different classes of neurotoxins bind to different sites on the sodium channels and alter channel functions. For example, pyrethroids cause prolonged opening of sodium channels primarily by inhibiting channel deactivation and inactivation and stabilizing the open configuration of the sodium channel (for review see Narahashi, 1988; 1996; 2000; Soderlund and Bloomquist, 1989; Raymond-Delpech et al., 2005).
Mammalian sodium channels, encoded by nine distinct genes, are known to exhibit different sensitivities to pyrethroid insecticides. For example, tetrodotoxin (TTX)-sensitive sodium channels in the rat dorsal root ganglion neurons are less sensitive to pyrethroids than TTX-resistant sodium channels in the same neurons (Ginsburg and Narahashi, 1993; Tatebayashi and Narahashi, 1994; Song and Narahashi, 1996; Tabarean and Narahashi, 1998). Rat Nav1.2 and Na1.4 channels are resistant to pyrethroids (Warmke et al., 1997; Vais et al, 1997; Smith and Soderlund, 1998; Wang et al., 2001), whereas rat Nav1.8 sodium channel is highly sensitive (Choi and Soderlund et al., 2006). However, the molecular basis of the differential sensitivities to pyrethroids remains unknown.
In contrast to multiple sodium channel genes in mammals, Drosophila melanogaster and other insects appears to have only a single sodium channel gene, para in Drosophila and BgNav in Blattella germanica (Warmke et al., 1997; Dong, 2007). However, electrophysiological studies showed that the sensitivity of the insect nervous system to pyrethroids varies greatly depending on nerve preparations, suggesting the presence of distinct subtypes of sodium channels (References in Dong, 2007). For example, permethrin affects the insect sensory neurons more profoundly than the neuromuscular synapses (Osborne and Hart, 1979). However, the molecular basis of the different sensitivities of insect sodium channels to pyrethroids was not known until recently. We found that the cockroach sodium channel gene BgNav undergoes extensive alternative splicing and RNA editing to produce at least twenty functionally distinct sodium channel variants (Tan et al., 2002; Song et al., 2004). This observation is consistent with what was found in the D. melanogaster para and housefly Vssc1 sodium channel transcripts. Most of these alternative splice sites are conserved in D. virilis (Thackeray and Ganetzky, 1995), the house fly Vssc1 gene (Lee et al, 2002), and the cockroach BgNav gene (Tan et al., 2002; Song et al., 2004). Interestingly, cockroach variants BgNav1-1 and BgNav2-1 showed greatly different sensitivities to pyrethroids: The BgNav2-1 channel variant is 100-fold less sensitive to deltamethrin than the BgNav1-1 variant (Tan et al., 2002).
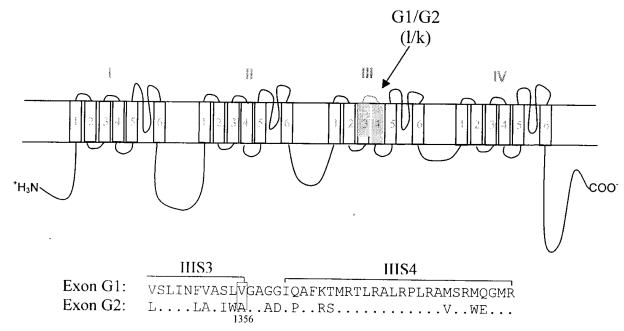
Like mammalian α-subunits, the insect sodium channel contains four repeated homologous domains (I–IV), each having six membrane-spanning segments (S1–6) connected by intracellular or extracellular loops of amino acid sequences. As shown in Fig. 5, two mutually exclusive exons G1/G2 encode IIIS3-IIIS4. The BgNav2-1 channel contains exon G2, whereas the BgNav1-1 channel contains exon G1. When the G1/G2 exons were swapped between BgNav1-1 and BgNav2-1, recombinant BgNav1-1 channel carrying exon G2 (BgNav1-1G2) was 10-fold more resistant to deltamethrin than BgNav1-1; whereas recombinant BgNav2-1 channel carrying exon G1 (BgNav2-1G1) was 10-fold more sensitive to deltamethrin than BgNav2-1 (Tan et al., 2002). Therefore, the different pyrethroid sensitivities between BgNav1-1 and BgNav2-1 channels can be partially attributed to the presence of exon G1 or G2. There are 14 amino acid differences between exons G1 and G2 (Fig. 5). To identify the residue(s) in exons G1/G2 that are responsible for the different deltamethrin sensitivities, we made single, double, or triple amino acid substitutions (depending on whether the amino acid differences are adjacent to each other or not) in BgNav1-1 or BgNav2-1. We found that a V to A change at the amino acid position 1356 (V1356A) in exon G2 was responsible for the exon G2-associated low sensitivity to pyrethroids (Du et al., 2006). This is the first example of involvement of alternative splicing of a sodium channel gene in differential sensitivity to neurotoxins. The G1/G2 exons are also conserved in D. melanogaster, which allows us to use powerful tools and resources for further genetic manipulation.
Figure 5.
A schematic diagram of the sodium channel protein, indicating four homologous domains (I-IV), each having six transmembrane segments (1 to 6). The locations of the sequences corresponding to the two mutually exclusive exons G1/G2 in the cockroach BgNav gene are indicated with solid blocks. The corresponding exons l and k in the Drosophila para gene are indicated in parenthesis. An alignment of amino acid sequences encoded by exons G1 and G2 are presented below the topology diagram. The residues in G2 that are identical to those in G1 are marked with dots.
Another excellent example of using insect sodium channels to study the molecular action of pyrethroids on sodium channels is the study of naturally occurring sodium channel mutations that confer knockdown resistant (kdr) to pyrethroids (Soderlund, 2005; Dong, 2007). Specifically, a large number of single amino acid mutations were found in the sodium channels of kdr mutant insects, many of which were later confirmed to confer reduced pyrethroid-sensitivity to sodium channels expressed in Xenopus oocytes. Because these mutations also confer insecticide resistance at the whole insect level, the biological relevance can be established beyond in vitro or cell culture systems. A similar combination of approaches should prove useful in using insects as models for the study of other classes of neurotoxins that target sodium channels.
Conclusions
Insect models have proven to be excellent in advancing our Conunderstanding of the molecular action of pyrethroids, which act on both mammalian and insect sodium channels. The pyrethroid-sodium channel research illustrates the great potential of using naturally occurring neurotoxin-resistant insects or target variants as excellent tools for understanding the mechanisms underlying mammalian neurotoxicological processes involving the same or similar insect targets.
Glutamate-activated chloride channel: unique chemical target present in insects but not in mammals (T.N.)
Most Insecticides are more toxic to insects than to mammals, and this is one of the important characteristics for a chemical to become a useful insecticide. In many cases, insects and mammals have the same type of target site for an insecticide, but with differential sensitivity. For example, the major target site of pyrethroids is the voltage-gated sodium channels in both insects and mammals, but the insect sodium channel is much more sensitive to pyrethroids than the mammalian sodium channel with a difference in EC50 of ~1000-fold (Ginsburg and Narahashi, 1993; Narahashi, 2001; Narahashi et al., 2007). However, in some other cases, an insecticide affects the same insect and mammalian target site almost equally. An example is endosulfan which blocks the GABA receptors of both cockroach neurons and rat dorsal root ganglion neurons with almost the equal potency, with IC50s of 5 nM and 10 nM, respectively ( Zhao et al., 2007).
It has become increasingly clear that invertebrates including insects and C. elegans have inhibitory glutamate-gated chloride channels (GluCls) which are not present in mammals and which are highly sensitive to insecticides. Whereas glutamatergic synaptic transmission in vertebrate is excitatory mediated by glutamate-activated cation channels, glutamate serves as both an excitatory (Gration et al., 1979; Patlak et al., 1979; Ultsch et al., 1992) and an inhibitory (Cleland, 1996) transmitter in invertebrates. Inhibitory glutamatergic synaptic transmission is mediated by glutamate-gated chloride channels (Cleland, 1996; Raymond and Sattelle, 2002) that are present only in invertebrates.
Because of the presence only in invertebrates, GluCls are a potentially important target of insecticides. Many studies of GluCls were performed with C. elegans, particularly as a target site of the anthelminthic/insecticide invermectin (Hejmadi et al., 2000; Burkhart, 2000; Arena et al, 1995). Studies of GluCls using insects in connection with ivermectin were limited (Kane et al., 2000), but increasing attentions are recently focused on insect GluCls (Ikeda et al., 2003; Zhao et al., 2004a,b; Ihara et al., 2005; Eguchi et al., 2006; Janssen et al., 2007).
We present the physiological and toxicological characteristics of GluCls. Whole-cell patch clamp experiments were performed using cockroach thoracic ganglion neurons. Neurons were isolated as described in our previous paper (Zhao et al., 2004a).
At least three types of currents could be recorded in response to glutamate application (Zhao et al., 2004b; Narahashi et al., 2007). One was a fast-desensitizing current (Fig. 6A). Some neurons generated a slow-desensitizing current (Fig. 6B). Other neurons produced a mixed type of current (Fig. 6C). As will be shown later, since fast- and slow-desensitizing currents exhibit different characteristics and different pharmacological responses, it is often necessary to analyze them separately. We have recently developed methods for recording them differentially (Zhao et al., 2007). Slow-desensitizing currents could be inhibited selectively by trypsin, whereas fast-desensitizing currents were blocked selectively by soybean trypsin inhibitor or polyvinylpyrrolidone. These selective inhibitors now make separate recordings of two types of currents much easier than before.
Figure 6.
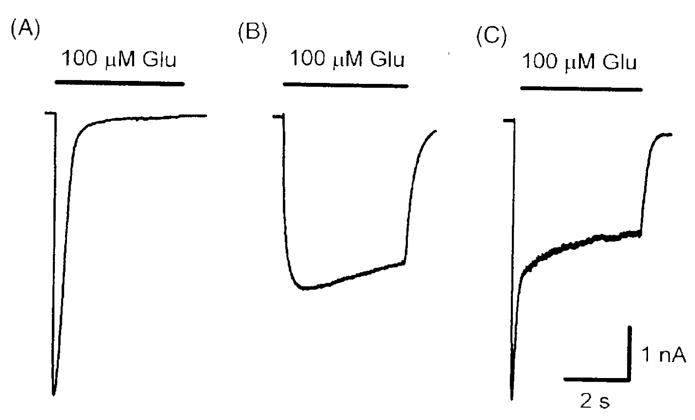
Glutamate-activated currents in cockroach thoracic ganglion neurons recorded by the whole-cell patch clamp method. Three types of currents were evoked following the U-tube applications of 100 μM glutamate for 8 sec at a holding potential of −60 mV, with the symmetrical chloride concentrations between internal and external solutions (Zhao et al., 2004b).
These glutamate-induced currents have been shown to be carried by chloride ions (Zhao et al., 2004b). With symmetrical chloride concentrations across the membrane, the reversal potential was estimated to be +2.5 mV for both fast- and slow-desensitizing currents. This value is very close to the calculated chloride equilibrium potential. When the external chloride concentration was reduced to a quarter, the reversal potential was shifted to +37.5 mV. This amount of shift agreed with the calculated shift of reversal potential. Thus, it is concluded that GluCls are carried by chloride ions.
Since cockroach GABA receptors and glutamate receptors are both accompanied by chloride channels, a question arises as to whether these two receptors are different entities. Several types of experiments have proven that this is indeed the case (Zhao et al., 2004b).
Fipronil is known to block GABA receptors thereby causing hyperexcitation of insects and mammals (Ikeda et al., 2001; Zhao et al., 2003). Fipronil has also been found to be a potent blocker of GluCls (Zhao et al., 2004a, b; 2005). The slow-desensitizing GluCls were much more sensitive to the blocking action of fipronil than the fast-desensitizing GluCls with IC50s of 10 nM and 800 nM, respectively (Fig. 7) (Zhao et al., 2004a; Narahashi et al., 2007).
Figure 7.
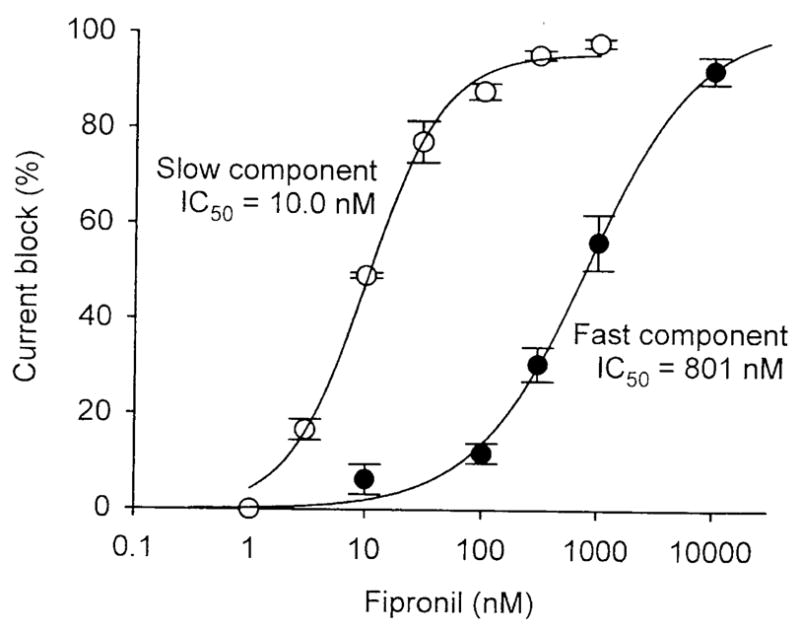
Dose-response relationships of fipronil block of slow- desensitizing and fast-desensitizing GluCls of cockroach neurons. The currents were evoked by 20-sec U-tube and bath application of 100 μM glutamate and various concentrations of fipronil at a holding potential of −60 mV. The maximum peak current was measured for the fast-desensitizing current and the steady-state current was measured for the slow-desensitizing current (Zhao et al., 2004a).
Fipronil is known to be degraded to fipronil sulfone via biotic/abiotic oxidation and to a desulfinyl photoproduct via photolysis (Bobe et al., 1998; Ngim et al., 2000). The biological conversion of fipronil to fipronil sulfone was blocked by piperonyl butoxide (Hainzl et al., 1998; Caboni et al., 2003). However, it was unlear whether the conversion of fipronil to its sulfone represented detoxication in mammals and insects. Fipronil sulfone blocked both slow- and fast-desensitizing GluCls (Zhao et al., 2005). The IC50 for slow- and fast-desensitizing currents were calculated to be 8.5 ± 0.4 nM (n = 4) and 25 ± 2 nM (n = 4), respectively. Thus, fipronil sulfone plays an important role in the toxicity caused by fipronil.
The blocking action of fipronil in cockroach neuron GluCls is much more potent than that in mammalian neurons. The IC50 values are: 10 nM in cockroach slow GluCls, 800 nM in cockroach fast GluCls, 30 nM in cockroach GABAergic chloride channels, and 1600 nM in rat GABAergic chloride channels. Thus, the high sensitivity of cockroach GluCls and GABAergic chloride channels plays a crucial role in selective fipronil toxicity in insects over mammals. Insect GluCls will become an excellent target site for development of new insecticides with a high degree of selectivity over mammals.
Conclusions
Glutamate-activated chloride channels, which are present in insects but not in mammals, are crucial target site responsible for the selective toxicity to insects over mammals for certain insecticides. Therefore, GluCls are a unique and important site for developments of new insecticides. This represents an excellent example in which non-mammals are irreplaceable materials for the study of neurotoxicology.
Overall Conclusions
Certain non-mammalian preparations have been shown to be excellent materials for the study of neurotoxicology. Recent advances in genetic techniques and the strong conservation between human and non-mammalian genomes make it possible to dissect out the molecular pathways involved in neurotoxicological responses using genetically tractable organisms. In many cases, toxicant’s target receptors are similar between mammals and non-mammals. However, in some other cases, non-mammals have a target receptor that is not present in mammals; this makes the receptor important for the selective toxicity of insecticides. Low costs and easiness for maintaining non-mammals in the laboratory are another great advantage.
Zebrafish offers several advantages: low costs, a variety of morphological and functional assays, and a high degree of correlation with mammalian systems. C. elegans provides remarkable opportunities to identify and characterize genes and proteins involved in Parkinson’s disease. It can also be utilized to screen and identify environmental agents and to develop several toxicological assays. An example is also given in which molecular target sites of sodium channels for pyrethroid insecticides can be analyzed using Drosophila and cockroach. Cockroach neurons also represent a unique material for the electrophysiological analysis of selective toxicity as they are endowed with glutamate-activated chloride channels which are highly sensitive targets of fipronil and which are present in insects but not in mammals. It is expected that use of non-mammals for a variety of neurotoxicological studies will become more important in the coming
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arena JP, Liu KK, Paress PS, Frazier EG, Cully DF, Mrozik H, Schaeffer JM. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. J Parasitol. 1995;81:286–294. [PubMed] [Google Scholar]
- Bang PI, Yelick PC, Malicki JJ, Sewell WF. High-throughput behavioral screening method for detecting auditory response defects in zebrafish. J Neurosci Methods. 2002;118:177–187. doi: 10.1016/s0165-0270(02)00118-8. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Becker RA, Borgert CJ, Webb S, Ansell J, Amundson S, Portier CJ, Goldberg A, Bruner LH, Rowan A, Curren RD, Stott WT. Report of an ISRTP workshop: progress and barriers to incorporating alternative toxicological methods in the U.S. Regul Toxicol Pharmacol. 2006;46:18–22. doi: 10.1016/j.yrtph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Bloom L. PhD Thesis. Massachusetts Institute of Technology; Cambridge, MA: 2003. Genetic and molecular analysis of genes required for axon outgrowthCaenorhabditis elegans. [Google Scholar]
- Bobe AP, Meallier J, Cooper F, Coste CM. Kinetics and mechanisms of abiotic degradation of fipronil (hydrolysis and photolysis) J Agr Food Chem. 1998;46:2834–2839. [Google Scholar]
- Boyd WA, McBride SA, Freedman JH. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: evaluation of a novel, high-throughput screening assay. PLoS ONE. 2007;2:e1259. doi: 10.1371/journal.pone.0001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM. Cystic canal mutants in Caenorhabtitis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev Biol. 1999;214:227–241. doi: 10.1006/dbio.1999.9398. [DOI] [PubMed] [Google Scholar]
- Burkhart CN. Ivermectin: an assessment of its pharmacology, microbiology and safety. Vet Hum Toxicol. 2000;42:30–35. [PubMed] [Google Scholar]
- Caboni P, Sammelson E, Casida JE. Phenylpyrazole insecticide photochemistry, metabolism and GABAergic action: ethiprole compared with fipronil. J Agr Food Chem. 2003;51:7055–7061. doi: 10.1021/jf030439l. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Chia SE, Foo SC, Gan SL, Jeyaratnam J, Tian CS. Neurobehavioral functions among workers exposed to manganese ore. Scand J Work Environ Health. 1993;19:264–720. doi: 10.5271/sjweh.1475. [DOI] [PubMed] [Google Scholar]
- Choi JS, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Nav 1.8 sodium channels expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2006;211:233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Cleland TA. Inhibitory glutamate receptor channels. Mol Neurobiol. 1996;13:97–136. doi: 10.1007/BF02740637. [DOI] [PubMed] [Google Scholar]
- Cole RD, Anderson GL, Williams PL. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol. 2004;194:248–56. doi: 10.1016/j.taap.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Dong K. Insect sodium channels and pyrethroid resistance. Invert Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Liu Z, Nomura Y, Khambay B, Dong K. An alanine in segment 3 of domain III (IIIS3) of the cockroach sodium channel contributes to the low pyrethroid sensitivity of an alternative splice variant. Insect Biochem Molec Biol. 2006;36:161–168. doi: 10.1016/j.ibmb.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Ihara M, Ochi E, Shibata Y, Matsuda K, Fushiki S, Sugama H, Hamasaki Y, Niwa H, Wada M, Ozoe F, Ozoe Y. Functional characterization of Musca glutamate- and GABA-gated chloride channels expressed independently and coexpressed in Xenopus oocytes. Insect Mol Biol. 2006;15:773–783. doi: 10.1111/j.1365-2583.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- Feng BY, Simeonov A, Jadhav A, Babaoglu K, Inglese J, Shoichet BK, Austin CP. A high-throughput screen for aggregation-based inhibition in a large compound library. J Med Chem. 2007;50:2385–90. doi: 10.1021/jm061317y. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Cox KJ, O’alley DM. Monitoring activity in neuronal populations with single-cell resolution in a behaving vertebrate. Histochem J. 1998;30:153–167. doi: 10.1023/a:1003243302777. [DOI] [PubMed] [Google Scholar]
- Ginsburg KS, Narahashi T. Differential sensitivity of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels to the insecticide allethrin in dorsal root ganglion neurons. Brain Res. 1993;627:239–248. doi: 10.1016/0006-8993(93)90326-i. [DOI] [PubMed] [Google Scholar]
- Gration KAF, Clark RB, Usherwood PNR. Three types of L-glutamate receptor on junctional membrane of locust muscle fibres. Brain Res. 1979;171:360–364. doi: 10.1016/0006-8993(79)90343-3. [DOI] [PubMed] [Google Scholar]
- Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- Hejmadi MV, Jagannathan S, Delany NS, Coles GC, Wolstenholme AJ. L–glutamate binding sites of parasitic nematodes: an association with ivermectin resistance? Parasitology. 2000;120:535–545. doi: 10.1017/s0031182099005843. [DOI] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Ishida C, Okuda H, Ozoe Y, Matsuda K. Differential blocking actions of 4′l-4-n-propylbicycloorthobenzoate (EBOB) and γ-hexachlorocyclohexane (γ-HCH) on γ-aminobutyric acid- and glutamate-induced responses of American cockroach neurons. Invert Neurosci. 2005;5:157–164. doi: 10.1007/s10158-005-0008-5. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Zhao X, Nagata K, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of γ-aminobutyric acidA receptors in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2001;296:914–921. [PubMed] [Google Scholar]
- Ikeda T, Zhao X, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of glutamate-induced chloride currents in cockroach thoracic ganglion neurons. Neurotoxicology. 2003;24:807–815. doi: 10.1016/S0161-813X(03)00041-X. [DOI] [PubMed] [Google Scholar]
- Janssen D, Derst C, Buckinx R, Van den Eynden J, Rigo J-M, Van Kerkhove E. Dorsal unpaired median neurons of Locusta migratoria express ivermectin- and fipronil-sensitive glutamate-gated chloride channels. J Neurophysiol. 2007;97:2642– 2650. doi: 10.1152/jn.01234.2006. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord. 1998;13:24–34. [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel target: C. elegans as a model organism. Nat Rev Drug Discover. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kane NS, Hirschberg B, Qian S, Hunt D, Thomas B, Brochu R, Ludmerer SW, Zheng Y, Smith M, Arena JP, Cohen CJ, Schmatz D, Warmke J, Cully DF. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc Natl Acad Sci USA. 2000;97:13949–13954. doi: 10.1073/pnas.240464697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khersonsky SM, Jung DW, Kang TW, Walsh DP, Moon HS, Jo H, Jacobson EM, Shetty V, Neubert TA, Chang YT. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J Am Chem Soc. 2003;125:11804–11805. doi: 10.1021/ja035334d. [DOI] [PubMed] [Google Scholar]
- Lakso M, Vartianen S, Moilanen AM, Sirvio J, Thomoas JH, Nass R, Blakely RD, Wong G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human α-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ingles PJ, Knipple DC, Soderlund DM. Developmental regulation of alternative exon usage in the house fly Vssc1 sodium channel gene. Invert Neurosci. 2002;4:125–133. doi: 10.1007/s10158-001-0014-1. [DOI] [PubMed] [Google Scholar]
- Link EM, Hardimann G, Sluder AE, Johnson CD, Liu LX. Therapeutic target discovery using Caenorhabditis elegans. Pharmacogenomics. 2000;1:203–217. doi: 10.1517/14622416.1.2.203. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Method Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- McKinley ET, Baranowski TC, Blavo DO, Cato C, Doan TN, Rubinstein AL. Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Brain Res Mol Brain Res. 2005;141:128–137. doi: 10.1016/j.molbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- Moon HS, Jacobson EM, Khersonsky SM, Luzung MR, Walsh DP, Xiong W, Lee JW, Parikh PB, Lam JC, Kang TW, Rosania GR, Schier AF, Chang YT. A novel microtubule destabilizing entity from orthogonal synthesis of triazine library and zebrafish embryo screening. J Am Chem Soc. 2002;124:11608–11609. doi: 10.1021/ja026720i. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Molecular and cellular approaches to neurotoxicology: past, present and future. In: Lunt GG, editor. Neurotox ‘88: Molecular Basis of Drug and Pesticide Action. New York: Elsevier; 1988. pp. 563–582. [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J Pharmacol Exp Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- Narahashi T. Recent progress in the mechanism of action of insecticides: pyrethroids, fipronil and indoxacarb. J Pestic Sci. 2001;26:277–285. [Google Scholar]
- Narahashi T, Zhao X, Ikeda T, Nagata K, Yeh JZ. Differential actions of insecticides on target sites: basis for selective toxicity. Human Exp Toxicol. 2007;26:361–366. doi: 10.1177/0960327106078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Blakely RB. The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration. Annu Rev Pharmacol. 2003;43:521–544. doi: 10.1146/annurev.pharmtox.43.100901.135934. [DOI] [PubMed] [Google Scholar]
- Nass R, Hahn MK, Jessen T, McDonald PW, Carvelli L, Blakely RD. A genetic screen in Caenorhabditis elegans for dopamine neuron insensitivity to 6-hydroxydopamine identifies dopamine transporter mutants impacting transporter biosynthesis and trafficking. J Neurochem. 2005;94:774–785. doi: 10.1111/j.1471-4159.2005.03205.x. [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorgabditis elegans. P Natl Acad Sci USA. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Miller DM, Blakely RD. C.elegans: A novel pharmacogenetic model to study Parkinson’s disease. Parkinsonism Relat D. 2001;7:185–91. doi: 10.1016/s1353-8020(00)00056-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Appendix C Signaling Pathways. Scientific Frontiers in Developmental Toxicology and Risk Assessment; Committee on Developmental Toxicology, Board on Environmental Studies and Toxicology, Commission on Life Sciences and National Research Council, editors; Washington, DC: National Academy Press; 2000. pp. 296–308. [Google Scholar]
- Ngim KK, Mabury SA, Crosby DG. Elucidation of fipronil photodegradation pathways. J Agr Food Chem. 2000;48:4661–4665. doi: 10.1021/jf9913007. [DOI] [PubMed] [Google Scholar]
- Osborne MP, Hart RJ. Neurophysiological studies of the effects of permethrin upon pyrethroid resistant (kdr) and susceptible strains of dipateran larvae. Pestic Sci. 1979;10:407–413. [Google Scholar]
- Patlak JB, Gration KAF, Usherwood PNR. Single glutamate-activated channels in locust muscle. Nature. 1979;278:643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Parng C, Anderson N, Ton C, McGrath P. Zebrafish apoptosis assays for drug discovery. Methods Cell Biol. 2004;76:75–85. doi: 10.1016/s0091-679x(04)76005-7. [DOI] [PubMed] [Google Scholar]
- Parng C, Roy NM, Ton C, Lin Y, McGrath P. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol. 2007;55:103–112. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Parng C, Ton C, Lin YX, Roy NM, McGrath P. A zebrafish assay for identifying neuroprotectants in vivo. Neurotoxicol Teratol. 2006;28:509–516. doi: 10.1016/j.ntt.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Peitsaro N, Kaslin J, Anichtchik OV, Panula P. Modulation of the histaminergic system and behaviour by alpha-fluoromethylhistidine in zebrafish. J Neurochem. 2003;86:432–1. doi: 10.1046/j.1471-4159.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- Pifl C, Khorchide M, Kattinger A, Reither H, Hardy J, Hornykiewicz O. alpha-Synuclein selectively increases manganese-induced viability loss in SK-N-MC neuroblastoma cells expressing the human dopamine transporter. Neurosci Lett. 2004;354:34–37. doi: 10.1016/j.neulet.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Pulak R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol Biol. 2006;351:275–86. doi: 10.1385/1-59745-151-7:275. [DOI] [PubMed] [Google Scholar]
- Raymond-Delpech V, Matsuda K, Sattelle BM, Rauh JJ, Sattelle DB. Ion channels: molecular targets of neuroactive insecticides. Invert Neurosci. 2005;5:119–133. doi: 10.1007/s10158-005-0004-9. [DOI] [PubMed] [Google Scholar]
- Raymond V, Sattelle DB. Novel animal-health drug targets from ligand-gated chloride channels. Nat Rev Drug Discov. 2002;1:427–436. doi: 10.1038/nrd821. [DOI] [PubMed] [Google Scholar]
- Reading RJ, Dunnett SB. 6-hydroxdopamine lesions of nigrostriatal neurons as an animal model of Parkinson’s Disease. In: Woodruff ML, Nonnemann AJ, editors. Toxin-induced Models of Neurological Disorders. New York: Plennum; 1994. pp. 89–119. [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C. elegans II. New York: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Shafizadeh E, Peterson RT, Lin S. Induction of reversible hemolytic anemia in living zebrafish using a novel small molecule. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138:245–249. doi: 10.1016/j.cca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. NeuroToxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- Soderlund D. Sodium channels. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Insect Science, Elsevier B.V. Vol. 5-Pharmacology. 2005. pp. 1–24. [Google Scholar]
- Soderlund DM, Bloomquist JR. Neurotoxic actions of pyrethroid insecticides. Ann Rev Entomol. 1989;34:77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]
- Song JH, Narahashi T. Modulation of sodium channels of rat cerebellar Purkinje neurons by the pyrethroid tetramethrin. J Pharmacol Exp Ther. 1996;277:445–453. [PubMed] [Google Scholar]
- Song W, Liu Z, Tan J, Nomura Y, Dong K. RNA editing generates tissue-specific sodium channels with distinct gating properties. J Biol Chem. 2004;279:2554–32561. doi: 10.1074/jbc.M402392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring DR, Krishnan S, Blackwell HE, Schreiber SL. Diversity-oriented synthesis of biaryl-containing medium rings using a one bead/one stock solution platform. J Am Chem Soc. 2002;124:1354–1363. doi: 10.1021/ja017248o. [DOI] [PubMed] [Google Scholar]
- Stern HM, Murphey RD, Amatruda JF, Straub CT, Pfaff KL, Weber G, Tallarico JA, King RW, Zon LI. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol. 2005;1:366–370. doi: 10.1038/nchembio749. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Louca JB, Wong JC, Schreiber SL. Split--pool synthesis of 1,3-dioxanes leading to arrayed stock solutions of single compounds sufficient for multiple phenotypic and protein-binding assays. J Am Chem Soc. 2001;123:1740–1747. doi: 10.1021/ja0036108. [DOI] [PubMed] [Google Scholar]
- Stredrick DL, Stokes AH, Worst TH, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the type II pyrethroid deltamethrin. J Pharmacol Exp Ther. 1998;284:958–65. [PubMed] [Google Scholar]
- Tan J, Liu Z, Nomura Y, Goldin AL, Dong K. Alternative splicing of an insect sodium channel gene generates pharmacologically distinct sodium channels. J Neurosci. 2002;22:5300–5309. doi: 10.1523/JNEUROSCI.22-13-05300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatara CP, Newman MC, McCloskey JT, Williams PL. Use of ion characteristics to predict relative toxicity of mono-, di- and trivalent metal ions: Caenorhabditis elegans LC50s. Aquat Toxicol. 1998;42:255–269. [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tertodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Thackeray JR, Ganetzky B. Conserved alternative splicing patterns and splicing signals in the Drosophila sodium channel gene para. Genetics. 1995;141:203–214. doi: 10.1093/genetics/141.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH, Lockery SH. Neurobiology. In: Hope IA, editor. C. elegans: A practical approach. New York: Oxford Univ. Press; 1999. pp. 143–179. [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Ultsch A, Schuster CM, Laube B, Schloss P, Schmitt B, Betz H. Glutamate receptors of Drosophila melanogaster: cloning of a Kainate-selective subunit expressed in the central nervous system. Proc Natl Acad Sci USA. 1992;89:10484–10488. doi: 10.1073/pnas.89.21.10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Hick CA, Eldursi N, Devonshire AL, Usherwood PNR. Functional analysis of a rat sodium channel carrying a mutation for insect knockdown resistance (kdr) to pyrehroids. FEBS Lett. 1997;413:327–332. doi: 10.1016/s0014-5793(97)00931-9. [DOI] [PubMed] [Google Scholar]
- Wang SY, Barile M, Wang GK. A phenylalanine residue at segment D3-S6 in Nav1.4 voltage-gated Na+ channels is critical for pyrethroid action. Mol Pharmacol. 2001;60:620–628. [PubMed] [Google Scholar]
- Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan RAG, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van der Ploeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels: Modulation by the membrane protein tipE and toxin Pharmacology. J Gen Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet. 2001;28:160–164. doi: 10.1038/88878. [DOI] [PubMed] [Google Scholar]
- Zhao X, Salgado VL, Yeh JZ, Narahashi T. Differential actions of fipronil and dieldrin insecticides on GABA-gated chloride channels in cockroach neurons. J Pharmacol Exp Ther. 2003;306:914–924. doi: 10.1124/jpet.103.051839. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yeh JZ, Salgado VL, Narahashi T. Fipronil is a potent open channel blocker of glutamate-activated chloride channels in cockroach neurons. J Pharmacol Exp Ther. 2004a;310:192–201. doi: 10.1124/jpet.104.065516. [DOI] [PubMed] [Google Scholar]
- Zhao X, Salgado VL, Yeh JZ, Narahashi T. Kinetic and pharmacological characterization of desensitizing and non-desensitizing glutamate-gated chloride channels in cockroach neurons. Neurotoxicology. 2004b;25:967–980. doi: 10.1016/j.neuro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yeh JZ, Salgado VL, Narahashi T. Salfone metabolite of fipronil blocks γ-aminobutyric acid- and glutamate-activated chloride channels in mammalian and insect neurons. J Pharmacol Exp Ther. 2005;314:363–373. doi: 10.1124/jpet.104.077891. [DOI] [PubMed] [Google Scholar]
- Zhao X, Salgado VL, Yeh JZ, Narahashi T. Mechanism of selective toxicity of α-endosulfan in insects and mammals; Society of Toxicology Annual Meeting; 2007. Abstr. #895. [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]