Abstract
IL-2 and IL-15 were tested for effects on responses to mechanical or thermal stimuli when spinally administered to male Sprague-Dawley rats with surgically implanted intrathecal catheters. Restricted doses of both IL-2 and IL-15 produced increased responsiveness to mechanical stimulation of the hindpaws. This effect lasted up to 48 hours. IL-2 had biphasic effects on thermal responses whereas IL-15 produced thermal hypalgesia alone. These effects dissipated within 24 hours. These results suggest that IL-2 and IL-15 may participate in the generation of hyperalgesia in some pain conditions.
Keywords: Hyperalgesia, hypalgesia, cytokines
Introduction
Cytokines are widely recognized as important mediators of many nociceptive processes [1;2]. For example, interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNFα) play key roles in the development and maintenance of chronic inflammatory diseases such as rheumatoid arthritis. Intradermal, intrathecal, or intraperitoneal injection of any of these alone or in combination cause mechanical hyperalgesia and increase various discharge properties of peripheral and dorsal horn neurons [3–7].
Less clear data are available on IL-2 and IL-15 in pain processes. IL-2 and IL-15 are functionally very similar pro-inflammatory cytokines that both bind and activate shared β and γ receptor subunits and similarly, though not identically, stimulate the proliferation of peripheral blood T, B and NK cells [8]. IL-2 and IL-15 and their receptors are also found in numerous nervous system structures including dorsal root ganglion, spinal cord, hippocampus, neostriatum, and hypothalamus [9]. TH1 cells are considered the main source of IL-2 in the periphery, though B cells also produce IL-2 and IL-2 has been detected in primary cultures of neural tissue [10]. The main sources for IL-15 include epithelial cells, monocytes, fibroblasts, dendritic cells and activated T cells [8]. Through their immunomodulatory effects both IL-2 and IL-15 are implicated in the pathogenesis, albeit perhaps indirectly, of numerous painful and inflammatory conditions that involve clonal expansion of T and B cell subsets or auto-reactive cytotoxic T cells and antibodies. Examples of these conditions might include multiple sclerosis, human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis, rheumatoid arthritis, sarcoiditis, ulcerative colitis, and inflammatory bowel disease [11–13]
Nevertheless, the direct effects of these cytokines on pain processes have either not been studied or been mixed. Intraplantar injection of recombinant IL-2 produced antinociception that was related to the binding of IL-2 to opioid receptors [14]. Similarly, the intrathecal administration of IL-2 and IL-2 gene transfection ameliorated thermal hyperalgesia in rats with chronic constriction injury of the sciatic nerve [15]. Contrary to these reports, the intravenous administration of IL-2 in humans for the treatment of various malignancies such as renal carcinoma, melanoma and neuroblastoma [11] have been associated with severe painful neuropathy refractory to analgesic medications, muscle aches, enhanced tumor-induced bone pain, and persistent localized pain at the site of the cytokine injection in humans [16].
Amidst this controversy, IL-15 has been found in synovial fluid of patients with rheumatoid arthritis [17] and was found to be increased in dorsal root ganglion and the spinal dorsal horn of rats with vinca alkaloid (vincristine)-induced neuropathic pain [18]. Similarly, the expression of IL-2 receptor was increased in the DRG of rats with vincristine induced chemoneuropathy [18]. IL-15 produced hyperalgesia when given intradermally in mice [19], but otherwise has not been tested for effects on pain processing. Hence, the goal of this study was to investigate the effects of intrathecal injection of IL-15 and to re-investigate the effects of intrathecal IL-2 on pain processing in rats.
Methods
A total of 58 male Sprague-Dawley rats (Harlan, Houston) weighing 350–400 g were used. Rats were individually housed at a room temperature of 22°. They were fed ad libitum and kept in a 12-h dark-light cycle. Behavioral experiments were conducted in a quiet room during the morning light period (9AM-12 Noon). The investigator was blinded to the treatment administered during all of the behavioral tests. All experiments were conducted with Institutional IACUC review and approval and were in accord with the NIH guidelines to minimize pain or discomfort and the numbers of animals used.
Intrathecal catheter implantation
A PE-10 catheter pre-filled with 7–8µl of artificial cerebrospinal fluid was placed in the intrathecal space overlying the L1 spinal segment using previously described techniques [20;21]. Briefly, rats were anesthetized with 50 mg/kg of pentobarbital. Supplemental oxygen was provided during surgery. The posterior aspect of the neck was shaved, cleansed, incised and the atlanto-occipital membrane was exposed. The membrane was carefully opened, and the catheter advanced to the lumbar enlargement. Animals were allowed to recover for a minimum period of 5 days. No animals in this series showed any signs of motor impairment as a consequence of the catheter placement. At seven to 10 days after surgery, the animals received a single i.t. injection of 10µL 1% lidocaine to induce a transient spinal anesthesia and thus verify proper catheter position. This was evidenced by flaccid hindlimbs and a lack of responses to toe pinch. At the end of all experiments the animals were sacrificed by pentobarbital overdose (300mg, i.p.) and a laminectomy was performed. Dye was then injected via the catheter to visualize the placement of the tip. All animals in this series showed both hindlimb paralysis with spinal injection of lidocaine and dye spread over the lumbar enlargement during the post-mortem visualization were included for statistical analysis.
Behavioral Testing
Behavioral testing began 10 to 14 days after surgery. Rats were previously acclimated to both the mechanical and thermal testing chambers by placing them into these chambers and allowing them to acclimate for 15–30 minutes on three consecutive days between recovery from the surgery and the initiation of any test procedures. Assessment of mechanical and thermal withdrawal data was pseudorandomized for each rat on each testing day. Rats were allowed to return to their home cage for a one hour rest between the first (mechanical or thermal) and the second assays on each day.
Mechanical withdrawal threshold
The rats were loosely restrained under clear acrylic boxes, placed on a wire mesh, and allowed to acclimate on each experimental day for at least 15 min. Von Frey filaments with bending forces of 0.12, 0.53, 1.05, 4.41, 8.86, 16.4, and 42g were presented from below in pseudorandom order and held in bent position for 1–2 sec on the mid-plantar surface of the hind paw. Each foot was stimulated with the same filament 5 times with an interstimulus interval of 3–5 minutes. The total number of paw withdrawals for each filament was recorded. A response was defined as brisk foot withdrawal in response to the mechanical stimulus [18].
Thermal nociceptive threshold
Thermal nociceptive thresholds were assessed using radiant heat [22]. The rats were loosely restrained under arcylic boxes placed on a glass surface for a conditioning period of at least 15 min. The heat source was positioned beneath the mid-plantar surface of the hind paw. Withdrawal latency was defined as the period of time from the beginning of the thermal stimulation to the brisk withdrawal of the hind paw. To avoid tissue damage, a cutoff time of 24 sec was set. Thermal stimulation was applied 3 times to each hind paw in pseudorandomized order at an interstimulus interval of 3–5 min [18].
Drug administration and treatment groups
IL-2 (recombinant rat) and IL-15 (recombinant human) were purchased from Biosource International (Carmarillo, CA, Catalog no.s PRC0024 and PHC0154, carrier-free lyophilized protein). Both cytokines were injected in a volume of 10 µL followed by the administration of 10 µL of artificial cerebrospinal fluid (ACSF). The dose ranges were based on precedent studies of IL-2 effects in the peripheral and central nervous system [14;23;24] and based on measurements of cytokine responses in neural tissue induced in models of experimental neuropathy . Two doses of IL-2 were used in reference to the earlier studies: 500 U and 2000 U. These are referred to here as the IL-2500U and IL-22000U groups. Given the limited knowledge of IL-15 effects in the CNS, three doses of this cytokine were used: 500, 1000, and 10,000 U. These are referred to here as the IL-15500U, IL-151000U, and the IL-1510,000U groups. Denatured IL-2 (2000U) and IL-15 (500U and 1000U) were injected to verify specific activity of the protein and to exclude non-specific effects of foreign protein. These groups are referred to as the IL2Denatured and the IL15Denatured groups, respectively. The composition of the ACSF was standard based on previous behavioral and physiological studies in the laboratory (e.g., [21]) and contained the following (in mM): NaCl 120; KCl 3; NaHCO3 25; CaCl2 2.5; MgCl2 0.5; glucose 12 (pH 7.4).
Data Analysis
The percent mechanical withdrawal threshold and the thermal withdrawal latencies are expressed as mean ± standard error. To evaluate statistical significance between treatment groups in mechanical withdrawal threshold the Kruskall-Wallis non-parametric analysis was used. The effects of each agent over time were analyzed using ANOVA. Post hoc evaluations in the latter analysis were performed using Tukey’s test. Significant difference was considered at p < 0.05.
Results
Mechanical stimulation
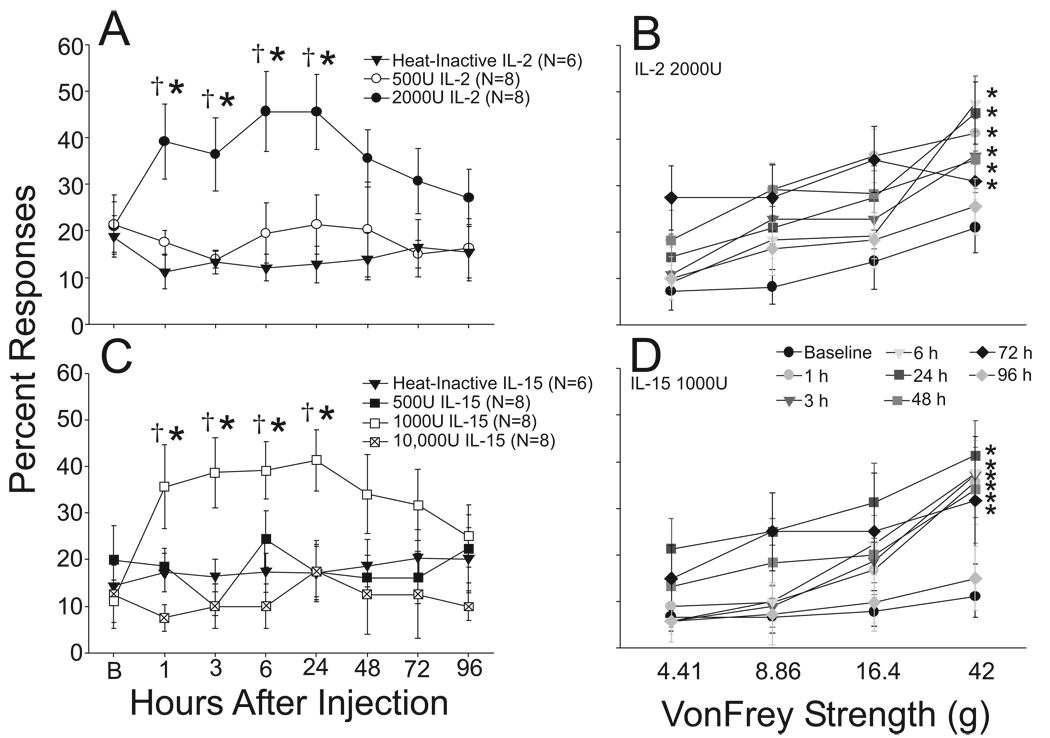
The spinal administration of denatured IL-2/IL-15 in ACSF did not produce any change in response to mechanical stimulation of the hindpaws. Figure 1 shows the mean responses for each treatment group to the highest threshold 42g von Frey filament in the test series over time in comparison with the data obtained appropriate control groups (A and C). This strength filament produced constant responses at approximately 15% of all trials throughout the experiment for the control group. The withdrawal responses in the control rats to other filaments were similarly stable albeit at lesser rates of response. The range of filaments where responses were obtained also remained constant down to the 4.41g filament. The mean response at this lowest strength filament was approximately 6% at all trials (mean 5.8 ± 2% for the control group at baseline). Intrathecal injection of 500 U IL-2 had no effects on mechanical withdrawal responses (Figure 1). These animals also showed overall responses over the same range of filaments as the control rats (4.41g to 42.0g filaments), and the rates of responses to all filaments were comparable to the control rats.
Figure 1.
Scatter line plots in 1A and 1C show the response rates of rats to a 42g von Frey filament at baseline (B) and then at several time points following intrathecal (i.t) injection of heat-inactivated protein (solid triangles, upper and lower panels), IL-2500U (open circles, upper panel), IL-22000U (filled circles, upper panel), IL-15500U (filled squares, lower panel), IL-151000U (open squares, lower panel) or IL-1510,000U (cross-hatched squares, lower panel). IL-22000U and IL-151000U both produced hyper-responsiveness to mechanical stimuli evidenced by the increased percent of positive withdrawal responses by one hour following i.t. injection and this effect persisted for 24 hours. Responses to mechanical stimuli remained elevated, though not significantly so, for up to three days and then slowly returned to baseline levels. Responses to all filaments for IL-22000 and IL-151000U, the doses of each cytokine that produced mechanical hyperalgesia, are shown over time in 1B and 1D. In A and C, *, p < 0.05 vs. combined baseline values, †, p < 0.05 vs. heat-inactivated protein control. In B and D, *, p < 0.05 vs. matching baseline (AUC Comparison).
In contrast, intrathecal IL-2 at a dose of 2000 U induced mechanical hyper-responsiveness. This is evidenced in Figure 1A by an increase in the percentage of hind paw withdrawal in response to the 42g force. The baseline responses were comparable in both groups; however, by one hour after injection the response of the IL-22000U group had increased to 39.1 ± 8.5% whereas the control rats maintained stable responses at 11.7± 3.5% (p < 0.05 vs. matching time IL-2Denatured, and IL-22000U baseline). The responses to mechanical stimulation remained elevated when measured at 3 (IL-2Denatured: 12.7 ± 3.1% vs IL-22000U: 35.5 ± 5.5%; p < 0.05 matching time IL2Denatured, and IL-22000U baseline), 6 (controls: 12.7 ± 3.5% vs IL-22000U 45.5 ± 8.5%, p<0.05 matching time IL2Denatured, and IL-22000U baseline) and 24 hours (IL-2Denatured: 15.45 ± 5.36% vs IL-2: 45.45 ± 8.04%; p < 0.05 matching time IL-2Denatured, and IL-22000U baseline) after cytokine injection. Responses were similarly elevated to the other filaments in the test series that were effective in evoking responses in the baseline tests (4.41 to 42g). These data are shown in Figure 1B. The filaments not effective at evoking responses in the baseline (0.12 to 1.05g filaments) remained ineffective following IL-22000U except for that the 1.1g filament evoked 3.6 ± 1.3% responses at 1 hour following cytokine injection (not shown). Mechanical responsiveness was found to be returning toward normal by 48 hours after cytokine injection and showed full recovery by one week.
Spinal administration of 500 U and 10 000 U IL-15 produced no change in the responses to mechanical stimulation. However, the intermediate dose of 1000 U IL-15 induced mechanical hyperalgesia in a pattern very similar to that observed in the IL-22000U group. The rate of response to the 42g filament was increased to 35.6 ± 9.0% one hour following cytokine injection (p < 0.05 matching time IL-15Denatured, and IL-15000U baseline) and this effect persisted when measured at 3, 6, and 24 hours. Similar increases in responses were observed in the other filaments effective at baseline (4.41 to 42g) and shown in Figure 1D. No responses developed in the filaments ineffective at baseline (0.12 to 1.05g). Finally, like observed in the IL-22000U group, the responses to mechanical stimulation returned toward baseline at 48 hours in the IL151000U group, and showed full recovery by one week.
Thermal stimulation
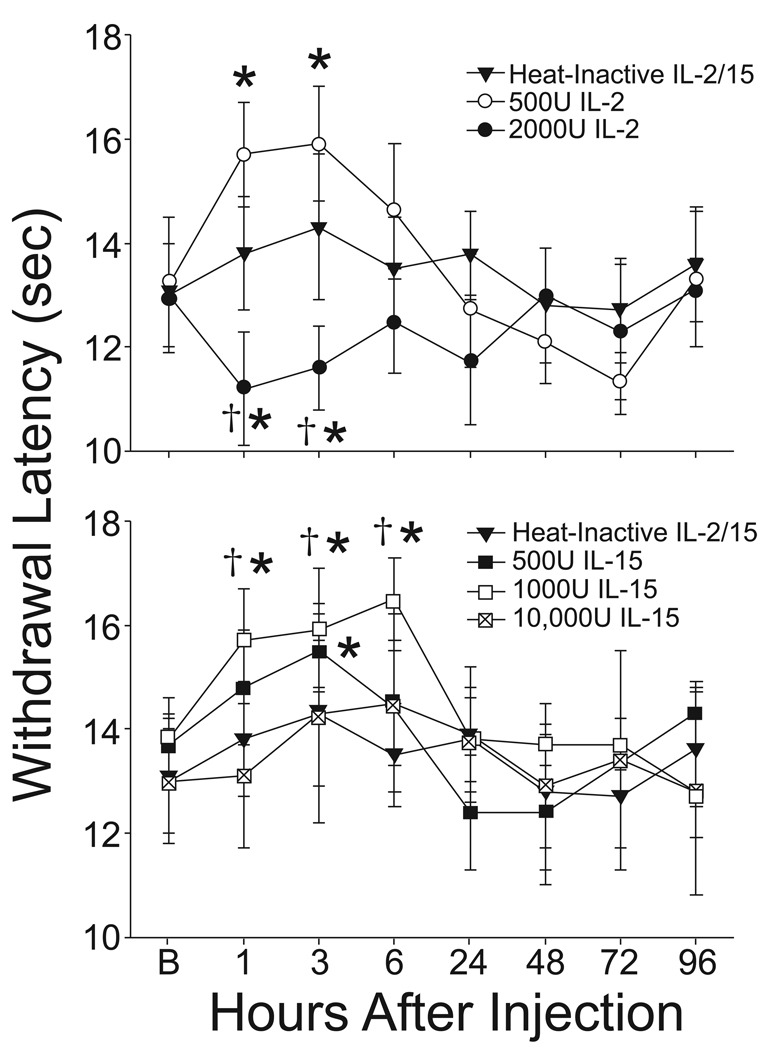
Denatured IL15/IL2-ACSF did not modify paw withdrawal latency to radiant heat. As shown in figure 2, paw withdrawal occurred at 13.0 ± 1s in the baseline assessment. Later assessments yielded values very close to the baseline ranging from a high of 14.3 ± 1.3s at 6 hours after injection to a low of 12.8 ± 1.0s at the 48 hr time point. Baseline paw withdrawal latencies for the remaining treatment groups also fell in this same range of times (Figure 2).
Figure 2.
The scatter line plots show the mean withdrawal latency to radiant heat at baseline (B) and then at several time points following intrathecal (i.t) injection of heat-inactivated protein (solid triangles, upper and lower panels), IL-2500U (open circles, upper panel), IL-22000U (filled circles, upper panel), IL-15500U (filled squares, lower panel), IL-151000U (open squares, lower panel) or IL-1510,000U (cross-hatched squares, lower panel). IL-22000U produced hyper-responsiveness to thermal stimuli evidenced by the decreased latency to paw withdrawal to radiant heat by one hour following i.t. injection and this effect persisted for 3 hours and then returned to baseline. In contrast, IL-2500U and IL-15500U and IL-151000U produced thermal hypalgesia (analgesia) that similarly was evidenced within one hour by an elevation in paw withdrawal latency to radiant heat (3 hours for IL-15500U) and this effect persisted between 3 and 6 hours. All responses returned to the baseline level by 24 hours following i.t. injection. *, p < 0.05 vs. combined baseline values, †, p < 0.05 vs. heat-inactivated protein control.
Intrathecal administration of IL2 produced two patterns of change in response to thermal stimulation. The higher dose of 2000U IL-2 produced a transient thermal hyperalgesia present when measured at 1 and at 3 hours after treatment. In contrast, intrathecal injection of 500U IL-2 produced a modest increase in paw withdrawal latency when measured at 1 and 3 hours after injection. This increase was significantly different by a within group comparison to baseline, but was not sufficiently large in magnitude to achieve statistical significance in comparison to the IL-2denatured group. Both effects, the IL-22000U induced hyperalgesia and the IL-2500U induced hypalgesia dissipated by 24 hours following cytokine injection.
Intrathecal injection of IL-15 produced only a single pattern of change in response to radiant heat. Animals in the IL-15500U and IL-151000U groups each showed increased withdrawal latencies to radiant heat at 1, 3 and 6 hours following cytokine injection. This increase was significantly different from the within group baseline and the IL-15denatured group for the IL-151000U group at each of these time points, but was only significantly different in the IL-155ooU from the within-group baseline at the 3 hour time point. These effects had resolved by 24 hours following cytokine injection and the responses to thermal stimulation remained in the normal range for the remainder of the experiment. No effect of intrathecal injection of 10,000U of IL-15 on the responses to radiant heat were observed.
Discussion
IL-2 has many well documented effects on forebrain neurons [9]. IL-2 modulates the maturation, survival and connectivity of forebrain neurons [25]. In the adult brain, IL-2 penetrates the blood-brain barrier [26] and exerts modulatory effects on forebrain-mediated neuroendocrine and behavioral functions. Thus, IL-2 alters the physiology of neurons in the ventromedial, supraoptic and paraventricular nuclei of the hypothalamus [27] resulting in alterations in the secretion of multiple hormones including oxytocin [28] and especially pro-opiomelanocortin-derived hormones and peptides [29;30]. IL-2 produces profound psychological and cognitive impairments in both experimental animals and human patients [23;31;32]. These effects may be mediated by modulation of forebrain dopamine systems [33] and suppression of hippocampal long-term potentiation [34], that in turn may be mediated by direct partial inhibition by IL-2 of calcium, NMDA and kainite receptor-mediated currents in hypothalamic, hippocampal and tegmental neurons [24;35;36]. Prolonged systemic or intrathecal infusion of IL-2 in adults results in toxicity to oligodendrocytes, promotes demyelination and toxicity to neurons [9;25].
There is less cumulative history, and as well less agreement, concerning the role of IL-2 in pain processing. One set of evidence indicate that increased levels of IL-2 in and around sensory nerve endings or in the central nervous system promote nociceptive behaviors. Systemic administration of IL-2 in cancer patients induces pain at the site of injection, bone pain, and painful peripheral neuropathy [37–39]. Experimental studies in animals replicate many of these clinical observations. Intraplantar injections of IL-2 in rats produced mechanical hyperalgesia that was inhibited by the previous administration of anti-IL-2 antibody [40]. Simiarly, intraarticular injections of IL-2 into the knee joints of rats produced mechanical hyperalgesia with a maximun peak effect at 1 hour after the injection that was prevented and reversed by the administration of a bradykinin receptor 1 antagonist [41]. These effects appear to be mediated by depolarization by IL-2 of discrete subpopulations of polymodal nociceptors [42]. Nevertheless, in another series of papers IL-2 induced thermal antinociception in rats that was naloxone-reversible, suggesting an interaction with opioid receptors (e.g.[43]). As noted above, these findings have support in other CNS studies wherein it was observed that IL-2 induces the release of opioid peptides [30] and IL-2 hyperpolarized DRG neurons by interactions at µ opioid receptors [14].
The results shown here help resolve the apparent conflict in the role of IL-2 in nociceptive processing in that our findings confirm both lines of study. Intrathecal injection of 500U of rat recombinant IL-2 produced thermal antinociception, whereas higher doses produced thermal and mechanical hyperalgesia. The discovery of this biphasic response to the species-specific protein, typical of many effects of cytokines [12], suggests that perhaps one set of responses are mediated by interactions of the protein with high-affinity receptors, whereas other responses, particularly those at higher doses and with cross-species proteins, may be mediated at low-affinity receptors. Thus, the analgesic dose required in previous studies [43] was significantly higher than the dose used in the present study possibly due to the use of cross-species protein. Similarly, the time course to the analgesic effects observed by this group was very short, on the order of minutes [43], whereas in this report the effects lasted several hours. These discrepancies may be accounted for by the fact that we used rat-IL-2 and whereas this other group used human recombinant protein. Interestingly our findings with recombinant human IL-15 are in accord with all the findings of this group with human IL-2 except in duration of effects. Presumably, the high-affinity effects of each cytokine would be mediated by the private α receptor subunits, whereas the lower-affinity sites would potentially be mediated by the shared βγ subunits [44].
In conclusion, the spinal administration of IL-2 and IL-15 induces transient changes in the responses of rats to mechanical and thermal stimuli. These results suggest that these cytokines should be considered as additional members of CNS-active immune-derived signal molecules that likely have important roles in inflammatory and neuropathic pain conditions.
Acknowledgments
This work was supported by NIH Grants CA-39933 and NS-109624.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 2.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 3.Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-α, interleukin-1β and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain. 2002;96:89–97. doi: 10.1016/s0304-3959(01)00433-x. [DOI] [PubMed] [Google Scholar]
- 4.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994;657:133–140. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 5.Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur. J. Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- 6.Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 9.Hanisch U-K, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain Res. Rev. 1995;21:246–284. doi: 10.1016/0165-0173(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 10.Kryworuchko M, Theze J. Interleukin-2: From T cell growth and homeostasis to immune reconstitution of HIV patients. Vit. Horm. 2006;74:531–547. doi: 10.1016/S0083-6729(06)74021-3. [DOI] [PubMed] [Google Scholar]
- 11.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Fact. Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 12.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends in Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 13.Azimi N, Jacobson S, Leist T, Waldmann TA. Involvement of IL-15 in the Pathogenesis of Human T Lymphotropic Virus Type I-Associated Myelopathy/Tropical Spastic Paraparesis: Implications for Therapy with a Monoclonal Antibody Directed to the IL-2/15R{beta} Receptor. J Immunol. 1999;163:4064–4072. [PubMed] [Google Scholar]
- 14.Song P, Lie-Chang W, Wang GD, Zhou Z, Zhao Z-Q. Interleukin-2 regulates membrane potentials and calcium channels via µ opioid receptors in rat dorsal root ganglion neurons. Neuropharmacology. 2002;43:1324–1329. doi: 10.1016/s0028-3908(02)00298-8. [DOI] [PubMed] [Google Scholar]
- 15.Yao M-Z, Gu J-F, Wang J-H, Sun L-Y, Lang M-F, Liu J, Zhao Z-Q, Liu X-Y. Interleukin-2 gene terapy of chronic neuropathic pain. Neuroscience. 2002;112:409–416. doi: 10.1016/s0306-4522(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau RF, Haight AE, Hirschmann-Jax C, Yvon ES, Rill DR, Mei Z, Smith SC, Inman S, Cooper K, Alcoser P, Grilley B, Gee A, Popek E, Davidoff A, Bowman LC, Brenner MK, Strother D. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 17.McInnes IB, Al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 18.Cata JP, Weng H-R, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anes. 2006;72:151–169. [PubMed] [Google Scholar]
- 19.Verri WA, Jr, Cunha TM, Parada CA, Wei X-Q, Ferreira SH, Liew FY, Cunha FQ. IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-γ, endothelin, and prostaglandin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9721–9725. doi: 10.1073/pnas.0603286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 21.Grabow TS, Dougherty PM. Cervicomedullary intrathecal injection of morphine produces antinociception in the orofacial formalin test in the rat. Anesthesiology. 2001;95:1427–1434. doi: 10.1097/00000542-200112000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves KM, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 23.Zalcman S, Murray L, Dyck DG, Greenberg AH, Nance DW. Interleukin-2 and -6 induce behavioral-activating effects in mice. Brain Res. 1998;811:111–121. doi: 10.1016/s0006-8993(98)00904-4. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Zhu L-J, Liu S-S, Zhou S-Y, Luo J-H. Interleukin-2 inhibits NMDA receptor-mediated currents directly and may differentially affect subtypes. Biochem. Biophys. Res. Comm. 2006;351:449–454. doi: 10.1016/j.bbrc.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 25.Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related 19 cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Waguespack PJ, Banks WA, Kastin AJ. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res. Bull. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 27.Bindoni M, Perciavalle V, Berretta S, Belluardo N, Diamantstein T. Intereukin 2 modifies the bioelectric activity of some neurosecretory nuclei in the rat hypothalamus. Brain Res. 1988;462:10–14. doi: 10.1016/0006-8993(88)90578-1. [DOI] [PubMed] [Google Scholar]
- 28.Pardy K, Murphy D, Carter D, Hui KM. The influence of interleukin-2 on vasopressin and oxytocin gene expression in the rodent hypothalamus. J. Neuroimmunol. 1993;42:131–138. doi: 10.1016/0165-5728(93)90002-g. [DOI] [PubMed] [Google Scholar]
- 29.Cambronero JC, Rivas FJ, Borrell J, Guaza C. Interleukin-2 induces corticotropin-releasing hormone release from superfused rat hypothalami: Influence of glucocorticoids. Endocrinology. 1992;131:677–683. doi: 10.1210/endo.131.2.1639014. [DOI] [PubMed] [Google Scholar]
- 30.Lapchak PA, Araujo DM. Interleukin-2 regulates monoamine and opioid peptide release from the hypothalamus. Neuroreport. 1993;4:303–306. doi: 10.1097/00001756-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Fent K, Zbinden G. Toxicity of interferon and interluekin. TIPS. 1987;8:100–105. [Google Scholar]
- 32.De Sarro GB, Masuda Y, Ascioti C, Audino MG, Nistico G. Behavioural and ECoG spectrum changes induced by intracerebral infusion of interferons and interleukin 2 in rats are antagonized by naloxone. Neuropharmacology. 1990;29:167–179. doi: 10.1016/0028-3908(90)90057-x. [DOI] [PubMed] [Google Scholar]
- 33.Petitto JM, McCarthy DB, Rinker CM, Huang Z, Getty T. Modulation of behavioral and neurochemical measures of forebrain dopamine fucntion in mice by species-specific interleukin-2. J. Neuroimmunol. 1997;73:183–190. doi: 10.1016/s0165-5728(96)00196-8. [DOI] [PubMed] [Google Scholar]
- 34.Tancredi V, Zona C, Velotti F, Eusebi F, Santoni A. Interleukin-2 suppresses established long-term potentiation and inhibits its induction in the rat hippocampus. Brain Res. 1990;525:149–151. doi: 10.1016/0006-8993(90)91331-a. [DOI] [PubMed] [Google Scholar]
- 35.Ye J-H, Zalcman SS, Tao L. Kainate-activated currents in the ventral tegmental arrea of neonatal rats are modulated by interleukin-2. Brain Res. 2005;1049:227–233. doi: 10.1016/j.brainres.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Plata-Salamán CR, FFrench-Mullen JMH. Interleukin-2 modulates calcium currents in dissociated hippocampal CA1 neurons. Neuroreport. 1993;4:579–581. doi: 10.1097/00001756-199305000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Atzpodien J, Kuchler T, Wandert T, Reitz M. Rapid deterioration in quality of life during interleukin-2- and alpha-interferon-based home therapy of renal cell carcinoma is associated with a good outcome. Br J Cancer. 2003;89:50–54. doi: 10.1038/sj.bjc.6600996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albertini MR, Hank JA, Schiller JH, Khorsand M, Borchert AA, Gan J, Bechhofer R, Storer B, Reisfeld RA, Sondel PM. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3:1277–1288. [PubMed] [Google Scholar]
- 39.Vivancos P, Granena AJ, Sarra J, Granena A. Treatment with interleukin-2 (IL-2) and interferon (IFN(alpha 2b)) after autologous bone marrow or peripheral blood stem cell transplantation in onco-hematological malignancies with a high risk of relapse. Bone Marrow Transplant. 1999;23:169–172. doi: 10.1038/sj.bmt.1701532. [DOI] [PubMed] [Google Scholar]
- 40.Pereira LS, Ferreira-Alves DL, de Resende MA, Romualdo VA, Dos Reis WG, Lopes MT, Santoro MM, Francischi JN. Reduced production of hyperalgesic substances by mononuclear cells from aged rats incubated with carrageenan: role of interleukin 2 and prostaglandins. Inflamm. Res. 2003;52:119–125. doi: 10.1007/s000110300024. [DOI] [PubMed] [Google Scholar]
- 41.Davis AJ, Perkins MN. The involvement of bradykinin B1 and B2 receptor mechanisms in cytokine-induced mechanical hyperalgesia in the rat. Br J Pharmacol. 1994;113:63–68. doi: 10.1111/j.1476-5381.1994.tb16174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin HA, Murphy PR. Interleukin-2 activates a sub-population of cutaneous C-fibre polymodal nociceptors in rat hairy skin. Arch. Physiol. Biochem. 1995;103:136–148. doi: 10.3109/13813459508996127. [DOI] [PubMed] [Google Scholar]
- 43.Song P, Liu XP, Zhao Z. Interleukin-2-induced antinociception in morphine-insensitive rats. Acta Pharmacol Sin. 2002;23:981–984. [PubMed] [Google Scholar]
- 44.Waldmann TA. The Il-2/IL-15 receptor systems: Targets for immunotherapy. J. Clin. Immunol. 2002;22:51–56. doi: 10.1023/a:1014416616687. [DOI] [PubMed] [Google Scholar]