Abstract
Statins are inhibitors of HMG-CoA reductase that have been recently recognized as anti-inflammatory and neuroprotective drugs. Herein, we investigated anti-excitotoxic and anti-seizure effects of statins by using kainic acid (KA)-rat seizure model, an animal model for temporal lobe epilepsy and excitotoxic neurodegeneration. We observed that pretreatment with Lipitor (atorvastatin) effeiciently reduced KA-induced seizure activities, hippocampal neuron death, monocyte infiltration and proinflammatory gene expression. In addition, we also observed that lovastatin treatment attenuated KA- or glutamate-induced excitotoxicity of cultured hippocampal neurons. These observations suggest a potential for use of statin treatment in modulation of seizures and other neurological diseases associated with excitotoxicity.
Keywords: Atorvastatin, excitotoxicity, inflammation, hippocampus, kainic acid, ovastatin and seizure
1. Introduction
Statins are inhibitors of HMG-CoA reductase and inhibit cellular synthesis of cholesterol and isoprenoids. We have previously demonstrated the anti-inflammatory and vasoprotective effects of statins on cultured brain cells (astrocytes and microglia) and endothelial cells, where statins exerted their effects by inhibiting synthesis of isoprenoids and thus interfering with isoprenylation and membrane attachment of small GTPases [21, 26]. Later, neuroprotective effects of statin were reported in various animal disease models and clinical studies. We have reported that statin treatment provided anti-inflammatory effects on experimental autoimmune encephalomyelitis (EAE), a rat spinal cord injury model, human multiple sclerosis and X-adrenoleukodystrophy [20, 23, 24, 31, 33–35, 38]. Moreover, other research groups also have reported the similar neuroprotective role of statins in other neurological disease models, such as traumatic brain injury, brain ischemia and Alzheimer’s disease [9, 13, 16]. However, whether all these neuroprotective activities of statins are a result of their anti-inflammatory and/or vascular protective properties is not clear at present.
Excitotoxicity is a major pathological mechanism underlying acute and delayed neuronal death induced by prolonged ischemia, traumatic brain and spinal cord injuries and Alzheimer’s disease as well as epilepsy/seizure [19, 25, 43]. Therefore, studies have been focused on the reduction of glutamate accumulation in synaptic clefts and/or on the inhibition of glutamate-induced neuronal excitation for prevention of neuronal cell death under various neurological disease conditions. Kainic acid (KA) is an agonist for a subtype of ionotropic glutamate receptor, AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propioni acid and kainic acid receptors). Systemic injection of KA induces progressive limbic seizures in rats, which resemble human temporal lobe epilepsy, the most common symptom of adult human epilepsy [4]. These culminate in status epilepticus (SE) leading to neuronal cell death by induction of reactive oxygen species production and mitochondrial dysfunction in many regions of the brain, particularly in limbic structures (i.e. hippocampal CA1 and CA3, and the hilus of dentate gyrus) [3]. Moreover, delayed induction of proinflammatory gene expression, such as TNF-α, IL-1β, IL-6, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), are regarded to induce prolonged neurodegeneration [1, 28]. For these reasons, KA has been adapted for studies various neurological disorders involving excitotoxicity and inflammation, such as epilepsy, Huntington’s chorea and Alzheimer’s disease [7].
Herein, we examined the potential efficacy of Lipitor (LP; atorvastatin) on modulation of KA-induced status epilepticus and related excitotoxicity and inflammation. We observed that pre-and post-treatment of LP attenuated the seizure activities induced by KA. Furthermore, KA-induced loss of body weight, neuronal damage and proinflammatory reactions in the hippocampal area (CA1 and CA3) were significantly reduced by LP treatment. Therefore, these results suggest the potential for use of statins in modulation of seizures and other neurological diseases associated with excitotoxicity.
Materials and Methods
Experimental animals and induction of seizure
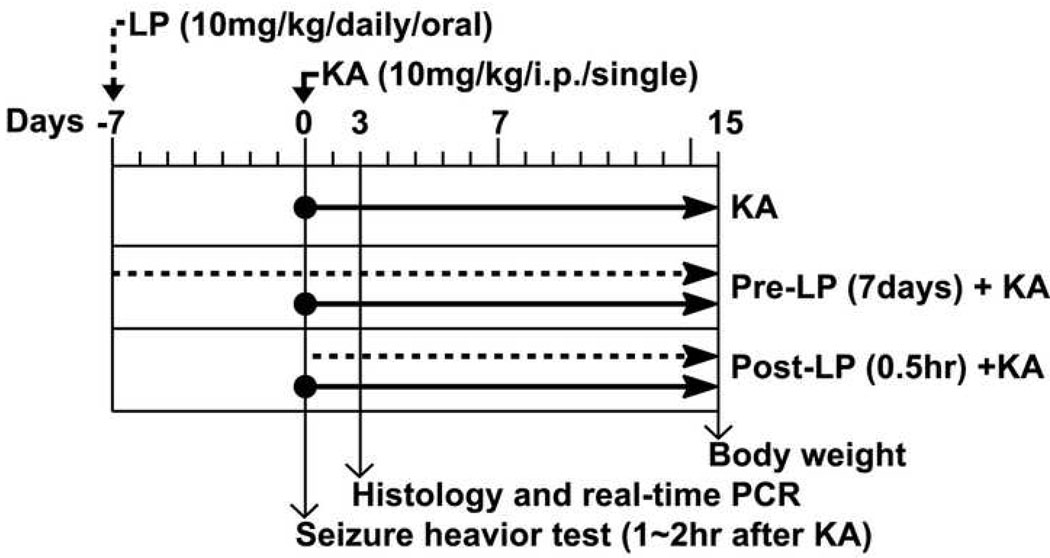
We used adult male Sprague-Dawley rat (270–280 g) maintained on a 12 h light/dark cycle in a temperature-controlled colony room at 22–24°C with free access to rodent pellets and tap water. They were handled according to the NIH Guide for the Care and Use of Laboratory Animals. Figure 1 describes the experimental design of this study. For evaluation of LP as a preventative, atorvastatin calcium (Lipitor®, Pfizer) suspended in 0.9% NaCl was given orally for seven days (10 mg/kg/day) before KA injection, thereafter, until the animals were sacrificed. For evaluation of LP as post-seizure treatment, LP was given 0.5hr after KA injection. For induction of seizure, the rats received a single intraperitoneal (i.p.) injection of kainic acid (Sigma Chemicals, St. Louis, MO) (10 mg/kg in 0.9% NaCl, pH 7.0) or vehicle, and the animals were analyzed for behavior analysis and sacrificed for histochemistry and quantitative real-time PCR analysis. The used Lipitor® (80mg atorvastatin) is an impure form and includes inactive ingredients, such as calcium carbonate, candelilla wax, croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, Opadry White YS-1-7040 (hypromellose, polyethylene glycol, talc, titanium dioxide), polysorbate 80, simethicone emulsion.
Fig. 1. Experimental design.
LP: Lipitor® (atorvastatin calcium), KA: Kainic acid.
Seizure activity was rated at 1 hr after KA injection for 2 hrs according to the scale devised by Racine (1972): Stage 1, facial clonus; Stage 2, nodding and wet dog shaking; Stage 3, forelimb clonus; Stage 4, forelimb clonus with rearing; Stage 5, rearing, jumping, and falling. Animals were scored after having had 3 consecutive events at each Stage. Number of wet dog shaking (WDS) behavior was counted at 0.5hr after intraperitoneal KA injection for 1 hr. Body weight (g) of rats was measured at 15 days after KA injection.
Histological Examination
The brain was fixed in 10% buffered formalin, embedded in paraffin and sectioned at 4 µm thickness. The brain tissue slides were deparaffinized and dehydrated and stained with cresyl violet (Sigma). Immunofluorescent labeling was performed on sections with primary antibody for macrophage marker ED-1 (Biosource International, Camarillo, CA). The sections were examined under a microscope (Olympus BX-60) with an Olympus digital camera (Optronics; Goleta, CA). TUNEL assay was performed using the ApopTag® plus peroxidase kit (Intergen Co.) and Methyl Green counter staining according to the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA purified from hippocampus by using TRIZOL® reagent (Gibco BRL) were subjected to quantitative real-time PCR (BIO-RAD Laboratories iCycler iQ Real-Time PCR Detection System). The primer sets used were GAPDH, forward primer: 5’-cctacccccaatgtatccgttgtg-3’ and reverse primer: 5’-ggaggaatgggagttgctgttgaa-3’; IL-1β, forward primer: 5’-gagagacaagcaacgacaaaatcc-3’ and reverse primer: 5’-ttcccatcttcttctttgggtattg-3’; TNF-α̣ forward primer: 5’-cttctgtctactgaacttcggggt-3’ and reverse primer: 5’-tggaactgatgagagggagcc-3’; iNOS, forward primer: 5’-ggaagaggaacaactactgctggt-3’ and reverse primer: 5’-gaactgagggtacatgctggagc-3’. IQTM SYBR Green Supermix was purchased from BIO-RAD (BIO-RAD Laboratories, Hercules, CA). Thermal cycling conditions were as follows: activation of iTaq™ DNA polymerase at 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 30 sec and 55–57.5°C for 1 min. The normalized expression of target gene with respect to GAPDH was computed for all samples using Microsoft excel data spreadsheet.
Hippocampal neuron culture and MTT assay
Highly enriched hippocampal neuron culture (<99% neuron) was prepared as described previously [42]. Briefly, hippocampi were removed from 17-day old rat mbryos of SD rats and incubated in 0.25% trypsin in HBSS at 37°C for 15 min and then triturated to form single-cell suspensions. The cells were plated onto poly-L-lysine (10 µg/ml) precoated 12-well culture plates at a density of 100,000 cells/cm2 in neurobasal medium with B27 supplements, 25 µM glutamate, 0.5 mM L-glutamine, and penicillin/streptomycin. The medium was changed 4 hr after the initial plating, and glutamate was removed after 4 days in culture. Neurons were cultured at an atmosphere of 95% air and 5% CO2 at 37°C for at least 12 days prior to use. The viability of neurons was determined by the mitochondrial conversion of MTT to formazan as detected by the change of optical density at 570 nm as described previously [41].
Statistical analysis
All graphs shown represent Means ± S.E.M of n determination, obtained from at least three independent experiments. Statistical analyses were performed by one-way ANOVA followed by the Student-Neunan-Keuls post test.
Results
Statin attenuates seizure behavior induced by kainic acid
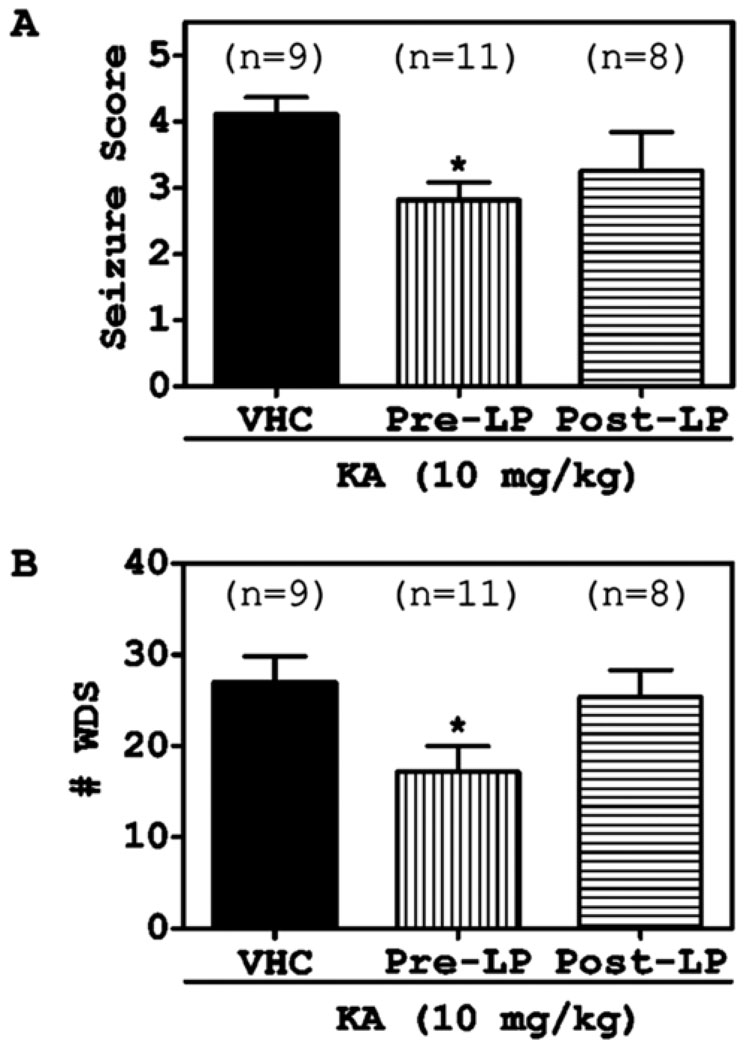
In the present study, we observed seizure behavior at 1hr after intraperitoneal KA injection for 2 hr. Pre-treatment of atorvastatin (LP) attenuated the seizure score significantly (Fig. 2A). We also counted the number of wet dog shaking (WDS) behavior at 0.5 hr after KA treatment for 1 hr. Pre-treatment with LP inhibited the number of WDS behavior induced by KA significantly (Fig. 2B). However, LP post-treatment marginally affected either seizure score or WDS number without any significance, indicating that only LP pre-treatment is effective on attenuation of KA-induced seizure.
Fig. 2. Atorvastatin attenuated KA-induced seizure behavior in rats.
The rats were orally pre-treated (7 days before) or post-treated (3 days after) with atorvastatin (LP; 10 mg/kg) prior to KA (10 mg/kg, i.p.). The seizure behavior test (seizure score (A) and counting of WDS behavior (B)) were performed during 1–3 hr after KA (10 mg/kg, i.p.) administration, according to the methods. The graphs indicate the S.E.M. (* P < 0.05 compare to saline treated group).
Statin inhibits hippocampal cell death induced by kainic acid
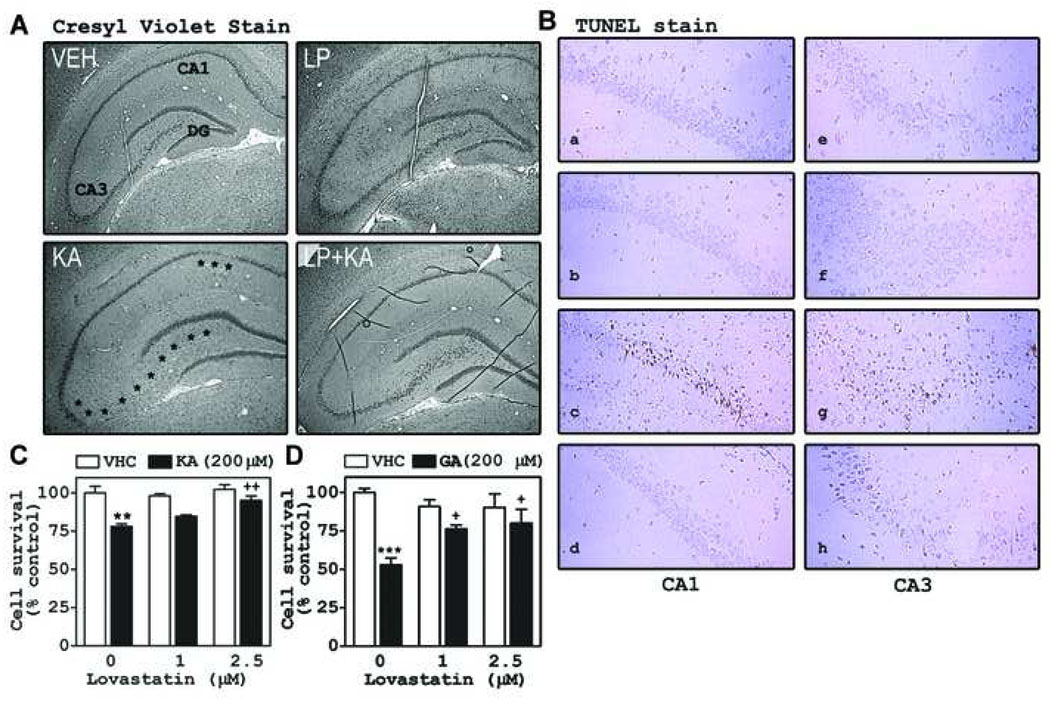
Based on the observed protective effect of LP on KA-induced seizure activity, we examined the effect of LP on neuronal death in hippocampus at 3 days after exposure to KA. As expected, KA caused cell death in CA1 and CA3 regions (Fig. 3A). Pre-treatment with LP inhibited hippocampal cell death induced by KA in the CA1 and CA3 regions (asterisk symbols indicate cell loss regions). To examine whether the observed cell death is apoptotic, we performed the TUNEL assay. As shown in Fig. 3B, KA increased TUNEL positive cells in pyramidal cell layer (CA1 and CA3 regions) of hippocampus, and this increase was inhibited by LP pre-treatment. To examine whether the LP exerts its neuroprotective role against KA through inhibiting excitotoxicity, we examined the effects of lovastatin on KA- or glutamate-induced cell death in cultured hippocampal neurons. For these in vitro studies, we used lovastatin (another inhibitor of HMG-CoA reductase), rather than LP (Lipitor pill), because of unavailability of pure atorvastatin compound. In agreement with in vivo studies, lovastatin also inhibited neuronal cell death induced by glutamate (200 µM) or KA (200 µM) (Fig. 3C and 3D).
Fig. 3. Statins inhibited hippocampal apoptotic cell death induced by KA.
The rats were orally pre-treated (7 days before) with atorvastatin (LP; 10 mg/kg) prior to KA (10 mg/kg, i.p.). At 3 days after KA injection, we examined neuronal cell death in hippocampus using cresyl violet stain (A) and DNA fragmentation as a marker for apoptosis (B). The asterisks show the cell-loss regions compared to the vehicle (VHC) treated group. In the hippocampal neuron cell, we examined the effect of lovastatin after treatment of glutamate (GA: 200 µM) or KA (200 µM ) on the cell survival using MTT assay (C and D). The graphs indicate the S.E.M. (** P <0.01, *** P < 0.001 vs. the vehicle treated group; + P < 0.05, ++ P < 0.01 vs. the KA or GA treated group; n = 4 independent experiments).
Statin inhibits the infiltration of macrophages and expression of inflammatory genes in brain of kainic-induced animal model
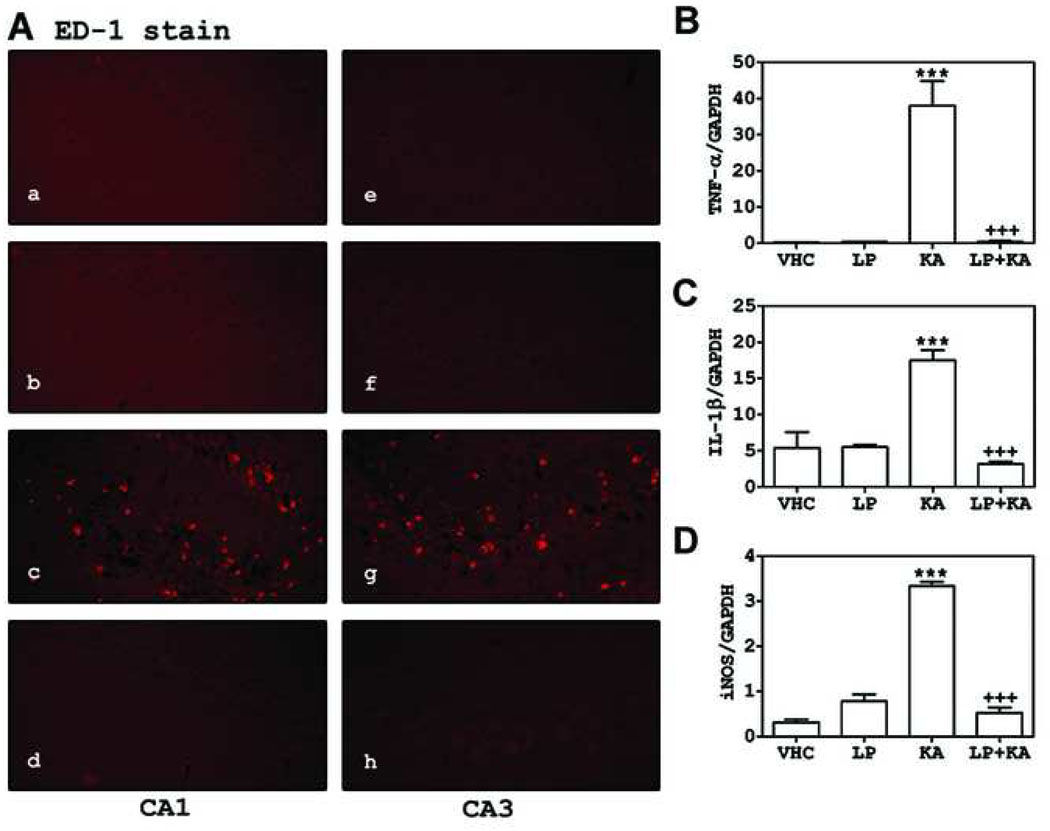
Studies have reported that the protection of endothelium/blood brain barrier [9, 13, 15, 16, 18, 20, 26] and attenuation of expression of proinflammatory cytokines [21, 24, 34, 35, 37] may contribute toward the observed statin mediated neuroprotection in various neurodegenerative disease conditions. Therefore, we investigated the effects of statin treatment on infiltration of ED-1 (cell surface marker of monocytes/macrophages lineage) positive cells and expression of cytokines (IL-1β, TNF-α) and inducible nitric oxide synthase (iNOS) in the KA animal model of epilepsy. As shown in Fig. 4, LP treatment decreased KA-induced increase in ED-1 positive cell infiltration in around of cell death regions of CA1 and CA3 (A) and mRNA expression of TNF-α, IL-1β, and iNOS in hippocampal area (B~D).
Fig. 4. Atorvastatin inhibited the macrophage infiltration and inflammatory gene expression induced by KA in hippocampus.
The rats were orally pre-treated (7 days before) with atorvastatin (LP; 10 mg/kg) prior to KA (10 mg/kg, i.p.). At 3 days after KA injection, we examined infiltration of macrophages (ED-1 positive cells) in CA1 and CA3 regions of hippocampus using immunofluorescent labeling (a and e: vehicle treated group; b and f: LP treated group; c and g: KA treated group; d and h: LP plus KA treated group) and expression of inflammatory genes, such as TNF-α (B), IL-1β̣(C), and iNOS (D) in hippocampus using real time PCR. The expression of each gene was normalized with GAPDH expression. The graphs indicate the S.E.M. (*** P < 0.001 vs. the vehicle injected group; +++ P < 0.001 vs. the KA injected group; n = 4 independent experiments).
Discussion
Systemic injection of rats with KA induces progressive limbic seizures mimicking human temporal lobe epilepsy [4]. These culminate in status epilepticus (SE) and lead to neuronal death and inflammatory reaction in areas of hippocampus and dentate gyrus [3]. Herein, we observed that pre-treatment with LP attenuated the seizure activities induced by KA. Further, LP inhibited KA-induced neuronal damage in the hippocampus (CA1 and CA3) and inflammation (i.e. expression of cytokines and iNOS and infiltration of ED-1 positive cells), thus suggesting a potential utility of statin treatment in modulation of seizures and other neurological diseases associated with excitotoxicity. Our data is well correlated with previously reported neuroprotective role of lovastatin in brain damages induced by pilocarpine-mediated SE [27].
Statins are inhibitors of HMG-CoA reductase, a rate limiting enzyme for synthesis of cholesterol and isoprenoids, thus, their biological roles are expected to be mediated by their inhibitory roles in cholesterol and isoprenoid synthesis. Indeed, the inhibitory role of statins in cholesterol biosynthesis previously reported to inhibit NMDA mediated excitotoxicity in vitro neuron culture study [44]. In the meantime, Hering et al. also reported that statin treatment of cultured neuron cells leads to instability of AMPA receptor by disruption of cholesterol and sphingolipid rich membrane microdomains called “detergent resistant/insoluble membrane domains (DRM/DIM)” or “lipid rafts” [11]. Therefore, these in vitro studies suggest the possible involvement of cholesterol biosynthesis in statin-mediated anti-excitotoxic activity. At present, whether cholesterol biosynthesis plays a role in statin-mediated anti-seizure and anti-excitotoxicity in the in vivo animal model is not known. In the brain, cholesterol has a very long half life (up to several months). Therefore, statins are not expected to change brain cholesterol level readily. Indeed, previous studies have reported that systemic statin treatment does not affect cholesterol levels in the brain of guinea pigs [10, 17]. Nevertheless, previously reported reduction in cholesterol levels in lipid raft fractions of synaptosomal plasma membrane [14] suggests that statin treatment may affect intercellular or intracellular distribution of cholesterol which is associated with glutamate receptor function in induction of status epilepticus and excitotoxicity.
Along with cholesterol, isoprenoids including farnesyl-pyrophosphate and geranylgeranyl-pyrophosphate are lipid byproducts produced by the mevalonate pathway and implicated in membrane attachment and function of small GTPases. Since the small GTPases (i.e. RhoA and H-Ras) are involved in various neuronal functions and excitotoxic signaling cascades [30, 40], statins are also expected to exert their anti-seizure and anti-excitotoxic activities through inhibition of isoprenoid synthesis and interfering with small GTPase signaling. Indeed, recent in vivo reports have demonstrated that inhibition of H-Ras farnesylation by franeyltransferase inhibitor (FTI) treatment inhibits NMDA-mediated excitotoxicity in the rat brain [29]. This report supports that statins may modulate KA-mediated seizure activity and excitotoxicity by down-regulation of H-Ras isoprenylation. However, whether statins exert their anti-seizure and anti-excitotoxic roles through inhibiting synthesis of isoprenoid or cholesterol or both of them is not known at present.
Recently, our laboratory and that of others have reported that neuroprotective efficacies of statins are mediated by their anti-inflammatory activity under various neurological disease conditions such as human multiple sclerosis [38] and its animal models [20, 23, 33–35], Spinal cord injury [24], X-linked adrenoleukodystrophy [22], Alzheimer’s disease [6], traumatic brain injury [39], and stroke [12]. In these studies, statins inhibited inflammatory signal cascades and gene expression through inhibition of isoprenylation of small GTPase (Ras and RhoA) [21, 24, 26]. A similar inhibitory role of statins in inflammatory reactions was observed in KA-treated rats. However, we do not expect that the observed statin-mediated reduction in inflammatory reactions as well as apoptotic cell death in KA-treated rat is mediated by its own anti-inflammatory activity observed in our previous studies. Since post-seizure inflammation and leakage of blood-brain barrier are known to directly correlate with activity and frequency of status epilepticus [32, 36], the decreased status epilepticus by statin treatment appears to attenuate inflammatory reaction in KA-treated rats. Clinically, however, we expect that anti-inflammatory activity of statins could be helpful for intervention of epilepsy as an off-setting efficacy. Human epilepsy is a condition in which a person has recurrent seizures with chronic inflammation [2–5]. Since such chronic inflammation in the CNS is implicated in progressive neurodegeneration [8], it will be interesting if statins will produce anti-inflammatory activity after seizure. Further studies would be required to answer this question.
Acknowledgements
This work was supported by Children’s Hospital Fund of Medical University of South Carolina and National Institute of Health (NS-22576, NS-34741, NS-37766, and NS-40144).
References
- 1.Ananth C, Gopalakrishnakone P, Kaur C. Protective role of melatonin in domoic acid-induced neuronal damage in the hippocampus of adult rats. Hippocampus. 2003;13:375–387. doi: 10.1002/hipo.10090. [DOI] [PubMed] [Google Scholar]
- 2.Beal MF. Mechanisms of excitotoxicity in neurologic diseases. Faseb J. 1992;6:3338–3344. [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 5.Bittigau P, Ikonomidou C. Glutamate in neurologic diseases. J Child Neurol. 1997;12:471–485. doi: 10.1177/088307389701200802. [DOI] [PubMed] [Google Scholar]
- 6.Cordle A, Landreth G. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25:299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature. 1976;263:244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Villoslada P, Oksenberg JR. Chronic inflammatory diseases of the nervous system. Curr Opin Neurol. 1998;11:235–240. doi: 10.1097/00019052-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong H, Zeng JS, Kreulen DL, Kaufman DI, Chen AF. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am J Physiol Heart Circ Physiol. 2006;291:H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kirsch C, Eckert GP, Koudinov AR, Muller WE. Brain cholesterol, statins and Alzheimer's Disease. Pharmacopsychiatry. 2003;36 Suppl 2:S113–S119. doi: 10.1055/s-2003-43058. [DOI] [PubMed] [Google Scholar]
- 14.Kirsch C, Eckert GP, Mueller WE. Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem Pharmacol. 2003;65:843–856. doi: 10.1016/s0006-2952(02)01654-4. [DOI] [PubMed] [Google Scholar]
- 15.Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- 16.Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 17.Lutjohann D, Stroick M, Bertsch T, Kuhl S, Lindenthal B, Thelen K, Andersson U, Bjorkhem I, Bergmann Kv K, Fassbender K. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 2004;69:431–438. doi: 10.1016/j.steroids.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Palma L, Pehar M, Cassina P, Peluffo H, Castellanos R, Anesetti G, Beckman JS, Barbeito L. Involvement of nitric oxide on kainate-induced toxicity in oligodendrocyte precursors. Neurotox Res. 2003;5:399–406. doi: 10.1007/BF03033168. [DOI] [PubMed] [Google Scholar]
- 19.Molinuevo JL, Llado A, Rami L. Memantine: targeting glutamate excitotoxicity in Alzheimer's disease and other dementias. Am J Alzheimers Dis Other Demen. 2005;20:77–85. doi: 10.1177/153331750502000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273–1286. doi: 10.4049/jimmunol.172.2.1273. [DOI] [PubMed] [Google Scholar]
- 21.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai GS, Khan M, Barbosa E, Key LL, Craver JR, Cure JK, Betros R, Singh I. Lovastatin therapy for X-linked adrenoleukodystrophy: clinical and biochemical observations on 12 patients. Mol Genet Metab. 2000;69:312–322. doi: 10.1006/mgme.2000.2977. [DOI] [PubMed] [Google Scholar]
- 23.Paintlia AS, Paintlia MK, Khan M, Vollmer T, Singh AK, Singh I. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. Faseb J. 2005;19:1407–1421. doi: 10.1096/fj.05-3861com. [DOI] [PubMed] [Google Scholar]
- 24.Pannu R, Christie DK, Barbosa E, Singh I, Singh AK. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J Neurochem. 2007;101:182–200. doi: 10.1111/j.1471-4159.2006.04354.x. [DOI] [PubMed] [Google Scholar]
- 25.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 26.Prasad R, Giri S, Nath N, Singh I, Singh AK. Inhibition of phosphoinositide 3 kinase-Akt (protein kinase B)-nuclear factor-kappa B pathway by lovastatin limits endothelial-monocyte cell interaction. J Neurochem. 2005;94:204–214. doi: 10.1111/j.1471-4159.2005.03182.x. [DOI] [PubMed] [Google Scholar]
- 27.Rangel P, Cysneiros RM, Arida RM, de Albuquerque M, Colugnati DB, Scorza CA, Cavalheiro EA, Scorza FA. Lovastatin reduces neuronal cell death in hippocampal CA1 subfield after pilocarpine-induced status epilepticus: preliminary results. Arq Neuropsiquiatr. 2005;63:972–976. doi: 10.1590/s0004-282x2005000600013. [DOI] [PubMed] [Google Scholar]
- 28.Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, Veliskova J, Moshe SL, De Simoni MG, Vezzani A. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003;14:494–503. doi: 10.1016/j.nbd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Ruocco A, Santillo M, Cicale M, Seru R, Cuda G, Anrather J, Iadecola C, Postiglione A, Avvedimento EV, Paterno R. Farnesyl transferase inhibitors induce neuroprotection by inhibiting Ha-Ras signalling pathway. Eur J Neurosci. 2007;26:3261–3266. doi: 10.1111/j.1460-9568.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- 30.Semenova MM, Maki-Hokkonen AM, Cao J, Komarovski V, Forsberg KM, Koistinaho M, Coffey ET, Courtney MJ. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat Neurosci. 2007;10:436–443. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]
- 31.Singh I, Pahan K, Khan M. Lovastatin and sodium phenylacetate normalize the levels of very long chain fatty acids in skin fibroblasts of X-adrenoleukodystrophy. FEBS Lett. 1998;426:342–346. doi: 10.1016/s0014-5793(98)00370-6. [DOI] [PubMed] [Google Scholar]
- 32.Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 33.Stanislaus R, Gilg AG, Singh AK, Singh I. Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by Lovastatin. Neurosci Lett. 2002;333:167–170. doi: 10.1016/s0304-3940(02)00943-6. [DOI] [PubMed] [Google Scholar]
- 34.Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci Lett. 1999;269:71–74. doi: 10.1016/s0304-3940(99)00414-0. [DOI] [PubMed] [Google Scholar]
- 35.Stanislaus R, Singh AK, Singh I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. J Neurosci Res. 2001;66:155–162. doi: 10.1002/jnr.1207. [DOI] [PubMed] [Google Scholar]
- 36.van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969–1973. doi: 10.1161/01.str.30.9.1969. [DOI] [PubMed] [Google Scholar]
- 38.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, Rizzo M, Singh I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Lynch JR, Song P, Yang HJ, Yates RB, Mace B, Warner DS, Guyton JR, Laskowitz DT. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L. Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol Neurobiol. 2004;29:1–14. doi: 10.1385/MN:29:1:01. [DOI] [PubMed] [Google Scholar]
- 41.Won JS, Im YB, Key L, Singh I, Singh AK. The involvement of glucose metabolism in the regulation of inducible nitric oxide synthase gene expression in glial cells: possible role of glucose-6-phosphate dehydrogenase and CCAAT/enhancing binding protein. J Neurosci. 2003;23:7470–7478. doi: 10.1523/JNEUROSCI.23-20-07470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Won JS, Im YB, Khan M, Contreras M, Singh AK, Singh I. Lovastatin inhibits amyloid precursor protein (APP) beta-cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Zacco A, Togo J, Spence K, Ellis A, Lloyd D, Furlong S, Piser T. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]