Abstract
Vascular endothelial growth inhibitor TNFSF15 (TL1 A), a ligand for TNFRSF25 (DR3) and decoy receptor TNFRSF6B (DcR3), is expressed in human pulmonary arterial (HPAEC) and lung microvascular (HMVEC) endothelial cells where it might modulate inflammation and sickle vasculopathy. Pulmonary disease, endothelial abnormalities and inflammation are prominent features of sickle cell disease (SCD). Butyrate has opposing effects on endogenous TNFSF15 expression in pulmonary endothelium, acting as an inhibitor in HPAEC and an inducer in HMVEC. Similar effects were observed with a known cytokine TNF-α in these two cell types. Furthermore the TNFSF15 promoter utilized different combinations of cis-elements for its expression in these two cell types. AP1-like and G-rich sequence elements were critical for promoter activity in large vessel HPAEC while AP1-like and NF-κB consensus sequence elements were required in small vessel HMVEC. The requirement of an NF-κB sequence element by the TNFSF15 promoter in HMVEC but not in HPAEC supported the notion that HMVEC might be a target of inflammation and vasoocclusion in SCD. The dual effects of butyrate-dependant TNFSF15 regulation in lung endothelium may help in identify inflammatory pathways and understand the role of pulmonary microvascular EC’s in pathogenesis of vasoocclusion in SCD.
Keywords: endothelium, butyrate, TNF-α, inflammation, sickle cell disease
1. Introduction
Modifications of the endothelial cell (EC) phenotype can occur after exposure to sickle erythrocytes (RBCs) and/or plasma (1,2). Exposure of cultured bovine, human pulmonary artery, or human lung microvascular endothelial cells (BPAEC, HPAEC and HMVEC) to plasma taken from SCD patients during the acute chest syndrome resulted in up-regulation of mRNA expression of the vasoconstrictor endothelin-1 (ET-1) and dysregulation of nitric oxide (NO) metabolism (1,3). Exposure of EC to RBCs from SCD patients without a recent acute vasoocclusive event (steady-state) induced expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin (2). These data suggest a role for chronic inflammation in sickle vasoocclusion as alterations in the EC phenotype occurred upon exposure to blood components from SCD patients even when they appeared clinically well. TNFSF15 encodes a cytokine belonging to the tumor necrosis factor ligand family that is predominantly expressed on endothelial cells and functions as an autocrine cytokine, inhibiting angiogenesis, cell proliferation, and tumor growth as well as stabilizing the vasculature (4). This cytokine plays a role in the induction of pro-inflammatory cytokines and extracellular matrix degrading enzymes in atherogenesis (5). Its expression is up-regulated in inflammatory bowel disease, suggesting a role in inflammation (6, 7). It also functions as a T-cell co-stimulator leading to increased secretion of pro-inflammatory cytokines (8).
Sickle vasculopathy is, at least in part, a result of endothelial damage and inflammation. Perturbation of the microvascular endothelium, because of its important role in inflammation and maintenance of vascular tone, may play a role in sickle vasoocclusion (9, 10). EC activation may differ between large vessels and the microvasculature; this heterogeneity could be a factor in the pathophysiology of diseases across different vascular beds. In earlier studies, human umbilical vein endothelial cells (HUVEC) were used for endothelial cell activation studies, but concerns about EC heterogeneity makes this cell type less suitable to study pulmonary complications of SCD. Since much of vasoocclusion occurs at the level of microvasculature, we hypothesized that the pulmonary microvascular endothelium could be a primary target for cytokines during the acute chest syndrome and possibly other types of sickle cell lung disease.
Sodium butyrate and similar compounds can induce fetal hemoglobin expression in SCD and these agents might be therapeutically useful (11, 12). Butyrate is also known to modulate gene expression in the endothelium. In HUVEC, exposure to butyrate induced expression of ICAM-1, E-selectin and endothelin-1 (13). Sodium phenyl butyrate increased VCAM-1 and ICAM-1 expression while down-regulating ET-1 in transformed human bone marrow endothelial cells (14). Butyrate’s effects are not restricted to the endothelium as it is also known to suppress pro-inflammatory cytokines in monocytes (15). These studies suggest that butyrate could potentially act as an immune modulator in endothelial cells. In the present study, we examined the effects of butyrate on TNFSF15 expression in HPAEC and HMVEC while comparing these effects to those of another known immune modulator, TNF-α. This study may provide new insights into the role of TNFSF15 in inflammation within microvascular endothelium.
2. Materials and Methods
2.1. Cell cultures
HPAEC and HMVEC (passage 6 to 9) were grown in EGM-2 and EGM-2 MV media supplemented with growth factors and 10% fetal bovine serum (Clonetics, Walkersville, MD) under humidified conditions (5% CO2) at 37°C. Sodium butyrate, 4mM (Sigma-Aldrich, USA) or recombinant human TNF-α, 40ng/ml (Millipore Corp, Temecula, CA) was added directly to the media. For experiments involving endogenous TNFSF15 expression, cells were incubated in presence of butyrate or TNF-α for 20 min, 40 min or 1, 2, 6 or 24 hrs. At the end of each incubation period, the cells were washed with PBS and lysed for RNA and protein isolation.
2.2. RNA isolation and RT-PCR assay
Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA) and treated with RNase-free DNase (Qiagen). DNA free RNA (0.25 μg) was used as the template for one-step RT-PCR (Invitrogen, Carlsbad, CA). The forward and reverse primers used were; a) TNFSF15 -5′-ATGGGCCGAGGATCTGGGACTGAGC-3′ and 5′-CTATAGTAAGAAGGTTTTATCTTC-3′ (750 bp fragment); b) TL1 -5′-GCAAAGTCTACAGTTTCCCAATGAGAAAA TTAATCC-3′ and the same reverse primer as used for TNFSF15 (522 bp fragment); c) GAPDH- 5′-ATGACATCAAGAAGGTGGTG-3′ and 5′-CATACCAGGAAATGAGCTGG-3′ (177 bp fragment). The following conditions were used: initial incubation, 30 min (53°C), 2 min (94°C), 40 sec denaturation (94°C), 40 sec annealing (55°C) and 1 min elongation (68°C) for a total of 30 cycles. The PCR products were run on 1.5% agarose gels and bands were visualized. Relative levels of TNFSF15 RNA expression were obtained from densitometry analysis; the intensity of the TNFSF15 RT-PCR band was divided by the corresponding GAPDH band and the values obtained were plotted against each time point. Densitometry image analysis was done by using software available at http://rsb.info.nih.gov/. Each experiment was performed in triplicate.
2.3 Western blot analysis of TNFSF15 protein
HPAEC and HMVEC were plated and incubated in the presence of butyrate (4mM) or TNF-α (40ng/ml) for 20 min, 40 min, 1 hr, 2 hr, 6 hr or 24 hr. Following incubation, cells were washed with PBS and lysed in RIPA buffer containing a cocktail of protease inhibitors (16). The lysate was cleared of cellular debris, protein concentration was determined by using the Bio-Rad Dc protein assay (Bio-Rad, Hercules, CA) and samples (40 μg) loaded onto a 10% SDS- PAGE gel. Gel-fractionated proteins were transferred to a nitrocellulose membrane and blotted with an anti-human TL1A primary antibody raised against human TNFSF15 peptide (1:200 dilution) (Biolegend, San Diego, CA), followed by a goat anti-mouse IgG-HRP secondary antibody (1:5000 dilution). Bound antibody was detected via an enhanced chemiluminescence fluorography reagent kit (Western lightning chemiluminescence reagent, Perkin Elmer, USA). Use of an anti-tubulin goat anti-mouse antibody (Santa Cruz Biotech, Santa Cruz, CA) served as an internal control for these experiments. Relative levels of TNFSF15 protein were determined via densitometry and corrected for tubulin expression. Each experiment was performed in triplicate.
2.4. Reporter plasmid constructs
HPAEC DNA was used to amplify the TNFSF15 promoter, a 1417 base pair fragment (−1390/+27 from initiation site, ref Gene NM_005118). The forward and reverse primers used were 5′-TCCAGGCTGTGACATAACCA-3′ and 5′-CAAAGCTCAGTCCCAGATCC-3′. The amplified product was cloned into p-GEM-T Easy vector (Promega, Madison, WI), releasing the insert with Not 1 and end-filling the fragment. The excised and end-filled fragment was cloned into the basic pGL3 (Promega) luciferase reporter vector at the Sma1 site in the multi-cloning site. The promoter orientation was confirmed by restriction enzyme analysis and sequencing. A series of 5′-end deletion constructs of the TNFSF15 promoter were generated. The deleted constructs were designated: p-89-luc, p-188-luc, p-444-luc, p-540-luc, p-912-luc, p-1056-luc, p-1228-luc and p-1390-luc.
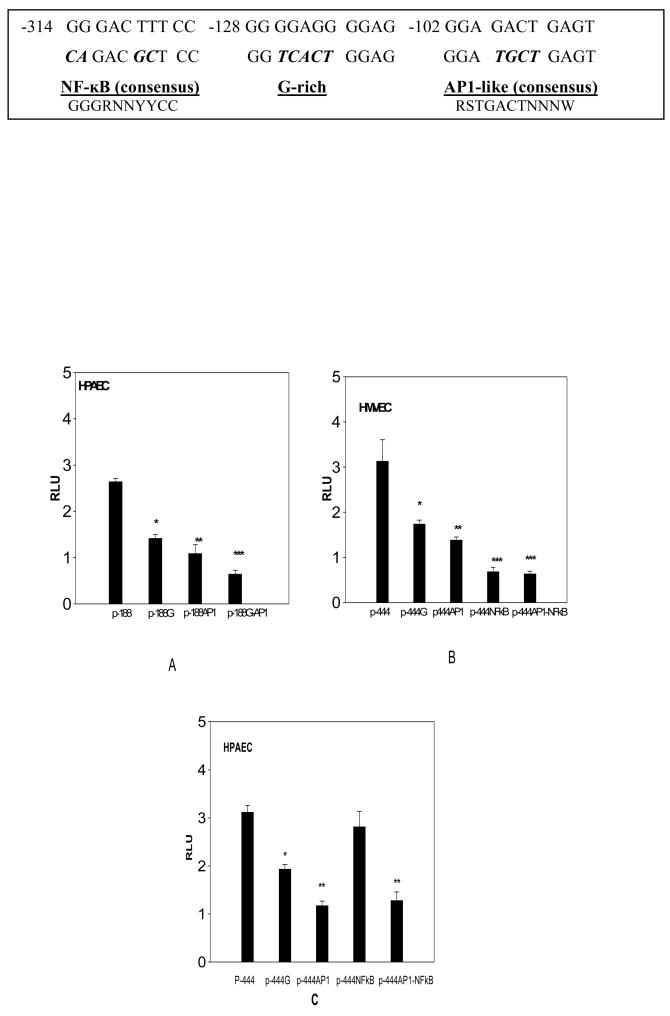
In the p-188-luc and p-444-luc constructs, we identified a modification of the classic AP1 site with 80 % homology; this sequence element was designated as AP1-like motif. The G-rich, AP1-like and NF-κB binding sequences were mutated individually and in combination (described in Fig. 4), using the Quick change multi-site-directed PCR based mutagenesis kit (Stratagene, La Jolla, CA). These mutations in consensus AP1 and NF-κB sequences have previously been reported to abolish the functional activity of these sequence elements (17, 18, 19). All the mutant constructs were confirmed by sequencing. Plasmids constructs were purified (Qiagen plasmid kit) before electroporation into the endothelial cells.
Fig. 4. Effect of mutated consensus binding sequences on the activity of p-188-luc and p-444-luc promoter constructs in HPAEC and HMVEC.
The p-188-luc promoter construct and the mutants (along with control pRL-SV40) were electroporated into HPAEC (n=3) At the end of 6hr incubation period, cells were lysed and assayed for luciferase activity, normalized to that of pRL-SV40 (A). The data are presented as relative luciferase units (RLU). While mutations in either G-rich region (p-188-G) (*p< 0.004 compared with p-188-luc) or the AP1-like region (p-188-AP) (**p<0.007 compared with p-188) resulted in a significant decrease in activity, the combination of the two sequences (p-188-G-AP1) had a greater effect suggesting synergy (***p<0.001 compared with HPAEC). The p-444-luc promoter construct and its mutants were similarly electroporated into HMVEC (B) and HPAEC (C). Mutations in the G-rich (p-444-G) (*p<0.02), AP1-like (p-444-AP) (**p<0.01), NF-κB (p-444-NF-κB) regions of p-444-luc (***p<0.005) were all significant when compared to unaltered p-444-luc in HMVEC. The sequence motifs and their mutants are shown at the top with mutations indicated in boldface italics
2.5. Cell transfection and luciferase assay
HPAEC and HMVEC were grown to a density of 1.5×106 cells per 100×15 mm plate, washed with PBS, trypsinized, centrifuged, and resuspended in media containing 10% FBS. A total of 1.25 to 1.5×106 cells were transfected with 12 μg of PGL-3 basic luciferase reporter plasmid linked to various constructs of the TNFSF15 promoter and 0.14 μg of pRL-SV40 Renilla luciferase (85:1 dilution) as an internal control reporter, in a final volume of 400 μl. Reporter plasmids and cells were incubated on ice for 10 min and electroporated (Bio-Rad Electroporator) at 200 V/975 μF for HPAEC and at 180 V/950 μF for HMVEC in a 0.4cm cuvette. Three electroporations were performed for each plasmid. After electroporation, cells were allowed to recover for 10 minutes at room temperature, pooled and equal volumes were placed into 6 or 24 well culture plates with 1 to 2 ml of media per well. In the preliminary promoter transfections studies, the luciferase activity was monitored up to 48 hrs. The promoter activity was found to peak at 6–8 hrs after transfection; hence 6 hrs of incubation time was selected for all transfection studies. At the end of incubation period, media was aspirated; cells were washed with PBS, lysed and assayed for luciferase activity using a dual-luciferase reporter assay system (Promega, Madison, WI) and a Bio-Rad Luminometer. Results presented as RLU, a ratio of PGL-3 promoter construct firefly luciferase values to the renilla luciferase internal control.
Statistical analysis
Data are expressed as mean ± SEM. Differences between groups were evaluated by paired t- tests using Σ-sigma plot 8.02, SPSS software package for Microsoft Windows. Results were considered significant when p ≤0.05.
3. Results
3.1. Effect of butyrate on endogenous TNFSF15 expression
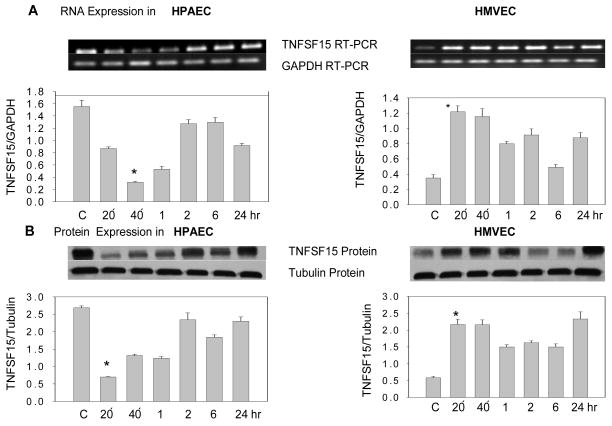
The expression pattern of TNFSF15 in HPAEC and HMVEC was studied using RT-PCR and Western analysis. The EC’s were incubated in presence or absence of butyrate (4mM) for 20 or 40 min or 1, 2, 6 or 24 hrs and at the end of each incubation period, RNA and protein were isolated. Using TNFSF15 gene specific primers, a 750 bp fragment was amplified in both cell types, and is expressed at high levels in HPAEC when compared to HMVEC (Fig. 1A, lane C). The presence of this fragment was indicative of full length TNFSF15 transcript. This transcript is a longer variant of TL1/VEGI, a protein with antiangiogenic activity. When TL1 specific primers were used, no amplified product of this transcript (522 bp) was detected in either cell types (data not shown). This observation suggests that TNFSF15 (TL1A) is predominantly expressed in the two pulmonary endothelial cell types studied. Using a TNFSF15 specific antibody, a 31 kDa band corresponding to TNFSF15 (TL1A) protein in both cell types was detected and baseline protein expression was higher in HPAEC as compared to HMVEC (Fig. 1B, lane C).
Fig. 1. Effect of butyrate on TNFSF15 expression in HPAEC and HMVEC.
(A) TNFSF15 RNA expression in untreated (C) and butyrate (4mM) treated HPAEC and HMVEC at 20 min, 40 min and at 1, 2, 6 or 24 hrs. The TNFSF15 gene specific PCR amplified product (750bp) and GAPDH amplified product, (177bp) are shown. Graphic representation of mean TNFSF15 expression corrected for GAPDH for each time point (± SEM) is presented (n=3); (B) Western analysis and graphic representation of mean TNFSF15 protein expression (31 kDa) corrected for tubulin (50 kDa) expression at each time point (± SEM) (n=3).
Next the effect of sodium butyrate on TNFSF15 RNA and protein expression in these two cell types was investigated. Butyrate’s effects on gene expression were observed after 20 min of exposure in both HPAEC and HMVEC. In HPAEC, butyrate inhibited RNA expression of after 20 min of exposure; this inhibitory effect was lost at 2 hr and at later time points. In HMVEC, co-incubation with butyrate for 20 min induced RNA expression and this effect persisted up to 24 hours. The effect of butyrate on TNFSF15 expression was more robust in HMVEC as compared to HPAEC. The effect of butyrate on gene expression was also observed at the protein level in these two cell types and followed a similar time course (*p<.05); (Fig. 1, A & B).
3.2. Effect of TNF-α on endogenous TNFSF15 expression
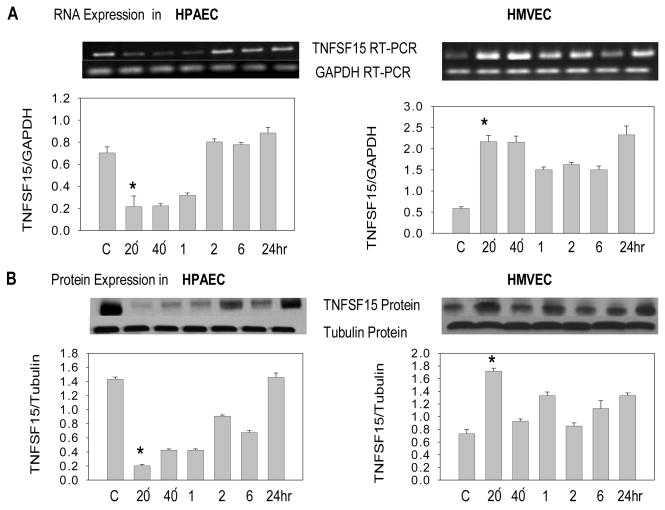
Co-incubating HPAEC and HMVEC with TNF-α (40ng/ml) produced effects on TNFSF15 expression similar to those observed with butyrate. In HPAEC, TNFSF15 RNA and protein expression was inhibited after 20 min, while in HMVEC, expression was induced at 20 min and continued to be higher up to 24 hrs of incubation (*p<.05); (Fig. 2, A& B). These data suggest that TNF-α and butyrate have similar effects on TNFSF15 expression in these two cell types.
Fig. 2. Effect of TNF-α on TNFSF15 expression in HPAEC and HMVEC.
(A) TNFSF15 RNA expression in untreated (C) and TNF-α (40 ng/ml) treated HPAEC and HMVEC at 20 min, 40 min and at 1, 2, 6 or 24 hrs. The TNFSF15 gene specific PCR amplified product (750bp) and GAPDH amplified product (177bp) are demonstrated. Graphic representation of mean TNFSF15 expression corrected for GAPDH for each time point (± SEM) is presented (n=3); (B) Western analysis of TNFSF15 (31 kDa) and tubulin (50 KDa) expression. Graphic representation of mean TNFSF15 protein expression corrected for tubulin expression at each time point (± SEM) is presented (n=3).
3.3 Identification of TNFSF15 minimal functional promoter element in HPAEC and HMVEC
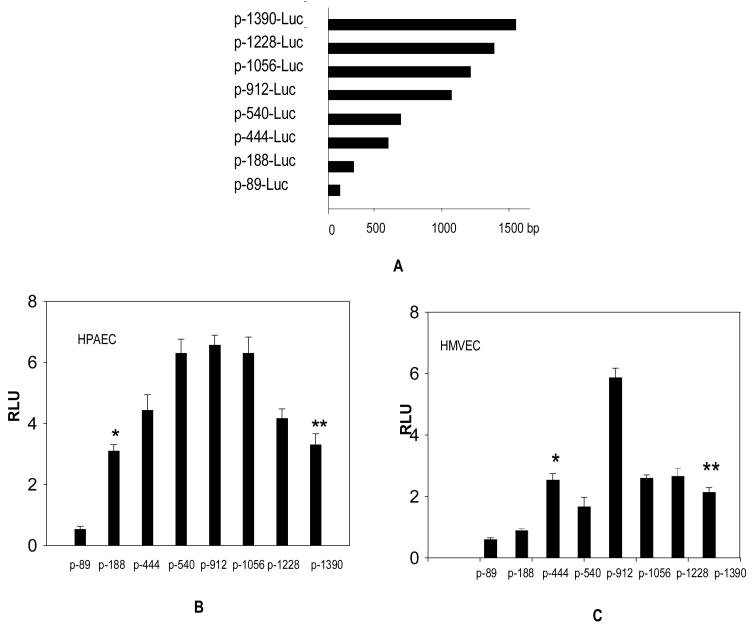
Having established a differential effect of butyrate on endogenous TNFSF15 expression in HPAEC and HMVEC, our next step was to investigate whether this effect could be attributed to the use of different sequence elements within the TNFSF15 promoter (−1390/+27). For this purpose, a series of 5′-deletion constructs of the TNFSF15 promoter were generated (Fig. 3A).
Fig. 3. Identification of TNFSF15 minimal promoter sequence elements active in HPAEC and HMVEC.
(A). Schematic diagram of TNFSF15 promoter-luciferase reporter constructs. Base pair scale is represented on the X-axis for each of the deleted promoters listed on the Y axis. These promoter constructs along with the control plasmid pRL-SV40 were electroporated into HPAEC (B) and HMVEC (C) and incubated for 6 hrs. At the end of incubation period, cells were lysed and assayed for luciferase activity. Luciferase activity was normalized to Renilla luciferase activity encoded by co-transfected pRL-SV40 control plasmid, mean luciferase activity expressed as relative luciferase units (RLU) is presented (n=3). The p-188-luc and p-1390-luc promoters have higher activity than the p-89-luc promoter in HPAEC (*p<0.01). In HMVEC, p-444-luc and p-1390-luc promoter activity was significantly higher than the p-188-luc promoter (**p<0.05).
In HPAEC, the promoter region sufficient for minimal promoter activity was identified between the −89 and −188 nucleotides, 5′ to the initiation codon (p-188-luc). Other promoter deletion constructs, p-444-luc, p-540-luc, p-912-luc, p-1056-luc and p-1228-luc exhibited higher activity than either p-188-luc or p-1390/+27-luc, suggesting the presence of some activator sequences located between 5′ to p-188-luc and p-1390-luc. Deletions at 3′ of p-188-luc (p-89-luc) reduced most of its activity (Fig. 3B). These results indicate the presence of critical minimal regulatory elements in p-188-luc, essential for its expression in HPAEC.
These p-1390-luc deletion constructs were also tested in HMVEC, p-188-luc exhibited little activity, implying that p-188-luc does not have all of the required sequence elements necessary for its expression in HMVEC. Nevertheless, the p-444-luc construct activity was comparable to that of p-1390-luc in HMVEC, suggesting that p-444-luc is the essential minimal promoter functional in HMVEC (Fig. 3C). From these observations, it is reasonable to conclude that the TNFSF15 promoter utilizes different promoter sequences for its expression in HPAEC and HMVEC.
3.4. G-rich and AP1- like sequence elements are necessary for TNFSF15 promoter activity in HPAEC
Our next goal was to identify the sequence elements critical for minimal promoter activity in each of these cell types. Several consensus sequences were identified in the p-188-luc promoter region (−188/+27) including a putative AP1-like binding domain (−102 to −92), a G-rich sequence (−128 to −118) as well as the MZF1, ADR1, GKLF and NF-AT sequence elements. The functional roles of the AP1-like and G-rich sequence in modulating p-188-luc construct activity was tested by mutating core nucleotide bases in these sequence elements (G-rich, AP1 and G-AP1 mutant constructs). The p-188-luc-AP1 mutant has significantly decreased activity and G-rich sequence alteration also reduced the promoter activity; mutations in the G-rich region also partially abolished the MFZ1 element (NGNGGGGA) located 5′ to the putative G-rich element. When both AP1-like and G-rich elements were mutated in p-188-luc, the inhibition was greater than with either mutated sequence element alone, suggesting a synergistic effect. Based on these results, it appears that both the G-rich and AP1-like sequences have critical roles in regulating the TNFSF15 promoter expression in HPAEC (Fig. 4A). Another consensus AP1-like binding site exists at −34 to −26 region in the construct p-89-luc, but since this promoter construct has little functional activity, this sequence is unlikely to contribute to the TNFSF15 promoter activity.
3.5. NF-κB sequence element is critical to TNFSF15 promoter activity in HMVEC
The lack of p-188-luc promoter activity in HMVEC suggests that the presence of AP1-like and G-rich sequence elements in this construct is not sufficient to allow its expression in HMVEC. It is likely that sequences 5′ to the p-188-luc region are critical to its expression in this cell type. A sequence motif search in this region led to identification of a NF-κB sequence in p-444-luc (−314–305 region). Altering this NF-κB sequence significantly reduced p-444-luc construct expression in HMVEC. Although G-rich and AP1-like sequence mutants also reduce the p-444-luc activity, these two sequence motifs alone can not drive the promoter in the absence of NF-κB element. Furthermore, altering both AP1-like and NF-κB sequence elements in p-444-luc did not produce a significant change in promoter activity compared with mutation of the NF-κB site alone (Fig. 4B). However, altering NF-κB sequence in p-444-luc did not inhibit its activity in HPAEC, but altering both AP1-like and NF-κB elements inhibited activity and produced effects similar to altering the AP1-like sequence element (Fig. 4C). Taken together, these observations imply that, in HMVEC, even though AP1-like and G-rich sequence elements may play a role, the presence of NF-κB sequence element is critical for TNFSF15 promoter activity. Conversely, in HPAEC, the AP1-like sequence element and not the NF-κB site is required for TNFSF15 minimal promoter activity.
4. Discussion
Our goal was to explore the expression pattern of TNFSF15 in HPAEC and HMVEC and define the effects of butyrate or TNF-α on its expression. Induction of TNFSF15 has been associated with inflammation, one of the proposed mediators of sickle vasoocclusion (6, 7, 8). The existence of heterogeneity in endogenous TNFSF15 expression amongst endothelial cell types suggests that differential effects on expression may be induced by butyrate. Vasoocclusive episodes and hemolytic anemia are hallmarks of the pathophysiology of SCD; the acute chest syndrome and pulmonary hypertension are prominent examples of vasoocclussive and hemolysis-related complications respectively. The endothelium appears to play a role in the pathogenesis of these major pulmonary complications of SCD. Because of this, we choose to extend previous studies centered on other vascular beds to an evaluation of the major endothelial cell types within the lung, HPAEC and HMVEC. These results suggest that TNFSF15 could play a role in vasoocclusion within the pulmonary vasculature.
Endogenous TNFSF15 expression at the base line was higher in HPAEC compared with HMVEC, supporting the notion that expression is heterogeneous amongst cell types. Moreover, co-incubation with either butyrate or TNF-α produced differing effects on TNFSF15 expression in these two cell types, even though the downstream effects were similar with butyrate in both. Butyrate or TNF-α inhibited TNFSF15 RNA and protein expression in HPAEC, indicating a possible anti-inflammatory role of these two agents in large vessel endothelium. In contrast, the induction of TNFSF15 RNA and protein expression in HMVEC by butyrate or TNF-α suggested a pro-inflammatory role of these agents in small vessel endothelium. We have not investigated the mechanism of butyrate’s effects on TNFSF15 expression in these endothelial cell types. Butyrate has been reported to regulate gene expression via the AP1 binding sequences and histone acetylation (20) SP1/SP3 multi-protein interactions (21, 22), a CRE motif (23), interaction with NF-κB signal transduction pathway (24) and inhibition of histone deacetylase (25). We found that TNFSF15 gene can be either induced or repressed in response to butyrate, depending upon the cell type studied.
Although the mechanisms for the TNFSF15 response to butyrate or TNF-α in these two lung EC types is not clear, it might relate to the selective use of promoter sequence elements as suggested by our promoter deletion studies. It would of interest to establish whether the TNFSF15 promoter uses the same or different cis-elements to regulate the gene expression in the two pulmonary endothelial cell types and this might help in understanding the mechanism of the differential effects of butyrate. To begin to address the mechanism responsible for the regulation of TNFSF15 expression, we identified different regions of the TNFSF15 promoter that are critical for gene expression in each cell type. The minimal promoter construct p-188-luc was functional in HPAEC but not in HMVEC; altering the core AP1-like sequence element along with G-rich sequence in p-188-luc eliminated most of the activity of this construct. This minimal promoter has two putative AP1-like and one G-rich sequences, but only one of these AP1-like sequence elements (−102 to−92) contributed to the minimal promoter activity. The NF-κB sequence element (−314 to −305) did not appear to be essential for minimal promoter activity in HPAEC as its deletion in p-188-luc or alteration in p-444-luc, respectively, did not affect the activity. In HMVEC, the promoter construct p-444-luc was functional and its activity appeared to be due to the NF-κB sequence element present in this construct. The G-rich and AP1-like sequence elements do appear to exert additional effects on promoter activity. These results suggest that NF-κB, possibly in tandem with AP1-like sequence elements, drive the TNFSF15 promoter in HMVEC. Interestingly, this NF-κB sequence element was also critical for TNFSF15 activity in murine cerebral endothelial cells suggesting an overlap with other endothelial cell types (26).
The use of an NF-κB sequence motif by the TNFSF15 promoter in HMVEC, and its pro-inflammatory response to butyrate suggests that HMVEC are a target of inflammation as NF-κB is a key transcription factor in the inflammatory response (27). Moreover, the recent description of TNFSF15 as a critical gene in gut inflammation (7) supports the notion that HMVEC are targets of inflammation and that TNFSF15 might play an important role in this process. Hence, TNFSF15 up-regulation in lung microvascular endothelium could provide a pro-inflammatory milieu favoring SCD vasoocclusion. During vasoocclusion, the endothelium in SCD becomes pro-inflammatory via the up-regulation of adhesion molecules (28, 29). Endothelial activation can occur in this setting by a variety of mechanisms. Pro-inflammatory cytokines such as TNF-α can be released by circulating monocytes and activate the endothelium (30). Low oxygen tension within the vasculature results in HbS polymerization, adherence of sickle cells to the vascular endothelium and microvasculature occlusion (31, 32). Co-incubation of sickle erythrocytes with HPAEC resulted in up-regulation of the adhesion molecules, VCAM-1, ICAM-1 and E-selectin (1, 2), suggesting a role for the sickle erythrocyte in the modulation of inflammation. Leukocyte and platelet adherence to the endothelium also occur and might further enhance this process (33, 34, 35). Abnormal interactions between sickle erythrocytes, leukocytes and platelets with the endothelium could trigger the expression of cytokines such as TNFSF15 that can activate or inactivate genes essential for normal endothelial function. The observation of a pro-inflammatory response to butyrate in HMVEC raises the possibility that pharmacological use of this agent to induce fetal hemoglobin might paradoxically perturb the endothelium.
We show that both butyrate and TNF-α produce differential effects on TNFSF15 expression in HPAEC and HMVEC. The induction of TNFSF15 gene expression in HMVEC by butyrate suggests that up-regulation of this gene could produce a pro-inflammatory environment that might contribute to the pathogenesis of sickle vasoocclusion.
Acknowledgments
The authors thank Dr. Douglas V. Faller for his review of this manuscript and helpful suggestions.
Grants
Supported in part NIH grants R01-HL68970, R01-HL68970-02-S1, U54HL70819 (MHS) and K23 HL079003 (ESK),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammerman SI, Klings ES, Hendra KP, Upchurch GR, Jr, Rishikof DC, Loscalzo J, et al. Endothelial cell nitric oxide production in acute chest syndrome. Am J Physiol Heart Circ Physiol. 1999;277:H1579– H1592. doi: 10.1152/ajpheart.1999.277.4.H1579. [DOI] [PubMed] [Google Scholar]
- 2.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 3.Hammerman SI, Kourembanas S, Conca TJ, Tucci M, Brauer M, Farber HW. Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 1997;156:280–285. doi: 10.1164/ajrccm.156.1.9611085. [DOI] [PubMed] [Google Scholar]
- 4.Jingyi Yu, Tian S, Metheny-Barlow L, Chew L-J, Hayes AJ, Pan H, et al. Modulation of endothelial cell growth arrest and apoptosis by vascular endothelial growth inhibitor. Circul Res. 2001;89:1161–1167. doi: 10.1161/hh2401.101909. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y-J, Kim W-J, Bae H-Uk, Kim DI-k, Park YB, Park J-E, et al. Involvement of TL1A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 2005;29:229–235. doi: 10.1016/j.cyto.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, Targan SR. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN- γ, in mucosal inflammation. Clin Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Nieves JR, et al. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci USA. 2006;103:8441– 8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migone T-S, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T-cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 9.Pober J, Cotran R. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hebbel RP, Osarogiagbon UR, Kaul D. The endothelial biology of Sickle cell disease: inflammation and chronic vasculopathy. Microcirculation. 2004;11:129– 152. [PubMed] [Google Scholar]
- 11.Perrine SP, Faller DV. Butyrate-induced reactivation of the fetal globin genes: a molecular treatment for the beta-hemoglobinopathies. Experientia. 1993;49:133–137. doi: 10.1007/BF01989417. [DOI] [PubMed] [Google Scholar]
- 12.Safaya S, Ibrahim A, Rieder RF. Augmentation of γ-globin gene promoter activity by carboxylic acids and components of the human β-globin locus control region. Blood. 1994;84:3929–3935. [PubMed] [Google Scholar]
- 13.Miller SJ, Zaloga GP, Hoggatt AM, Carlos LC, Faulk WP. Short-chain fatty acids modulate gene expression for vascular endothelial cell adhesion molecules. Nutrition. 2005;21:740–748. doi: 10.1016/j.nut.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Odièvre MH, Brun M, Krishnamoorthy R, Lapouméroulie C, Elion J. Sodium phenylbutyrate down regulates endothelin-1 expression in cultured human endothelial cells: relevance to sickle-cell disease. Am J Hematol. 2007;82:357–362. doi: 10.1002/ajh.20709. [DOI] [PubMed] [Google Scholar]
- 15.Saemann MD, Bohmig GA, Österreicher CH, Burtscher H, Parolini O, Diakos C, et al. Anti- inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL 12 and up- regulation of IL-10 production. FASEB J. 2000;14:2380–2382. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Watanabe K, Yamashita H, Abe M, Yoshimatsu H, Ohta H, et al. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem Biophys Res Commun. 2005;327:700–706. doi: 10.1016/j.bbrc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 17.Lui W-Y, Sze K-L, Lee WM. Nectin-2 expression in testicular cells is controlled via the functional cooperation between transcription factors of the Sp1, CREB, and AP-1 families. J cell Physiol. 2006;207:144–157. doi: 10.1002/jcp.20545. [DOI] [PubMed] [Google Scholar]
- 18.Marreirosa A, Czolijc R, Yardleya G, Crossleyc M, Jackson P. Identification of regulatory regions within the KAI1 promoter: a role for binding of AP1, AP2 and p53. Gene. 2003;302:155–164. doi: 10.1016/s0378-1119(02)01101-0. [DOI] [PubMed] [Google Scholar]
- 19.Ganster RW, Taylor BS, Shao L, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yutaka K, Shimizu T, Kuwano K. Sodium butyrate up- regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol Immunol. 2006;43:1972– 1981. doi: 10.1016/j.molimm.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Walker GE, Wilson EM, Powell D, Oh Y. Butyrate, a histone deacetylase inhibitor, activates the human IGF binding protein-3 promoter in breast cancer cells: molecular mechanism involves anSp1/Sp3 multiprotein complex. Endocrinology. 2001;142:3817– 3827. doi: 10.1210/endo.142.9.8380. [DOI] [PubMed] [Google Scholar]
- 22.Jianjiang Ye, Shedd D, Miller G. An Sp1 response element in the Kaposi’s Sarcoma-Associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J Virol. 2005;79:1397–1408. doi: 10.1128/JVI.79.3.1397-1408.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel P, Nankova BB, LaGamma EF. Butyrate, a gut-derived environmental signal, regulates tyrosine hydroxylase gene expression via a novel promoter element. Dev Brain Res. 2005;160:53–62. doi: 10.1016/j.devbrainres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-κB activation and cellular proteasome activity. J Biol Chem. 2001;276:44641– 44646. doi: 10.1074/jbc.M105170200. [DOI] [PubMed] [Google Scholar]
- 25.Yoo EJ, Chung JJ, Choe SS, Kim KH, Kim JB. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J Biol Chem. 2006;281:6608–6615. doi: 10.1074/jbc.M508982200. [DOI] [PubMed] [Google Scholar]
- 26.Xiao Q, Hsu CY, Chen H, Xiucui MA, Xu J, Lee J-M. Characterization of cis-regulatory elements of the vascular endothelial growth inhibitor gene promoter. Biochem J. 2005;388:913–920. doi: 10.1042/BJ20041739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebban H, Courtois G. NF-κB and inflammation in genetic disease. Biochem Pharmacol. 2006;72:1153–1160. doi: 10.1016/j.bcp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288:H2715–H2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 29.Mahaseth H, Vercellotti GM, Welch TE, Bowlin PR, Sonbol KM, Hsia CJ, et al. Polynitroxyl albumin inhibits inflammation and vasoocclusion in transgenic sickle mice. J Lab Clin Med. 2005;145:204–211. doi: 10.1016/j.lab.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Setty BN, Stuart MJ. Vascular cell adhesion molecule-1 is involved in mediating hypoxia- induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood. 1996;88:2311–2320. [PubMed] [Google Scholar]
- 31.Kaul DK, Fabrey ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndrome. Blood Rev. 1996;10:29–44. doi: 10.1016/s0268-960x(96)90018-1. [DOI] [PubMed] [Google Scholar]
- 32.Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Invest. 2000;106:337– 338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaul DK, Liu XD, Choong S, Belcher JD, Vercellotti GM, Hebbel RP. Anti-inflammatory therapy ameliorates leukocyte adhesion and Microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2004;287:H293–H301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- 34.Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Curr Opin Hematol. 2006;13:40–44. doi: 10.1097/01.moh.0000190108.62414.06. [DOI] [PubMed] [Google Scholar]
- 35.Covas DT, de Lucena Angulo I, Vianna Bonini PP, Zago MA. Effects of hydroxyurea on the membrane of erythrocytes and platelets in sickle cell anemia. Haematologica. 2004;89:273–80. [PubMed] [Google Scholar]