Abstract
Objectives
Tooth eruption requires the presence of a dental follicle (DF), alveolar bone resorption for an eruption pathway, and alveolar bone formation at the base of the bony crypt. The objectives of our investigations have been to determine how the DF regulates both the osteoclastogenesis and osteogenesis needed for eruption.
Material & Methods
Multiple experimental methods have been employed.
Results
The DF regulates osteoclastogenesis and osteogenesis by regulating the expression of critical genes in both a chronological and spatial fashion. In the rat 1st mandibular molar there is a major burst of osteoclastogenesis at day 3 postnatally and a minor burst at day 10. At day 3, the DF maximally expresses colony-stimulating factor-1 (CSF-1) to down-regulate the expression of osteoprotegerin such that osteoclastogenesis can occur. At day 10, the minor burst of osteoclastogenesis is promoted by upregulation of VEGF and RANKL in the DF. Spatially, the bone resorption is in the coronal portion of the bony crypt and genes such as RANKL are expressed more in the coronal region of the DF than in its basal one-half. For osteogenesis, bone formation begins at day 3 at the base of the bony crypt and maximal growth is at days 9–14. Osteo-inductive genes such as BMP-2 appear to promote this and are expressed more in the basal half of the DF than in the coronal.
Conclusion
The osteoclastogenesis and osteogenesis needed for eruption are regulated by differential gene expression in the DF both chronologically and spatially.
Keywords: dental follicle, osteoclastogenesis, osteogenesis, tooth eruption
Introduction
In order for a tooth to erupt, two obvious requirements are needed. First, there has to be alveolar bone resorption of the bone overlying the crown of the tooth such that an eruption pathway is formed. Second, there has to be a biological process that will result in the tooth moving through this eruption pathway. This review will focus on the molecules needed to initiate and regulates these two events, as well as consider what cells and tissues are involved.
When studying these biological events of eruption, it is important to keep in mind that tooth eruption is a localized event. Thus, in teeth of limited eruption, be it human dentition or rat molars, the time of eruption is different for different teeth. In rat molars (our experimental model), the first molar usually erupts around day 18 postnatally whereas the second molar erupts around day 25. The processes that bring about the eruption are the same for each tooth but the timing is different. This can be dramatically seen in scanning electron micrograph images (Fig. 1) comparing the bases of the alveolar bony crypts of the rat 1st mandibular molar and adjacent 2nd molar at day 14 in which significant bone growth is visible in the crypt of the 1st molar but bone growth is just beginning in the 2nd molar (2).
Figure 1.
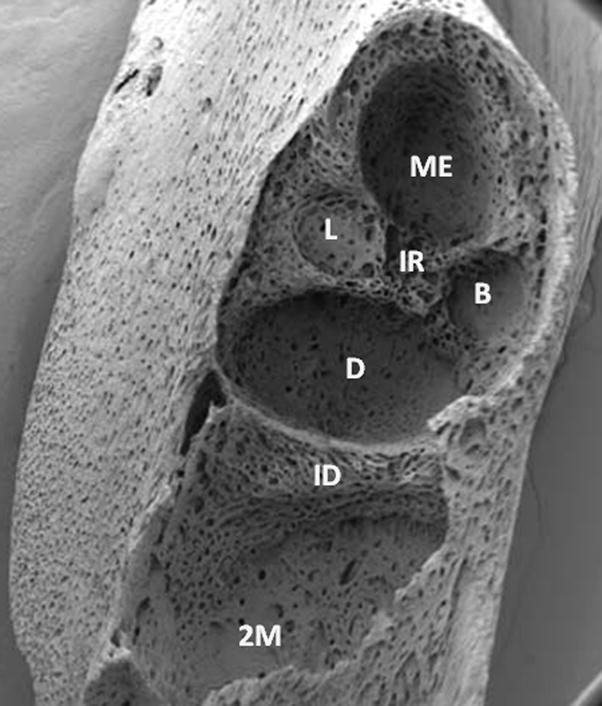
Comparison of the alveolar bony crypts (sockets) of the rat first mandibular molar and second mandibular molar (2M) at day 14 postnatally. The differences between the two sockets emphasize that tooth eruption is a localized event and, as such, alveolar bone formation in the socket of the first molar (which erupts 7–8 days earlier) is far advanced over that of the socket of the 2nd molar. The two sockets are separated by the interdental septum (ID). The socket of the 1st molar is almost filled with new alveolar bone other than in the regions where the roots reside –mesial (ME), distal (D), mesiolabial (L) and mesiobuccal (B).
Alveolar bone resorption for eruption
To study the cellular and molecular events that lead to alveolar bone resorption and the formation of an eruption pathway could be a daunting task given that several tissues and cell types comprise the tooth. Fortunately, studies in the early 1980’s delineated which tissue was needed for eruption. Specifically, experiments in which the dental follicle, a loose connective tissue sac that surrounds the unerupted tooth, was surgically removed from the tooth resulted in the tooth not erupting (3). More dramatically, if the dental follicle (DF) was left intact but the tooth removed and replaced with a metallic replica, that replica would erupt (4). In addition to demonstrating that the DF was required for eruption (at least for the intra-osseous phase), the study eliminated the possibility of many other tissues and/or structures being required; e.g., dental pulp and roots.
Further surgical studies with dog premolars demonstrated regional differences in the DF. Specifically, if the coronal one-half of the follicle were removed but the basal (apical) one-half was left intact, alveolar bone resorption did not occur and the tooth did not erupt (5). Conversely, if the basal one-half of the DF were removed and the coronal one-half left intact, alveolar bone resorption occurred but the tooth did not erupt because of the absence of alveolar bone formation at the base of the crypt. This requirement of bone formation for eruption will be discussed later. Regardless, studies such as these suggested that the coronal region of the DF regulates the osteoclastogenesis and bone resorption needed for eruption whereas the basal one-half regulates the osteogenesis needed for eruption.
The spatial effects of the follicle likely are the result of regional differences in gene expression. Using laser capture microdissection (LCM) to isolate the coronal and basal one-halves from the DFs of rat first mandibular molars, RNA was then extracted from the halves. The expression of a marker gene for osteoclastogenesis for resorption, receptor activator of nuclear factor kappa B (RANKL), and the expression of a marker gene for osteogenesis for bone formation, bone morphogenetic protein-2 (BMP-2), were measured by real-time RT-PCR. The expression of RANKL was greater in the coronal half than in the basal half whereas the expression of BMP-2 was greater in the basal half than in the coronal half (6). Thus, at the molecular level, spatial localization of different levels of gene expression appears to be one means by which the DF regulates the osteoclastogenesis and osteogenesis needed for tooth eruption.
At the ultrastructural level, the bony crypt reflects the spatial effects of the DF. In the unerupted 3rd premolar of the dog, Marks and Cahill (7) showed by scanning electron microscopy (SEM) that the architecture of the bone in the crypt is scalloped in the coronal region above and around the crown; smooth in a narrow region in the middle of the crypt; and, trabecular as the basal or apical region of the crypt. Given that the bone architecture/morphology reflects the physiological state of the bone (8), the scalloped bone in the coronal region is bone undergoing resorption, the smooth bone undergoing neither resorption nor formation, and the trabecular region is forming bone. The bony crypt of the first mandibular molar of the rat has a similar morphology (2).
Cellular and molecular events in the DF that regulate bone resorption for eruption
To determine the molecular regulation of a given process, one has to know what cells are involved to regulate or be regulated. Again, because the DF was known to be required for eruption, one could focus on the cellular events in the DF. In that vein, it was shown that in the 3rd and 4th mandibular permanent premolars of the dog, at a specific time prior to the onset of eruption, there was a major influx of mononuclear cells into the follicle along with an increase of osteoclasts in the alveolar bony crypt (9). Subsequent studies in the rat first mandibular molars showed that a major influx of TRAP-positive mononuclear cells occurred at day 3 postnatally along with a maximal number of osteoclasts seen on the bony crypt (10, 11). A minor burst of osteoclastogenesis also occurs at day 10 before the first mandibular molar erupts at day 18 (11).
The mononuclear cells recruited to the DF are osteoclast precursors as seen by their TRAP-positive staining (10) and by their fusion to form osteoclasts as seen by electron microscopy (12). In turn, what signals are produced by the DF to recruit these mononuclear cells to the DF? Targeted RT-PCR studies showed that colony-stimulating factor-one (CSF-1) and monocyte chemotactic protein-1 (MCP-1) were maximally expressed in the DF at day 3, the time of maximal influx of mononuclear cells (13, 14). This correlation is strengthened by studies in mice in which the maximal influx of mononuclear cells is at day 5 (15) and the maximal expression of CSF-1 and MCP-1 is at day 5 in the mouse DF (16). In vitro, both MCP-1 and CSF-1 are secreted by the DF cells and are chemotactic for monocytes (14).
Recent microarray studies suggest that in addition to MCP-1 and CSF-1 being expressed in the DF, another cytokine with a chemokine domain, endothelial monocyte-activating polypeptide (EMAP-II), also is expressed in the DF (17). Moreover, it is expressed maximally at days 1–3 in the DF and in vitro studies in which EMAP-II expression is knocked down by siRNA in DF cells reduces the ability of the conditioned medium from those cells to recruit bone marrow mononuclear cells (18). EMAP-II has been shown to have a chemotactic effect on mononuclear cells in other studies (19, 20) and thus it is possible that EMAP-II may aid in recruiting the mononuclear cells to the DF for the major burst of osteoclastogenesis. In addition to acting as a chemokine, EMAP-II upregulates the gene expression of both CSF-1 and MCP-1 which, in turn, would indirectly promote mononuclear cell recruitment (18).
The mononuclear cells recruited to the DF must fuse to form osteoclasts for resorption of alveolar bone for the eruption pathway. This major burst of osteoclastogenesis occurs at day 3 in the rat first mandibular molar and the molecular regulation of this by the DF is critical for eruption. In essence, two molecules known to promote osteoclastogenesis, CSF-1 and RANKL, are required for this major burst (1). Yet it is the down-regulation of a molecule that inhibits osteoclastogenesis, osteoprotegerin (OPG), that enables the major burst of osteoclastogenesis to occur. Early studies showed that in vivo the gene expression of OPG was down-regulated at day 3 in the DF of the first mandibular molar of the rat and at day 5 in the mouse (21). Each time correlates with the maximal burst of osteoclastogenesis in each species.
More recently, we have shown that in the rat it is CSF-1, maximally expressed at day 3, that down-regulates the expression of OPG to enable osteoclastogenesis to occur (22). Comparing the expression of OPG in the DF of osteopetrotic rats that have defective or absent CSF-1 versus the expression in their normal littermates of the same age showed that OPG expression was upregulated in the DF of the osteopetrotic rats as compared to their normal phenotype littermates (22). Moreover, inhibiting CSF-1 expression in DF cells in vitro using siRNAs targeted against CSF-1 results in OPG expression being upregulated in such cells (22). Although RANKL also is expressed in the DF at day 3, its gene expression is not upregulated at this time (23). However, the down-regulation of OPG at day 3 would result in a ratio of RANKL/OPG that would favor osteoclastogenesis. The maximal expression of CSF-1 at this time would also promote osteoclastogenesis, given that CSF-1 upregulates the expression of RANK in the osteoclast precursors to enhance cell-to-cell signaling of RANKL and RANK (24) and that CSF-1 promotes the survival and proliferation of osteoclast precursors (e.g., see 25, 26).
Microarray studies show that one other molecule that inhibits osteoclastogenesis, secreted frizzled-related protein-1 (SFRP-1) also has its gene expression down-regulated in the DF at day 3 (time of major burst of osteoclastogenesis) and at day 10 (time of minor burst of osteoclastogenesis) [17]. In vitro osteoclastogenic assays suggest that SFRP-1 inhibits via a different inhibitory pathway than does OPG (17). Regardless, as with OPG, it is the negative regulation of its expression that would enable promotion of osteoclast formation.
In the absence of either CSF-1, as seen in osteopetrotic rats that have defective CSF-1 (27) or in the absence of RANKL, as seen in knockout mice (28), teeth do not erupt. Thus, the differential chronological expression of these genes in the DF, as well as the spatial expression regarding RANKL, is critical for initiating and promoting the osteoclastogenesis needed for eruption.
The studies by Marks and Cahill (5) showing that removal of the coronal one-half of the DF prevented both alveolar bone resorption and tooth eruption demonstrates expression of genes in the DF that affect osteoclastogenesis would regulate the alveolar bone resorption needed for eruption. This is also supported by the studies showing that in RANKL knockout mice rescued with a RANKL transgene expressed in both B and T lymphocytes, there still is no tooth eruption just as is the case with RANKL knockouts (29, 30). In the RANKL rescued transgenics, osteoclasts and bone resorption occur in the endosteum of long bones but not in alveolar bone (30). Thus, the RANKL needed for alveolar bone resorption (and hence tooth eruption) has to come from another source; i.e., the DF.
The minor burst of osteoclastogenesis at day 10 prior to eruption appears to require one or two new genes, as well as an alternation of expression of genes also expressed at day 3 (major burst). Specifically, CSF-1 expression is reduced at day 10 but its function, in part, appears to be replaced by vascular endothelial growth factor (VEGF) which is maximally expressed in the DF at days 9–11 (31). VEGF upregulates the expression of RANK on osteoclast precursors (32), as does CSF-1. In conjunction with this, tumor necrosis factor-alpha (TNF-α) also is maximally expressed in the DF at day 9 and it enhances the gene expression of VEGF in the DF cells (33). TNF-α itself also promotes osteoclastogenesis perhaps either independent of the need for RANKL (e.g., see 34) or by doing so after the osteoclast precursors have been treated with RANKL (e.g., see 35).
A marked contrast of the minor burst of osteoclastogenesis with the major burst is the levels of OPG and RANKL. Unlike the major burst at day 3, OPG levels are high at day 10 (21) but at day 10 the expression of RANKL is upregulated such that it is the time of maximal expression (23). Thus, due to the upregulation of RANKL, a favorable RANKL/OPG ratio would be created to promote osteoclast formation
The major chronological changes of gene expression in the DF that bring about the osteoclastogenesis needed for alveolar bone resorption to create an eruption pathway are summarized in Table 1.
Table 1.
Expression of Genes in the Rat DF at the Time of Major (day 3) and Minor (day 10) Bursts of Osteoclastogenesis
| OPG | RANKL | CSF-1 | VEGF | TNF-α | EMAP-II | MCP-1 | SFRP-1* | |
|---|---|---|---|---|---|---|---|---|
| Day 3 | + | ++ | +++ | + | + | +++ | +++ | + |
| Day 10 | ++ | +++ | + | +++ | +++ | + | + | + |
low level
basal level
high level
Downregulated (days 3 and 10) but high levels all other days
Cellular and molecular events in the DF that regulate bone formation for eruption
The concept that alveolar bone formation at the base of the tooth plays a role in eruption, especially during the intra-osseous phase of eruption, is not new. Sicher (36) noticed alveolar bone growth in the crest of the interradicular septum of human molars. On a more experimental level, Cahill (37) impacted unerupted premolars in dogs with transmandibular wires and then observed eruption after removal of the wires. The released teeth erupted at a rate exceeding the normal rate and the eruption was characterized by an increase in trabecular bone at the base of each tooth. Although not proof that such alveolar bone growth was causal for eruption, the correlation of the onset of rapid bone growth with a rapid rate of eruption was striking.
Subsequent studies implicated the DF in this bone growth, as well as showing that the bone growth was necessary for eruption. As mentioned earlier, surgical removal of the basal one-half of the DF from the unerupted tooth inhibits both eruption and the alveolar bone growth in the base of the bony crypt (5). Scanning electron microscopy studies (SEM) demonstrate bone growth in the base of the crypt in erupting premolars of the dog (7) and in the rat mandibular molars (2). Regarding the latter, the growth is so extensive in the forming interradicular septum during the intra-osseous phase of eruption such that the tooth has no place to move but through the eruption pathway.
Other evidence of alveolar bone formation being required for eruption comes from studies of knockout mice deficient in membrane-type 1 matrix metalloproteinase (MT1-MMP) in which it was observed that eruption was delayed (38, 39). Although alveolar bone resorption occurs in these mice, alveolar bone growth does not. MT1-MMP affects the remodeling of bone by degrading collagens and periodontal ligament fibroblasts of these MT1-MMMP mice have a large accumulation of phagosomes packed with collagen fibrils (38). Thus, remodeling at the connective tissue bone interface likely does not occur and alveolar bone formation is inhibited.
Given that the DF, especially the basal one-half, is required for bone formation and eruption, how does it regulate the osteogenesis needed for eruption? As alluded to earlier, it appears to do so by expression of specific genes in both a chronological and spatial manner. For example, BMP-2 is expressed more in the basal one-half of the DF than in the coronal half (6), and chronologically, gene expression of BMP-2 begins to increase at day 3 with maximal expression at day 9 postnatally (40). These expression times correlate with the beginning of alveolar bone formation at day 3 at the base of the socket and with rapid bone formation by day 9 (2). Thus, BMP-2 may be regulating the osteogenesis of the basal bone growth in the crypt. Future studies injecting siRNA’s targeted against BMP-2 into the DF may determine if this is so. In the interim, we currently are conducting gene microarray studies examining other osteogenic genes to determine if any of these genes are upregulated during the times of alveolar bone growth at the base of the socket.
Whether or not, this alveolar bone growth as a motive force of eruption continues past the intra-osseous phase of eruption is unknown. In the rat molar, the DF becomes organized into a periodontal ligament (PDL) and attaches the tooth to the bony socket at the end of the intra-osseous phase (2) and this is also true for dog premolars (41). Thus, at the point, perhaps the PDL helps lift the tooth to its occlusal plane during the supra-osseous phase of eruption just as it may do so in teeth of continuous eruption (42, 43).
Finally, the presence of stem cells in the DF (44–46), raises the question as to their potential role in tooth eruption. Given that these stem cells exhibit pluripotency in being able to differentiate under appropriate conditions into adipocytes, osteoblasts/cementoblasts or neurons (46), perhaps they also contribute to formation of some of the osteoclasts and osteoblasts needed for tooth eruption.
In conclusion, tooth eruption is a localized event in which specific genes in the DF that surrounds the unerupted tooth are either upregulated or downregulated at critical times to bring about the osteoclastogenesis and osteogenesis needed for eruption. In addition to the chronological regulation of these genes, there is a spatial localization of some of them in the DF to help promote alveolar bone resorption in the coronal portion of the socket and alveolar bone formation at the base of the socket.
Acknowledgments
This work was supported by an R01 NIH Grant DE008911-16 to G.E.W. The author thanks Ms. Cindy Daigle for the processing of this manuscript.
Footnotes
Clinical Relevance
To understand and correct eruption disorders, elucidation of the cellular and molecular requirements needed for normal eruption is necessary. Many tooth eruption disorders are a result of errors or deficits in gene expression. Thus, development of a molecular approach to get impacted teeth to erupt could be a cost effective means of treatment. The dental follicle develops into the periodontal ligament (PDL), and many of the osteoclastogenic molecules expressed for eruption in the follicle also are expressed in the PDL. Alteration of their normal expression in the PDL could result in unwanted alveolar bone resorption, as seen in periodontitis (1).
References
- 1.Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise GE, Yao S, Henk WG. Bone formation as a potential motive force of tooth eruption in the rat molar. Clin Anat. 2007;20:632–639. doi: 10.1002/ca.20495. [DOI] [PubMed] [Google Scholar]
- 3.Cahill DR, Marks SC., Jr Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 4.Marks SC, Jr, Cahill DR. Experimental study in the dog of the non-active role of the tooth in the eruptive process. Arch Oral Biol. 1984;29:311–322. doi: 10.1016/0003-9969(84)90105-5. [DOI] [PubMed] [Google Scholar]
- 5.Marks SC, Jr, Cahill DR. Regional control by the dental follicle of alterations in alveolar bone metabolism during tooth eruption. J Oral Pathol. 1987;16:164–169. doi: 10.1111/j.1600-0714.1987.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 6.Wise GE, Yao S. Regional differences of expression of BMP-2 and RANKL in the rat dental follicle. Eur J Oral Sci. 2006;114:512–516. doi: 10.1111/j.1600-0722.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 7.Marks SC, Jr, Cahill DR. Ultrastructure of alveolar bone during tooth eruption in the dog. Am J Anat. 1986;177:427–438. doi: 10.1002/aja.1001770311. [DOI] [PubMed] [Google Scholar]
- 8.Boyde A, Hobdell MH. Scanning electron microscopy of primary membrane bone. Z Zellforsch Mikrosk Anat. 1969;99:98–108. doi: 10.1007/BF00338800. [DOI] [PubMed] [Google Scholar]
- 9.Marks SC, Jr, Cahill DR, Wise GE. The cytology of the dental follicle and adjacent alveolar bone during tooth eruption in the dog. Am J Anat. 1983;168:277–289. doi: 10.1002/aja.1001680303. [DOI] [PubMed] [Google Scholar]
- 10.Wise GE, Fan W. Changes in the tartrate-resistant acid phosphatase cell population in dental follicles and bony crypts of rat molars during tooth eruption. J Dent Res. 1989;68:150–156. doi: 10.1177/00220345890680021001. [DOI] [PubMed] [Google Scholar]
- 11.Cielinski MJ, Jolie M, Wise G, Ando D, Marks SJ. Colony-stimulating factor-1 (CSF-1) is a potent stimulator of tooth eruption in the rat. In: Davidovitch Z, editor. The biological mechanisms of tooth eruption, resorption and replacement by implants. Birmingham, AL: EBSCO Media; 1994. pp. 429–436. [Google Scholar]
- 12.Wise GE, Marks SC, Jr, Cahill DR. Ultrastructural features of the dental follicle associated with formation of the tooth eruption pathway in the dog. J Oral Pathol. 1985;14:15–26. doi: 10.1111/j.1600-0714.1985.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 13.Wise GE, Lin F, Zhao L. Transcription and translation of CSF-1 in the dental follicle. J Dent Res. 1995;74:1551–1557. doi: 10.1177/00220345950740090801. [DOI] [PubMed] [Google Scholar]
- 14.Que BG, Wise GE. Colony-stimulating factor-1 and monocyte chemotactic protein-1 chemotaxis for monocytes in the rat dental follicle. Arch Oral Biol. 1997;42:855–860. doi: 10.1016/s0003-9969(97)00072-1. [DOI] [PubMed] [Google Scholar]
- 15.Volejnikova S, Laskari M, Marks SC, Jr, Graves DT. Monocyte recruitment and expression of monocyte chemoattractant protein-1 are developmentally regulated in remodeling bone in the mouse. Am J Pathol. 1997;150:1711–1721. [PMC free article] [PubMed] [Google Scholar]
- 16.Wise GE, Huang H, Que BG. Gene expression of potential tooth eruption molecules in the dental follicle of the mouse. Eur J Oral Sci. 1999;106:1–5. doi: 10.1046/j.0909-8836.1999.eos107610.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Wise GE. A DNA microarray analysis of chemokine and receptor genes in the rat dental follicle--role of secreted frizzled-related protein-1 in osteoclastogenesis. Bone. 2007;41:266–272. doi: 10.1016/j.bone.2007.04.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Wise GE. Expression of endothelial monocyte-activating polypeptide II in the rat dental follicle and its potential role in tooth eruption. Eur J Oral Sci. 2008;116:334–340. doi: 10.1111/j.1600-0722.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao J, Ryan J, Brett G, Chen J, Shen H, Fan YG, et al. Endothelial monocyte-activating polypeptide II. A novel tumor-derived polypeptide that activates host-response mechanisms. J Biol Chem. 1992;267:20239–20247. [PubMed] [Google Scholar]
- 20.Journeay WS, Janardhan KS, Singh B. Expression and function of endothelial monocyte-activating polypeptide-II in acute lung inflammation. Inflamm Res. 2007;56:175–181. doi: 10.1007/s00011-006-6162-3. [DOI] [PubMed] [Google Scholar]
- 21.Wise GE, Lumpkin SJ, Huang H, Zhang Q. Osteoprotegerin and osteoclast differentiation factor in tooth eruption. J Dent Res. 2000;79:1937–1942. doi: 10.1177/00220345000790120301. [DOI] [PubMed] [Google Scholar]
- 22.Wise GE, Yao S, Odgren PR, Pan F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J Dent Res. 2005;84:837–841. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Yao S, Pan F, Wise GE. Chronology and regulation of gene expression of RANKL in the rat dental follicle. Eur J Oral Sci. 2005;113:404–409. doi: 10.1111/j.1600-0722.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 24.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wesenbeeck L, Odgren PR, MacKay CA, D’Angelo M, Safadi FF, Popoff SN, et al. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: Evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci USA. 2002;99:14303–14308. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Shin J, Li R, Cheong C, Kim K, Kim S. A novel anti-tumor cytokine contains an RNA binding motif present in aminoacyl-tRNA synthetases. J Biol Chem. 2000;275:27062–27068. doi: 10.1074/jbc.C000216200. [DOI] [PubMed] [Google Scholar]
- 30.Odgren PR, Kim N, MacKay CA, Mason-Savas A, Choi Y, Marks SC., Jr The role of RANKL (TRANCE/TNFSF11), a tumor necrosis factor family member, in skeletal development: effects of gene knockout and transgenic rescue. Connect Tissue Res. 2003;44 (Suppl 1):264–271. [PubMed] [Google Scholar]
- 31.Wise GE, Yao S. Expression of vascular endothelial growth factor in the dental follicle. Crit Rev Eukaryot Gene Expr. 2003a;13:173–180. [PubMed] [Google Scholar]
- 32.Yao S, Liu D, Pan F, Wise GE. Effect of vascular endothelial growth factor on RANK gene expression in osteoclast precursors and on osteoclastogenesis. Arch Oral Biol. 2006;51:596–602. doi: 10.1016/j.archoralbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Wise GE, Yao S. Expression of tumor necrosis factor-alpha in the rat dental follicle. Arch Oral Biol. 2003b;48:47–54. doi: 10.1016/s0003-9969(02)00153-x. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–285. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sicher H. Tooth eruption: axial movement of teeth with limited growth. J Dent Res. 1942;21:395–402. [Google Scholar]
- 37.Cahill DR. The histology and rate of tooth eruption with and without temporary impaction in the dog. Anat Rec. 1970;166:225–238. doi: 10.1002/ar.1091660211. [DOI] [PubMed] [Google Scholar]
- 38.Beertsen W, Holmbeck K, Niehof A, Bianco P, Chrysovergis K, Birkedal-Hansen H, et al. On the role of MT1-MMP, a matrix metalloproteinase essential to collagen remodeling, in murine molar eruption and root growth. Eur J Oral Sci. 2002;110:445–451. doi: 10.1034/j.1600-0722.2002.21384.x. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett JD, Zhou Z, Skobe Z, Dobeck JM, Tryggvason K. Delayed tooth eruption in membrane type-1 matrix metalloproteinase deficient mice. Connect Tissue Res. 2003;44:300–304. [PubMed] [Google Scholar]
- 40.Wise GE, Ding D, Yao S. Regulation of secretion of osteoprotegerin in rat dental follicle cells. Eur J Oral Sci. 2004;112:439–444. doi: 10.1111/j.1600-0722.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 41.Cahill DR, Marks SC., Jr Chronology and histology of exfoliation and eruption of mandibular premolars in dogs. J Morphol. 1982;171:213–218. doi: 10.1002/jmor.1051710208. [DOI] [PubMed] [Google Scholar]
- 42.Berkovitz BK, Thomas NR. Unimpeded eruption in the root-resected lower incisor of the rat with a preliminary note on root transection. Arch Oral Biol. 1969;14:771–780. doi: 10.1016/0003-9969(69)90168-x. [DOI] [PubMed] [Google Scholar]
- 43.Moxham BJ, Berkovitz BK. The effects of root transection on the unimpeded eruption rate of the rabbit mandibular incisor. Arch Oral Biol. 1974;19:903–909. doi: 10.1016/0003-9969(74)90053-3. [DOI] [PubMed] [Google Scholar]
- 44.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kuhn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Luan X, Ito Y, Dangaria S, Diekwisch TGH. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15:595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao S, Pan F, Prpic V, Wise GE. Differentiation of stem cells in the dental follicle. J Dent Res. 2008;87:767–771. doi: 10.1177/154405910808700801. [DOI] [PMC free article] [PubMed] [Google Scholar]


