Abstract
Purpose
To assess the role of vascular space occupancy (VASO) MRI, a non-invasive CBV-weighted technique, for evaluating CBV reactivity in patients with internal carotid artery (ICA) stenosis.
Materials and Methods
VASO reactivity, defined as signal change in response to hypercapnic stimulus (4s exhale, 14s breath hold), was measured in the left and right ICA flow territories in patients (n=10) with varying degrees of unilateral and bilateral ICA stenosis and in healthy volunteers (n=10).
Results
Percent VASO reactivity was more negative (P<0.01) bilaterally in patients (ipsilateral: −3.6±1.5%; contralateral: −3.4±1.2%) compared to age-matched controls (left: −1.9±0.6%; right: −1.9±0.8%). Owing to the nature of the VASO contrast mechanism, this more negative VASO reactivity was attributed to autoregulatory CBV effects in patients. A post-breath-hold overshoot, which was absent in healthy-volunteers, was observed unilaterally in a subset of patients.
Conclusions
More negative VASO reactivity was observed in patients with ICA stenosis and may be a marker of autoregulatory effects. Furthermore, the post-breath-hold overshoot observed in patients is consistent with compensatory microvascular vasoconstriction and may be a marker of hemodynamic impairment. Based on the results of this feasibility study, VASO should be useful for identifying CBV adjustments in patients with steno-occlusive disease of the ICA.
Keywords: VASO, stenosis, CBV, CBF, autoregulation, stroke
Introduction
In patients with cervical or intracranial artery stenosis or occlusion, cerebral blood flow (CBF) may be maintained through an autoregulatory increase in cerebral blood volume (CBV), referred to as stage I hemodynamic impairment1. More significant decreases in perfusion pressure will exhaust autoregulatory capacity, resulting in decreased CBF and a concomitant rise in oxygen extraction fraction (OEF), termed stage II hemodynamic impairment1. Previous studies have demonstrated that patients with internal carotid artery (ICA) disease may have either no impairment with adequate compensation mechanism, such as collateral blood flow2,3, or stage I or II hemodynamic impairment. An increased risk of stroke has been found in patients with stage II hemodynamic impairment4,5 and in patients with abnormal CBF reactivity to a hypercapnic stimulus6, however the relationship between abnormal regulation of CBV and stroke risk is unclear4,7. It has been demonstrated that patients with elevated CBV and OEF are at higher risk for ischemic stroke than are patients with elevated OEF and normal CBV4. However, currently available imaging approaches for measuring CBV generally require administration of contrast agents1,7,8 or the use of cerebral vasodilatory agents7,9 rendering them logistically complex. Thus, there is a need for novel CBV-weighted approaches that do not require the injection of contrast agents, and for non-pharmacological cerebral vasodilatory testing mechanisms.
Recently, the vascular space occupancy (VASO) MRI approach was introduced as a method to obtain rapid assessments of CBV changes without contrast injection10. The physiological relevance of VASO contrast has been substantiated in functional MRI (fMRI) studies11–13, and in glioma patients14, but has not yet been tested in patients with steno-occlusive cerebrovascular disease. Owing to the role of CBV in autoregulation, VASO may provide important information on hemodynamic reactivity in patients with steno-occlusive disease and may be useful for identifying patients at higher risk for stroke who are most likely to benefit from revascularization procedures. In this feasibility study, VASO reactivity during a brief hypercapnic stimulus was measured in healthy controls and in patients with varying degrees of unilateral and bilateral ICA stenosis. As VASO reactivity increases with increasing autoregulatory CBV effects, we hypothesized that if autoregulatory effects are present, VASO reactivity would be greater in patients compared to healthy controls.
Materials and Methods
Theory of VASO Contrast
VASO is a novel MRI sequence that can detect CBV changes non-invasively in vivo10. A detailed account of the VASO contrast mechanism can be found in the literature10,13. In brief, intravascular blood signal is nulled and changes in tissue water signal are detected. Imaging is performed following a non-selective inversion pulse, when intravascular blood magnetization is zero, yet tissue magnetization is slightly positive. This is possible due to the shorter longitudinal relaxation time of tissue water (T1~1100–1200 ms at 3.0T) compared to blood water (T1~1600–1700 ms at 3.0T)12. When CBV increases, such as during breath hold, smooth muscle cells surrounding arterioles, and pericytes lining capillaries, facilitate vasodilatation of the cerebral microvasculature. This vasodilatation will increase the intravascular blood compartment within an imaging voxel, whose signal is nulled in the VASO experiment. The result is a decrease in the measurable tissue signal; the magnitude of the CBV increase can then be inferred from the tissue signal decrease. VASO signal changes (ΔS/S), or VASO reactivity, between a baseline and vasodilatory state such as breath hold can be expressed using the following proportionality:
| (1) |
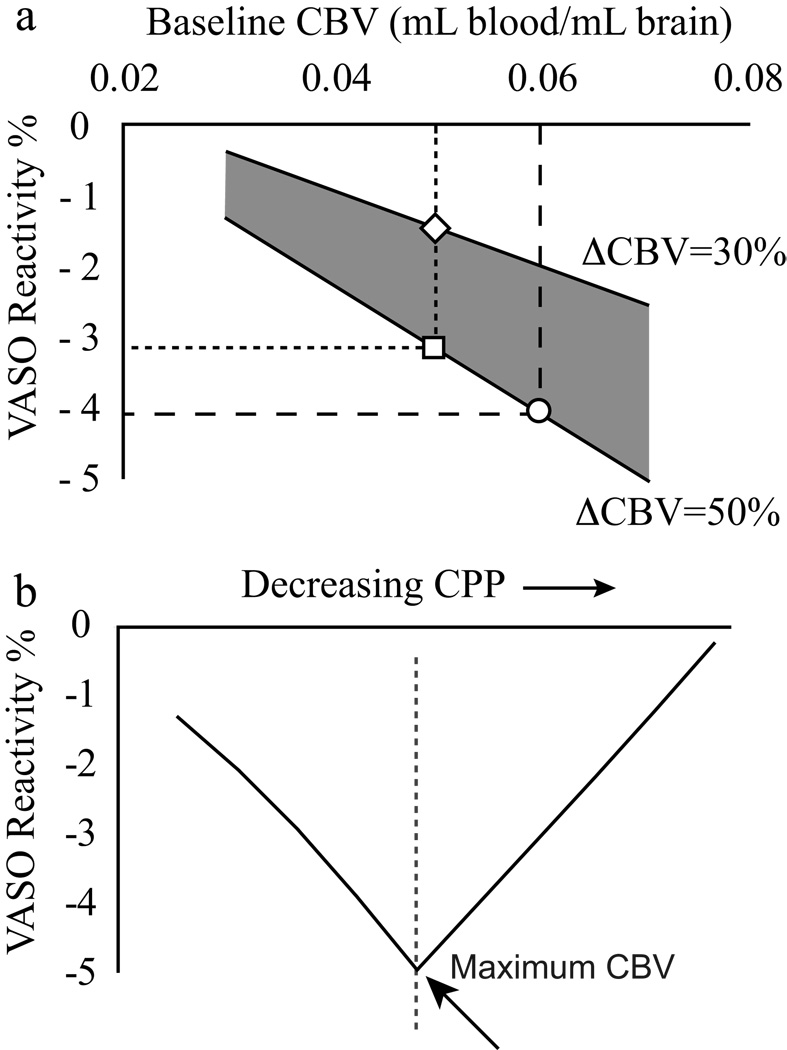
, where S is MRI signal intensity, CBVbase is baseline CBV and CBVbreath-hold is the CBV during the breath-hold period. Since breath hold is associated with vasodilatation (CBVbreath-hold > CBVbase), the VASO reactivity is negative. Two principal effects influence VASO reactivity. First, as can be seen in the denominator of Eq. 1, a larger CBVbase causes the VASO reactivity to become more negative. Second, an increase in the difference between CBVbreath-hold and CBVbase will similarly cause the VASO reactivity to become more negative. An elevated CBVbase may occur in patients with reduced flow capacity, leading VASO reactivity to become more negative in patients with stage I hemodynamic impairment compared to healthy controls. An even more negative VASO reactivity may arise from an additional influence of elevated CBV reactivity (larger CBV change between baseline and breath hold). For stage II impairment, VASO reactivity may become less negative if patients are functioning at maximal autoregulatory capacity (CBVbreath-hold ≈ CBVbase). Fig. 1 simulates the effect of CBV on VASO reactivity.
Figure 1.
(a) Simulated gray matter VASO reactivity as a function of baseline CBV, including a 15% CSF fraction13. For increases in baseline CBV (e.g. square to circle transition), or in the CBV alteration between a baseline and vasodilatory period (e.g. diamond to circle transition), the measured VASO reactivity becomes more negative. (b) Theoretical prediction of VASO reactivity changes with decreasing cerebral perfusion pressure (CPP). In the autoregulatory range (stage I impairment), VASO reactivity becomes more negative when baseline CBV increases. Eventually, vessels will approach maximal vasodilatory capacity (dashed vertical line) and the CBV change between breath hold and baseline (ΔCBV) will begin to decrease. Beyond this autoregulatory threshold, VASO reactivity will become less negative and will eventually reduce to zero. Therefore, patients with stage I hemodynamic impairment may have more negative VASO reactivity, whereas patients with very advanced hemodynamic impairment may have less negative VASO reactivity. Tracking patients over a time course of disease progression may therefore be useful.
Additional effects from blood and tissue water exchange13 as well as CSF partial volume contributions13,15,16 have recently been shown to contribute to the VASO contrast as well. These and other additional contributions have been largely controlled for in the present study and are addressed in the Discussion.
Experiment
The study protocol was approved by the Institutional Review Board and written, informed consent was obtained from all patients and volunteers. Ten patients and ten control subjects were enrolled. Patients were included if they had symptomatic bilateral (n=4) or unilateral (n=6) ICA stenosis (6 male, 4 female, age 68±9 yr). Symptoms of carotid artery disease included transient monocular blindness (n=1), hemispheric transient ischemic attacks (n=7) or minor ischemic strokes (Rankin Scale17 1–3) (n=2). The control group comprised five young volunteers (age: 27±2 yr) and five older, age-matched volunteers (age: 63±10 yr).
The protocol consisted of three breath-hold tasks each comprising 56s normal breathing, 4s exhalation, and 14s of breath holding. Experiments were performed on a 3.0T MRI scanner (Philips Medical Systems, Best, The Netherlands), using body coil transmission and SENSE head coil reception. A single slice was acquired parallel to the anterior commissure/posterior commissure line and 5 mm superior to the corpus callosum. MRI scan parameters: TR/TI/TE=5000/1054/15 ms, FOV=240×240 mm2, spatial resolution=3×3×5 mm3, SENSE-factor= 2.5, single-shot gradient-echo EPI.
Recent work has considerably expanded on the nature of the VASO contrast mechanism13,16, as well as the effectiveness of multi-slice VASO approaches16,18. In this feasibility study, we have implemented long-TR VASO for specific CBV sensitivity13 and have maintained a single-slice protocol to maximize the intravascular blood nulling ability with little tissue signal loss18. Recently, it has been shown that CSF may influence the VASO signal changes13,15,16. We have therefore scanned age-matched volunteers who are expected to have similar CSF fractions as to the patients, in an effort to control for this contribution.
VASO reactivity between baseline and breath holding was calculated using a cross-correlation test. Voxels showing a statistical (P<0.05; SNR≥20; cluster size≥3) correlation with breath hold were considered activated. For these voxels, VASO reactivity was calculated in each hemisphere and grouped ipsilateral/contralateral to symptoms in patients or left/right in controls. Due to the superior location of the slice, the left and right hemispheres approximately corresponded to the left and right ICA flow territories. A t-test was used to identify statistically significant (P<0.05) differences in VASO reactivity between hemispheres and between patients and controls. It may be argued that a better method for assessing regional CBV changes would be to analyze all voxels over the entire ICA territory. Averaging over an entire territory may lead to a misleading mean signal change if positive and negative signal changes are present, as has been shown to be the case in some brain regions19. Therefore, consistent with previous work10,16, we have studied only brain regions with a statistical CBV increase.
Results
Fig. 2 shows representative VASO reactivity maps and hemodynamic changes for a healthy control (Fig. 2a) and for patients with unilateral (Fig. 2b) and bilateral stenosis (Fig. 2c). Note that the VASO reactivity is more negative in the patients compared to the control, which is consistent with autoregulatory CBV effects as was outlined in the previous section. A post-breath-hold overshoot is apparent in the ipsilateral hemisphere in Fig. 2b and to a lesser degree in Fig. 2c, while no overshoot is visible in the control (Fig. 2a). Interestingly, the small overshoot after cessation of the breath hold shown in Figs. 2b,c is apparent in a subgroup of patients only, most noticeably in the symptomatic hemisphere. No post-breath-hold overshoot was found in any control.
Figure 2.
VASO breath-hold reactivity maps and averaged hemodynamic time courses for (a) a healthy volunteer (YC5; Table 1), (b) a unilateral ICA stenosis patient (PT2; Table 1), and (c) a bilateral ICA stenosis patient (PT8; Table 1). The red line indicates ipsilateral to symptoms in patients, or left in control; the black line indicates contralateral to symptoms in patients, or right in the control. The light gray boxes indicate the period of breath hold (14s) and the dark gray box indicates the exhale period (4s). Notice the more negative VASO reactivity in the patient as well as the asymmetric post-task overshoot.
Fig. 3 shows the average VASO time courses for all young controls (Fig. 3a), age-matched controls (Fig. 3b), patients with a unilateral stenosis (Fig. 3c) and patients with bilateral stenoses (Fig. 3d). Again, it can be seen that VASO reactivity is more negative (P<0.01) in both unilateral patients (ipsilateral: −3.7±1.1%; contralateral: −3.4±1.3%) and bilateral patients (ipsilateral: −3.5±2.2%; contralateral: −3.2±1.1%) compared to age-matched controls (left: −1.9±0.6%; right: −1.9±0.8%). Age-matched controls were found to have a more negative VASO reactivity compared to young controls (left: −1.4±0.4%; right: −1.4±0.3%), however this small change was not found to be statistically significant over this volunteer population (P=0.33). VASO reactivity values for all volunteers are given in Table 1. The top half of Table 1 details the control VASO reactivity and the bottom half of Table 1 details the clinical symptoms, reactivity and overshoot values for all patients.
Figure 3.
The averaged VASO reactivity time courses over the three breath hold periods for young controls (a; n=5), age-matched controls (b; n=5), patients with a unilateral stenosis (c; n=6) and patients with bilateral stenoses (d; n=4). Error bars indicate 95% confidence level. Black indicates ipsilateral to symptoms in patients or left in healthy volunteers; gray indicates contralateral to symptoms in patients or right in volunteers. The light gray box demarcates the region of breath hold (14s) and the dark gray box outlines the exhale period (4s). VASO reactivity is found to be more negative in patients (c,d) compared to controls (a,b) with a small average overshoot evident in patients with a unilateral stenosis.
Table 1.
Individual VASO reactivity and overshoot values in controls and patients.
| Age (yrs) |
VASO Reactivity % | Overshoot % | |||
|---|---|---|---|---|---|
| Young Controls |
L | R | L | R | |
| YC1 | 30 | −1.8±0.1 | −1.5±0.1 | 0.1±0.1 | 0.1±0.1 |
| YC2 | 28 | −0.9±0.1 | −1.0±0.1 | 0.0±0.1 | 0.0±0.1 |
| YC3 | 25 | −1.4±0.1 | −1.2±0.1 | 0.1±0.1 | 0.1±0.1 |
| YC4 | 26 | −1.4±0.1 | −1.4±0.1 | 0.1±0.1 | 0.1±0.1 |
| YC5 | 25 | −1.7±0.1 | −1.7±0.1 | 0.0±0.1 | 0.0±0.1 |
| Mean | 27±2 | −1.4±0.4 | −1.4±0.3 | 0.1±0.1 | 0.1±0.1 |
| Older Controls |
|||||
| OC1 | 74 | −1.5±0.1 | −1.2±0.1 | 0.1±0.1 | 0.1±0.1 |
| OC2 | 73 | −2.7±0.1 | −3.0±0.1 | 0.1±0.1 | 0.1±0.1 |
| OC3 | 54 | −2.4±0.1 | −2.1±0.1 | 0.1±0.1 | 0.1±0.1 |
| OC4 | 53 | −1.5±0.1 | −1.1±0.1 | 0.1±0.1 | 0.1±0.1 |
| OC5 | 60 | −1.4±0.2 | −2.3±0.1 | 0.2±0.1 | 0.1±0.1 |
| Mean | 63±10 | −1.9±0.6 | −1.9±0.8 | 0.1±0.1 | 0.1±0.1 |
| Age (yrs) |
Stenosis Type |
Stenosis % | Symptoms | ||
| Patients | I | C | |||
| PT1 | 61 | UL | 50–70 | 0–30 | Hemispheric TIA |
| PT2 | 56 | UL | 70–90 | 0–30 | Minor ischemic stroke |
| PT3 | 59 | UL | 70–90 | 0–30 | Transient blindness |
| PT4 | 62 | UL | 50–70 | 0–30 | Hemispheric TIA |
| PT5 | 80 | UL | 50–70 | 0–30 | Hemispheric TIA |
| PT6 | 79 | UL | 70–90 | 0–30 | Minor ischemic stroke |
| PT7 | 75 | BL | 90–100 | 70–90 | Hemispheric TIA |
| PT8 | 76 | BL | 70–90 | 70–90 | Hemispheric TIA |
| PT9 | 69 | BL | 70–90 | 70–90 | Hemispheric TIA |
| PT10 | 62 | BL | 100 | 70–90 | Hemispheric TIA |
| Mean | 68±9 | ||||
| VASO Reactivity % | Overshoot % | ||||
| Patients | I | C | I | C | |
| PT1 | −3.4±0.3 | −2.8±0.2 | −0.1±0.2 | −0.1±0.2 | |
| PT2 | −2.9±0.2 | −2.3±0.2 | 1.1±0.1 | 0.2±0.1 | |
| PT3 | −3.5±0.3 | −3.9±0.2 | 2.3±0.1 | 2.4±0.1 | |
| PT4 | −4.7±0.3 | −4.2±0.2 | 0.6±0.1 | 0.0±0.1 | |
| PT5 | −2.4±0.1 | −2.0±0.1 | 0.0±0.1 | 0.0±0.1 | |
| PT6 | −5.4±0.4 | −5.4±0.4 | −0.1±0.1 | −0.2±0.1 | |
| PT7 | −1.4±0.1 | −3.2±0.2 | −0.1±0.1 | 0.0±0.1 | |
| PT8 | −4.7±0.4 | −4.9±0.4 | 1.6±0.2 | 0.7±0.2 | |
| PT9 | −6.0±0.3 | −2.6±0.2 | −0.1±0.3 | 0.5±0.1 | |
| PT10 | −1.9±0.2 | −2.2±0.2 | 0.0±0.2 | 0.0±0.0 | |
| Mean | −3.6±1.5 | −3.4±1.2 | 0.5±0.9 | 0.4±0.8 | |
All signal changes: mean±STD over all voxels meeting activation criteria; left (L); right (R) brain hemispheres; young controls (YC); older controls (OC); Ipsilateral (I); contralateral (C); patient (PT). For stenosis type, UL=unilateral and BL=bilateral.
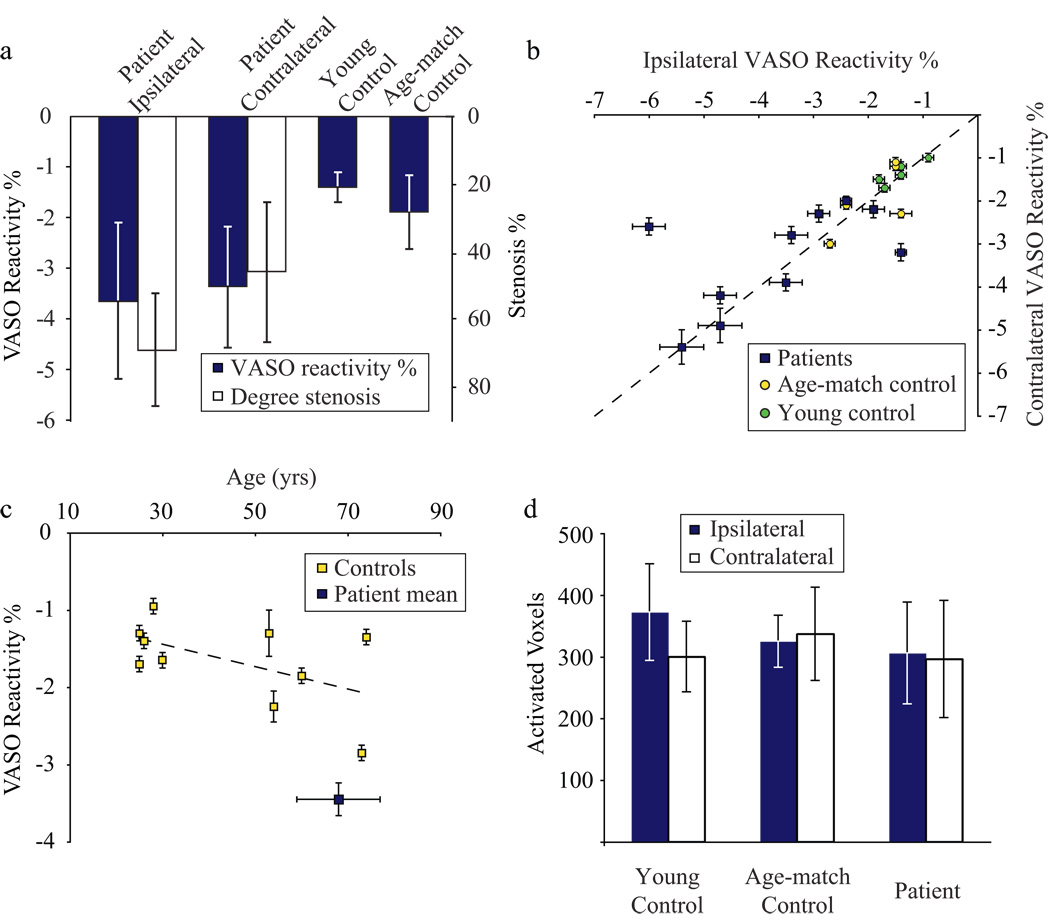
Fig. 4 shows the averaged VASO reactivity and degrees of stenosis (Fig. 4a), flow territory differences in VASO reactivity (Fig. 4b), effect of age on VASO reactivity (Fig. 4c) and number of voxels meeting activated criteria in ipsilateral vs. contralateral brain regions (Fig. 4d). First, mean VASO reactivity was significantly more negative in patients than in controls (Fig. 4a); this more negative patient reactivity was found bilaterally (Fig. 4b) in almost all patients except the two patients with most severe stenosis (Patients 9,10). The increased VASO reactivity in patients was observed in bilateral hemispheres regardless of whether the carotid stenosis was unilateral or bilateral. Fig 4c shows a slight downward trend of VASO reactivity with age in healthy volunteers. Statistically, no difference (P=0.33) is found between VASO reactivity in young and age-matched controls, however there is clearly a larger variation in VASO reactivity for older volunteers. For comparison, the average patient VASO reactivity and age is represented by the blue square. The number of voxels meeting activation criteria did not differ between brain hemispheres in either controls or patients (Fig. 4d) and no statistical difference in the number of voxels meeting activation criteria between volunteer groups was found.
Figure 4.
(a) Mean VASO reactivity (blue) and mean stenosis degree (white) for patients and controls. The patient VASO reactivity is significantly more negative (P<0.01) than the control reactivity. (b) Comparison of reactivity between hemispheres. In patients (blue squares), VASO reactivity ipsilateral vs. contralateral to symptoms is plotted, whereas for young controls (green circles) and age-matched controls (yellow circles) this is left vs. right. The dashed line is a line of unity. No significant difference (P>0.05) is seen between hemispheres, neither in patients nor controls. (c) The effect of VASO reactivity on age for healthy controls (yellow squares). For comparison, the mean VASO reactivity and age of patients is shown by the blue square. Error bars represent standard deviation of age, as well as between flow territories in the patient square. (d) The number of voxels meeting activation criteria in different flow territories for patients and controls. Blue signifies ipsilateral to symptoms in patients or left in controls; white signifies contralateral to symptoms in patients or right in controls. No significant trend is found either between brain regions or volunteer groups.
Discussion
The goal of this feasibility study was to use VASO MRI, a non-invasive CBV-weighted imaging technique, to assess cerebrovascular function and possible altered CBV in patients with symptomatic ICA stenosis. Consistent with the hypothesis, it was observed that VASO reactivity was significantly more negative in patients than in controls, suggesting that more negative VASO reactivity may be a marker of hemodynamic impairment. These preliminary results suggest that VASO MRI with breath hold might constitute a new test that could be performed without the use of contrast agents.
Several aspects of these data merit discussion. First, VASO reactivity was statistically more negative in patients with ICA stenosis than in controls, which would be consistent with a functioning cerebrovascular reserve with increased baseline CBV. This likely corresponds to the autoregulatory effects expected with a reduction in perfusion pressure in stage I hemodynamic impairment4. Importantly, this response occurred bilaterally in eight of ten patients, six of whom had unilateral ICA lesions, suggesting that abnormally increased CBV may be distributed globally in certain brain regions of patients with ICA stenosis. This is not unexpected since imaging was performed in a single slice above the Circle of Willis, where the influence of collateral flow mechanisms would be apparent. In the remaining two patients (Patients 9,10), the increase in vasodilatation upon breath hold was found to be similar to controls in the ipsilateral territory. As these patients had the most severe ICA stenosis, it is possible that these cases represent an exhaustion of the autoregulatory reserve with maximal vasodilatation (Fig. 1b).
An important concern is that in patients functioning at or near maximal autoregulatory capacity, VASO signal changes may become less negative and may even give a “pseudo-normal” value similar to what is obtained in a healthy control, as in Fig. 1b. While this is certainly a potential limitation, two important points should be noted. First, VASO MRI is a non-invasive approach and therefore offers the possibility of serially measuring cerebrovascular reserve over a time course of disease progression. Second, an unexpected finding was a post-breath-hold VASO reactivity overshoot (Figs. 2b,c; Table 1) that occurred in four out of ten patients, predominantly in the ipsilateral hemisphere. Such a positive VASO response is similar to that found in hyperventilation experiments on healthy controls and likely represents relative vasoconstriction10. This overshoot may indicate a response to increased perfusion pressure after breath hold and could be a further indication of impairment. Therefore, future studies might try to determine whether the overshoot is a marker of the severity of the cerebrovascular impairment, perhaps even more so than the signal change itself.
Three additional potential causes of the more negative VASO signal changes, in additional to increased baseline CBV, should be noted. First, vascular dilation in response to stimulation may be enhanced in some patients, which is counterintuitive in view of current literature clearly showing reduced flow in patients8,20. However, even though CBF reactivity is impaired, it is still possible that CBV will increase during CBF-demanding tasks (e.g. breath holding). Additional experiments will be required to determine if this is a possibility. A second cause of the more negative signal changes could be a larger CSF voxel fractions in the patients compared with controls. It has previously been shown that CSF will cause VASO signal changes to become more negative at long TR13 . In older individuals with increased tissue atrophy, voxels may be composed of a higher fraction of CSF, which will make the signal changes more negative. We scanned age-matched controls to attempt to control for this effect; however, in patients with disease, CSF fractions could have been increased further. Improvements to the VASO sequence, including VASO-FLAIR, in which blood and CSF is nulled simultaneously, as well as additional sequences where CSF is nulled separately may be beneficial in the future for controlling for CSF effects13,15,16. Finally, changes in vascular flow velocities may be present in patients compared to controls. However, since VASO utilizes a non-selective inversion pulse, it is relatively insensitive to the velocity of inflowing blood so long as the flow velocity is not so high as to introduce fresh blood from outside the labeling bolus. Therefore, unlike many other CBF and CBV approaches, the arterial input function is always instantaneous in VASO and decreased flow velocities should not contribute to the reactivity measurement.
Several limitations of this study should be considered. Similar to other MRI methods, the VASO signal change will depend on the particular MRI acquisition parameters and on the hardware used, e.g. body coil versus head coil. It is very important to remember that VASO signal changes generally become larger with reduced voxel size10,13. Thus when using VASO reactivity studies, data between sites may only be comparable when acquired at the same spatial resolution. In the current study, voxel size was rather large, namely 3×3×5mm. In a recent breath-hold study using a voxel size of 3×3×3 mm11, VASO reactivities were found to be much increased. Interestingly, this latter study also showed a counterintuitive relationship between CBF and CBV changes during breath hold when compared to visual activation, which is in line with the findings here of VASO reactivity not necessarily reflecting literature CBF reactivity. This discrepancy is under investigation. Similar to fMRI studies, voxel selection in VASO reactivity studies is based on the presence of a statistical change in signal, thresholding out some other voxels. Thus, VASO reactivity reported will always be higher than when including all voxels in gray matter. In the current study, only a single slice above the Circle of Willis was acquired and it is possible that other brain regions may show different reactivity. Also, since patients with asymptomatic carotid stenosis or patients not undergoing revascularization were not included, it was not possible to investigate the relationship between VASO reactivity and stroke risk. Longitudinal studies using a larger patient population are currently being designed with the intent of correlating VASO breath-hold reactivity with the degree stenosis and with patient symptoms. The purpose of this initial feasibility study was simply to determine if VASO was sensitive enough to detect reactivity differences in stenosis patients vs. controls, which appears to be the case. To more thoroughly understand the meaning of the more negative bilateral VASO signal changes in patients, additional functional imaging data from blood-oxygenation-level-dependent (BOLD) and arterial spin labeling (ASL) approaches would be useful. Additionally, longer functional paradigms with more breath-hold repetitions and slices may allow for statistically significant regional effects to be found. A larger study incorporating these additional techniques along with follow-up studies would be useful.
In conclusion, a feasibility study was undertaken to measure VASO reactivity in ICA stenosis patients. Overall, VASO reactivity was more negative in patients compared to controls, consistent with autoregulatory CBV effects. Importantly, a post-stimulus overshoot, indicative of vasoconstrictive compensation, was observed unilaterally in a subgroup of patients. This overshoot was not observed in healthy controls and its physiological significance should be a focus for future clinical studies. While changes in CBF and OEF have been studied in ICA stenosis patients, there is a lack of imaging methodology sensitive to small CBV adjustments associated with autoregulation. This observation, combined with the fact that VASO does not require the injection of a contrast agent, indicates that VASO MRI is a promising neuro-imaging protocol to assess cerebrovascular reserve in patients with carotid artery disease.
Acknowledgments
Grant support: NIH-NCRR P41-RR15241, NIH-NIBIB R01-EB004130, and The Netherlands Organization for Scientific Research Grant 916.76.035.
References
- 1.Derdeyn CP, Grubb RL, Jr., Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53(2):251–259. doi: 10.1212/wnl.53.2.251. [DOI] [PubMed] [Google Scholar]
- 2.Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke. 2000;31(1):128–132. doi: 10.1161/01.str.31.1.128. [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 4.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125(Pt 3):595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 5.Grubb RL, Jr., Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. Jama. 1998;280(12):1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner RW, Regard M. Role of impaired CO2 reactivity in the diagnosis of cerebral low flow infarcts. J Neurol Neurosurg Psychiatry. 1994;57(7):814–817. doi: 10.1136/jnnp.57.7.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen A, Shyr MH, Chen TY, Lai HY, Lin CC, Yen PS. Dynamic CT perfusion imaging with acetazolamide challenge for evaluation of patients with unilateral cerebrovascular steno-occlusive disease. AJNR Am J Neuroradiol. 2006;27(9):1876–1881. [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee P, Kang HC, Videen TO, McKinstry RC, Powers WJ, Derdeyn CP. Measurement of cerebral blood flow in chronic carotid occlusive disease: comparison of dynamic susceptibility contrast perfusion MR imaging with positron emission tomography. AJNR Am J Neuroradiol. 2003;24(5):862–871. [PMC free article] [PubMed] [Google Scholar]
- 9.Detre JA, Samuels OB, Alsop DC, Gonzalez-At JB, Kasner SE, Raps EC. Noninvasive magnetic resonance imaging evaluation of cerebral blood flow with acetazolamide challenge in patients with cerebrovascular stenosis. J Magn Reson Imaging. 1999;10(5):870–875. doi: 10.1002/(sici)1522-2586(199911)10:5<870::aid-jmri36>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Golay X, Pekar JJ, Van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50(2):263–274. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- 11.Donahue MJ, Stevens RD, de Boorder M, Pekar JJ, Hendrikse J, van Zijl PC. Hemodynamic changes after visual stimulation and breath holding provide evidence for an uncoupling of cerebral blood flow and volume from oxygen metabolism. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H, van Zijl PC. Experimental measurement of extravascular parenchymal BOLD effects and tissue oxygen extraction fractions using multi-echo VASO fMRI at 1.5 and 3.0 T. Magn Reson Med. 2005;53(4):808–816. doi: 10.1002/mrm.20379. [DOI] [PubMed] [Google Scholar]
- 13.Donahue MJ, Lu H, Jones CK, Edden RA, Pekar JJ, van Zijl PC. Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med. 2006;56(6):1261–1273. doi: 10.1002/mrm.21072. [DOI] [PubMed] [Google Scholar]
- 14.Donahue MJ, Blakeley JO, Zhou J, Pomper MG, Laterra J, van Zijl PC. Evaluation of human brain tumor heterogeneity using multiple T1-based MRI signal weighting approaches. Magn Reson Med. 2008;59(2):336–344. doi: 10.1002/mrm.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scouten A, Constable RT. VASO-based calculations of CBV change: Accounting for the dynamic CSF volume. Magn Reson Med. 2008;59(2):308–315. doi: 10.1002/mrm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scouten A, Constable RT. Applications and limitations of whole-brain MAGIC VASO functional imaging. Magn Reson Med. 2007;58(2):306–315. doi: 10.1002/mrm.21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamford JM, Sandercock PAG, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20:828. doi: 10.1161/01.str.20.6.828. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, van Zijl PC, Hendrikse J, Golay X. Multiple acquisitions with global inversion cycling (MAGIC): a multislice technique for vascular-space-occupancy dependent fMRI. Magn Reson Med. 2004;51(1):9–15. doi: 10.1002/mrm.10659. [DOI] [PubMed] [Google Scholar]
- 19.Donahue MJ, R S, M dB, J.J P, van Zijl PC. Cerebral blood volume decreases demonstrated during breath-hold with vascular-space-occupancy (VASO) and VASO-FLAIR fMRI; The International Society for Magnetic Resonance in Medicine 16th Scientific Meeting and Exhibition; Berlin. 2007. Abstract 617. [Google Scholar]
- 20.Hendrikse J, van Osch MJ, Rutgers DR, et al. Internal carotid artery occlusion assessed at pulsed arterial spin-labeling perfusion MR imaging at multiple delay times. Radiology. 2004;233(3):899–904. doi: 10.1148/radiol.2333031276. [DOI] [PubMed] [Google Scholar]