Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS). In MS, myeloid dendritic cells (mDCs) secrete elevated amounts of IL-23, a potent pro-inflammatory cytokine, compared to healthy donors. Here, we examined the role of CD46, a complement binding factor, in mDCs by analyzing cytokine and chemokine production in healthy donors and patients with MS. There were striking differences between these groups with increased IL-23p19, CCL2 and CCL5 production, but decreased CCL2 levels in patients. This demonstrates major differences of DC activation upon CD46 activation, with a potential role in the pathogenesis of MS.
Keywords: Multiple sclerosis, human dendritic cells, chemokines, IL-23, CD46
1-Introduction
Multiple sclerosis is a chronic inflammatory demyelinating disease of the CNS (Hafler et al., 2005; Weiner, 2004b) in which both the release of inflammatory mediators and chemotaxis are important (Adorini, 2004; Elhofy et al., 2002). DCs are professional antigen-presenting cells (APC) which secrete cytokines and chemokines upon maturation and play a key role in the induction of immune responses by activating naïve T cells. We previously demonstrated that mDCs from patients with MS secrete elevated amounts of IL-23 compared to healthy controls (Vaknin-Dembinsky et al., 2006). IL-23 is a proinflammatory cytokine that consists of a specific p19 subunit associated with the shared IL-12p40 subunit (also called IL-12 beta1 subunit which associates with IL-12p35 to form IL-12 (p70)) (Frucht, 2002; Oppmann et al., 2000; Trinchieri et al., 2003). IL-23 promotes the expansion of a specific subset of T cells secreting IL-17, a potent inflammatory cytokine (Bettelli et al., 2007) involved in several autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), a murine model of MS (Komiyama et al., 2006). Previous MS studies have also demonstrated increased amounts of IL-12p40 in the nervous system and increased IL-12 production by PBMC (Balashov et al., 1997; Comabella et al., 1998; Soldan et al., 2004). Therefore, production of both IL-12 and IL-23 are dysregulated in patients with MS (Gran et al., 2004).
The engagement of CD46 at the surface of APC has been shown to modulate the production of IL-12 (p70) and/or p40 subunit, either increasing or decreasing their expression depending on the cell type and stimulus used (Karp et al., 1996; Kurita-Taniguchi et al., 2000; Schnorr et al., 1997; Smith et al., 2003). CD46 is a widely expressed transmembrane protein initially identified as a complement regulatory protein (Seya et al., 1986). It has then been described as a ‘magnet for pathogens’ (Cattaneo, 2004), acting as a receptor for several virus and bacteria. More recently, it was identified as a co-stimulatory molecule for T cell activation (Astier et al., 2000; Marie et al., 2002; Zaffran et al., 2001) and it induces a Tr1 regulatory phenotype with considerable secretion of IL-10 and granzyme B (Grossman et al., 2004; Kemper et al., 2003). This Tr1 differentiation is altered in patients with MS, characterized by a lack of IL-10 production upon CD46 activation (Astier and Hafler, 2007; Astier et al., 2006). Herein, we further investigated the role of CD46 in MS by analyzing its role on mDCs and comparing healthy donors and patients with MS. There were striking differences between these two groups with increased IL-23 production and modulation of CCL2, CCL3 and CCL5 secretion.
2-Material and Methods
Subjects
Local Ethical Committee approval has been received for this study and the informed consent of all participating subjects was obtained. All patients were seen at the Partners MS Center at the Brigham and Women's Hospital in Boston. Peripheral blood was obtained from healthy subjects (10 donors; average age = 35yrs±6.6, 6 females/4 males) and patients with MS (10 untreated patients with MS (41yrs±8; EDSS: 1.44±0.95, 9 females/1 male) in the relapsing–remitting stage. No patient was in relapse at the time of the blood draw. None of the patients had received steroids in the 2 months prior to blood drawing, and were not treated with interferon beta 1a in the 10 months prior to blood drawing or with immunosuppressive therapy in the 3 years prior to blood drawing. None of the patients were treated with glatiramer acetate prior to blood drawing.
Purification of mDCs and T cells
mDCs were directly isolated from the blood using CD1c beads (Miltenyi Biotec) and matured by adding 100ng/ml LPS with or without CD46 (5µg/ml, mAb 20.6). Forty-eight hours later, cells were collected for gene expression assays. For IL-17 expression, CD4+ T cells were isolated as described before (Astier et al., 2006) and cultured at 2×106 cells/ml in 48-well plates coated with anti-CD3 mAb (OKT3, 2.5 µg/ml) for 48h with and without mDCs supernatants matured with LPS or LPS plus anti-CD46.
Detection of cytokines by ELISA
Secretion of cytokines was determined in cell culture supernatants by ELISA as previously described (Astier et al., 2006; Vaknin-Dembinsky et al., 2006). IL-23/12p40, IL-12p70, and IL-17 ELISA were predesigned kits from R&D Systems. According to the manufacturer's specifications, the IL-12 p70 ELISA does not cross-react with recombinant human IL-12 p40 (rhIL-12) p40 or rhIL-12 p35 monomers or homodimers.
Real time (TaqMan) RT-PCR analysis
Total RNA was extracted from 0.5*105 cells using an RNeasy kit (Qiagen) according to the manufacturer's instructions. cDNA was synthesized using Applied Biosystems kit. The primers and probes for the TaqMan qPCR assays for IL-12p35, IL-23p19, CCL2, CCL3, and CCL5 were obtained from Applied Biosystems. Human GAPDH (Applied Biosystems) was used to normalize each sample.
Statistics
The groups were analyzed using the Prism software (GraphPad Software Inc.) and compared using the Wilcoxon matched pair test to assess CD46 stimulation effect or the Mann-Whitney test to assess significance between healthy donors and patients, two non-parametric tests that do not assume Gaussian variation.
3-Results
CD46 modulates LPS-mediated maturation of mDCS
We first analyzed the cytokine profiles produced by CD46 engagement on mDCs. A role of CD46 in IL-12p40 and p70 expression have been previously reported (Karp et al., 1996; Kurita-Taniguchi et al., 2000; Schnorr et al., 1997; Smith et al., 2003). Hence, we analyzed the role of CD46 in both IL-12 and IL-23 inductions. mDCs from 10 healthy donors were matured with LPS with or without anti-CD46 antibodies for 2 days. Supernatants and cells were then harvested, and cytokine production was assessed by quantitative real-time PCR (qPCR) or ELISA (Figure 1). We observed a significant increase of the specific p35 IL-12 chain by qPCR (p< 0.05)(Fig. 1A) upon CD46 activation. We next assessed the levels of both p40 and p70 by ELISA (Figure 1B). The levels of IL-12 p70 detected were barely detectable (average p70: 39 vs. 43 pg/ml, p=0.11). An increase in p40 could be detected (average p40: 1933 vs. 15873 pg/ml, **p=0.0078). Hence, we next examined whether CD46 stimulation could affect IL-23 secretion. An increased expression of the specific IL-23p19 subunit was observed (Fig. 1A, p< 0.05). IL-23 triggers the expansion and survival of a novel T cell subset characterized by the secretion of IL-17, a potent proinflammatory cytokine (Bettelli et al., 2006). Hence, to confirm the induction of IL-23 by CD46-stimulated mDCs, we next evaluated by ELISA the amounts of IL-17 secreted by activated T cells co-cultured with the supernatants of CD46-stimulated mDCs. Addition of CD46-activated DC supernatant significantly augmented IL-17 secretion (Fig. 1B; average IL-17: 477 vs. 772 pg/ml **p=0.0078), which was inhibited by the addition of sIL-23R (Fig. 1B). The addition of sIL-23R also led to significantly lower IL-23 levels than with LPS alone (p=0.0007), indicating that low amounts of IL-23 are secreted by mDCs matured by LPS alone, which are increased by concomitant stimulation with CD46.
Figure 1. IL-12 and IL-23 production by CD46-activated mDCs.
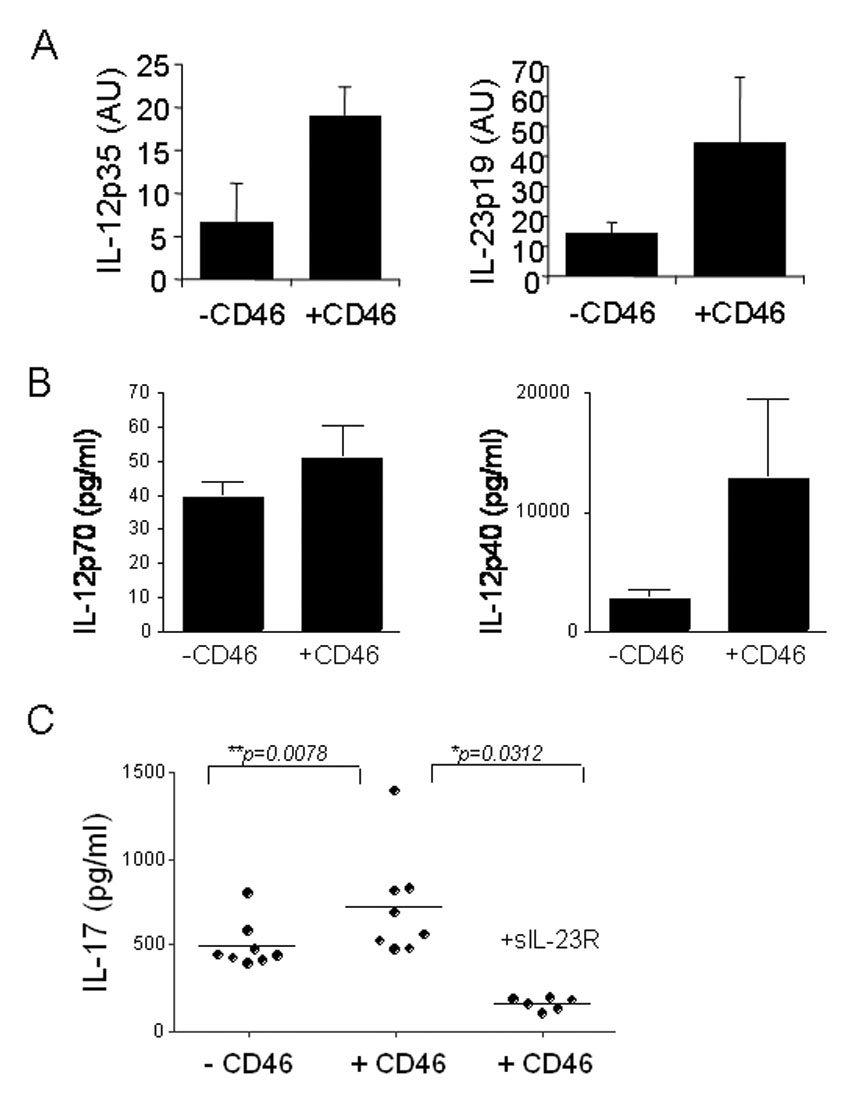
(A) The production of IL-12 and IL-23 by CD46-activated mDCs was quantified by qPCR. Isolated mDCs from 10 healthy donors were matured in presence of LPS in presence or absence of anti-CD46 antibodies. After 48h, RNA were isolated and cytokine production examined by qPCR. (B) The amounts of IL-12p40 and IL-12 p70 secreted by mDC in presence of absence of CD46 were also quantified by ELISA (C) The amount of IL-17 produced by activated T cells cultured in presence of supernatants from mature mDCs with or without anti-CD46 was determined by ELISA. The addition of sIL-23R in the T cell culture blocked IL-17 induction.
IL-23p19 increase in patients with MS
mDCs from patients with MS secrete increased amounts of IL-23 compared to healthy donors (Vaknin-Dembinsky et al., 2006). Therefore, we next determined whether the specific chains for IL-12 and IL-23, p35 and 19 respectively, were differently modulated upon CD46 in patients with MS compared to healthy donors. mDCs obtained from a cohort of 10 patients with MS in the relapsing-remitting stage were compared to mDCS from a subset of 10 age-matching healthy donors. mDCs were cultured in presence of LPS with or without CD46 antibodies and cytokine production were determined by qPCR 48hrs later (Fig.2). The IL-12p35 specific chain was induced in both groups, and no significant difference was observed between these groups (Fig. 2A). An ELISA assessing IL-12p70 was also performed but very low levels were detected and no difference between these two groups was observed (not shown). However, a marked increase in IL-23p19 expression was detected in patients compared to healthy controls (Fig. 2B, **p=0.0043; >4.5fold induction).
Figure 2. Increased IL-23p19 production in patients with MS.
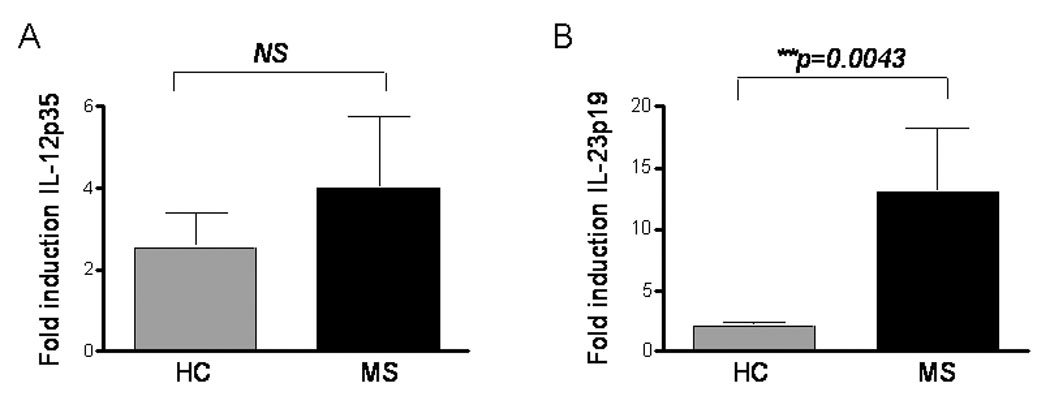
The induction of IL-12p35 (A) and IL-23p19 (B) upon CD46 activation was assessed in healthy controls (HC) and patients with MS (MS). Isolated mDCs from 10 untreated patients with MS in the relapsing-remitting phase and of 10 healthy donors were matured in presence of LPS in presence or absence of anti-CD46 antibodies. After 48h, RNA were isolated and cytokine production examined by qPCR. The induction of both IL-12 and IL-23 upon CD46 activation was calculated.
Chemokine modulation by CD46 stimulation
Among the pathogenic mechanisms that contribute to the inflammation observed in MS, the production of inflammatory mediators and leukocyte chemotaxis into the CNS are both important (Sorensen et al., 1999; Zhang et al., 2000). We next examined whether CD46 could modulate chemokine secretion by mDCs, and compared patients with MS and healthy donors. mDCs were matured in presence of LPS with or without CD46 antibodies for 48hrs and the relative expression of CCL2 (MCP-1), CCL3 (MIP-1α) and CCL5 (Rantes) compared to their expression in immature mDCs was determined by qPCR (Fig. 3A). While LPS induced CCL2 secretion, CD46 engagement led to a significant decrease of CCL2 expression. Furthermore, a significant difference was observed between healthy donors and patients with MS. While ~50% of CCL2 expression was inhibited by CD46 in mDCs from healthy donors, ~90% inhibition was observed in patients. Alternatively, CD46 stimulation induced a significant increase of CCL3 and CCL5 in patients with MS. These data were further confirmed by ELISA (Figure 3B): while LPS increased CCL2 production, CD46 led to a decrease in CCL2 secretion in both healthy donors and patients with MS. However, the decrease observed in patients was much more pronounced as the levels of CCL2 were similar to those obtained with immature DC. Conversely, CD46 stimulation led to an increase of both CCL3 and CCL5, which was significantly increased in patients when compared to healthy donors.
Figure 3. Chemokine production by CD46-activated mDCs.
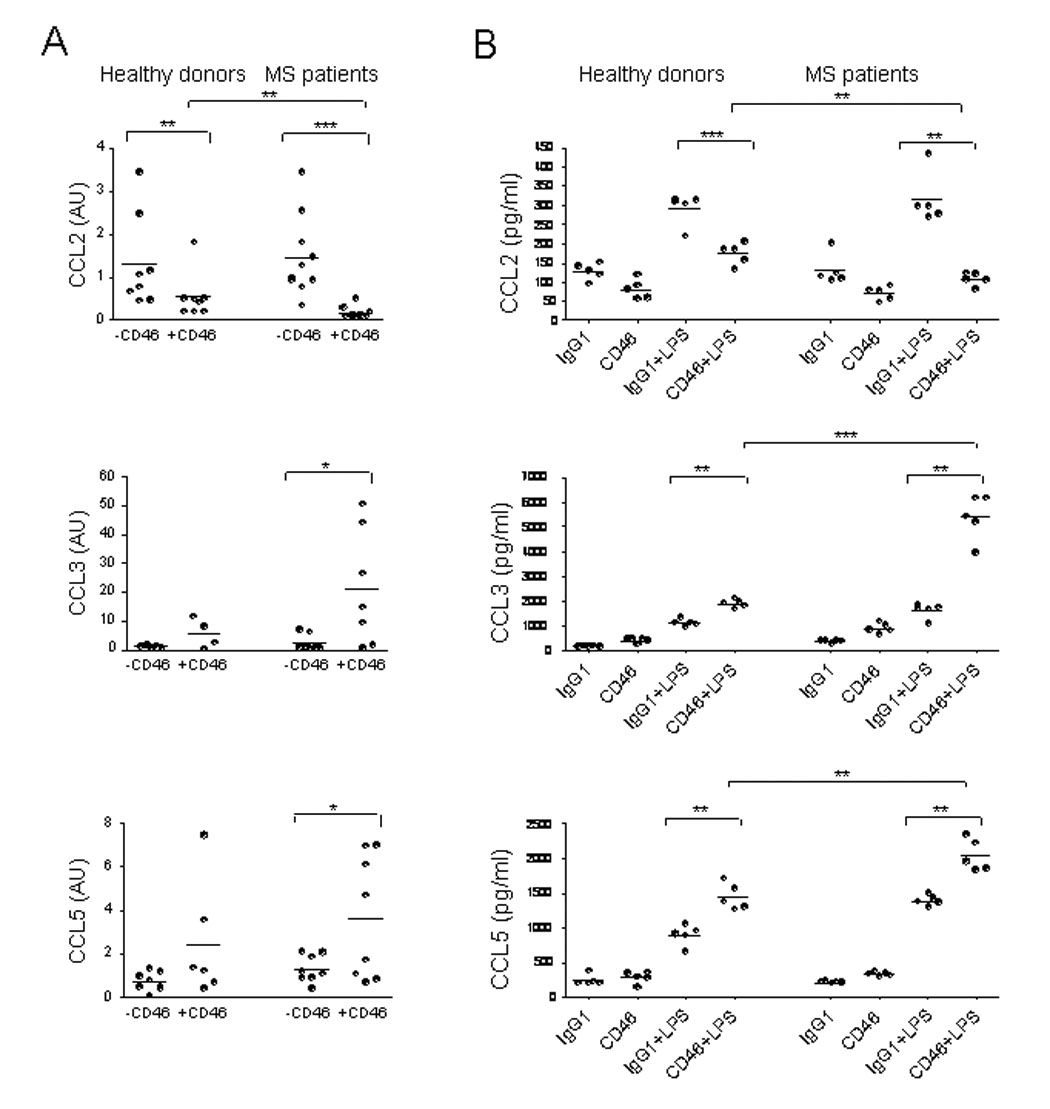
(A) The productions of CCL2, CCL3 and CCL5 by mature mDCs obtained by culture with LPS in presence or absence of CD46 antibodies were measured by qPCR (A) in a cohort of 8 healthy donors and 9 untreated patients with MS in the relapsing-remitting phase. Their relative expression compared to immature mDCs is plotted. (B) The amounts of CCL2, CCL3 and CCL5 secreted by mDC activated by CD46 (or irrelevant IgG1) were assessed by ELISA.
4-Discussion
MS is a demyelinating disease with a chronic inflammation of the central nervous system (Weiner, 2004a). Dendritic cells are professional APC that initiate the immune response by activating naive T cells. Upon maturation, they then secrete cytokines such as IL-12 and IL-23 that regulate inflammation. In particular, IL-23 is a potent inflammatory cytokine which plays a crucial role in the inflammation of the brain (Cua et al., 2003). The expression of the specific IL-23p19 subunit in transgenic mice also results in systemic inflammation (Wiekowski et al., 2001). Moreover, IL-23 triggers the expansion and survival of a novel T cell subset characterized by the secretion of the proinflammatory cytokine IL-17 (Bettelli et al., 2007; Kolls and Linden, 2004). Production of IL-12p70 by APC can be modulated by cross-linking of CD46 at their surface, either increasing or decreasing IL-12 depending on the cell type and stimulus used. An inhibition of IL-12 production by monocytes is observed after CD46 ligation by antibodies or C3b binding (Karp et al., 1996), and a posttranscriptional inhibition of IL-12p70 was also observed when CD46 was triggered by HHV6 (Smith et al., 2003). On the other hand, IL-12p40 was increased by CD46 ligation on GM-CSF treated monocytes (Kurita-Taniguchi et al., 2000), and infection of dendritic cells by measles virus led to an increase in IL-12p40 (Schnorr et al., 1997). In this report, we demonstrate that activation of mDCs through CD46 increases the expression of both p35 and p19, specific chains for IL-12 and IL-23 respectively. However, no significant secretion of IL-12p70 could be detected, while the secretion of IL-23 was further confirmed by the proliferation of T cells secreting IL-17 when co-cultured with the supernatant of CD46-activated DCs. Furthermore, this was blocked by the addition of sIL-23R in the culture. The addition of sIL-23R also led to lower IL-17 levels than with LPS alone, indicating that low amounts of IL-23 are secreted by mDCs matured by LPS alone, which are increased by concomitant stimulation with CD46 (Fig. 1B). We had previously shown that increased amounts of IL-23 were secreted by DCs in patients when compared to healthy donors (Vaknin-Dembinsky et al., 2006). Herein, we show that the main increase in IL-23 detected in patients might be due to an alteration of CD46, as CD46-activated DCs from patients express much more p19 than healthy donors do (>4.5 fold induction). No significant differences in IL-12 were observed.
Among the pathogenic mechanisms that contribute to the inflammation observed in MS, the production of inflammatory mediators and leukocyte chemotaxis into the CNS are both important. We therefore investigated the chemokine profile secreted after CD46 ligation on DCs. We demonstrated that CD46 activation modulated the profile of chemokines expressed by DCs. First, although LPS induced CCL2 expression, CD46 engagement induced a significant decrease of CCL2 expression (Fig. 2A). Of note, the differences observed on CCL2 secretion between LPS and CD46 indicated that the effects observed with CD46 were not due to endotoxins, but were specific to CD46. Furthermore, a significant difference was observed between healthy donors and patients with MS. While CD46 activation led to a ~50% inhibition in healthy donors, CCL2 production was almost totally inhibited in patients with MS (~90%). In MS, the level of CCL2 in CSF during relapses is reduced but increases during remission periods (Bartosik-Psujek and Stelmasiak, 2005; Sorensen et al., 2004). None of the patients analyzed in this study were in relapse. A systematic study by Sorensen and colleagues on the level of CCL2 in both serum and CSF showed that there is a significant decrease in CCL2 in CSF from patients compared to controls (Sorensen et al., 2004). Furthermore, CCL2 levels are higher in PBMC from untreated patients in stable phase, and are modulated by IFNβ or methylprednisolone treatments (Iarlori et al., 2002; Moreira et al., 2006). Altogether, these data argue in favor of a negative correlation between the levels of CCL2 and active MS. We show that CCL2 levels are markedly reduced in patients with MS upon CD46 stimulation, likely contributing to MS pathogenesis.
Alternatively, CD46 stimulation led to increased CCL3 and CCL5 levels compared to LPS alone, and both chemokines were significantly further increased in patients with MS. A role of CCL3 in EAE has been previously described (Karpus et al., 1995). Indeed, the blockade of CCL3 with antibodies prevents the development of disease and inhibits the infiltration of mononuclear cells into the CNS. Increased expressions of CCL5 and of its receptors, CCR1 and CCR5, have also been described in MS lesions (Balashov et al., 1999; Ubogu et al., 2006), and correlated to increased IL-12 concentrations in serum from patients (Bartosik-Psujek and Stelmasiak, 2005). Furthermore, MS is an autoimmune disease with environmental influences and genetic predisposition (Hafler et al., 2005). Genetic linkage studies in multiple sclerosis have identified the chromosome 17q11 as a susceptibility locus, which encodes a cluster of genes for beta-chemokines or CC chemokine ligands (CCLs) including CCL2 and CCL3 (Vyshkina and Kalman, 2005; Vyshkina et al., 2005). We show here that CD46 activation modulates the secretion of these chemokines with differential expressions in patients with MS. Altogether, these data suggests that CD46 activation is altered in patients with MS with decreased CCL2 production and increased CCL3 and CCL5 secretion that might contribute to the development of MS lesions.
Hence, our data illustrate novel roles of CD46 in mDC function that are altered in MS. We show that CD46 controls the release of chemokines and of both IL-12 and IL-23 by activated mDCs. IL-23 production was drastically increased in patients with MS, along with significant differences in chemokine production which likely contribute to the inflammation and subsequent development of MS lesions. Altogether with our recent data on CD46 in T cells from patients with MS, this report underlines the importance of CD46 in the control of the immune response and how its dysfunction may contribute to MS pathogenesis.
Acknowledgments
The authors thank Prof. Chantal Rabourdin-Combe for the kind gift of the anti-CD46 antibody (20.6). This work was supported by a National Institutes of Health Grant (NS23132) and an NMSS advanced fellowship to ALA. The authors have no financial conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5-References
- Adorini L. Immunotherapeutic approaches in multiple sclerosis. J Neurol Sci. 2004;223:13–24. doi: 10.1016/j.jns.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- Astier AL, Hafler DA. Abnormal Tr1 differentiation in multiple sclerosis. J Neuroimmunol. 2007;191:70–78. doi: 10.1016/j.jneuroim.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci U S A. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Stelmasiak Z. Correlations between IL-4, IL-12 levels and CCL2, CCL5 levels in serum and cerebrospinal fluid of multiple sclerosis patients. J Neural Transm. 2005;112:797–803. doi: 10.1007/s00702-004-0225-9. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comabella M, Balashov K, Issazadeh S, Smith D, Weiner HL, Khoury SJ. Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J Clin Invest. 1998;102:671–678. doi: 10.1172/JCI3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Elhofy A, Kennedy KJ, Fife BT, Karpus WJ. Regulation of experimental autoimmune encephalomyelitis by chemokines and chemokine receptors. Immunol Res. 2002;25:167–175. doi: 10.1385/IR:25:2:167. [DOI] [PubMed] [Google Scholar]
- Frucht DM. IL-23: a cytokine that acts on memory T cells. Sci STKE. 2002:PE1. doi: 10.1126/stke.2002.114.pe1. [DOI] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Rostami A. Role of the IL-12/IL-23 system in the regulation of T-cell responses in central nervous system inflammatory demyelination. Crit Rev Immunol. 2004;24:111–128. doi: 10.1615/critrevimmunol.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Hafler DA, Slavik JM, Anderson DE, O'Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Iarlori C, Reale M, De Luca G, Di Iorio A, Feliciani C, Tulli A, Conti P, Gambi D, Lugaresi A. Interferon beta-1b modulates MCP-1 expression and production in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2002;123:170–179. doi: 10.1016/s0165-5728(01)00487-8. [DOI] [PubMed] [Google Scholar]
- Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4(+) cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kurita-Taniguchi M, Fukui A, Hazeki K, Hirano A, Tsuji S, Matsumoto M, Watanabe M, Ueda S, Seya T. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J Immunol. 2000;165:5143–5152. doi: 10.4049/jimmunol.165.9.5143. [DOI] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- Moreira MA, Tilbery CP, Monteiro LP, Teixeira MM, Teixeira AL. Effect of the treatment with methylprednisolone on the cerebrospinal fluid and serum levels of CCL2 and CXCL10 chemokines in patients with active multiple sclerosis. Acta Neurol Scand. 2006;114:109–113. doi: 10.1111/j.1600-0404.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Schnorr JJ, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci U S A. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45–70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102:2877–2884. doi: 10.1182/blood-2002-10-3152. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Dysregulation of IL-10 and IL-12p40 in secondary progressive multiple sclerosis. J Neuroimmunol. 2004;146:209–215. doi: 10.1016/j.jneuroim.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Sorensen TL, Ransohoff RM, Strieter RM, Sellebjerg F. Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur J Neurol. 2004;11:445–449. doi: 10.1111/j.1468-1331.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Callahan MK, Tucky BH, Ransohoff RM. Determinants of CCL5-driven mononuclear cell migration across the blood-brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol. 2006;179:132–144. doi: 10.1016/j.jneuroim.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- Vyshkina T, Kalman B. Haplotypes within genes of beta-chemokines in 17q11 are associated with multiple sclerosis: a second phase study. Hum Genet. 2005;118:67–75. doi: 10.1007/s00439-005-0003-2. [DOI] [PubMed] [Google Scholar]
- Vyshkina T, Shugart YY, Birnbaum G, Leist TP, Kalman B. Association of haplotypes in the beta-chemokine locus with multiple sclerosis. Eur J Hum Genet. 2005;13:240–247. doi: 10.1038/sj.ejhg.5201295. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Immunosuppressive treatment in multiple sclerosis. J Neurol Sci. 2004a;223:1–11. doi: 10.1016/j.jns.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 2004b;61:1613–1615. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–6785. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Baker CM, Kolson DL, Rostami AM. Chemokines and chemokine receptors in the pathogenesis of multiple sclerosis. Mult Scler. 2000;6:3–13. doi: 10.1177/135245850000600103. [DOI] [PubMed] [Google Scholar]


