Abstract
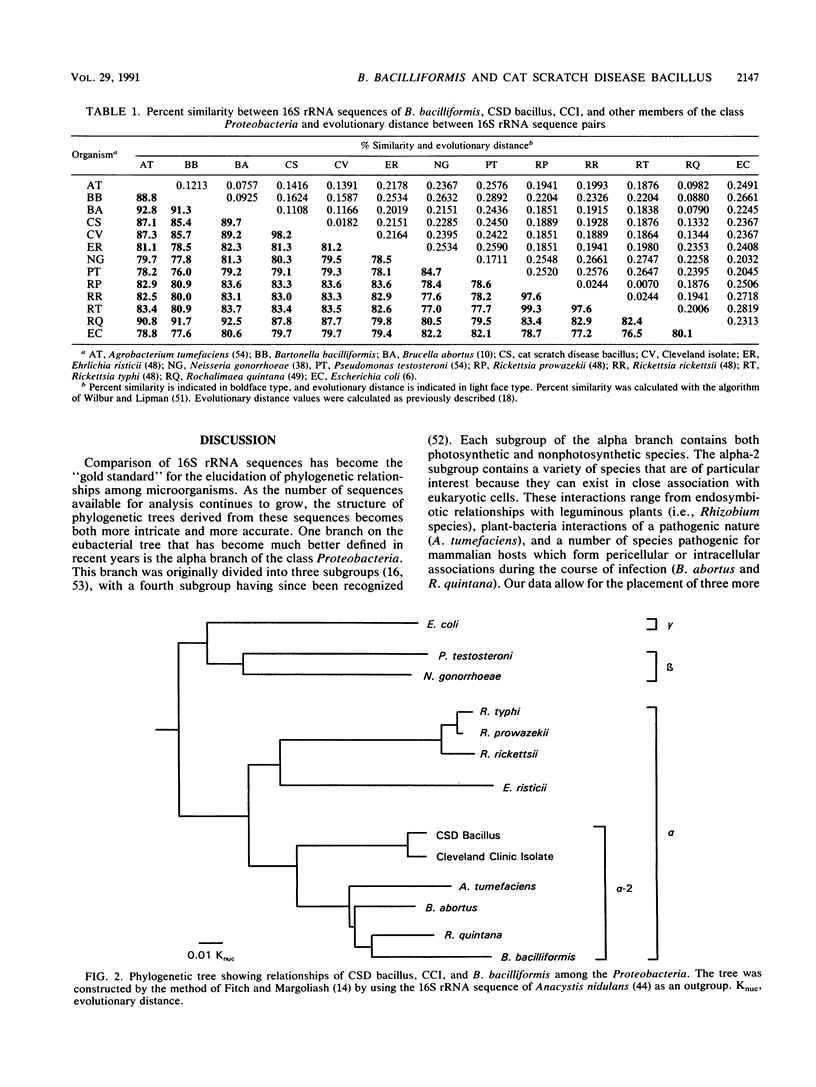
The primary structures of 16S rRNAs of Bartonella bacilliformis, an isolate of the cat scratch disease (CSD) bacillus, and a strain phenotypically similar to the CSD bacillus were determined by reverse transcriptase sequencing. These microorganisms were found to be members of the alpha-2 subgroup of the class Proteobacteria. The sequence from B. bacilliformis was most closely related to the rRNA of Rochalimaea quintana (91.7% homology), the etiologic agent of trench fever. The sequence from the isolate of the CSD bacillus showed the greatest homology with Brucella abortus (89.7%) and, when compared with oligonucleotide catalog data, formed a cluster with Rhodopseudomonas palustris, Pseudomonas carboxidovorans, Nitrobacter species, and Bradyrhizobium species. The 16S rRNA sequence was also determined for the Cleveland Clinic isolate, which was previously shown to be phenotypically similar to and approximately 30% related, by DNA hybridization, to the CSD bacillus. The Cleveland Clinic isolate was isolated from a patient not diagnosed with CSD. The rRNAs from these bacteria exhibited 98.2% homology, confirming that this isolate is a second species in the same genus as the CSD bacillus. Our data suggest that neither B. bacilliformis nor the CSD bacillus is the etiologic agent of bacillary epithelioid angiomatosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilofsky H. S., Burks C. The GenBank genetic sequence data bank. Nucleic Acids Res. 1988 Mar 11;16(5):1861–1863. doi: 10.1093/nar/16.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., O'Connor S. P., Hollis D. G., Weaver R. E., Steigerwalt A. G. Molecular characterization and proposal of a neotype strain for Bartonella bacilliformis. J Clin Microbiol. 1991 Jul;29(7):1299–1302. doi: 10.1128/jcm.29.7.1299-1302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerell C. J., Friedman-Kien A. E. Epithelioid angiomatosis and cat scratch disease bacillus. Lancet. 1988 Jun 11;1(8598):1334–1335. doi: 10.1016/s0140-6736(88)92146-0. [DOI] [PubMed] [Google Scholar]
- Cockerell C. J., Whitlow M. A., Webster G. F., Friedman-Kien A. E. Epithelioid angiomatosis: a distinct vascular disorder in patients with the acquired immunodeficiency syndrome or AIDS-related complex. Lancet. 1987 Sep 19;2(8560):654–656. doi: 10.1016/s0140-6736(87)92442-1. [DOI] [PubMed] [Google Scholar]
- DeBorde D. C., Naeve C. W., Herlocher M. L., Maassab H. F. Resolution of a common RNA sequencing ambiguity by terminal deoxynucleotidyl transferase. Anal Biochem. 1986 Sep;157(2):275–282. doi: 10.1016/0003-2697(86)90626-3. [DOI] [PubMed] [Google Scholar]
- Dorsch M., Moreno E., Stackebrandt E. Nucleotide sequence of the 16S rRNA from Brucella abortus. Nucleic Acids Res. 1989 Feb 25;17(4):1765–1765. doi: 10.1093/nar/17.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons R. W., Riggs J. L., Schachter J. Continuing search for the etiology of cat scratch disease. J Clin Microbiol. 1976 Jul;4(1):112–114. doi: 10.1128/jcm.4.1.112-114.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English C. K., Wear D. J., Margileth A. M., Lissner C. R., Walsh G. P. Cat-scratch disease. Isolation and culture of the bacterial agent. JAMA. 1988 Mar 4;259(9):1347–1352. doi: 10.1001/jama.259.9.1347. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gerber M. A., Sedgwick A. K., MacAlister T. J., Gustafson K. B., Ballow M., Tilton R. C. The aetiological agent of cat scratch disease. Lancet. 1985 Jun 1;1(8440):1236–1239. doi: 10.1016/s0140-6736(85)92311-6. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Kim C. S., Heberling R. L. Herpes-like virus particles associated with cat scratch disease. Nature. 1969 Oct 11;224(5215):190–190. doi: 10.1038/224190a0. [DOI] [PubMed] [Google Scholar]
- Kitchell C. C., DeGirolami P. C., Balogh K. Bacillary organisms in cat scratch disease. N Engl J Med. 1985 Oct 24;313(17):1090–1091. doi: 10.1056/NEJM198510243131715. [DOI] [PubMed] [Google Scholar]
- Koehler J. E., LeBoit P. E., Egbert B. M., Berger T. G. Cutaneous vascular lesions and disseminated cat-scratch disease in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Ann Intern Med. 1988 Sep 15;109(6):449–455. doi: 10.7326/0003-4819-109-6-449. [DOI] [PubMed] [Google Scholar]
- Kreier J. P., Ristic M. The biology of hemotrophic bacteria. Annu Rev Microbiol. 1981;35:325–338. doi: 10.1146/annurev.mi.35.100181.001545. [DOI] [PubMed] [Google Scholar]
- LeBoit P. E., Berger T. G., Egbert B. M., Yen T. S., Stoler M. H., Bonfiglio T. A., Strauchen J. A., English C. K., Wear D. J. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat scratch disease bacillus infection? Lancet. 1988 Apr 30;1(8592):960–963. doi: 10.1016/s0140-6736(88)91779-5. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Lester S., Parsonnet J. Unusual presentation of cat scratch disease in a patient positive for antibody to the human immunodeficiency virus. Rev Infect Dis. 1989 Sep-Oct;11(5):793–803. doi: 10.1093/clinids/11.5.793. [DOI] [PubMed] [Google Scholar]
- Margileth A. M., Wear D. J., English C. K. Systemic cat scratch disease: report of 23 patients with prolonged or recurrent severe bacterial infection. J Infect Dis. 1987 Mar;155(3):390–402. doi: 10.1093/infdis/155.3.390. [DOI] [PubMed] [Google Scholar]
- Margileth A. W., Wear D. J., Hadfield T. L., Schlagel C. J., Spigel G. T., Muhlbauer J. E. Cat-scratch disease. Bacteria in skin at the primary inoculation site. JAMA. 1984 Aug 17;252(7):928–931. doi: 10.1001/jama.252.7.928. [DOI] [PubMed] [Google Scholar]
- Milam M. W., Balerdi M. J., Toney J. F., Foulis P. R., Milam C. P., Behnke R. H. Epithelioid angiomatosis secondary to disseminated cat scratch disease involving the bone marrow and skin in a patient with acquired immune deficiency syndrome: a case report. Am J Med. 1990 Feb;88(2):180–183. doi: 10.1016/0002-9343(90)90471-o. [DOI] [PubMed] [Google Scholar]
- Miller-Catchpole R., Variakojis D., Vardiman J. W., Loew J. M., Carter J. Cat scratch disease. Identification of bacteria in seven cases of lymphadenitis. Am J Surg Pathol. 1986 Apr;10(4):276–281. [PubMed] [Google Scholar]
- Moreno E., Stackebrandt E., Dorsch M., Wolters J., Busch M., Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990 Jul;172(7):3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Holzer G., Wallace P. L., Hollis D. G. Cellular fatty acid compositions of an unidentified organism and a bacterium associated with cat scratch disease. J Clin Microbiol. 1990 May;28(5):1071–1074. doi: 10.1128/jcm.28.5.1071-1074.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS D., WIGAND R. Bartonellaceae. Bacteriol Rev. 1955 Sep;19(3):150–159. doi: 10.1128/br.19.3.150-159.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon V. A., Echols R. M. Cat-scratch disease in a patient with AIDS. Am J Clin Pathol. 1989 Aug;92(2):236–240. doi: 10.1093/ajcp/92.2.236. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Loutit J. S., Schmidt T. M., Falkow S., Tompkins L. S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990 Dec 6;323(23):1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- Rossau R., Heyndrickx L., Van Heuverswyn H. Nucleotide sequence of a 16S ribosomal RNA gene from Neisseria gonorrhoeae. Nucleic Acids Res. 1988 Jul 11;16(13):6227–6227. doi: 10.1093/nar/16.13.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossberg D., Morad Y., Krouse T. B., Wear D. J., English C. K., Littman M. Culture-proved disseminated cat-scratch disease in acquired immunodeficiency syndrome. Arch Intern Med. 1989 Jun;149(6):1437–1439. [PubMed] [Google Scholar]
- Stackebrandt E., Charfreitag O. Partial 16S rRNA primary structure of five Actinomyces species: phylogenetic implications and development of an Actinomyces israelii-specific oligonucleotide probe. J Gen Microbiol. 1990 Jan;136(1):37–43. doi: 10.1099/00221287-136-1-37. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Bonfiglio T. A., Steigbigel R. T., Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983 Nov;80(5):714–718. doi: 10.1093/ajcp/80.5.714. [DOI] [PubMed] [Google Scholar]
- Szaniawski W. K., Don P. C., Bitterman S. R., Schachner J. R. Epithelioid angiomatosis in patients with AIDS. Report of seven cases and review of the literature. J Am Acad Dermatol. 1990 Jul;23(1):41–48. doi: 10.1016/0190-9622(90)70183-i. [DOI] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Waldo E., Sidhu G. S., Stahl R., Zolla-Pazner S. Histiocytoid hemangioma with features of angiolymphoid hyperplasia and Kaposi's sarcoma. A study by light microscopy, electron microscopy, and immunologic techniques. Am J Dermatopathol. 1983 Dec;5(6):525–538. doi: 10.1097/00000372-198312000-00003. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Margileth A. M., Hadfield T. L., Fischer G. W., Schlagel C. J., King F. M. Cat scratch disease: a bacterial infection. Science. 1983 Sep 30;221(4618):1403–1405. doi: 10.1126/science.6612349. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Woese C. R., Dobson M. E., Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985 Nov 1;230(4725):556–558. doi: 10.1126/science.3931222. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]



