Abstract
Background/Aim
Previous studies on “Black seed” or “Black Cumin” Nigella sativa (NS) have reported a large number of pharmacological activities including its anti-ulcer potential. These studies employed either fixed oil, volatile oil components or different solvent extracts. In folkloric practices, NS seeds are taken as such, in the form of coarse dry powder or the powdered seeds are mixed with water. This study examines the effect of NS aqueous suspension on experimentally induced gastric ulcers and basal gastric secretion in rats to rationalize its use by herbal and Unani medicine practitioners.
Materials and Methods
The study was conducted at the Medicinal, Aromatic and Poisonous Plants Research Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Acute gastric ulceration was produced by various noxious chemicals (80% ethanol, 0.2 M NaOH, 25% NaCl and indomethacin) in Wistar albino rats. Anti-secretory studies were undertaken in a separate group of rats. Gastric wall mucus contents and non-protein sulfhydryl concentration were estimated, and gastric tissue was examined histopathologically.
Results
An aqueous suspension of Black seed significantly prevented gastric ulcer formation induced by necrotizing agents. It also significantly ameliorated the ulcer severity and basal gastric acid secretion in pylorus-ligated Shay rats. Moreover, the suspension significantly replenished the ethanol-induced depleted gastric wall mucus content levels and gastric mucosal non-protein sulfhydryl concentration. The anti-ulcer effect was further confirmed histopathologically.
Conclusion
These findings validate the use of Black seed in gastropathies induced by necrotizing agents. The anti-ulcer effect of NS is possibly prostaglandin-mediated and/or through its antioxidant and anti-secretory activities.
Keywords: Habbatul-Barakah, Nigella sativa, stomach, ulcer, gastric acid
Gastric ulcers, one of the most widespread diseases, is believed to be due to an imbalance between aggressive and protective factors.[1] The gastric mucosa is continuously exposed to potentially injurious agents such as acid, pepsin, bile acids, food ingredients, bacterial products (Helicobacter pylori) and drugs.[2] These agents have been implicated in the pathogenesis of gastric ulcer, including enhanced gastric acid and pepsin secretion, inhibition of protaglandin synthesis and cell proliferation growth, diminished gastric blood flow and gastric motility.[3] Drug treatment of peptic ulcers is targeted at either counteracting aggressive factors (acid, pepsin, active oxidants, platelet aggravating factor “PAF,” leukotrienes, endothelins, bile or exogenous factors including NSAIDs) or stimulating the mucosal defenses (mucus, bicarbonate, normal blood flow, prostaglandins (PG), nitric oxide).[4] The goals of treating peptic ulcer disease are to relieve pain, heal the ulcer and prevent ulcer recurrence. Currently, there is no cost-effective treatment that meets all these goals. Hence, efforts are on to find a suitable treatment from natural product sources. A large percentage of world population relies on natural remedies to treat a variety of diseases. Due to socio-economic factors, faith and ancestral experience, medicinal herbs and spices are considered as an indispensable part of traditional medicine.[5] Besides this, a large number of spices and herbs have been evaluated by various researchers for their anti-ulcer effects to achieve a favorable outcome.[6–12] The seeds of Nigella sativa L. (Rananculacene) (NS) known as Black seed or Black cumin (“Al Habbah Al Sawda” or “Habbatul-Barakah”) have long been used in folk medicine in the Middle East, Far East and in the Indian subcontinent as a traditional medicine. The Ayurvedic, Unani and herbal medicine practitioners extensively used this age old spice for a wide range of illnesses, including bronchial asthma, headache, dysentery, infections, obesity, back pain, hypertension and gastrointestinal problems as well as a diuretic, and to promote menstruation and increase lactation.[13–15] Its use in skin conditions as eczema has also been recognized worldwide.[16] NS contains more than 30% of a fixed oil and 0.40-0.45 w/w of a volatile oil. The volatile oil has been shown to contain 18-24% thymoquinone (TQ) and 46% monoterpenes.[16] Recent clinical and experimental studies have shown several therapeutic effects of NS extracts including its antioxidant hepatoprotective,[17] immunomodulatory,[18] anti-inflammatory[19] and anti-tumor[20] activities. However, recently the active principle of NS oil TQ has shown to possess a gastroprotective activity in rats.[16] In other studies, TQ has demonstrated an antimicrobial activity[21] and was beneficial for treating doxorubicin-induced nephropathy in rats.[22] Although much work has been done on crude extract of NS and its constituents, to our knowledge, there is no report available on its aqueous suspension dosage form. NS seeds are customarily consumed either in their intact or powdered forms. Therefore, the present study was undertaken to determine the effect of an aqueous suspension of NS seeds against various necrotizing agents and indomethacin-induced gastric ulcer in rats. Anti-secretory assessment was performed on pylorus-ligated Shay rats. Gastric wall mucus and non-protein sulfhydryl contents were estimated and gastric tissue was histopathologically examined.
MATERIALS AND METHODS
The present study was carried out at the Medicinal, Aromatic and Poisonous Plants Research Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Plant material and preparation of aqueous suspension
NS seeds were purchased from a local herb dealer in Riyadh, were identified under expert guidance and preserved for future reference. The seeds were ground to a very fine powder and used as an aqueous suspension for treatment in different experiments. Wistar albino rats of either sex, approximately of same age, weighing 150-200 g were obtained from the Animal Care Center, College of Pharmacy, King Saud University. They were maintained under standard conditions of temperature, humidity and light (12 h dark, 12 h light) and provided with Purina chow and free access to water. Before testing, the animals were fasted for 36 h with access to water ad libitum. All experimental procedures were approved by the Ethics Committee of the Experimental Animal Care Society, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Gastric lesions induced by necrotizing agents
Each rat in the test group was given 1 ml of different necrotizing agents (80% ethanol, 0.2 M NaOH and 25% NaCl) that are known to produce gastric lesions.[23] NS suspension was given orally 30 min before administering the necrotizing agents. Animals were killed under ether anesthesia 1 h after treatment with the ulcerogenic agents. The stomach was excised and opened along the greater curvature. After washing with normal saline, the gastric lesions were quantified using a binocular magnifier and ulcers were scored.[24] Lesions were also assessed by two observers blinded to experimental protocols.
Histopathological evaluation
The gastric tissue samples were fixed in neutral buffered formalin for 24 h. Sections of tissue from stomachs were examined histopathologically to study the ulcerogenic and/or anti-ulcerogenic activity of NS. The tissues were fixed in 10% buffered formalin and were processed using a VIP tissue processor. The processed tissues were embedded in paraffin blocks and about 5-µm thick sections were cut using an American optical rotary microtome. These sections were stained with hemotoxylin and eosin using routine procedures.[25] The slides were examined microscopically for pathomorphological changes such as congestion, hemorrhage, edema and erosions using an arbitrary scale for the assessment of severity of these changes.[26]
Determination of anti-secretory activity
To determine anti-secretory activity, the 36-h fasted rats were anesthetized under light ether and the pylorus was ligated. Care was taken to avoid bleeding or occlusion of the blood vessels. Aqueous suspension of NS was administered intraperitoneally, immediately after pyloric ligation.[27] The animals were sacrificed 6 h after the pyloric ligation. The stomachs were removed, contents collected, measured, centrifuged and subjected to analysis for titratable acidity against 0.01 N NaOH at pH 7 and the total acid output was calculated.
Gastric wall mucus determination
The glandular segments of the stomach were removed and weighed, to determine gastric wall mucus in rats after receiving 80% ethanol only or ethanol plus NS suspension.[28] Each segment was transferred immediately to 1% Alcian blue solution (in sucrose solution, buffered with sodium acetate at pH 5), and the excess dye was removed by rinsing with sucrose solution. The dye complexed with gastric wall mucus was extracted with magnesium chloride solution. A 4-ml aliquot of blue extract was then shaken with an equal volume of diethyl ether. The resulting emulsion was centrifuged and the absorbance of the aqueous layer was recorded at 580 nm. The quantity of Alcian blue extracted per gram of glandular tissue (net) was then calculated.
Indomethacin-induced gastric mucosal ulceration
Indomethacin was suspended in 1.0% carboxymethyl cellulose in water (6 mg/ml) and administered orally to the fasted rats at 30 mg/kg body weight (0.5 ml/100 g). Control rats were treated similarly with an equivalent amount of the vehicle.[29] The animals were sacrificed 6 h after the treatment. The stomachs were excised, rinsed with normal saline and ulcers were scored.
Estimation of non-protein sulfhydryl (NP-SH) groups
Gastric mucosal NP-SH was measured to analyze the oxidant/antioxidant balance. The glandular stomach was removed and homogenized in ice-cold 0.02 M ethylene-diamine-tetraacetic acid. The homogenate was mixed with distilled water and 50% (w/v) aqueous tri-chloroacetic acid and centrifuged; the supernatants were mixed with phosphate buffer (pH 8), 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) was added and the sample was shaken. The absorbance was read within 5 min of addition of DTNB at 1/12 nm, against a reagent blank with no homogenate.[30]
Statistical analysis
The readings shown are mean ± standard error of means. The mean determination of treatment groups was compared statistically with that of control by using ANOVA followed by Duncan's multiple range test.
RESULTS
Aqueous suspension of NS significantly reduced gastriculcers induced by ethanol (P < 0.0001), NaOH (P < 0.0001) and NaCl (P < 0.006) at 250 and 500 mg/kg body weight compared to the values obtained in the control [Table 1]. Pretreatment with NS suspension (250 and 500 mg/kg body weight) completely protected the gastric mucosa against different histopathological changes (congestion, hemorrhage, edema, necrosis, inflammatory and dysplastic changes, erosions and ulceration) caused in ethanol-treated rats [Table 2 and Figures 1–4]. In addition, treatment with NS aqueous suspension resulted in a significant decrease in the volume of basal gastric secretion and titratable acidity after pyloric ligation for 6 h (P < 0.0001 and P < 0.005, respectively, at 250 and 500 mg/kg body weight), while NS treatment did not influence the ulcer index at high dose (P 0.57) [Table 3]. Treatment with ethanol (80%) caused a significant (P < 0.0001) decrease in the mucus content of the gastric wall in untreated animals [Table 4]. The depleted gastric mucus was significantly replenished (P < 0.0001) after pretreatment with NS suspension at both doses. Table 5 shows a significant dose-dependent reduction (P < 0.002) of stomach ulceration produced by indomethacin after pretreatment with NS suspension at both doses. In addition, ethanol (80%) induced a significant decrease (P < 0.0001) in NP-SH concentrations in the gastric tissue as compared to the control and NS-treated (normal) animals [Table 4]. An increased level of NP-SH concentration was observed after pretreatment with NS aqueous suspension at250 and 500 mg/kg body weight.
Table 1.
Effect of Nigella sativa on the various necrotizing agents in rats
| Ulcer Index (Mean ± S.D.) | Treatment (Dose) | ||||
|---|---|---|---|---|---|
| Control | Nigella sativa (250) | Nigella sativa (500) | F-value | P-value | |
| 80% EtOH | 8.0 ± 0.0* | 4.0 ± 1.8* | 2.17 ± 16* | 27.77 | <0.0001 |
| 0.2 M NaOH | 7.83 ± 0.41* | 6.0 ± 1.67* | 1.83 ± 1.72* | 26.68 | <0.0001 |
| 25% NaCI | 7.0 ± 1.67** | 3.83 ± 1.33 | 3.83 ± 1.94 | 7.22 | 0.006 |
Statistically significantly different from each other (ANOVA followed by Duncan's multiple range test)
Statistically significantly higher than other two groups (ANOVA followed by Duncan's multiple range test)
Table 2.
Effect of N. sativa on ethanol-induced histopathological lesions in gastric mucosa of rats
| Treatment and dose (mg/kg., body weight) | Histopathological Lesions | |||||||
|---|---|---|---|---|---|---|---|---|
| Congestion | Haemorrhage | Edema | Necrosis | Inflammatory changes | Dysplastic changes | Erosions | Ulceration | |
| Control (distilled water) | − | − | − | − | − | − | − | − |
| Ethanol, 80% | +++ | ++ | + | + | + | + | ++ | ++ |
| N. sativa (250) + ethanol, 80% | − | − | − | − | − | − | − | − |
| N. sativa (500) + ethanol, 80% | − | − | − | − | − | − | − | − |
− = Normal, + = Moderate effect, ++ = severe effect, +++ = Intensely severe effect
Figure 1.
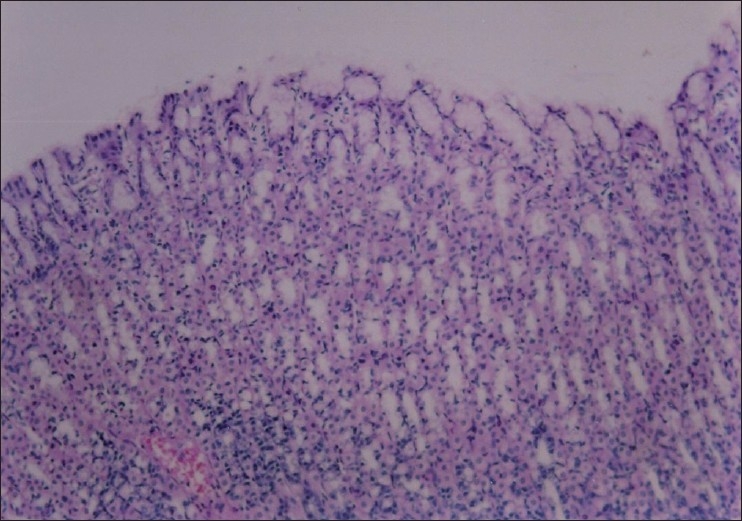
Section through gastric mucosa of control rat showing normal appearance (H&E, ×100)
Figure 4.
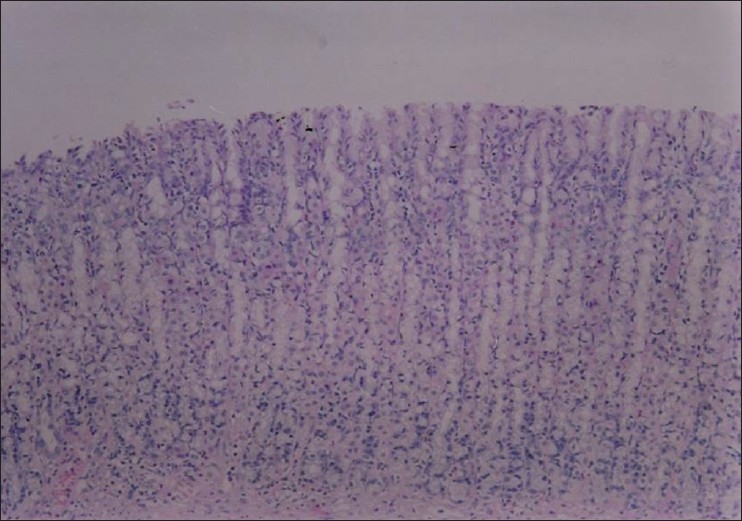
Section through gastric mucosa of rat treated with Nigella sativa (500 mg/kg) and ethanol (80%, 1 ml). No significant histopathological changes are seen in the mucosal layer (H&E, ×100)
Table 3.
Effect of Nigella sativa on gastric secretion, titratable acidity and ulceration in 6-hr pylorus ligated (Shay) rats
| Outcome variables (Mean ± S.D.) | Treatment (Dose) | ||||
|---|---|---|---|---|---|
| Control | Nigella sativa (250) | Nigella sativa (500) | F-value | P-value | |
| Volume of gastric content | 8.0 ± 1.3* | 1.8 ± 0.8 | 2.0 ± 2.9 | 18.41 | <0.0001 |
| Titratable acid | 135.0 ± 4.0* | 58.9 ± 64.9 | 32.2 ± 49.9 | 7.7 | 0.005 |
| Ulcer Index | 0.33 ± 0.5 | 0 | 0.33 ± 0.8 | 0.59 | 0.57 |
Statistically significantly higher than the two treatment groups (ANOVA followed by Duncan's multiple range test)
Table 4.
Effect of Nigella sativa on gastric wall mucus and non-protein sulfhydryl concentrations in rats
| Outcome variables (Mean ± S.D.) | Treatment (Dose) | |||||
|---|---|---|---|---|---|---|
| Control | 80% Ethanol only | Nigella sativa (250) | Nigella sativa (500) | F-value | P-value | |
| GWM concentration (μg/g) | 450.8 ± 47.5* | 291.9 ± 8.2 | 311.6 ± 16.0 | 321.9 ± 24.2 | 35.71 | <0.0001 |
| Alician blue g net tissue | ||||||
| NP-SH (μmol/100mg concentration | 8.5 ± 0.80* | 5.89 ± 0.39** | 7.47 ± 0.44 | 7.97 ± 0.88 | 53.22 | <0.0001 |
Statistically siginficantly higher than the 3 treatment groups (ANOVA followed by Duncan's multiple range test)
Statistically siginficantly lower than the other 2 treatment groups and control group (ANOVA followed by Duncan's multiple range test), GWM = Gastric wall mucus, NP-SH = Non-protein sulfhydryl
Table 5.
Effect of Nigella sativa on the gastric damage induced by indomethacin in rats
| Treatment | Dose (mg/kg, p.o.) | Ulcer index (Mean ± S.D.) | F-value | P-value | |
|---|---|---|---|---|---|
| Control | - | 30.50 ± 5.9* | |||
| Nigella sativa | 250 | 14.00 ± 12.7 | 9.56 | 0.002 | |
| Nigella sativa | 500 | 4.60 ± 14.4 |
Statistically significantly higher than the 2 treatment groups (ANOVA followed by Duncan's multiple range test)
Figure 2.
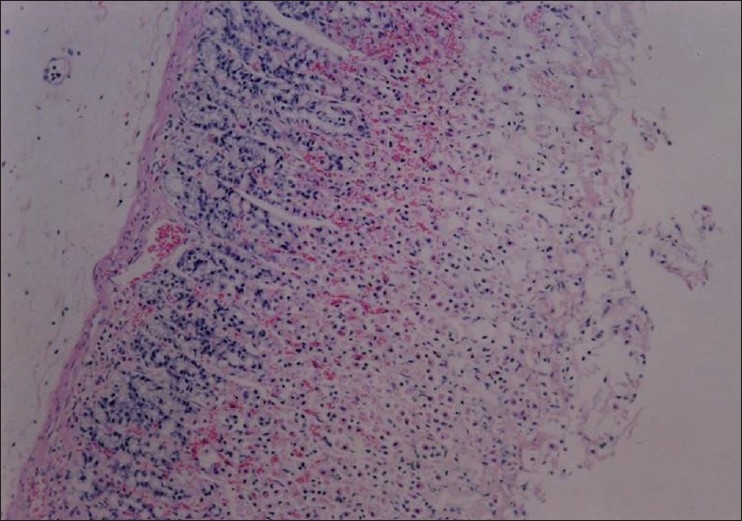
Section through gastric mucosa of rat treated with ethanol (80%, 1 ml) showing mucosal ulceration, inflammation and haemorrhage (H&E, ×100)
Figure 3.
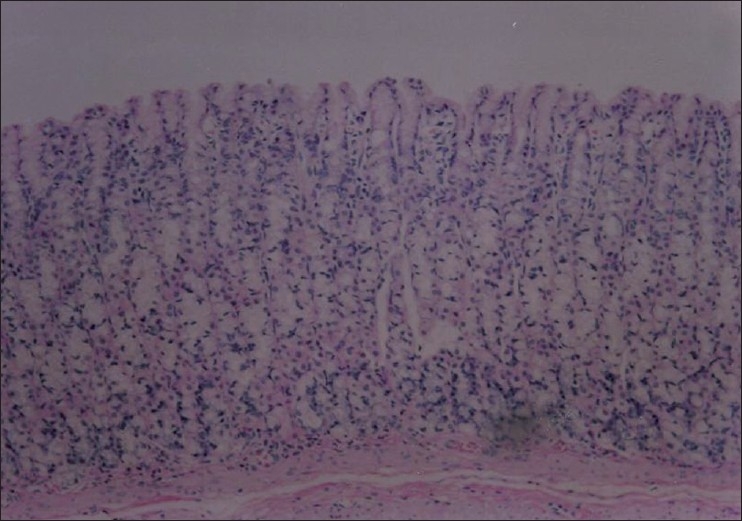
Section through gastric mucosa of rat treated with Nigella sativa (250mg/kg) and ethanol (80%,1 ml). There are no significant histopathological changes (H&E,×100)
DISCUSSION
The results of this study showed that oral administration of an aqueous suspension of NS prevents gastric mucosal injuries caused by ethanol and strong alkalies, the most commonly employed tests in the evaluation of anti-ulcer and cytoprotective activities.[31–34] It is suggested that oxygen radicals may contribute to the formation of ethanol-induced gastric mucosal lesions and antioxidants are protective against the damage caused by these oxidants.[35] The cytoprotective effect of black cumin observed in the present study, which could be attributed to the endogenous generation of PG, was responsible for maintaining the cellular integrity of the gastric epithelium; therefore, such endogenous release of PG would play a physiological role in protecting the gastric mucosa.[23] On the other hand, it has been reported that plants and/or spices sometimes exhibit their cytoprotective action through their mild irritant property.[6,9,10] Furthermore, Robert et al,[23] have also described the ability of a mild irritant in protecting gastric mucosa against strong irritants. This protection is called “adaptive cytoprotection.” These results were further supported by histopathological findings. Pretreatment with NS suspension prevented histopathological changes in the form of congestion, hemorrhage, edema, necrosis, inflammatory and dysplastic changes, erosions and ulceration caused by the destructive stimuli in the gastric tissue. This confirms the cytoprotective ability of the NS suspension, which may be attributed to chemical components such as TQ, a major and active constituent of NS oil.[36] In some earlier studies, TQ has been reported to exhibit a significant protective action on gastric mucosal lesions induced by different necrotic agents.[37,38] A significant reduction in basal gastric secretion and inhibition of gastric ulcers by NS suspension may involve direct reduction of gastric secretion through one or more of possible mechanisms.[39–41] Moreover, gastric acid is an important factor for the genesis of pyloric ligation ulcer in rats.[27] However, gastric hypersecretion is unlikely to be the sole major mechanism that contributes to the development of gastric mucosal lesions.[42,43]
Our findings are in agreement with previous data reporting that an aqueous extract of Black cumin did not only inhibit gastric secretion but also reduced the ulcer index in rats.[44,45] The current data clearly demonstrated that, at the very least, NS suspension inhibited gastric acid secretion. The gastroprotective effect observed in the present study might be due to a possible relationship between mucosal injury, inhibition of acid secretion and the antioxidant nature of NS.[46]
It has been reported that certain anti-ulcer drugs increase the amount of gastric mucus secretion in gastric mucosa.[47,48] This mucus consists of mucin-type glycoproteins, which can be detected by amounts of Alcian blue.[49] The gastric wall mucus depletion induced by 80% ethanol is also one of the pathogenic mechanisms responsible for gastric lesions.[50] Alcian blue dye binds with negatively charged materials. The increase in bound Alcian blue indicates the protective effect of orally administered NS suspension, which may occur via the formation of protecting complexes between the aqueous suspension and mucus that act as a barrier against several necrotizing agents introduced in the stomach.[51] In the present study, NS suspension replenished the ethanol-induced decreased concentration of gastric wall mucus. Thus, the possible mechanism of gastric mucosal protection by NS suspension is due to the reinforcement of resistance of the mucosal barrier by a protective coating. Administration of indomethacin caused gastric side effects ranging from endothelial microvascular damage to development of macroscopic gastric mucosal lesions, which is attributed mainly to the inhibition of biosynthesis of cytoprotective PG, resulting in overproduction of leukotrienes and other products of the 5-lipoxygenase pathway.[52] These agents break the mucosal barrier, provoke an increase in gastric mucosal permeability to H+ and Na+ ions reducing the transmucosal potential difference and induce formation of erosions and ulcers.[53–54] In this assay, NS suspension was able to produce a significant reduction of the gastric mucosal damage induced by indomethacin, indicating a probable local increase in PG synthesis. Additionally, NP-SH is thought to be involved in protecting gastric mucosa against various chemical agents.[55] Our observations showed a significant reduction in the NP-SH content of gastric mucosa after 80% ethanol administration. However, pretreatment with NS aqueous suspension prevented this depletion. An elevated NP-SH level is reported to protect gastric damage against various noxious chemicals.[56] These findings clearly showed that the possible involvement of NP-SH in the ulcer protective potential is through the antioxidant activities of NS suspension. Thus, NS treatment appears to strengthen the mucosal barrier, which is the first line of defense against endogenous and exogenous ulcerogenic agents.
The results of the present study establish the anti-gastric ulcer and anti-secretory properties of NS aqueous suspension, substantiates its use against gastric disorders in Unani and Arab traditional medicine. The effects of NS suspension is possibly PG-mediated and/or through its free radical scavenging and anti-secretory properties.
Earlier reports have indicated that 88 mg/kg/day administration of NS oil for two weeks significantly increased glutathione levels in gastric mucosa but failed to suppress acidity.[37] However, in the current study a single dose of aqueous suspension induced a significant elevation in NP-SH levels and suppressed basal gastric secretion and titratable acidity. It appears therefore, that the aqueous suspension of NS is more effective than oil suspensions. Similarly, in another study, administration of high doses of NS oil and its major compound TQ at 50 and 100 mg/kg body weight demonstrated a gastroprotective effect, which was attributed to its free radical scavenging effect.[57] To our knowledge, the present investigation is the first reporting that NS aqueous suspension possesses cytoprotective, anti-ulcer and anti-secretory capacity. In comparison with different dosage forms, the dynamics of the whole seed aqueous suspension with all chemical constitutes and their synergistic effect was both advantageous and convenient to consume.
Acknowledgments
We are grateful to King Abdul-Aziz City for Science and Technology (KACST), Riyadh, Saudi Arabia, for providing a research grant to conduct experimental studies on spices (Project No. AR-16-37).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.AlKofahi A, Atta AH. Pharmacological screening of the antiulcerogenic effects of some Jordanian Medicinal Plants in rats. J Ethnopharmacol. 1999;65:341–5. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 2.Peskar BM, Maricic N. Role of prostaglandins in gastroprotection. Dig Dis Sci. 1998;43:S23–9. [PubMed] [Google Scholar]
- 3.Toma W, Hiruma-Lima CA, Guerrero RO, Souza Brito AR. Preliminary studies of Mammea Americana L (Guttiferae) bark/latex extract point to an effective antiulcer effect on gastric ulcer models in mice. Phytomedicine. 2005;12:345–50. doi: 10.1016/j.phymed.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Borelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Satyanarayan ND, Purohit MG. Antiulcer activity of Acalypha indica (Euphorbiaceae on ethanol induced gastric ulcers. J Ethnopharmacol. 2002;84:1–8. [Google Scholar]
- 6.Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Al-Said MS, Tariq M. Gastric antisecretory, antiulcer and cytoprotective properties of ethanol extract of Alpinia galanga willd in rats. Phytother Res. 1990;4:112–4. [Google Scholar]
- 7.Al-Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Qureshi S, Rafatullah S. Pharmacological studies on “clove” Eugenia caryophyllata. Pharmacog Mag. 2005;3:1055–9. [Google Scholar]
- 8.Al-Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Rafatullah S, Qureshi S. Inhibition of gastric mucosal damage by Piper nigrum (Black pepper) pretreatment in Wistar albino rats. Pharmacog Mag. 2005;2:64–8. doi: 10.1016/j.etap.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Rafatullah S, Tariq M, Al-Yahya MA, Mossa JS, Ageel AM. Evaluation of turmeric (Curcuma longa) for gastric and duodenal anti-ulcer activity in rats. J Ehthnopharmacol. 1990;29:25–34. doi: 10.1016/0378-8741(90)90094-a. [DOI] [PubMed] [Google Scholar]
- 10.Rafatullah S, Galal AM, Al-Yahya MA, Al-Said MS. Gastric antiulcer and cytoprotective effects of Aframomum melegueta in rats. Int J Pharmaco. 1995;33:311–6. [Google Scholar]
- 11.Alhaider AA, Al-Mofleh IA, Mossa JS, Al-Sohaibani MO, Qureshi S, Rafatullah S. Pharmacological and safety evaluation studies on “Cardamom” Elettaria Cardamomum: An important ingredient of Gahwa (Arabian coffee) Arab J Pharmaceut Sci. 2005;3:47–58. [Google Scholar]
- 12.Al-Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Rafatullah S, Qureshi S. Protection of gastric mucosal damage by Coriandrum sativum L. Pretreatment in Wistar albino rats. Environ Toxicol Pharmacol. 2006;22:64–9. doi: 10.1016/j.etap.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Schleicher P, Saleh M. Black seed cumin: The magical Egyptian herb for allergies, asthma, and immune disorders. Rochester, Vermont: Healing Arts Press; 1998. p. 90. [Google Scholar]
- 14.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Al-Rowais NA. Herbel medicine in the treatment of diabetes mellitus. Saudi Med J. 2002;23:1327–31. [PubMed] [Google Scholar]
- 16.Arslan SO, Gelir E, Armutcu F, Coskun O, Gurel A, Sayan H, et al. The protective effect of thymoquinone on ethanol-induced acute gastric damage in the rats. Nutrit Res. 2005;25:673–80. [Google Scholar]
- 17.Kanter M, Coskun O, Budankamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11:6684–8. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalouirus infection. Int J Immunopharmacol. 2000;22:729–40. doi: 10.1016/s0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 19.Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother Res. 2004;18:195–9. doi: 10.1002/ptr.1390. [DOI] [PubMed] [Google Scholar]
- 20.Salem ML. Immunomodulatory and antitumor effects of simultaneous treatment of mice with n-3 and n-6 polyunsaturated fatty acids. J Union Arab Biol. 2000;14:489–505. [Google Scholar]
- 21.Badry OA, Abdel-Naim AB, Abdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic hephropathy in rats. Toxicology. 2000;143:219–26. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 22.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herb and spices. Current Med Chem. 2004;11:1451–60. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- 23.Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through adaptive cytoprotection mediated by prostaglandins. Am J Physiol. 1983;245:G113–G21. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 24.Valcavi U, Caponi R, Brambilla A, Palmira M, Minoja F, Bernini F, et al. Gastric antisecretory, anti-ulcer and cytoprotective properties of 9-hydroxy-19, 20-bis-nor-prostanoic acid in experimental animals. Arzneim Forsch/Drug Res. 1982;32:657–63. [PubMed] [Google Scholar]
- 25.Culling CF. Handbook of histopathological and histochemical techniques. 3rd ed. London: Butterworth and Co; 1974. p. 37. [Google Scholar]
- 26.Al-Howiriny T, Al-Sohaibani M, Al-Said M, Al-Yahya M, El-Tahir K, Rafatullah S. Effect of Commiphora opobalsamum (L.) Engl (Balessan) on experimental gastric ulcers and secretion in rats. J Ethnopharmacol. 2005;98:287–94. doi: 10.1016/j.jep.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 27.Shay H, Komarov SA, Fels SS, Meranza D, Grunstein M, Siplet H. A simple method for assessing the extent of experimental erosions and ulcers. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 28.Corne SJ, Morrisey SM, Wood RJ. A method for quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116–7P. [PubMed] [Google Scholar]
- 29.Bhargava KP, Gupta MB, Tangri KK. Mechanism of ulcerogenic acitivity of indomethacin and oxyphenbutazone. Eur J Pharmacol. 1973;22:191–5. doi: 10.1016/0014-2999(73)90012-5. [DOI] [PubMed] [Google Scholar]
- 30.Sedlak J, Lindsay RH. Estimation of total protein-bound and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 31.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Wallace JL. Mechanisms of protection and healing: Current knowledge and future research. Am J Med. 2001;110:S19–22. doi: 10.1016/s0002-9343(00)00631-8. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita M, Tsunehisa N, Tamaki H. Effect of a comnbination of ecabet sodium and cimitidine on experimentally induced gastric-lesions and gastric-mucosal resistance to ulcerogenic agents in rats. Biol Pharm Bull. 1995;18:223–6. doi: 10.1248/bpb.18.223. [DOI] [PubMed] [Google Scholar]
- 34.Konturek PC, Brzowski T, Sliwowski Z, Pajido R, Stachura J, Hahn EG, et al. Involvement of nitric oxide and prostaglandins in gastroprotection induced by bacterial lipopolysaccharide. Scand J Gastroenterol. 1998;33:691–700. doi: 10.1080/00365529850171611. [DOI] [PubMed] [Google Scholar]
- 35.Toma W, Hiruma-Lima CA, Guerrero RO, Souza Brito AR. Preliminary studies of Mammea Americana L (Gutti ferae) bark/latex extract point to an effective antiulcer effect on gastriculcer models in mice. Phytomedicine. 2005;12:345–50. doi: 10.1016/j.phymed.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Fattah AM, Matsumoto K, Watanabe H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol. 2000;400:89–97. doi: 10.1016/s0014-2999(00)00340-x. [DOI] [PubMed] [Google Scholar]
- 37.El-Dakhakhny M, Barakat M, El-Halim A, Aly SM. Effect of Nigella sativa oil on gastric secretion and ethanol induced ulcer in rats. J Ethnopharmacol. 2000;72:299–304. doi: 10.1016/s0378-8741(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 38.El-Kadi A, Kandil O, Tabuni AM. Nigella cell mediated immunity. Arch AIDS Res. 1987;1:232–3. [Google Scholar]
- 39.Parmar NS, Parmar S. Antiulcer potential of flavonoids. Indian J Physiol Pharmacol. 1998;42:343–51. [PubMed] [Google Scholar]
- 40.Rafatullah S, Al-Yahya MA, Al-Said MS, Abdul Hameed Taragan KU, Mossa JS. Gastric anti-ulcer and cytoprotective effects of Cyamopsis tetragonoloba (Guar) in rats. Int J Pharmacog. 1994;32:163–70. [Google Scholar]
- 41.Njar VC, Adesanwo JK, Raji Y. Methyl angolensate: The antiulcer agent of the stem bark of Etandrophagma angolensee. Planta Med. 1995;61:91–2. doi: 10.1055/s-2006-958015. [DOI] [PubMed] [Google Scholar]
- 42.Murakami M, Lam SK, Inada M, Miyake T. Pathophysiology and pathogenesis of acute gastric lesions after hypothermic restraint stress in rats. Gastroenterology. 1985;88:660–5. doi: 10.1016/0016-5085(85)90133-7. [DOI] [PubMed] [Google Scholar]
- 43.Howden CW. Approprite acid suppression in the treatment of acid related conditions. Pharmacol Ther. 1994;63:123–34. doi: 10.1016/0163-7258(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 44.Akhtar MS, Riffat S. Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children. J Pak Med Assoc. 1991;41:185–7. [PubMed] [Google Scholar]
- 45.Chakravarty N. Inhibition of histamine release from mast cells by nigellone. Ann Allergy. 1993;70:237–42. [PubMed] [Google Scholar]
- 46.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 47.Bolton JP, Palmer D, Cohen MM. Effect of the E2 prostaglandins on gastric mucus production in rats. Surg For. 1976;27:402–3. [PubMed] [Google Scholar]
- 48.Robert A, Bottcher W, Golanska E, Kuffman GL. Lack of correlation between mucus gel thickness and gastric cytoprotection in rats. Gastroenterology. 1984;89:670–4. [PubMed] [Google Scholar]
- 49.Bolton JP, Palmer D, Cohen MM. Stimulation of mucus and non parietal cell secretion by the E2 prostaglandins. Dig Dis Sci. 1978;23:359–64. doi: 10.1007/BF01072421. [DOI] [PubMed] [Google Scholar]
- 50.Koo MWL, Ogle CW, Cho CH. Effect of verapamil, carbenoxolone and N-acetyl-cystein on gastric wall mucus ulceration in stressed rats. Pharmacology. 1986;32:326–34. doi: 10.1159/000138188. [DOI] [PubMed] [Google Scholar]
- 51.Clamp JR, Allen A, Gibbons RA, Roberts GP. Chemical aspects of mucus. Br Med Bull. 1978;34:25–41. doi: 10.1093/oxfordjournals.bmb.a071455. [DOI] [PubMed] [Google Scholar]
- 52.Nasuti C, Gabbianelli R, Falcioni G, Cantalamessa F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nutr Res. 2006;26:130–7. [Google Scholar]
- 53.Rainsford KD. Gastric ulcerogenecity of non-steroidal anti-inflammatory drugs in mice with mucosa sensitized by cholinomimetic treatment. J Pharm Pharmacol. 1978;39:669–72. [PubMed] [Google Scholar]
- 54.Droy-Lefaix MT. In: “Prostaglandins”, biology and chemistry of prostaglandins and related eicosanoids. Curtis-Prior PB, editor. New York: Churchill Livingstone; 1988. pp. 345–60. [Google Scholar]
- 55.Miller TA, Kuo YJ, Schmidt KL, Shambour LL. Non protein sulfhydryl compounds in canine gastric mucosa: Effect of PGE2 and ethanol. Am J Physiol. 1985;249:G137–42. doi: 10.1152/ajpgi.1985.249.1.G137. [DOI] [PubMed] [Google Scholar]
- 56.Szabo S, Trier JS, Frank PW. Sulfhydryl compounds may mediate gastric protection. Science. 1981;214:200–2. doi: 10.1126/science.7280691. [DOI] [PubMed] [Google Scholar]
- 57.El-Abhar HS, Abdallah DM, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituents, thymoquinone, against gastric mucosal injury induced by ischemia/reperfusion in rats. J Ethnopharmacol. 2003;84:251–8. doi: 10.1016/s0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]


