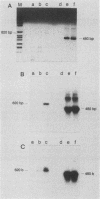
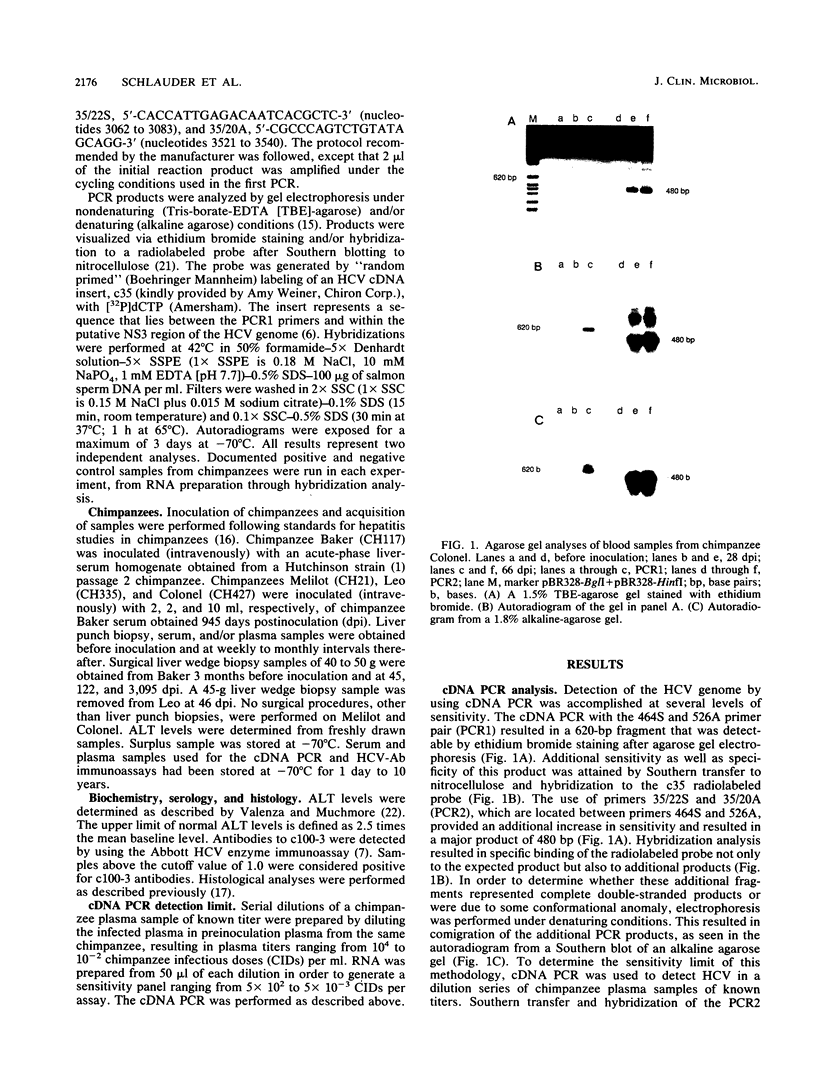
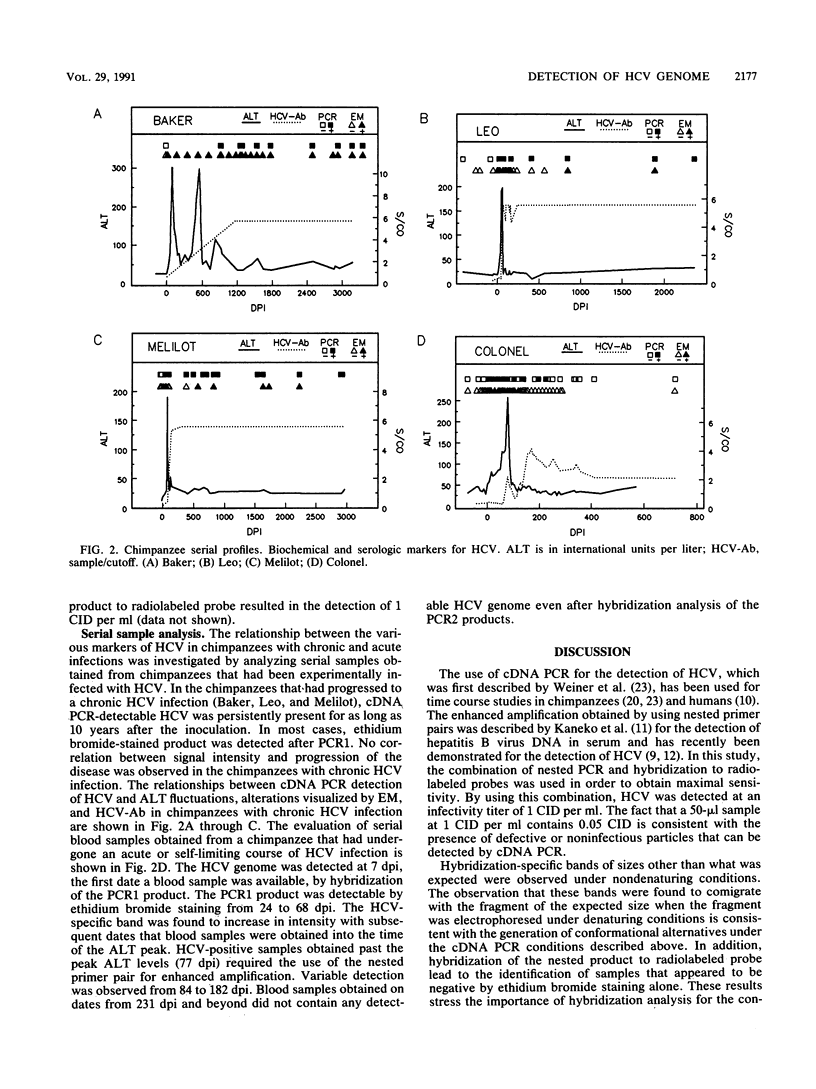
Abstract
In order to gain an understanding of the relationship of various markers of hepatitis C virus (HCV) infection in acute and chronic cases of the disease, serial blood samples obtained from chimpanzees before and after infection with HCV were analyzed for the presence of the HCV genome by using polymerase chain reaction (PCR) amplification of cDNA (cDNA PCR) synthesized from plasma- and serum-derived RNA. In a chimpanzee with acute hepatitis C, signals detectable by cDNA PCR appeared 1 week before characteristic ultrastructural changes visualized by electron microscopy, persisted throughout the peak alanine aminotransferase levels, and diminished with the disappearance of alterations visualized by electron microscopy. This was in contrast to the results obtained from chimpanzees with chronic HCV infection, in which the HCV genome was consistently detectable for up to 10 years after infection. The results indicate the usefulness of detection of HCV RNA by cDNA PCR as a sensitive and semiquantitative method for monitoring the course of HCV infection and as a potential marker for differentiating between chronic and acute cases of disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Holland P. V., Morrow A. G., Purcell R. H., Feinstone S. M., Moritsugu Y. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975 Nov 1;2(7940):838–841. doi: 10.1016/s0140-6736(75)90234-2. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L. non-A, Non-B hepatitis. II. Experimental transmission, putative virus agents and markers, and prevention. Gastroenterology. 1983 Sep;85(3):743–768. [PubMed] [Google Scholar]
- Garson J. A., Tedder R. S., Briggs M., Tuke P., Glazebrook J. A., Trute A., Parker D., Barbara J. A., Contreras M., Aloysius S. Detection of hepatitis C viral sequences in blood donations by "nested" polymerase chain reaction and prediction of infectivity. Lancet. 1990 Jun 16;335(8703):1419–1422. doi: 10.1016/0140-6736(90)91446-h. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Tuke P. W., Makris M., Briggs M., Machin S. J., Preston F. E., Tedder R. S. Demonstration of viraemia patterns in haemophiliacs treated with hepatitis-C-virus-contaminated factor VIII concentrates. Lancet. 1990 Oct 27;336(8722):1022–1025. doi: 10.1016/0140-6736(90)92487-3. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Feinstone S. M., Miller R. H. Rapid and sensitive method for the detection of serum hepatitis B virus DNA using the polymerase chain reaction technique. J Clin Microbiol. 1989 Sep;27(9):1930–1933. doi: 10.1128/jcm.27.9.1930-1933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Unoura M., Kobayashi K., Kuno K., Murakami S., Hattori N. Detection of serum hepatitis C virus RNA. Lancet. 1990 Apr 21;335(8695):976–976. doi: 10.1016/0140-6736(90)91042-9. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Takeuchi K., Boonmar S., Katayama T., Choo Q. L., Kuo G., Weiner A. J., Bradley D. W., Houghton M., Saito I. A cDNA fragment of hepatitis C virus isolated from an implicated donor of post-transfusion non-A, non-B hepatitis in Japan. Nucleic Acids Res. 1989 Dec 25;17(24):10367–10372. doi: 10.1093/nar/17.24.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Muchmore E. Hepatitis surveillance standards for hepatitis studies in a chimpanzee colony. Dev Biol Stand. 1980;45:13–21. [PubMed] [Google Scholar]
- Muchmore E., Popper H., Peterson D. A., Miller M. F., Lieberman H. M. Non-A, non-B hepatitis-related hepatocellular carcinoma in a chimpanzee. J Med Primatol. 1988;17(5):235–246. [PubMed] [Google Scholar]
- Pfeifer U., Thomssen R., Legler K., Böttcher U., Gerlich W., Weinmann E., Klinge O. Experimental non-A, non-B hepatitis: four types of cytoplasmic alteration in hepatocytes of infected chimpanzees. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33(3):233–243. doi: 10.1007/BF02899184. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Weiner A. J., Rosenblatt J., Wong D. C., Shapiro M., Popkin T., Houghton M., Alter H. J., Purcell R. H. Early events in hepatitis C virus infection of chimpanzees. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6441–6444. doi: 10.1073/pnas.87.16.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]