Abstract
The role of UVA radiation in the formation of human nuclear cataract is not well understood. We have previously shown that exposing guinea pigs for 5 months to a chronic low level of UVA light produces increased lens nuclear light scattering and elevated levels of protein disulfide. Here we have used the technique of dynamic light scattering (DLS) to investigate lens protein aggregation in vivo in the guinea pig/UVA model. DLS size distribution analysis conducted at the same location in the lens nucleus of control and UVA-irradiated animals showed a 28% reduction in intensity of small diameter proteins in experimental lenses compared with controls (P < 0.05). In addition, large diameter proteins in UVA-exposed lens nuclei increased five-fold in intensity compared to controls (P < 0.05). The UVA-induced increase in apparent size of lens nuclear small diameter proteins was three-fold (P < 0.01), and the size of large diameter aggregates was more than four-fold in experimental lenses compared with controls. The diameter of crystallin aggregates in the UVA-irradiated lens nucleus was estimated to be 350 nm, a size able to scatter light. No significant changes in protein size were detected in the anterior cortex of UVA-irradiated lenses. It is presumed that the presence of a UVA chromophore in the guinea pig lens (NADPH bound to zeta crystallin), as well as traces of oxygen, contributed to UVA-induced crystallin aggregation. The results indicate a potentially harmful role for UVA light in the lens nucleus. A similar process of UVA-irradiated protein aggregation may take place in the older human lens nucleus, accelerating the formation of human nuclear cataract.
Introduction
Cataract, an opacity of the lens of the eye, is the most frequent cause of vision loss in people (1,2), accounting for approximately 50% of all blindness worldwide (3,4). Nuclear cataract, an opacification in the center of the lens, is the most common type in older adults (5,6), and the type most likely to require surgery (3). In the United States, an episode of cataract surgery costs around $2600 (7). With more than 1.35 million cataract operations performed annually (8), this equates to a total cost of more than $3.4 billion nationwide (7,9). Finding a means of delaying or preventing maturity-onset human cataract is a major goal of lens and cataract research.
Although the etiology of cataract is still unknown, increasing evidence suggests that oxidation of lens crystallins to form protein aggregates, able to scatter light, is a possible cause (1,10–12). Crystallins present in the center of the lens do not regenerate as do other cellular proteins, making the proteins in this region as old as the individual (13). This may account for the accumulation of protein modifications that are known to occur in the human lens with age (10,14). The aging lens also experiences diminishing levels of antioxidants such as gluta-thione (GSH) (15), and activities of antioxidant enzymes such as GSH reductase and GSH peroxidase (16–18), which are required to protect sulfhydryl (-SH) groups of lens proteins. In advanced nuclear cataracts, >90% of protein thiols and ≥45% of methionines have become oxidized to disulfides and sulfoxides, respectively (19,20). The formation of disul-fides produces cross-linking of crystallins, which can lead to protein aggregation, protein insolubility and lens opacifica-tion (19,21).
Photo-oxidation by UV light is reportedly associated with the formation of maturity-onset cataract (22,23); however, the specific type of cataract that is produced, as well as the mechanism and wavelengths that are involved, and the targets of irradiation, are not well understood. A number of epide-miological studies have linked sunlight exposure with the formation of cataract, but only for the wavelengths of UVB radiation, not UVA (24–26). The exclusion of UVA light as a cause of cataract has been challenged by Dillon (27). The potentially damaging nature of UVA light has been well documented (28), and as we know that, in contrast to UVB, a considerable amount of potentially damaging UVA light can penetrate deep into the human lens (28–30), it is logical to carefully evaluate UVA-induced effects on the lens.
Most, but not all, epidemiological studies, have failed to find a link between sunlight and nuclear cataract (negative association: [24–26]; positive association: [31–34]). A recent study conducted in Australia did find a strong positive association between occupational sun exposure and nuclear cataract (35). It has been suggested that the lens nuclei of only middle-aged and older humans may be susceptible to sunlight (because of GSH loss), and that the process may take place in too short a time for epidemiological studies to detect an association (36,37). It is known that old human lenses, as well as nuclear cataracts, contain a collection of protein-bound UVA sensitizers that produce potentially damaging amounts of H2O2, superoxide anion and singlet oxygen (30,38–40). Changes induced in lens crystallins by singlet oxygen and UVA light are similar to those found in crystallins from human nuclear cataracts (41–43). We have previously established a UVA/guinea pig in vivo model for studying early nuclear cataract (44). The guinea pig lens mimics the old human lens by possessing a high level of a protein-bound toxic chromophore—not protein-bound kynurenine as in the old human lens (45), but protein-bound NADPH in the form of zeta crystallin, which makes up 10% of total lens protein in the guinea pig (46,47).
In the current study, we have used dynamic light scattering (DLS) to investigate UVA-induced protein aggregation in the guinea pig lens in vivo. We have previously employed this technique to evaluate lens protein aggregation in hyperbaric oxygen-treated guinea pigs (21). DLS has been used for nearly three decades to study aggregation of lens proteins, both in vitro and in vivo (48–50). This technique, which employs a weak incident laser beam to measure Brownian movement of proteins (51), is emerging as a valuable diagnostic tool for early detection of ocular diseases such as cataract (52,53). The results of our study provide in vivo evidence to link UVA-induced protein aggregation with an increased level of nuclear light scattering (NLS) in the guinea pig lens.
Materials and Methods
Animals
Male Hartley guinea pigs, initially 17 months old, were obtained from Kuiper Rabbit Ranch (Indianapolis, IN). The animals were held for 4 weeks before the start of UVA light treatment. The guinea pigs were 18 months old when UVA light treatment began. Before the start of the study, lenses of the animals were examined by slit-lamp biomicroscopy, and guinea pigs with cortical or nuclear opacities were excluded. The diet used throughout the study was Purina guinea pig Chow 5025 (Purina Mills, Richmond, IN), containing 0.1% ascorbic acid. Animal care and work performed in this study conformed to the US Department of Agriculture standards and the ARVO statement for the use of animals in ophthalmic and vision research.
In vivo exposure of guinea pigs to UVA light
The procedure for exposing guinea pigs to UVA light was modeled after Giblin et al. (44). Briefly, UVA irradiation of animals was conducted with use of Sylvania black light blue fluorescent lamps (F15T8/BLB; Osram Sylvania, Inc., Danvers, MA) with a spectral distribution of 340–410 nm wavelength, and maximum intensity at 365 nm. The lamps were about 5 cm above the caged animals. The irradiance on the animal corneas was approximately 0.5 mW cm−2, and the lamps were kept on nearly continuously for 5 months. The room temperature was 20°C and the temperature inside the cage was 23–24°C. Guinea pigs kept under these conditions showed no hair loss or damage to the skin or cornea. There was slight drying of the outer ears which was treated once per week with a small amount of moisturizing sun block lotion (Coppertone SPF50; Schering-Plough, Inc., Memphis, TN) (control animals were not treated similarly). Age-matched controls were kept under normal lighting conditions. Lens transparency of control and UVA-exposed guinea pigs was assessed using Zeiss slit-lamp photo-microscopy after full dilation of the eyes with 1% tropicamide and 10% phenylephrine.
Light scattering measurements
Static light scattering (SLS) and DLS were performed in vivo on eyes of control and experimental guinea pigs using a fiber optic laser probe. Procedures for this measurement have been described in detail by Simpanya et al. (21). Initially, the eyes of guinea pigs were dilated with drops of 1% solution of tropicamide (Alcon Pharmaceutical Co., Fort Worth, TX), and the animals were anesthetized intramuscularly with xylazine (8 mg kg−1) and ketamine (40 mg kg−1), administered 10 min apart. A laser power of 100 μW with a wavelength of 640 nm was employed for the measurements. Guinea pigs were positioned in such a way that the laser beam passed through the center of the eye along the optical axis of the lens. Scattered light was collected at 154°. The corneal surface was kept moist by topical application of 0.9% saline.
To conduct SLS measurements, the cornea was first detected by moving the laser light probe toward the eye and detecting reflected light from the cornea. At a threshold of 10,000 counts s−1, the movement of the motorized probe came to an automatic stop. The cornea surface was used as the origin for SLS. The laser probe was moved at a velocity of 0.01 mm s−1 from the cornea to the posterior capsule of the lens, a total distance of approximately 6 mm.
To determine the relative sizes of proteins in lenses of experimental animals compared with controls, DLS analysis was conducted immediately at the end of each SLS scan in the same position within the eye. The anterior capsule was used as the zero position for DLS analysis. DLS measurements were conducted every 0.1 mm across the ∼5.0 mm optical axis of the guinea pig lens for a total of approximately 50 measurements per lens. At the 50 locations in the lens, the instrument recorded 10–15 measurements of protein diameter in 5 s per location. The instrument was calibrated against suspensions of polystyrene beads with 80 nm diameter. Measured protein sizes are presented as relative sizes using arbitrary units to compare protein sizes in various regions of lenses of control and UVA-treated animals. The reason for this is that in order to measure absolute protein sizes, proteins must be present in dilute solutions of ≤0.1% by weight, with no interactions (54). The presence in an intact lens of highly packed proteins at concentrations >300 mg mL−1 (21) causes in addition to ionic and hydrophobic interactions, significant slowing of the diffusion of proteins, increasing their apparent size. For these reasons, it is not possible to measure absolute protein sizes accurately in intact lenses (54).
Results
Increased lens NLS was apparent in animals exposed to UVA light for 5 months, compared with age-matched controls, as evidenced by slit-lamp biomicroscopy (Fig. 1). The increase in NLS appeared as a nuclear haze, which produced a clear separation between the lens cortex and nucleus, compared with the control.
Figure 1.
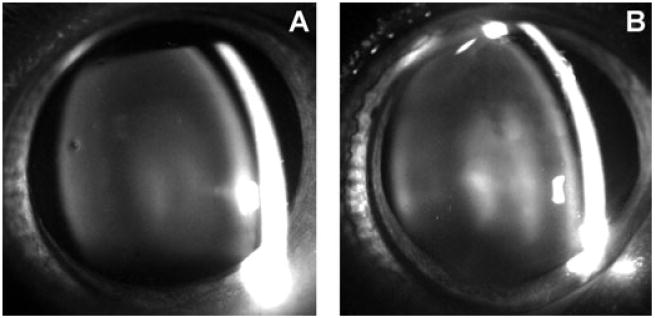
Typical slit-lamp biomicroscopy photographs of guinea pig eyes of a control (23 months old) and UVA-treated animal (23 months old) after 5 months of in vivo exposure. Note the increased lens nuclear light scattering in the UVA-treated lens (B), compared with the control (A), particularly in the center of the nucleus.
Analysis of control guinea pig eyes in vivo by SLS showed clearly the anatomic arrangement of the eye, viz. the cornea, aqueous humor, three regions of the lens (anterior cortex, nucleus, posterior cortex) and the vitreous humor (Fig. 2A). The cornea showed a high level of light scatter due to the reflection of laser light to the detector from this tissue. The control lens showed a low level of SLS for the entire axial diameter (∼5.0 mm) (Fig. 2A). In contrast, lenses of guinea pigs treated with UVA radiation for 5 months showed an increased level of SLS, especially in the center of the nucleus, 2.0–3.5 mm from the surface of the cornea (Fig. 2B).
Figure 2.
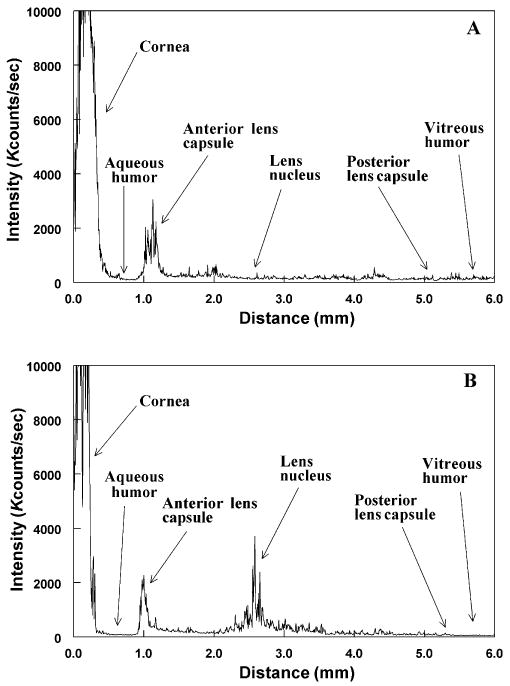
Typical static light scattering (SLS) analyses in vivo of eyes of control and UVA-treated guinea pigs. Each analysis was conducted along the optical axis of the lens, measuring 4.5–5.0 mm in diameter. Kcounts/s: 1000 photon counts per second of collected light intensity. (A) Control, 23-month-old untreated animal; (B) animal treated over a period of 5 months with UVA light. Note the increase in SLS intensity in the UVA-exposed lens, mainly in the central region, compared with that of an age-matched control animal.
To assess changes in lens protein size in vivo after UVA radiation of guinea pigs, DLS analysis was performed. Measurements were made every 0.1 mm across the optical axis of the guinea pig lens. Figure 3 shows a representative DLS profile of average protein sizes across the lens of a control and UVA-exposed animal. Guinea pigs kept under UVA light showed larger apparent protein sizes in the lens nuclear region, compared with proteins in the lenses of control animals; however, the increased sizes were not always uniform across the entire lens nuclear region, as is shown in the representative example of Fig. 3. For this reason, apparent protein diameters were averaged for two separate regions of the lenses: anterior cortex (0–1.0 mm from the anterior capsule) and nucleus (1.1–4.4 mm from the anterior capsule) (Fig. 4). The total numbers of measurements made for each region are stated in the legend of Fig. 4. The average apparent size of proteins in the lens nucleus of UVA-irradiated guinea pigs was 2.8 times larger than comparable proteins in the lens nucleus of age-matched controls (P < 0.001) (Fig. 4). The anterior lens cortex showed no significant difference in average protein size between control and UVA-exposed animals (P > 0.1).
Figure 3.
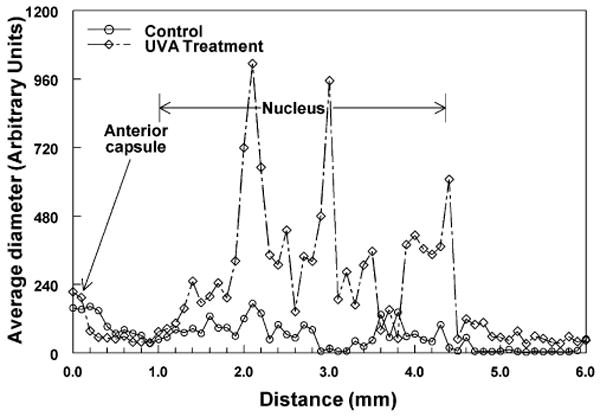
Representative dynamic light scattering (DLS) analysis profiles in vivo across lenses of control and UVA-treated guinea pigs. Fifty measurements of average protein diameter were made every 0.1 mm across the optical axis of each lens. The instrument made 10–15 measurements of protein diameter at each location and provided an average value. DLS data are not expressed as absolute sizes of lens proteins but as arbitrary units of diameter (see Materials and Methods for an explanation). (○) 23-month-old control animal; (⋄) 23-month-old experimental animal after 5 months of UVA light exposure.
Figure 4.
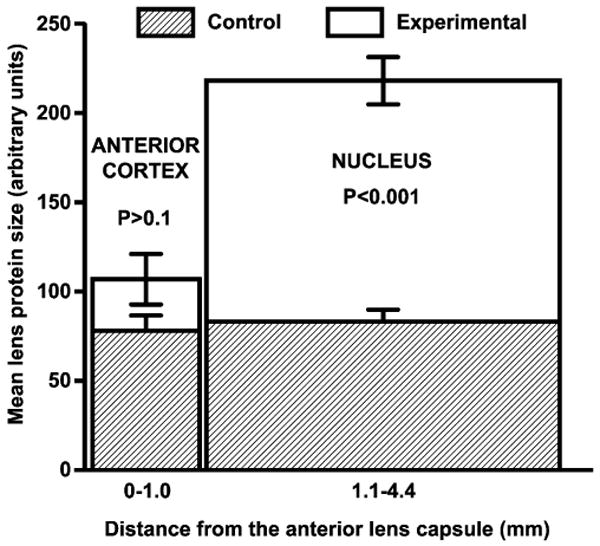
Analysis of average in vivo dynamic light scattering data for measurements taken across the lenses of control and UVA-treated guinea pigs. Measurements of average protein diameter were made every 0.1 mm across the optical axis of each lens (see legend of Fig. 3). These data were then averaged for two regions of each lens for control animals and animals exposed to UVA light. Analyses were conducted at 3 and 5 months of UVA exposure and the data combined (n = 6, control; n = 5, UVA-exposed). The total numbers of measurements made for each region were: control anterior cortex: 66; experimental anterior cortex: 88; control nucleus: 204; and experimental nucleus: 270. Each result is expressed as the mean ± SEM.
A more detailed analysis of protein size distribution was conducted at two specific locations in the lenses of control and UVA-exposed guinea pigs, located in the anterior cortex and in the approximate center of the nucleus (Table 1). The intensity (level) of small diameter proteins (<50 arbitrary units) at one location in the lens nucleus was 28% lower for UVA-exposed animals compared with controls (67% intensity compared with 93%, P < 0.05). In contrast, the level of large diameter proteins (>50 arbitrary units) at the same location in the nucleus was nearly five-fold higher for UVA-exposed animals compared with controls (33% intensity compared with 7%, P < 0.05). In addition, the size of small diameter proteins and the size of large diameter proteins were three-fold and 4.5-fold larger, respectively, in the nucleus of UVA-exposed animals, compared with controls (Table 1). No significant changes in either intensity or size of small diameter or large diameter proteins were observed in the lens anterior cortex as a result of UVA exposure (Table 1).
Table 1.
Protein size distribution analysis conducted in vivo for lenses of control and UVA-exposed guinea pigs.
| Intensity (%) | Protein diameter (arbitrary units) | |||||
|---|---|---|---|---|---|---|
| Control | UVA | P-value | Control | UVA | P-value | |
| Nucleus | ||||||
| Small diameter | 93 ± 7 | 67 ± 26 | <0.05 | 9 ± 5 | 26 ± 9 | <0.01 |
| Large diameter | 7 ± 7 | 33 ± 26 | <0.05 | 140 ± 147 | 638 ± 678 | >0.1 |
| Anterior cortex | ||||||
| Small diameter | 96 ± 6 | 93 ± 7 | >0.1 | 12 ± 4 | 12 ± 4 | >0.1 |
| Large diameter | 4 ± 6 | 6 ± 7 | >0.1 | 158 ± 298 | 201 ± 356 | >0.1 |
In vivo dynamic light scattering analyses were conducted in lenses of guinea pigs exposed to 0.5 mW cm−2 of UVA light. Analyses were conducted at 3 and 5 months of UVA exposure and the data combined. Data were collectedat one specific location each in the anterior cortex and in the approximate center of the nucleus for UVA-irradiated (n = 5) and age-matched control animals (n = 6). Small and large diameter proteins were classified as being <50 or >50 arbitrary units, respectively (see Materials and Methods for an explanation of why the data are expressed as arbitrary units of protein diameter). Results are expressed as the mean ± SD.
Discussion
A major finding of this study was that chronic exposure of guinea pigs to a low level of UVA light produced aggregation of proteins in the center of the lens with a concomitant increase in lens NLS. The intensity of UVA light employed, 0.5 mW cm−2, was about one-tenth the level of ambient UVA present in sunlight in New York City at noon on a clear day in August (55). Presumably, the observed protein aggregation was related to the absorption of UVA light by the UVA chromophore NADPH which is present in the guinea pig lens at high concentration (100 times higher than the human lens [46,47]). NADPH is known to produce reactive oxygen species including singlet oxygen, superoxide anion and H2O2 when irradiated with UVA light in the presence of oxygen (56,57), and we have detected trace amounts of oxygen (about 1 mmHg) present in the guinea pig lens nucleus (F. J. Giblin, unpublished result). Much of the NADPH present in the guinea pig lens is bound to the protein zeta crystallin (accounting for 10% of total guinea pig lens protein [46,47]), which has been reported to be in higher concentration in the guinea pig lens nucleus compared with the cortex (58). This presence of a toxic protein-bound UVA filter in the guinea pig lens nucleus mimics the aging human lens nucleus which contains various UVA-absorbing kynurenine compounds bound to crystallins (59). The binding of kynurenines to lens crystallins has been shown to produce lens crystallin damage (45), and it has been suggested that this binding may accelerate the progression of human nuclear cataract (10,12).
Distinct peaks and troughs were seen for DLS profiles for the lens nuclear regions of the UVA-treated guinea pigs. For example, in Fig. 3, particle sizes in certain regions of the UVA-exposed lens nucleus are two to three times higher than those in other regions of the nucleus. This was typically seen for each of the UVA-exposed lenses, and was also observed previously for the lens nuclei of hyperbaric oxygen-treated guinea pigs (21). The reason for this is not clear, but it is possible that early UVA-induced protein aggregation in the lens nucleus may not progress homogenously, resulting in the presence of larger aggregates in certain regions and smaller aggregates in others. The peaks and troughs do not appear to be due to the presence of lens membranes or sutures as they are not observed during analysis of control lenses. It is also possible, as discussed previously (21), that if aggregates become too large, multiple light scattering (a single photon scattering many times in the lens) may occur, causing absorption of light, diffusion broadening and the appearance of apparently lower particle sizes.
Although not measured in the current study, protein disulfide was presumably a major constituent of UVA-induced high molecular weight aggregates detected in the guinea pig lens nucleus. We have previously shown the presence of increased levels of protein disulfide in the lens nucleus of UVA-exposed guinea pigs (44). In addition, Barron et al. (60,61) have also shown an increase in lens nuclear disulfide in guinea pigs exposed to UVA light for months, and this increase in disulfide was also linked with an increase in lens NLS. Each of the guinea pig lens crystallins, and particularly the beta, gamma and zeta crystallins, possess high levels of -SH groups, contributing to the high level of -SH present in the guinea pig lens nucleus of nearly 50 mm. Disulfide-crosslinked aggregates have also been found in increased amounts in the lens nucleus of oxygen-treated guinea pigs (21) as well as in human nuclear cataracts (19,20). Thus, protein disulfide appears to be a common feature associated with loss of transparency of the lens nucleus. The similarity between UVA-induced lens effects observed in this study and previously determined O2-induced lens effects (21) is consistent with a role for oxidation in the UVA irradiation of guinea pig lenses.
We have shown previously that about 85% of UVA light that strikes the guinea pig cornea is able to reach the lens, and that UVA radiation can penetrate deep into the guinea pig lens nucleus (44). Although considerably more UVA light is absorbed in the guinea pig lens cortex than in the nucleus (44), we observed in the present study that UVA-induced aggregate formation occurred only in the nucleus. One reason for this is most likely the relatively low level of reduced GSH present in the guinea pig lens nucleus (<3 mm) compared with the cortex (nearly 25 mm). Long-term exposure of guinea pigs to UVA light has been shown to decrease the level of GSH in the lens nucleus by about 30%, with no significant effect on GSH levels in the cortex (44). GSH is known to be protective against UVA light in vitro (62,63), possibly due to its ability to scavenge reactive oxygen species. The low level of GSH in the guinea pig lens nucleus, combined with trace amounts of molecular oxygen present in this region, creates a favorable environment for UVA-induced oxidation of protein -SH groups to form disulfide-crosslinked aggregates. The older human lens nucleus also suffers from a low level of GSH, approaching 1 mm (10,64), leading to the potentially damaging binding of UVA-absorbing kynurenine compounds to lens crystallins as mentioned above (10,12). The results of the present study conducted with guinea pigs suggest that the lens nuclei of older humans may be more susceptible to UVA-induced aggregation of lens crystallins, possibly leading to nuclear cataract.
Based on our previous gel electrophoresis data involving the UVA/guinea pig model (44), we would expect that the composition of the aggregates observed in this study would include each of the lens crystallins, including alpha, beta, gamma and zeta. All of the lens crystallins except alpha-B crystallin contain -SH groups, and thus can participate in disulfide crosslinking. Zeta crystallin would be expected to be a major component of the aggregates as we have found that it is highly susceptible to both disulfide-crosslinking and oxidation-induced precipitation (21) and, in addition, zeta is the guinea pig lens crystallin that contains the bound UVA chromophore, NADPH. It also cannot be ruled out that portions of certain cytoskeletal proteins, such as actin or the α- or β-tubulins, may also have been included in the aggregates as we have demonstrated previously a nearly complete UVA-induced loss of lens nuclear cytoskeletal proteins after long-term exposure of guinea pigs to UVA light (44).
The average diameter of protein aggregates found in the lens nucleus of the UVA-exposed guinea pigs (638 arbitrary units, Table 1) was 70 times greater than that of control small diameter proteins (9 arbitrary units, Table 1). If it is assumed that the control small diameter proteins were 5 nm in diameter (about 40% of this fraction would be composed of 10–12 nm diameter alpha crystallin), it can be estimated that the diameter of the UVA-induced lens nuclear aggregates would be about 350 nm. Aggregates of this size would have produced significant scattered light (48) in the guinea pig lens nucleus, as was observed in the current study (Fig. 1). Proteins of similar sizes, 5 nm for small proteins and nearly 700 nm for large aggregates, have been found to be present in young and old human lenses, respectively, using light scattering techniques (65,66). Electron microscopic analysis of protein aggregates in human cataractous lenses have shown sizes of 200–500 nm (67,68), while similar studies of X-irradiated rabbits have shown lens nuclear protein aggregates of up to 1000 nm in diameter (69).
In summary, we have shown by DLS analysis in vivo that exposure of guinea pigs to a physiologically relevant level of UVA light can induce increased protein aggregation in the center of the lens. Presumably this result is related to the high level of UVA chromophore (NADPH bound to zeta crystallin) present in the guinea pig lens nucleus, along with trace amounts of molecular oxygen, leading to UVA-induced generation of reactive oxygen species. A similar process of UVA-induced protein aggregation may take place in the older human lens nucleus, possibly accelerating the formation of human nuclear cataract.
Acknowledgments
This work was supported in part by NASA Award NAG3-2892, NIH EY EY02027 and NIH EY 014803. We thank James King of NASA for help with in vivo analysis of the guinea pig lenses by DLS, and Li-Ren Lin, M.D., for slit-lamp examination of the eyes of the animals. We appreciate the professional long-term care of the animals provided by Cliff Snitgen, Janet Schofding and Joyce Schram.
References
- 1.Spector A. Review: Oxidative stress and disease. J Ocul Pharmacol Ther. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- 2.Ebert C, Foster S, Gibbs MJ, Hearle K, Hicks B, Lill PH, Longstreth J, Patel N, Pitcher HM, Teramura AF, Tirpak D, Titus J, Wells JB, Whitten GZ, Worrest R. Cataracts and other eye disorders. In: Hoffman JS, editor. Assessing the Risks of Trace Gases that can Modify the Stratosphere, Chapter 10. Vol. 2. Environmental Protection Agency; Washington, D.C.: 1987. pp. 1–32. [Google Scholar]
- 3.Javitt JC, Wang F, West SK. Blindness due to cataract: Epidemiology and prevention. Annu Rev Public Health. 1996;17:159–177. doi: 10.1146/annurev.pu.17.050196.001111. [DOI] [PubMed] [Google Scholar]
- 4.Midelfart A. Ultraviolet radiation and cataract. Acta Ophthalmol Scand. 2005;83:642–644. doi: 10.1111/j.1600-0420.2005.00595.x. [DOI] [PubMed] [Google Scholar]
- 5.Adamsons I, Muñoz B, Enger C, Taylor HR. Prevalence of lens opacities in surgical and general populations. Arch Ophthalmol. 1991;109:993–997. doi: 10.1001/archopht.1991.01080070105046. [DOI] [PubMed] [Google Scholar]
- 6.Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population. The Beaver Dam Eye Study. Ophthalmology. 1992;99:546–552. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto L, Andley U, Applegate R, Beyer EC, Clark J, Garland D, Goldman J, Grainger R, Horwitz J, Lou MF, Overbeek P, Rae J, Sperduto R, Stambolian D, Zelenka P, Liberman ES. National Eye Institute; Bethesda, MD: 2006. [May 27, 2008]. Report of the Lens and Cataract Panel: Program Overview and Goals. http://www.nei.nih.gov/resources/strategicplans/neiplan/frm_lens.asp. [Google Scholar]
- 8.Asbell PA, Dualan I, Mindel J, Brocks D. Age-related cataract. Lancet. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg EP, Javitt JC, Sharkey PD, Zuckerman A. The content and cost of cataract surgery. Arch Ophthalmol. 1993;111:1041–1049. doi: 10.1001/archopht.1993.01090080037016. [DOI] [PubMed] [Google Scholar]
- 10.Truscott RJW. Age-related nuclear cataract—Oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Spector A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 12.Davies MJ, Truscott RJW. Photo-oxidation of proteins and its role in cataractogenesis. J Photochem Photobiol B. 2001;63:114–125. doi: 10.1016/s1011-1344(01)00208-1. [DOI] [PubMed] [Google Scholar]
- 13.Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214:86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- 14.Truscott RJ. Age-related nuclear cataract: A lens transport problem. Ophthalmic Res. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]
- 15.Giblin FJ. Glutathione: A vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 16.Ohrloff C, Hockwin O, Olson R, Dickman S. Glu-tathione peroxidase, glutathione reductase and superoxide dismutase in the aging lens. Curr Eye Res. 1984;3:109–115. doi: 10.3109/02713688408997191. [DOI] [PubMed] [Google Scholar]
- 17.Linton S, Davies MJ, Dean RT. Protein oxidation and ageing. Exp Gerontol. 2001;36:1503–1518. doi: 10.1016/s0531-5565(01)00136-x. [DOI] [PubMed] [Google Scholar]
- 18.Linetsky M, Chemoganskiy VG, Hu F, Ortwerth BJ. Effect of UVA light on the activity of several aged human lens enzymes. Invest Ophthalmol Vis Sci. 2003;44:264–274. doi: 10.1167/iovs.02-0597. [DOI] [PubMed] [Google Scholar]
- 19.Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- 20.Garner MH, Spector A. Selective oxidation of cys-teine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpanya MF, Ansari RR, Suh KI, Leverenz VR. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: Dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerman S. Radiant Energy and the Eye. Macmillan; New York: 1980. pp. 131–164. [Google Scholar]
- 23.Zigman S. Photobiology of the lens. In: Maisel H, editor. The Ocular Lens: Structure, Function and Pathobiology. Marcel Dekker, Inc.; New York: 1985. pp. 301–347. [Google Scholar]
- 24.Taylor HR, West SK, Rosenthal FS, Muñoz B, Newland HS, Abbey H, Emmett EA. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988;319:1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- 25.Cruickshank KJ, Klein BE, Klein R. Ultraviolet light exposure and lens opacities: The Beaver Dam Eye Study. Am J Public Health. 1992;82:1658–1662. doi: 10.2105/ajph.82.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West SK, Duncan DD, Muñoz B, Rubin GS, Fried LP, Bandeen-Roche K, Schein OD. Sunlight exposure and risk of lens opacities in a population-based study: The Salisbury Eye Evaluation project. JAMA. 1998;280:714–718. doi: 10.1001/jama.280.8.714. [DOI] [PubMed] [Google Scholar]
- 27.Dillon J. Sunlight exposure and cataract. JAMA. 1999;281:229. [PubMed] [Google Scholar]
- 28.Dillon J, Zheng L, Merriam JC, Gaillard ER. The optical properties of the anterior segment of the eye: Implications for cortical cataract. Exp Eye Res. 1999;68:785–795. doi: 10.1006/exer.1999.0687. [DOI] [PubMed] [Google Scholar]
- 29.Gaillard ER, Zheng L, Merriam JC, Dillon J. Age-related changes in the absorption characteristics of the primate lens. Invest Ophthalmol Vis Sci. 2000;41:1454–1459. [PubMed] [Google Scholar]
- 30.Balasubramanian D. Photodynamics of cataract: An update on endogenous chromophores and antioxidants. Photochem Photobiol. 2005;81:498–501. doi: 10.1562/2004-11-01-RA-354. [DOI] [PubMed] [Google Scholar]
- 31.Mohan M, Sperduto RD, Angra SK, Milton RC, Mathur RL, Underwood BA, Jaffery N, Pandya CB, Chhabra VK, Vajpayee RB, Kalra VK, Sharma YR. India–US case–control study of age-related cataracts. India–US Case–Control Study Group. Arch Ophthalmol. 1989;107:670–676. doi: 10.1001/archopht.1989.01070010688028. [DOI] [PubMed] [Google Scholar]
- 32.Zigman S, Datiles M, Torczynski E. Sunlight and human cataracts. Invest Ophthalmol Vis Sci. 1979;18:462–467. [PubMed] [Google Scholar]
- 33.Dolezal JM, Perkins ES, Wallace RB. Sunlight, skin sensitivity, and senile cataract. Am J Epidemiol. 1989;129:559–568. doi: 10.1093/oxfordjournals.aje.a115168. [DOI] [PubMed] [Google Scholar]
- 34.Wong L, Ho SC, Coggon D, Cruddas AM, Hwang CH, Ho CP, Robertshaw AM, MacDonald DM. Sunlight exposure, antioxidant status, and cataract in Hong Kong fisher-men. J Epidemiol Community Health. 1993;47:46–49. doi: 10.1136/jech.47.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neale RE, Purdie JL, Hirst LW, Green AC. Sun exposure as a risk factor for nuclear cataract. Epidemiology. 2003;14:707–712. doi: 10.1097/01.ede.0000086881.84657.98. [DOI] [PubMed] [Google Scholar]
- 36.Truscott RJW. Human cataract: The mechanism responsible, light and butterfly eyes. Int J Biochem Cell Biol. 2003;35:1500–1504. doi: 10.1016/s1357-2725(03)00145-6. [DOI] [PubMed] [Google Scholar]
- 37.Bova LM, Sweeney MH, Jamie JF, Truscott RJ. Major changes in human ocular UV protection with age. Invest Ophthalmol Vis Sci. 2001;42:200–205. [PubMed] [Google Scholar]
- 38.Linetsky M, Ortwerth BJ. The generation of hydrogen peroxide by the UVA irradiation of human lens proteins. Photochem Photobiol. 1995;62:87–93. doi: 10.1111/j.1751-1097.1995.tb05243.x. [DOI] [PubMed] [Google Scholar]
- 39.Linetsky M, Ortwerth BJ. Quantitation of the reactive oxygen species generated by the UVA irradiation of ascorbic acid-glycated lens proteins. Photochem Photobiol. 1996;63:649–655. doi: 10.1111/j.1751-1097.1996.tb05669.x. [DOI] [PubMed] [Google Scholar]
- 40.Linetsky M, Ortwerth BJ. Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem Photobiol. 1997;65:522–529. doi: 10.1111/j.1751-1097.1997.tb08598.x. [DOI] [PubMed] [Google Scholar]
- 41.Zigler JS, Jr, Goosey JD. Singlet oxygen as a possible factor in human senile nuclear cataract development. Curr Eye Res. 1984;3:59–65. doi: 10.3109/02713688408997187. [DOI] [PubMed] [Google Scholar]
- 42.Zigler JS, Jr, Goosey JD. Photosensitized oxidation in the ocular lens: Evidence for photosensitizers endogenous to the human lens. Photochem Photobiol. 1981;33:869–874. doi: 10.1111/j.1751-1097.1981.tb05505.x. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramanian D, Du X, Zigler JS., Jr The reaction of singlet oxygen with proteins, with special reference to crystallins. Photochem Photobiol. 1990;52:761–768. doi: 10.1111/j.1751-1097.1990.tb08679.x. [DOI] [PubMed] [Google Scholar]
- 44.Giblin FJ, Leverenz VR, Padgaonkar VA, Unakar NJ, Dang L, Lin LR, Lou MF, Reddy VN, Borchman D, Dillon JP. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp Eye Res. 2002;75:445–458. [PMC free article] [PubMed] [Google Scholar]
- 45.Parker NR, Jamie JF, Davies MJ, Truscott RJ. Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic Biol Med. 2004;37:1479–1489. doi: 10.1016/j.freeradbiomed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Zigler JS, Jr, Rao PV. Extremely high levels of NADPH in guinea pig lens: Correlation with zeta-crystallin concentration. Biochem Biophys Res Commun. 1990;167:1221–1228. doi: 10.1016/0006-291x(90)90654-6. [DOI] [PubMed] [Google Scholar]
- 47.Zigler JS, Jr, Rao PV. Enzyme/crystallins and extremely high pyridine nucleotide levels in the eye lens. FASEB J. 1991;5:223–225. doi: 10.1096/fasebj.5.2.2004667. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Benedek GB. Observation of protein diffusivity in intact human and bovine lenses with application to cataract. Invest Ophthalmol. 1975;14:449–456. [PubMed] [Google Scholar]
- 49.Jedziniak JA, Nicoli DF, Baram H, Benedek GB. Quantitative verification of the existence of high molecular weight protein aggregates in the intact normal human lens by light-scattering spectroscopy. Invest Ophthalmol Vis Sci. 1978;17:51–57. [PubMed] [Google Scholar]
- 50.Latina M, Chylack LT, Jr, Fagerholm P, Nishio I. Dynamic light scattering in the intact rabbit lens. Its relation to protein concentration. Invest Ophthalmol Vis Sci. 1987;28:175–183. [PubMed] [Google Scholar]
- 51.Nelson E. [May 27, 2008];Dynamical Theories of Brownian Motion. (2nd). 2001 [ http://www.math.princeton.edu/∼nelson/books.html] (PDF version)
- 52.Datiles MB, 3rd, Ansari RR, Reed GF. A clinical study of the human lens with a dynamic light scattering device. Exp Eye Res. 2002;74:93–102. doi: 10.1006/exer.2001.1106. [DOI] [PubMed] [Google Scholar]
- 53.Ansari RR. Ocular static and dynamic light scattering: A noninvasive diagnostic tool for eye research and clinical practice. J Biomed Opt. 2004;9:22–37. doi: 10.1117/1.1626663. [DOI] [PubMed] [Google Scholar]
- 54.Dhadwal HS, Wittpenn J. In vivo dynamic light scattering characterization of a human lens: Cataract index. Curr Eye Res. 2000;20:502–510. [PubMed] [Google Scholar]
- 55.Merriam JC. The concentration of light in the human lens. Trans Am Ophthalmol Soc. 1996;94:803–918. [PMC free article] [PubMed] [Google Scholar]
- 56.Cunningham ML, Krinsky NI, Giovanazzi SM, Peak MJ. Superoxide anion is generated from cellular metabolites by solar radiation and its components. Free Radic Biol Med. 1985;1:381–385. doi: 10.1016/0748-5514(85)90150-3. [DOI] [PubMed] [Google Scholar]
- 57.Czochralska B, Bojarska E, Pawlicki K, Shugar D. Photochemical and enzymatic redox transformations of reduced forms of coenzyme NADP+ Photochem Photobiol. 1990;51:401–410. doi: 10.1111/j.1751-1097.1990.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 58.Simpanya MF, David LL, Padgaonkar VA, Giblin FJ. Glutathiolated crystallins in the guinea pig lens: Effects of age and exposure to oxidative stress in vivo. Invest Ophthalmol V is Sci (Suppl) 2006;47:S2527. [Google Scholar]
- 59.Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound UV filters in normal human lenses: The concentration of bound UV filters equals that of free UV filters in the center of older lenses. Invest Ophthalmol Vis Sci. 2007;48:1718–1723. doi: 10.1167/iovs.06-1134. [DOI] [PubMed] [Google Scholar]
- 60.Barron BC, Yu NT, Kuck JF., Jr Tryptophan Raman/457.9-nm-excited fluorescence of intact guinea pig lenses in aging and ultraviolet light. Invest Ophthalmol Vis Sci. 1987;28:815–821. [PubMed] [Google Scholar]
- 61.Barron BC, Yu NT, Kuck JF., Jr Raman spectroscopic evaluation of aging and long-wave UV exposure in the guinea pig lens: A possible model for human aging. Exp Eye Res. 1988;46:249–258. doi: 10.1016/s0014-4835(88)80082-4. [DOI] [PubMed] [Google Scholar]
- 62.Tobi SE, Paul N, McMillan TJ. Glutathione modulates the level of free radicals produced in UVA-irradiated cells. J Photochem Photobiol B. 2000;57:102–112. doi: 10.1016/s1011-1344(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 63.Petersen AB, Gniadecki R, Vicanova J, Thorn T, Wulf HC. Hydrogen peroxide is responsible for UVA-induced DNA damage measured by alkaline comet assay in HaCaT keratinocytes. J Photochem Photobiol B. 2000;59:123–131. doi: 10.1016/s1011-1344(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 64.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 65.Dierks K, Dieckmann M, Niederstrasser D, Schwartz R, Wegener A. Protein size resolution in human eye lenses by dynamic light scattering after in vivo measurements. Graefes Arch Clin Exp Ophthalmol. 1998;236:18–23. doi: 10.1007/s004170050037. [DOI] [PubMed] [Google Scholar]
- 66.van den Berg TJ. Light scattering by donor lenses as a function of depth and wavelength. Invest Ophthalmol Vis Sci. 1997;38:1321–1332. [PubMed] [Google Scholar]
- 67.Freel CD, Gilliland KO, Wesley LC, Giblin FJ, Costello JM. Fourier analysis of cytoplasmic texture in nuclear fiber cells from transparent and cataractous human and animal lenses. Exp Eye Res. 2002;74:689–702. doi: 10.1006/exer.2001.1166. [DOI] [PubMed] [Google Scholar]
- 68.Ringens PJ, Liem-The KN, Hoenders HJ, Wollensak J. Normal and cataractous human eye lens crystallins. Inter-discip Top Gerontol. 1978;13:193–211. [Google Scholar]
- 69.Liem-The KN, Stols AL, Jap PH, Hoenders HJ. X-ray induced cataract in rabbit lens. Exp Eye Res. 1975;20:317–328. doi: 10.1016/0014-4835(75)90114-1. [DOI] [PubMed] [Google Scholar]


