Abstract
Prolongation of cell survival through prevention of apoptosis is considered to be a significant factor leading to anabolic responses in bone. The current studies were carried out to determine the role of the small GTPase, RhoA, in osteoblast apoptosis, since RhoA has been found to be critical for cell survival in other tissues. We investigated the effects of inhibitors and activators of RhoA signaling on osteoblast apoptosis. In addition, we assessed the relationship of this pathway to parathyroid hormone (PTH) effects on apoptotic signaling and cell survival. Rho A is activated by geranylgeranylation, which promotes its membrane anchoring. In serum-starved MC3T3-E1 osteoblastic cells, inhibition of geranylgeranylation with geranylgeranyl transferase I inhibitors increased activity of caspase-3, a component step in the apoptosis cascade, and increased cell death. Dominant negative RhoA and Y27632, an inhibitor of the RhoA effector Rho kinase, also increased caspase-3 activity. A geranylgeranyl group donor, geranylgeraniol, antagonized the effect of the geranylgeranyl tranferase I inhibitor GGTI-2166, but could not overcome the effect of the Rho kinase inhibitor. PTH 1–34, a potent antiapoptotic agent, completely antagonized the stimulatory effects of GGTI-2166, dominant negative RhoA, and Y27632, on caspase-3 activity. The results suggest that RhoA signaling is essential for osteoblastic cell survival but that the survival effects of PTH 1–34 are independent of this pathway.
Keywords: osteoblast, RhoA, apoptosis, parathyroid hormone
Anti-apototic actions are a mechanism through which a number of stimuli have been proposed to increase osteoblastic activity, leading to anabolic responses in bone. These anti-apoptotic factors include parathyroid hormone (PTH) [Jilka, 2007; Jilka et al., 1999; Sowa et al., 2003; Wang et al., 2007], insulin-like growth factor-I [Grey et al., 2003], endothelin [Van Sant et al., 2007], mechanical loading, [Bran et al., 2008; Pavalko et al., 2003; Weyts et al., 2003] and matrix environment [Buxton et al., 2008]. Activation of protein kinase A appears to be a critical component of the anti-apoptotic response to parathyroid hormone [Chen et al., 2007; Swarthout et al., 2002]. However, other signaling pathways could contribute to anti-apoptotic actions. Our previous studies had shown that parathyroid hormone activates the small G protein RhoA in osteoblastic cells [Radeff et al., 2004]. RhoA signaling promotes survival in other tissues, [Gallagher, 2004; Kobayashi et al., 2004; Reuveny et al., 2004; Stepan et al., 2004; Zhu et al., 2008]. RhoA inactivation by statins leads to apoptosis of human osteosarcoma cells [Fromigue et al., 2006]. However, in some tissues RhoA can induce apoptosis [Del Re et al., 2007; Wu et al., 2006]. In view of these diverse findings, we were interested in determining whether the pathway is important for survival of osteoblastic cells, and whether it plays a role in the effects of PTH to promote osteoblast survival.
RhoA signaling can be manipulated with genetic and pharmacologic tools. Translocation of RhoA to the plasma membrane and its activation are dependent upon the lipid modification, geranylgeranylation. A number of agents have been developed that inhibit geranylgeranyltransferase I and prevent the geranylgeranylation of RhoA. Exogenous geranylgeranyl groups can be provided through the donor molecule geranylgeraniol. The downstream RhoA effector Rho kinase can be inhibited pharmacologically. Actions of RhoA can also be prevented through the stable expression of a dominant negative RhoA. These tools were used to assess the importance of the pathway in MC3T3-E1 osteoblastic cells.
The findings demonstrate that RhoA, through its downstream effects, promotes survival of MC3T3-E1 osteoblastic cells. Interruption of RhoA signaling or interference with the activation of RhoA increases caspase-3 activity and cell death. The effects of interfering with the RhoA pathway could be overcome by PTH treatment. However, blocking the pathway in the MC3T3-E1 cells did not significantly attenuate the effects of PTH, suggesting that these are independent pathways mediating osteoblast survival.
METHODS
Cell Culture
MC3T3-E1 pre-osteoblastic cells were obtained from RIKEN Cell Bank (Tsukuba, Japan), and maintained in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37°C, 5% CO2. Media were replaced every three days, and the cells were subcultured weekly for use in experiments within 20 passages. For serum starvation, 0.1% bovine serum albumin (Calbiochem, fraction V) was substituted for the bovine serum. For the caspase-3 assay, cells were seeded at 250,000 cells/well on a 6-well plate. For the LIVE/DEAD assay, cells were seeded at 55,000 cells/well on a 24-well plate.
Stable transfection of MC3T3-E1 cells
MC3T3-E1 cells were transfected with constitutive active RhoA63L, dominant negative RhoA19N, or pcDNA (empty vector) using a CalPhos mammalian transfection kit (Clontech, Palo Alto, CA). The RhoA construct and pcDNA were generously provided by Dr. Said Sebti, Moffitt Cancer Center. Briefly, 5 μg of plasmid DNA was added to 12.4 μl of 2M calcium solution to make 100 μl solution A. Solution A was then added dropwise into 100-μl 2x HEPES-buffered saline (Solution B) with slow vortexing to make the transfection solution. After incubation at room temperature for 20 min, the transfection solution was added dropwise to cell culture plates. After 3 hr at 37°C, calcium phosphate-containing medium was removed and replaced with 2 ml fresh medium for 24 hr. The stable transfectants were selected and maintained with culture medium containing 400 μg/ml G418 (SIGMA, St. Louis, MO).
Treatments
To inhibit geranylgeranylation, a process by which RhoA is modified to permit its translocation to the plasma membrane, a geranylgeranyl transferase I inhibitor, GGTI-2166, was used. This reagent was kindly provided by Dr. Said Sebti, Moffitt Cancer Center and Dr. Andrew Hamilton, Yale University. To inhibit the activity of the downstream target of RhoA, Rho kinase, the inhibitor Y-27632 (Calbiochem, San Diego, CA) was used. Geranylgeraniol (BIOMOL, Plymouth Meeting, PA) was used as a precursor to increase cellular geranylgeranylpyrophosphate. bPTH 1–34 peptide (PTH 1–34) was from Bachem (Philadelphia, PA).
Protein assay and Caspase-3 assay
Caspase-3 activity was measured using a caspase-3 colorimetric assay (R&D systems, Minneapolis, MN) according to the manufacturer’s instructions. At the end of the experiments, MC3T3-E1 cells and the culture media were harvested and centrifuged at 300 × g for 10 min. The supernatant was removed and the cell pellet was lysed by the addition of lysis buffer that was provided in the kit. The lysate was incubated on ice for 10 min. During the incubation the lysate was voltexed for 10 sec twice to homogenize. At the end of the incubation, the lysate was centrifuged at 10,000 × g for 1 min, and the supernatant was saved for the further experiments. The enzymatic reaction was carried out on a 96-well plate. 40 μl of lysate, 40 μl of reaction buffer 3 and 5 μl of substrate (DEVD-pNA) were mixed, incubated at 37°C for 2 hr, and the absorbance was read at 410 nm. After subtraction of the absorbance of reaction mix in which water was substituted for the cell lysate, the value was adjusted by the protein concentration determined with a BCA protein assay kit (Pierce, Rockford, IL).
LIVE/DEAD assay
For assay of cell survival, MC3T3-E1 cells grown on 96-well plates were rinsed with phosphate buffered saline (PBS), and stained with a LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. Briefly, 0.4 μM ethidium homodimer and 0.4 μM Calcein-AM dissolved in PBS were added to the wells, and the mixture was incubated for 45 min at room temperature. Microscope images were recorded and quantification of cell numbers was achieved with a “cell counter” plug-in of Image J software.
Statistics
Significance was determined by analysis of variance and Tukey’s post-hoc test.
RESULTS
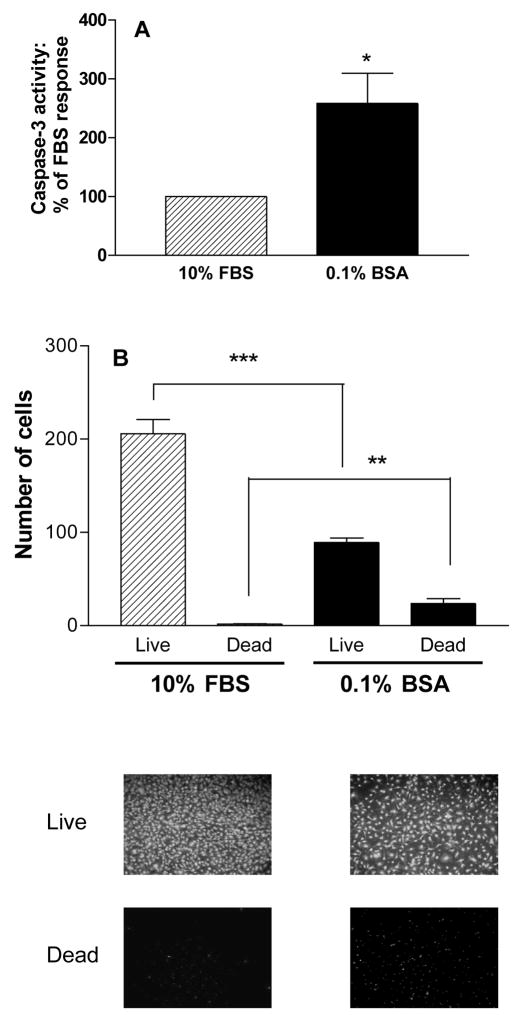
For the current investigation, two assays were used to determine the importance of RhoA for MC3T3-E1 osteoblastic cell survival. All treatments were assessed for their effects on the activity of caspase-3, a downstream effector caspase that reflects activity of both the mitochondrial and death receptor apoptotic mechanisms. However, recent work demonstrates that caspases are involved in processes in addition to apoptosis [Nhan et al., 2006] including osteoclast differentiation [Szymczyk et al., 2006], and therefore for most treatments, effects were also evaluated with a fluorescence-based LIVE/DEAD (InVitrogen) assay which is dependent on the esterase activity of live cells and the increased permeability of dead and dying cells. The assay identifies living cells based on their esterase activity, which allows them to release green fluorescent calcein from the nonfluorescent cell-permeant calcein acetoxymethylester (calcein-AM). Dead cells and dying cells are identified with the compound ethidium homodimer, which enters cells that have damaged membranes and undergoes a marked increase in red fluorescence when bound to nucleic acids. To demonstrate the effects of cell death in these assays, control experiments were carried out in which cells were subjected to serum starvation (24 hr culture in medium containing 0.1% bovine serum albumin (BSA)) in place of culture in 10% fetal bovine serum (FBS). Figure 1 shows that serum starvation of MC3T3-E1 cells resulted in increased caspase-3 activity (Figure 1A) and increased cell death (Figure 1B).
Figure 1.
Serum starvation (0.1% BSA for 24 hr) increases caspase-3 activity in MC3T3-E1 cells (Figure 1A) and decreases the number of live cells and increases the number of dead cells in a LIVE/DEAD assay (Figure 1B, graphs and images). Values are means +/− SE of 3 replicates for the caspase assay and 6 replicates for the Live/Dead assay. * p < 0.05; ** p < 0.01; *** p < 0.001 vs. FBS.
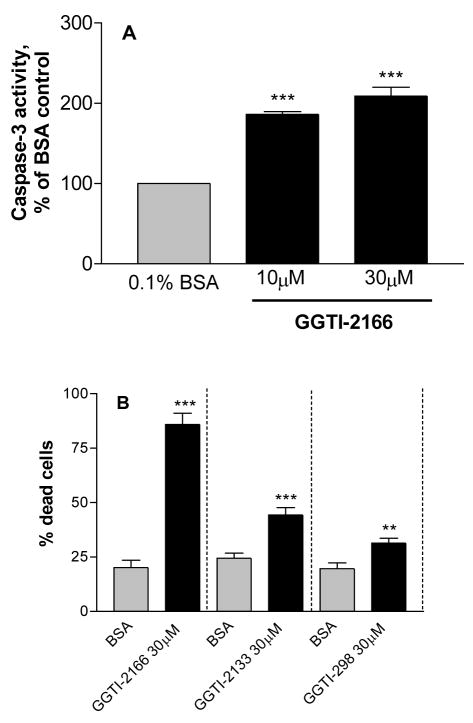
The Rho GTPases requires geranylgeranylation for membrane translocation and activation. This geranylgeranylation is catalyzed by the enzyme geranylgeranyl transferase I. The geranylgeranyl transferase I inhibitor GGTI-2166 increased caspase-3 activity (Figure 2A) and cell death (Figure 2B) in the serum-starved cells. Two other geranylgeranyl transferase I inhibitors, GGTI-2133 and GGTI 298 also increased the percent of dead cells, although they were less potent than GGTI-2166 (Figure 2B). GGTI-2166 did not increase caspase-3 activity or cell death in the cells cultured in 10% FBS (data not shown).
Figure 2.
Treatment of MC3T3-E1 cells for 6 hr with geranygeranyltransferase I inhibitors increases caspase-3 activity (Figure 2A) and the percentage of dead cells (Figure 2B). Basal caspase-3 activity in the serum-starved cells = .483 +/− .090 A410 absorbance units/mg protein. Values are means +/− SE of 3 replicates for the caspase assay and 6 replicates for the LIVE/DEAD assay. ** p < 0.01; p < 0.001 vs. BSA control.
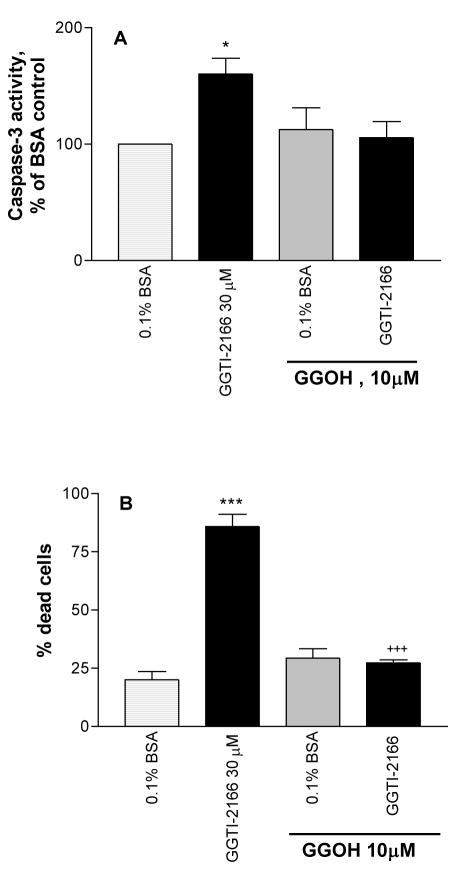
Geranylgeraniol (GGOH), a precursor of geranylgeranyl pyrophosphate, increases the amount of substrate available to geranylgeranylate Rho GTPases. GGOH was able to overcome the effect of the geranylgeranyl transferase I inhibitor GGTI-2166 in both the caspase-3 assay (Figure 3A) and the live/dead assay (Figure 3B). GGOH alone did not have significant effects in either assay.
Figure 3.
Co-treatment of MC3T3-E1 cells for 6 hr with geranylgeraniol (GGOH) protects against the apoptotic effect of geranylgeranyl transferase I inhibitor GGTI-2166 as shown by effects on caspase-3 activity (Figure 3A) and the percentage of dead cells (Figure 3B). Basal caspase-3 activity in the serum-starved cells = .525 +/− .018 A410 absorbance units/mg protein. Values are means +/− SE of 4 replicates for the caspase assay and 6 replicates for the LIVE/DEAD assay. * p < 0.01 vs. BSA control; *** p < 0.001 vs BSA control; +++ p < 0.001 vs. GGTI alone.
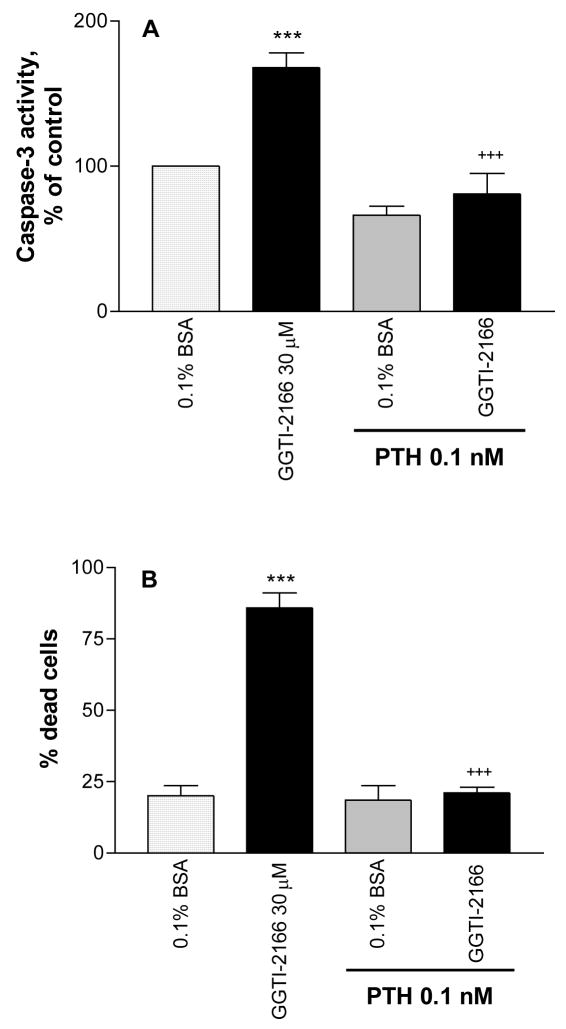
The effect of GGTI-2166 to increase caspase-3 activity in the serum-starved MC3T3-E1 cells was prevented by PTH (Figure 4A). PTH also prevented the increase in the number of dead cells resulting from treatment with GGTI-2166 (Figure 4B).
Figure 4.
Treatment of MC3T3-E1 cells for 6 hr with PTH 1–34 protects against the apoptotic effect of the geranylgeranyl transferase I inhibitor GGTI-2166 as shown by effects on caspase-3 activity (Figure 4A) and the percentage of dead cells (Figure 4B). Basal caspase-3 activity in the serum-starved cells = .455 +/− .026 A410 absorbance units/mg protein. Values are means +/− SE of 3 replicates for the caspase assay and 6 replicates for the Live/Dead assay. *** p < 0.001 vs. control; +++ p < 0.001 vs. GGTI alone.
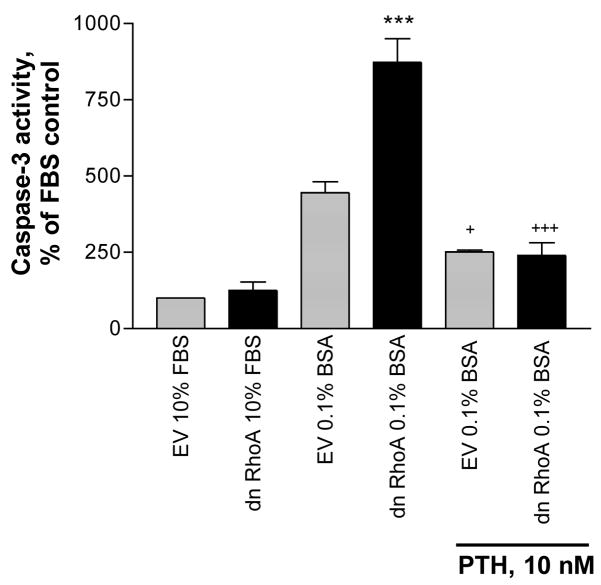
To directly test the role of the RhoA GTPase on survival of the MC3T3-E1 cells, the cells were stably transfected with either a dominant negative RhoA construct (Rho A19N) or empty vector (EV). Stable expression of the dominant negative RhoA increased caspase-3 activity and the effect was antagonized by PTH (Figure 5).
Figure 5.
Stable expression of dominant negative RhoA in MC3T3-E1cells increases caspase-3 activity in serum-starved cells; the effect is antagonized by 6 hr treatment with PTH 1–34. Basal caspase-3 activity in the serum-starved cells = 2.31 +/− .10 A410 absorbance units/mg protein. Values are means +/− SE of 3 replicates. *** p < 0.001 vs BSA control; + p < 0.05 vs. dn RhoA without PTH; +++ p < 0.001 vs. dn RhoA without PTH. EV = empty vector.
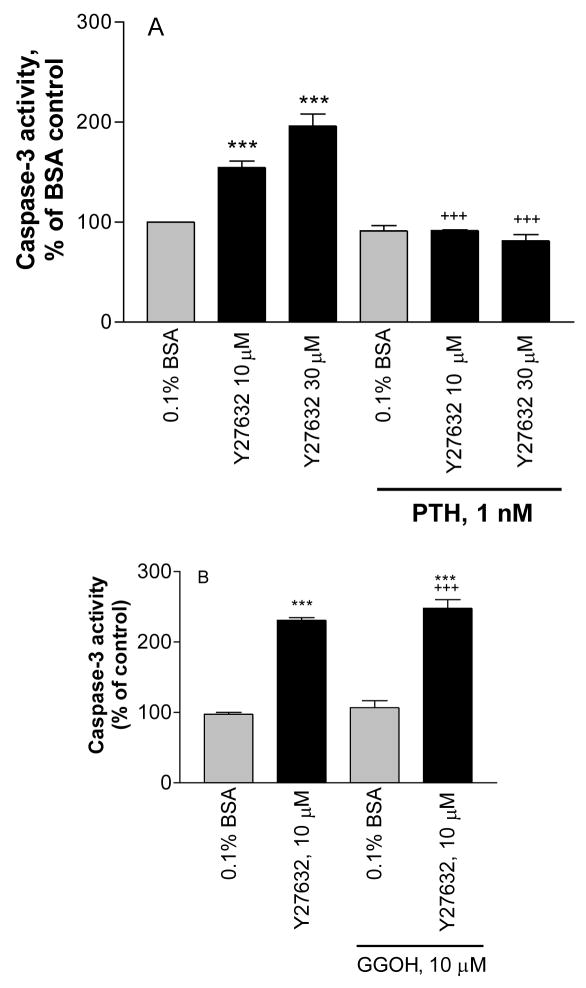
One of the effectors of RhoA is the downstream kinase, Rho kinase. A Rho kinase inhibitor, Y27632, increased caspase-3 activity. PTH prevented the effect of the Rho kinase inhibitor (Figure 6A), whereas GGOH did not prevent the effect of the downstream inhibitor Y27632 on caspase-3 activity (Figure 6B).
Figure 6.
Treatment of MC3T3-E1 cells for 6hr with the Rho kinase inhibitor Y27632 increases caspase-3 activity in serum starved cells; the effect is antagonized by co-treatment with PTH 1–34 (Figure 6A) but not by co-treatment with geranylgeraniol (GGOH) (Figure 6B). Basal caspase-3 activity in the serum-starved cells = A: .638 +/− .038 A410 absorbance units/mg protein; B: 1.40 +/− .28 A410 absorbance units/mg protein. Values are means +/−SE of 2 replicates for A and 3 replicates for B. *** p < 0.001 vs. BSA control; +++ p < 0.001 vs. Y27632 alone.
DISCUSSION
The current studies reveal that RhoA signaling plays an important role in osteoblast survival by inhibiting osteoblast apoptosis. Apoptosis, or programmed cell death, appears to function as a limiting factor in bone formation. A number of bone anabolic agents and conditions are able to prevent osteoblast apoptosis, including parathyroid hormone (PTH) [Jilka, 2007; Jilka et al., 1999; Sowa et al., 2003; Wang et al., 2007], insulin-like growth factor-I [Grey et al., 2003], endothelin [Van Sant et al., 2007], mechanical loading, [Bran et al., 2008; Pavalko et al., 2003; Weyts et al., 2003] and matrix environment [Buxton et al., 2008]. Thus it is important to understand the mechanisms responsible for osteoblast apoptosis. Apoptosis can be initiated through membrane receptor-associated and mitochondrial-initiated pathways that converge and mediate their downstream effects to degrade cellular proteins and organelles through the cysteine protease caspase 3 [Hengartner, 2000]. A family of proteins, the Bcl-2 family, which contains both pro-apoptotic (e.g. Bad, Bax, Bim) and anti-apoptotic members (Bcl-2, Bcl-xl), facilitates or inhibits the process [Hengartner, 2000]. Multiple signaling intermediates, including phosphatidylinositol 3-kinase, p42/p44 MAP kinase and protein kinase A interact with various Bcl-2 familymembers to regulate the pathway in response to stimuli [Danilkovitch et al., 2000; Sastry et al., 2006; van de Donk et al., 2005].
Rho small G proteins, molecular switches that function through binding and hydrolyzing GTP can promote cell survival and survival-related signaling [Gallagher, 2004; Kobayashi et al., 2004; Reuveny et al., 2004; Stepan et al., 2004; Zhu et al., 2008], likely through increased Bcl-2 expression [Gomez et al., 1997; Zhu et al., 2008]. Conditional knockout of RhoA or the Rho effector Rho kinase results in apoptosis of motor neurons in the spinal cord [Kobayashi et al., 2004]. The signaling pathway through which RhoA prevents apoptosis varies in different tissues. RhoA binds to the regulatory domain of mitogen-activated protein kinase kinase kinase [Gallagher, 2004]. The Mek/Erk pathway has been found to be crucial for anti-apoptotic effects during zebrafish embryogenesis [Zhu et al., 2008], but does not appear to be the critical pathway in AR4-2J pancreatic acinar cells [Stepan et al., 2004]. In the acinar cells and in myoblasts, survival involves the PI-3-kinase/Akt pathway [Reuveny et al., 2004; Stepan et al., 2004]. Interestingly, in some in vitro models Rho signaling promotes apoptosis [Aznar and Lacal, 2001; Del Re et al., 2007; Wu et al., 2006]. In cardiac myocytes this involves upregulation of Bax [Del Re et al., 2007].
The use of PTH as a therapeutic agent in the treatment of osteoporosis reflects its impressive effects on bone density and fracture prevention in clinical studies [Finkelstein et al., 1998; Hodsman et al., 1997; Lane et al., 1998; Lindsay et al., 1997; Neer et al., 2001] and responses in animal models [Dempster et al., 1993; Hock et al., 1989; Hodsman and Steer, 1993; Lane et al., 1996]. One of the mechanisms that is likely to be crucial to this anabolic effect is the ability of PTH to inhibit osteoblast apoptosis, resulting in increased bone formation. Signaling pathways considered to have a role in this response are p42/p44 MAP kinase [Chen et al., 2004; Gentili et al., 2001; Kaiser and Chandrasekhar, 2003; Swarthout et al., 2001] and PI-3-kinase/Akt [Gentili et al., 2002; Gentili et al., 2003] activation. At the level of G proteins, receptor association with Gαs containing heterotrimeric G proteins, leading to increased cyclic AMP and activation of protein kinase A have been strongly implicated as initiation events in the signaling [Chen et al., 2007; Swarthout et al., 2002]. Our previous studies showed that PTH treatment of osteoblastic cells could promote RhoA translocation to a cell membrane fraction and increase GTP-bound (activated) RhoA [Radeff et al., 2004]. Since both PTH and RhoA promote cell survival through MAP kinase and PI-3-kinase/Akt signaling, it was of interest to determine the interactions of PTH and agents that interfered with RhoA signaling, first to determine whether PTH could overcome the effects of inhibition of RhoA signaling, and second, whether RhoA signaling could contribute partially to the anti-apoptotic effects of PTH, i.e. whether attenuation of RhoA signaling decreased the antiapoptotic effect of PTH. The studies reported here show that PTH signaling is able to bypass or overcome the inhibitory effects of GGTI-2166, Y-27632 and dominant negative RhoA. However, the effects of PTH were not antagonized by the inhibitors of the pathway. This finding differed from what we observed in earlier studies (2007 ASBMR meeting abstract # 1192), in which there was a small but significant inhibition by GGTI-2166 of the effect of PTH on caspase-3 in the MC3T3-E1 cells. The reason for this is unclear. PTH itself did not have a significant antiapoptotic effect under basal conditions (i.e., serum starvation), except in transfected cells. The transfection procedure itself resulted in a higher basal caspase-3 activity. The role of conditions that can affect basal caspase activity on PTH-stimulated survival signaling merits further study. Also, the role of Rho signaling in the antiapoptotic effects of other treatments affecting bone, such as endothelin, other growth factors, and mechanical stimuli, needs to be investigated. In future studies we will examine the role of the pathway in primary osteoblasts. In preliminary studies using trypan blue uptake as an indicator of cell apoptosis, as previously applied by Jilka et al. [Jilka et al., 1998] we found that GGTI-2166 increased the percentage of neonatal mouse osteoblasts taking up trypan blue, and that this was antagonized by PTH, findings consistent with the current observations.
Acknowledgments
The research described in this manuscript was supported by NIH grant AR 11262 to PHS. Dr. Said Sebti, Moffitt Cancer Center and Research Institute, and Dr. Andrew Hamilton, Yale University, kindly donated the GGTI-2166 reagent. Dr. Sebti also provided the dominant negative RhoA plasmid and the pcDNA. We thank Dr. Jun Wang for his reading of the manuscript, and Ms. Shirley Foster for technical support.
Grant sponsor: National Institutes of Health
Grant number: AR11262
References
- Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165:1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- Bran GM, Stern-Straeter J, Hormann K, Riedel F, Goessler UR. Apoptosis in bone for tissue engineering. Arch Med Res. 2008;39:467–82. doi: 10.1016/j.arcmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Buxton PG, Bitar M, Gellynck K, Parkar M, Brown RA, Young AM, Knowles JC, Nazhat SN. Dense collagen matrix accelerates osteogenic differentiation and rescues the apoptotic response to MMP inhibition. Bone. 2008;43:377–85. doi: 10.1016/j.bone.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D’Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem. 2004;279:29121–9. doi: 10.1074/jbc.M313000200. [DOI] [PubMed] [Google Scholar]
- Chen X, Song IH, Dennis JE, Greenfield EM. Endogenous PKI gamma limits the duration of the anti-apoptotic effects of PTH and beta-adrenergic agonists in osteoblasts. J Bone Miner Res. 2007;22:656–64. doi: 10.1359/jbmr.070122. [DOI] [PubMed] [Google Scholar]
- Danilkovitch A, Donley S, Skeel A, Leonard EJ. Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells. Mol Cell Biol. 2000;20:2218–27. doi: 10.1128/mcb.20.6.2218-2227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–78. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34): a randomized controlled trial. Jama. 1998;280:1067–73. doi: 10.1001/jama.280.12.1067. [DOI] [PubMed] [Google Scholar]
- Fromigue O, Hay E, Modrowski D, Bouvet S, Jacquel A, Auberger P, Marie PJ. RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Differ. 2006;13:1845–56. doi: 10.1038/sj.cdd.4401873. [DOI] [PubMed] [Google Scholar]
- Gallagher E, Gutowski S, Sternweis PC, Cobb MH. RhoA binds to the amino terminus of MEKK1 and regulates its kinase activity. J Biol Chem. 2004;279:1872–1877. doi: 10.1074/jbc.M309525200. [DOI] [PubMed] [Google Scholar]
- Gentili C, Morelli S, Boland R, de Boland AR. Parathyroid hormone activation of map kinase in rat duodenal cells is mediated by 3′,5′-cyclic AMP and Ca(2+) Biochim Biophys Acta. 2001;1540:201–12. doi: 10.1016/s0167-4889(01)00134-3. [DOI] [PubMed] [Google Scholar]
- Gentili C, Morelli S, Russo De Boland A. Involvement of PI3-kinase and its association with c-Src in PTH-stimulated rat enterocytes. J Cell Biochem. 2002;86:773–83. doi: 10.1002/jcb.10264. [DOI] [PubMed] [Google Scholar]
- Gentili C, Picotto G, Morelli S, Boland R, de Boland AR. Effect of ageing in the early biochemical signals elicited by PTH in intestinal cells. Biochim Biophys Acta. 2003;1593:169–78. doi: 10.1016/s0167-4889(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Gomez J, Martinez C, Giry M, Garcia A, Rebollo A. Rho prevents apoptosis through Bcl-2 expression: implications for interleukin-2 receptor signal transduction. Eur J Immunol. 1997;27:2793–9. doi: 10.1002/eji.1830271108. [DOI] [PubMed] [Google Scholar]
- Grey A, Chen Q, Xu X, Callon K, Cornish J. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology. 2003;144:4886–93. doi: 10.1210/en.2003-0350. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hock JM, Hummert JR, Boyce R, Fonseca J, Raisz LG. Resorption is not essential for the stimulation of bone growth by hPTH-(1–34) in rats in vivo. J Bone Miner Res. 1989;4:449–58. doi: 10.1002/jbmr.5650040321. [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ, Watson PH, Ostbye T, Stitt LW, Adachi JD, Taves DH, Drost D. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 1997;82:620–8. doi: 10.1210/jcem.82.2.3762. [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Steer BM. Early histomorphometric changes in response to parathyroid hormone therapy in osteoporosis: evidence for de novo bone formation on quiescent cancellous surfaces. Bone. 1993;14:523–7. doi: 10.1016/8756-3282(93)90190-l. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Chandrasekhar S. Distinct pathways of extracellular signal-regulated kinase activation by growth factors, fibronectin and parathyroid hormone 1–34. Biochem Biophys Res Commun. 2003;305:573–8. doi: 10.1016/s0006-291x(03)00820-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci. 2004;24:3480–8. doi: 10.1523/JNEUROSCI.0295-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NE, Kimmel DB, Nilsson MH, Cohen FE, Newton S, Nissenson RA, Strewler GJ. Bone-selective analogs of human PTH(1–34) increase bone formation in an ovariectomized rat model. J Bone Miner Res. 1996;11:614–625. doi: 10.1002/jbmr.5650110509. [DOI] [PubMed] [Google Scholar]
- Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102:1627–33. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–5. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nhan TQ, Liles WC, Schwartz SM. Physiological functions of caspases beyond cell death. Am J Pathol. 2006;169:729–37. doi: 10.2353/ajpath.2006.060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Gerard RL, Ponik SM, Gallagher PJ, Jin Y, Norvell SM. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol. 2003;194:194–205. doi: 10.1002/jcp.10221. [DOI] [PubMed] [Google Scholar]
- Radeff JM, Nagy Z, Stern PH. Rho and Rho kinase are involved in parathyroid hormone-stimulated protein kinase C alpha translocation and IL-6 promoter activity in osteoblastic cells. J Bone Miner Res. 2004;19:1882–91. doi: 10.1359/JBMR.040806. [DOI] [PubMed] [Google Scholar]
- Reuveny M, Heller H, Bengal E. RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathway. FEBS Lett. 2004;569:129–34. doi: 10.1016/j.febslet.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Sastry KS, Smith AJ, Karpova Y, Datta SR, Kulik G. Diverse antiapoptotic signaling pathways activated by vasoactive intestinal polypeptide, epidermal growth factor, and phosphatidylinositol 3-kinase in prostate cancer cells converge on BAD. J Biol Chem. 2006;281:20891–901. doi: 10.1074/jbc.M602928200. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Iu MF, Tsukamoto T, Sugimoto T, Chihara K. Parathyroid hormone-Smad3 axis exerts anti-apoptotic action and augments anabolic action of transforming growth factor beta in osteoblasts. J Biol Chem. 2003;278:52240–52. doi: 10.1074/jbc.M302566200. [DOI] [PubMed] [Google Scholar]
- Stepan V, Ramamoorthy S, Pausawasdi N, Logsdon CD, Askari FK, Todisco A. Role of small GTP binding proteins in the growth-promoting and antiapoptotic actions of gastrin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G715–25. doi: 10.1152/ajpgi.00169.2003. [DOI] [PubMed] [Google Scholar]
- Swarthout JT, D’Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- Swarthout JT, Doggett TA, Lemker JL, Partridge NC. Stimulation of extracellular signal-regulated kinases and proliferation in rat osteoblastic cells by parathyroid hormone is protein kinase C-dependent. J Biol Chem. 2001;276:7586–92. doi: 10.1074/jbc.M007400200. [DOI] [PubMed] [Google Scholar]
- Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. Active caspase-3 is required for osteoclast differentiation. J Cell Physiol. 2006;209:836–44. doi: 10.1002/jcp.20770. [DOI] [PubMed] [Google Scholar]
- van de Donk NW, Lokhorst HM, Bloem AC. Growth factors and antiapoptotic signaling pathways in multiple myeloma. Leukemia. 2005;19:2177–85. doi: 10.1038/sj.leu.2403970. [DOI] [PubMed] [Google Scholar]
- Van Sant C, Wang G, Anderson MG, Trask OJ, Lesniewski R, Semizarov D. Endothelin signaling in osteoblasts: global genome view and implication of the calcineurin/NFAT pathway. Mol Cancer Ther. 2007;6:253–61. doi: 10.1158/1535-7163.MCT-06-0574. [DOI] [PubMed] [Google Scholar]
- Wang YH, Liu Y, Rowe DW. Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab. 2007;292:E594–603. doi: 10.1152/ajpendo.00216.2006. [DOI] [PubMed] [Google Scholar]
- Weyts FA, Bosmans B, Niesing R, van Leeuwen JP, Weinans H. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif Tissue Int. 2003;72:505–12. doi: 10.1007/s00223-002-2027-0. [DOI] [PubMed] [Google Scholar]
- Wu EH, Tam BH, Wong YH. Constitutively active alpha subunits of G(q/11) and G(12/13) families inhibit activation of the pro-survival Akt signaling cascade. Febs J. 2006;273:2388–98. doi: 10.1111/j.1742-4658.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- Zhu S, Korzh V, Gong Z, Low BC. RhoA prevents apoptosis during zebrafish embryogenesis through activation of Mek/Erk pathway. Oncogene. 2008;27:1580–9. doi: 10.1038/sj.onc.1210790. [DOI] [PubMed] [Google Scholar]