Abstract
Several neurotrophic factors (NTFs) are effective in protecting retinal photoreceptor cells from the damaging effects of constant light and slowing the rate of inherited photoreceptor degenerations. It is currently unclear whether, if continuously available, all NTFs can be protective for many or most retinal degenerations (RDs). We have used transgenic mice that continuously overexpress the neurotrophin, NT-3, from lens fibers under the control of the αA-crystallin promoter to test for neuroprotection in light-damage experiments and in four naturally occurring or transgenically induced RDs in mice. Lens-specific expression of NT-3 mRNA was demonstrated both by in situ hybridization in embryos and by RT-PCR in adult mice. Furthermore, NT-3 protein was found in abundance in the lens, ocular fluids and retina by ELISA and immunocytochemistry. Overexpression of NT-3 had no adverse effects on the structure or function of the retina for up to at least 14 months of age. Mice expressing the NT-3 transgene were protected from the damaging effects of constant light to a much greater degree than those receiving bolus injections of NT-3. When the NT-3 transgene was transferred into rd/rd, Rds/+, Q344ter mutant rhodopsin or Mertk knockout mice, overexpression of NT-3 had no protective effect on the RDs in these mice. Thus, specificity of the neuroprotective effect of NT-3 is clearly demonstrated, and different molecular mechanisms are inferred to mediate the protective effect in light-induced and inherited RDs.
Keywords: Neurotrophic factor, rd, Q344ter, Rds, Mertk
Inherited and age-related retinal degenerations (RDs) of photoreceptor (PR) cells have little or no effective treatment. The slowing of PR degeneration by the application of neurotrophic factors (NTFs) to degenerating retinas has raised the possibility that a pharmaceutical approach may be developed. This was demonstrated first by the significant slowing of PR degeneration following the intravitreal injection of bFGF (FGF-2) into RCS rats with inherited retinal dystrophy (Faktorovich et al., 1990). Soon thereafter, it was shown that a number of growth factors, neurotrophins and cytokines can protect the retina from the damaging effects of light (Faktorovich et al., 1992; LaVail et al., 1992).
The question of which survival factors, if any, would be effective in slowing PR cell death in inherited RDs was partially addressed by the demonstration that some agents could slow the progression of PR degeneration in three different mouse models, two of which had the same gene defects as different forms of human retinitis pigmentosa (LaVail et al., 1998). Neuronal specificity in localization and action of NTFs in the nervous system is widely accepted, but determining the selectivity of survival-promoting action in different RDs in mice is hampered by the inability to inject consistent doses intravitreally into the small eye of young mice (LaVail et al., 1998) and the relatively short half-life of the NTFs relative to the rate of PR degeneration (Cayouette et al., 1998). In fact, when CNTF is injected intravitreally into the eye of Rds/Rds (Rds2) mice, no protective effect is seen (Cayouette et al., 1998; LaVail et al., 1998), yet when the same agent is delivered continuously by retinal cells transduced with cDNA for CNTF by either adenovirus (Cayouette et al., 1998) or adeno-associated virus (AAV) (Bok et al., 2002; Liang et al., 2001a) vectors, the degeneration in Rds/Rds mice is slowed.
Continuous delivery of NTFs appears to have a significantly greater survival-promoting effect in several RDs than does direct injection of the proteins. For example, in experiments where bFGF, GDNF or BDNF were injected into the eyes of transgenic (Tg) rats with either P23H or S334ter mutant rhodopsin, virtually no protection from PR cell death was seen (W.M. Peterson, M.M. LaVail, J.G. Flannery and W.W. Hauswirth, unpublished observations). In contrast, continuous, gene-based delivery using AAV vectors to express bFGF (Lau et al., 2000), GDNF (McGee Sanftner et al., 2001) or BDNF (W.M. Peterson, M.M., LaVail, J.G., Flannery and W.W. Hauswirth, unpublished observations) gave clear PR protection. Similarly, intravitreal injection of BDNF in Q344ter mutant rhodopsin Tg mice gave no protection (LaVail et al., 1998), but transgenically overexpressed BDNF gave definite protection in the same line of mice (Okoye et al., 2003). These observations, along with the fact that continuous delivery of CNTF has protected PRs in every form of RD that has been examined in several different species (Bok et al., 2002; Cayouette et al., 1998; Cayouette and Gravel, 1997; Liang et al., 2001a; Liang et al., 2001b; Tao et al., 2002; Wang et al., 2002), raises the question of whether specificity exists and whether any continuously available NTF will show survival-promoting activity in various PR degenerations. We have tested this hypothesis by the use of Tg mice that continuously overexpress the neurotrophin, NT-3, from the lens in light-damage experiments and in four mouse models of inherited RD.
MATERIALS AND METHODS
Generation and screening of NT-3 Tg mice
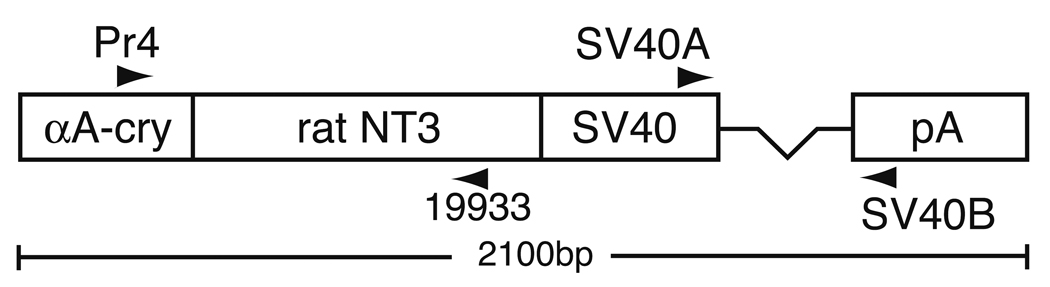
All procedures with mice adhered to the Policies on the Use of Animals in Neuroscience Research and the IACUC and animal research committees at our respective institutions. Tg mice expressing NT-3 from the lens were generated using an αA-crystallin NT-3 minigene. To generate this minigene, a plasmid containing a rat NT-3 clone, pSP70/rNT3, was obtained from Murray Robinson (Amgen, Inc., Thousand Oaks, CA). (Maisonpierre et al., 1990). The 800bp XhoI NT-3 fragment from pSP70/rNT-3 was subcloned into the Sal I site of the crystallin promoter vector CPV2. CPV2 contains the −288/+43 αA-crystallin promoter at the 5’ end and SV40 intron and polyadenylation sequences at the 3’ end (Robinson et al., 1995b). Correct orientation of the insert was confirmed by restriction mapping. The 2133 bp microinjection fragment was gel isolated following digestion with Sst II (Gibco/BRL).
FVB/N strain mouse embryos were injected with DNA at a concentration of 2 ng/µl as described by Robinson et al. (1995b). Potential Tg mice were screened using the polymerase chain reaction (PCR) on genomic DNA from tail biopsies. The primers used for PCR include the following: Pr4 (GCATTCCAGCTGCTGACGGT), a sense primer to the murine αA-crystallin promoter and 19933 (TTGACTGGCCTGGCTTCTTTACACCT), an antisense primer to rat NT-3. Mice were also screened using primers SV40A (GTGAAGGAACCTTACTTCTGTGGTG) and SV40B (GTCCTTGGGGTCTTCTACCTTTCTC), which span the intron in the SV40-derived sequences. Eight independent lines of Tg mice were produced, of which two (OVE613 and OVE614) were maintained for analysis.
RT-PCR
Transgenic mice on an FVB/N (rd/rd) genetic background were crossed to non-transgenic C57BL/6 mice to generate F1-hybrid mice that were heterozygous for the recessive rd mutation. Seven-week-old mice were euthanized and eyes were removed and dissected into lens and eye-without-lens fractions. Total RNA was isolated from each of these fractions using Trizol reagent (Invitrogen). Two µg of total lens RNA from each sample were reverse transcribed using Superscript II (Invitrogen), and the resultant cDNA was amplified by PCR using primers SV40A and SV40B (described above) as described previously (Robinson et al., 1995b). These primers amplify a 236 bp fragment diagnostic for correctly spliced transgene transcripts that is distinct from the 300 bp genomic band.
In situ hybridization to demonstrate localization of NT-3 mRNA expression
Embryonic heads were collected from timed pregnancies at 14.5 days post-coitus (E14.5), fixed in paraformaldehyde and embedded in paraffin wax. Tissue sections of 5 µm thickness were collected on Superfrost-Plus (Fisher Scientific) charged slides and hybridized to 35S-labeled riboprobes containing the SV40 sequences as described previously (Robinson et al., 1995b).
Measurement of NT-3 by ELISA
Standard ELISA methods were used to measure the amount of NT-3 in the lens, ocular fluids (vitreous and aqueous humor combined due to the very small vitreous of the mouse eye) and retina. Both lenses from a single mouse were pooled for measurement, as were the retinas and ocular fluids. The lenses and retinas were homogenized in 100 µl of buffer to extract the NT-3, so the values are expressed in ng/ml tissue in all cases. The values for each animal were the mean of duplicates determined from a standard curve in each ELISA run. The limits of quantification for the standard curve were 5 ng/ml (upper) and 0.04 ng/ml (lower). Measurements of NT-3 concentration were made both when the mice were on the original FVB/N strain and after they had been crossed to BALB/cByJ mice (see below). NT-3 ELISA measurements were performed by the Cell Biology Group at Regeneron Pharmaceuticals, Inc. without knowledge of the genotype.
Immunocytochemical localization of NT-3 in the lens and retina
Tg and non-Tg mice were intracardially perfused with 4% paraformaldehyde for 4 minutes immediately after euthanasia. Whole eyes were then taken and fixed in 4% paraformaldehyde for 1 hour. Tissues were cryoprotected with 30% sucrose in 0.1M phosphate buffer (pH 7.4), embedded in OCT, and sectioned at 10 µm on a cryostat. Specimens were labeled with goat anti-human NT-3 antibody of the IgG isotype (R&D Systems, Minneapolis, MN, catalog #AF-267-NA) at 10 µg/ml at 4°C overnight. This antibody was produced in goats immunized with purified, insect cell line Sf 21-derived, recombinant human Neurotrophin-3 (rhNT-3). NT-3 specific IgG was purified by NT-3 affinity chromatography. Based on the manufacturer’s datasheet, human, rat and mouse NT-3 propeptides are 91% identical in amino acid sequence, and the antibody is demonstrated to cross-react by immunocytochemistry with rat cerebellum. The secondary antibody was a Cy3-conjugated AffiniPure donkey anti-goat IgG (Jackson ImmunoResearch, West Grove, PA, catalog #705–165–147), incubated at room temperature for 1 hour at a dilution of 1:500. Blocking reagent consisting of 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS was applied prior to antibody incubations. Controls for the specificity of the primary antibody to NT-3 included 1) the staining pattern of NT-3 in the retina and lens was identical to that described previously in the mouse by Bennett et al. (1999); 2) Purkinje cells of mouse cerebella were examined at the same time and were found to be intensely stained (data not shown), which matched the pattern described for NT-3 staining of the rat cerebellum in the manufacturer’s datasheet; and 3) the lenses of the NT-3 Tg mice showed intense staining, whereas those of non-Tg mice showed none. Immunostaining was also performed with omission of the primary antibody to test for nonspecific binding of secondary antibody. Specimens were examined with a Zeiss LSM 5 Pascal confocal microscope.
Crosses of NT-3 Tg mice
FVB/N mice are homozygous for the rd mutation (now Pde6brd1), so the NT-3 transgene in OVE613 mice was moved onto an albino BALB/cByJ (Jackson Laboratory, Bar Harbor, ME) genetic background prior to doing studies of the retina. At each generation of backcrossing, mice carrying the NT-3 transgene were identified by PCR and backcrossed to BALB/cByJ mice. Litters of albino mice, each with approximately 50% NT-3 Tg and 50% non-Tg mice, were used for constant-light experiments after 1–3 backcrosses to BALB/cByJ mice. Some of these NT-3 Tg mice were mated to the mice with inherited RDs.
Q344ter Tg mice express a truncated rhodopsin missing the last 5 residues from the carboxy terminus (Sung et al., 1994), which results in a dominant loss of PR cells between postnatal day (P) 10 to P20. At P20, the ONL is reduced from 9–10 rows of PR nuclei in wild-type mice to 0–1 row in Q344ter mutants. To study the protective effect of NT-3 overexpression on Q344ter mice, NT-3 Tg mice were crossed with Q344ter Tg mice. This cross resulted in 4 genotypes that were distinguished by PCR, using the method described above to identify the NT-3 transgene and the primers and protocol described elsewhere for the Q344ter transgene (Sung et al., 1994). Only those mice carrying the Q344ter transgene were studied (approximately 50% of the total). Of these, approximately 50% carried the NT-3 transgene and the other 50% did not and served as controls. The retinas from P20 animals were examined histologically to determine if overexpression of NT-3 slowed the rate of RD in the Q344ter/+ mice. The Q344ter mice had been maintained by backcrossing to the pigmented C57BL/6J strain for at least 5 generations, and the NT-3 Tg mice were from the first backcross generation to BALB/cByJ so all of these experimental mice were pigmented.
The rd/rd mouse (Pde6brd1) has a similar rate of degeneration to that of the Q344ter Tg mouse, with only 0–1 rows of PR nuclei remaining at P20 (LaVail and Sidman, 1974). PR cell death results from mutations in the gene encoding the beta subunit of cGMP-PDE (Reviewed in Chang et al., 2002). NT-3 Tg mice were mated to rd/rd mice. Since the rd gene is recessive, the F1 mice that carried the NT-3 transgene were backcrossed to rd/rd mice, which resulted in 4 predicted genotypes that were distinguished by PCR to identify those carrying the NT-3 transgene (which were almost exactly 50% of the progeny) and eye histology at P20 to identify those homozygous for the rd gene (also almost exactly 50% of the progeny). Of the rd/rd progeny, almost exactly 50% were NT-3 Tg and 50% non-Tg. Thus, the rd/rd phenotype provided a reliable indicator for the rd/rd genotypes, as it is highly unlikely that NT-3 overexpression would have slowed the RD in the rd/rd retinas to the extent they could have been misidentified as normal, wild-type retinas, since this is unprecedented in the literature of RDs and neuroprotective agents. Moreover, this would have led to a deficit in the expected 50% of rd/rd animals, which was not the case. The rd/rd mice used for the experimental crosses were on a pigmented background congenic with C57BL/6J at the F25 generation. The NT-3 Tg mice were at the N4 backcross generation with BALB/cJ. Both experimental crosses produced all pigmented progeny.
The Rds mouse (Prph2Rd2), originally named retinal degeneration slow because of its slow PR degeneration over the course of a year (Sanyal et al., 1980), shows semidominant inheritance (Hawkins et al., 1985), as heterozygotes have an intermediate degree of RD. Homozygotes fail to produce the outer segment protein, Rds/peripherin (Travis et al., 1991) and develop no mature PR outer segments, whereas Rds/+ heterozygotes develop disorganized outer segments (Hawkins et al., 1985). NT-3 Tg mice were mated to Rds/Rds mice to produce all Rds/+ offspring. The offspring were genotyped for NT-3 by PCR, and retinas were examined histologically at P55-P60, when a significant difference can be seen between ONL thickness of Rds/+ and wild-type mice in the peripheral retina (Hawkins et al., 1985). The albino Rds/Rds mice used for the experimental cross were at the F38 generation on the O20/A strain, and albino NT-3 Tg mice were from the first backcross generation to BALB/cByJ. All experimental mice were albinos.
The Mertk knockout mouse (mertkkd/mertkkd) has a defect in the phagocytosis of rod outer segments by the retinal pigment epithelium, which leads to secondary death of PRs (Duncan et al., 2003). The knockout shows recessive inheritance, so the crosses to produce mertkkd homozygotes with 50% NT-3 Tg and 50% non-Tg, and the methods to identify the different genotypes, were the same as described above for the rd/rd mouse. The retinas were examined at P40, when only 2–3 rows of PR nuclei remain. The Mertk knockout mice were maintained on a mostly C57BL/6J background, with the experimental crosses using mice at the N4 backcross generation, and the NT-3 Tg mice were at the N12 backcross generation to BALB/cByJ. All of the progeny used for experiments were pigmented.
Light-damage experiments
Light-induced RD was produced in albino mice by exposing them to 2 or 3 wks of constant fluorescent light at an illuminance of approximately 115–150 ft-c as described elsewhere (Faktorovich et al., 1992). In the case of NT-3 Tg and non-Tg mice, littermates were exposed in the same cage to insure that the mice of both genotypes received the same irradiance. To compare Tg overexpression of NT-3 with intravitreal injection of NT-3, BALB/cByJ mice were injected intravitreally with 1 µl NT-3 (6.25 mg/ml; courtesy of Regeneron Pharmaceuticals, Inc., Tarrytown, NY) into one eye, as described elsewhere (LaVail et al., 1998), with the other eye either uninjected or injected with PBS buffer as a control. Two days after injection, these wild-type mice were exposed to constant light using the same duration and intensity of light as with the NT-3 Tg mice.
Electroretinography, histology and morphometric analysis
Full-field scotopic and photopic electroretinograms were recorded from selected anesthetized mice following appropriate flashes of light to determine the functional status of the retina using standard methods as described elsewhere (Bok et al., 2002; Duncan et al., 2003).
At different ages, mice were killed with carbon dioxide inhalation and immediately perfused intracardially with a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde. The eyes were removed, bisected along the vertical meridian, postfixed in osmium tetroxide and embedded in an Epon-Araldite mixture. Sections of the entire retina were cut at 1-µm thickness and stained with toluidine blue, as described elsewhere (LaVail and Battelle, 1975). The thickness of the outer nuclear layer (ONL) was taken as a measure of PR number (Michon et al., 1991), and the mean ONL thickness was obtained from 54 measurements distributed uniformly around the retina, as described elsewhere (Duncan et al., 2003; LaVail et al., 1987b). As described below, in the case of the Rds/+ mice, only the 6 measurements in the far peripheral retina in both the superior and inferior hemispheres (i.e., a total of 12 measurements in each eye) were used for comparison. Statistical comparisons of Tg and non-Tg retinas were done with a two-tailed, unpaired Student’s t-test.
For comparison of the protection afforded by injection of NT-3 versus overexpression of NT-3 in Tg mice, we used a relative measure of protection. This is necessary because the degree of light damage can vary significantly in animals of different genetic backgrounds (Danciger et al., 2000; LaVail et al., 1987a) (Reviewed in LaVail et al., 1987b). The Tg mice and injected mice were housed in different lighting conditions prior to constant light exposure, and light-damage experiments of the two groups were done about 8 mo apart. Since light damage is symmetrical in the two eyes of a given animal (LaVail et al., 1992), the mean ONL thickness in the injected eye was compared to that of the uninjected control eye of the same mouse, and the degree of protection in the injected eye was expressed as percent greater than control ONL. Statistical comparisons of the injected and control retinas were done with a two-tailed, paired Student’s t-test, and an unpaired t-test was used to compare the percent differences of protection afforded by the injected NT-3 and the transgenic NT-3.
For the preparation of photomicrographic figures, Adobe Photoshop CS (Adobe Systems, San Jose, CA) was used to adjust the contrast, brightness and sharpness.
RESULTS
Creation and initial characterization of transgenic lines
To increase the ocular concentration of NT-3 in the developing mouse eye, a transgene expression construct was designed by cloning a cDNA for rat NT-3 into the lens expression vector CPV2 (Robinson et al., 1995b), between the murine αA-crystallin promoter and the intron and polyadenylation sequences derived from SV-40 virus (Fig. 1). Eight transgenic founders were identified by PCR. RNA was isolated from one lens of each founder and transgene expression was assessed by RT-PCR using primers SV40A and SV40B. All eight founders demonstrated evidence of correctly spliced Tg transcripts (data not shown). None of the founder mice had cataracts, but the founders for the transgenic lines OVE613 and OVE614 exhibited corneal abnormalities (small blebs near the limbus of the eye) that became more prominent with age. Since both of these independent transgenic lines exhibited a similar ocular phenotype, we reasoned that the phenotype was almost certainly the result of transgene expression and not caused by random insertional mutations. Furthermore, since the founders for OVE613 and OVE614 were the only ones that exhibited an obvious eye phenotype, we reasoned that these founders most likely had the highest expression of NT-3 protein. Therefore, transgenic lines were established for both the OVE613 and OVE614 founders. The corneal phenotypes were maintained in Tg offspring from both lines. This corneal phenotype will be described in detail elsewhere.
Figure 1.
The NT-3 construct used to generate transgenic mice. The murine αA-crystallin promoter was linked to an 800 bp rat NT-3 cDNA followed by SV40 virus derived sequences including a 64 bp intron (represented by the line connecting the SV40-labeled box to the pA-labeled box) and polyadenylation site (SV40pA). Locations of primers used for genotyping and RT-PCR are indicated by labeled arrowheads.
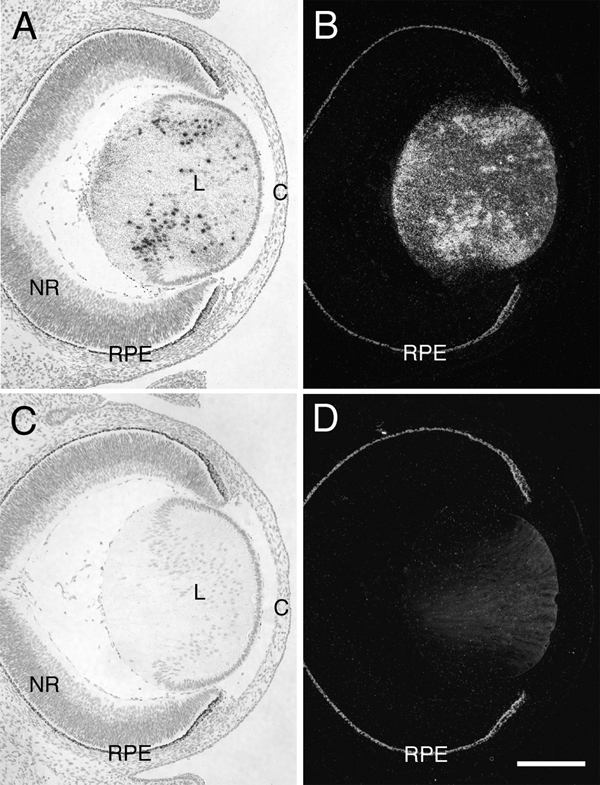
Transgene expression in E14.5 embryos was evaluated in lines OVE613 and OVE614 by in situ hybridization using 35S-labeled riboprobes specific for the transgenic transcripts. Ocular transgene expression in OVE613 was confined to the lens fiber cells, with no transgene expression detected in the neural retina (Fig. 2). The pattern of transgene expression in OVE614 was identical to that of OVE613 (data not shown). Fiber cell-specific expression is typical in transgenes under the regulation of the murine αA-crystallin promoter (Robinson et al., 1995a; Robinson et al., 1995b; Shirke et al., 2001). Southern hybridizations showed that lines OVE613 and OVE614 have a similar number of copies of the transgene integrated at a single genomic location (data not shown).
Figure 2.
Lens-specific transgene expression. Histological sections of E14.5 OVE613 Tg embryos were used for in situ hybridizations to riboprobes specific for the SV40 sequences present in the transgene. Hybridizations were done using an antisense riboprobe (A and B) and a sense riboprobe (C and D). Results are shown in bright-field (A and C) and corresponding dark-field (B and D) photomicroscopy. Transgene transcripts are indicated by silver grains. Hybridization signals were confined to the lens (L) with no hybridization detected in the neural retina (NR) or cornea (C). Non-Tg embryos showed no hybridization to either the antisense or sense riboprobes (data not shown). The melanin in the pigmented retinal pigment epithelium (RPE) appears bright in dark-field illumination (B and D). The lens sections demonstrate a non-specific glow with dark-field illumination that is unrelated to the hybridization signals (D). Hybridization signals were confined to the lens (L) with no hybridization detected in the neural retina (NR) or cornea (C). Scale bar = 200 µm.
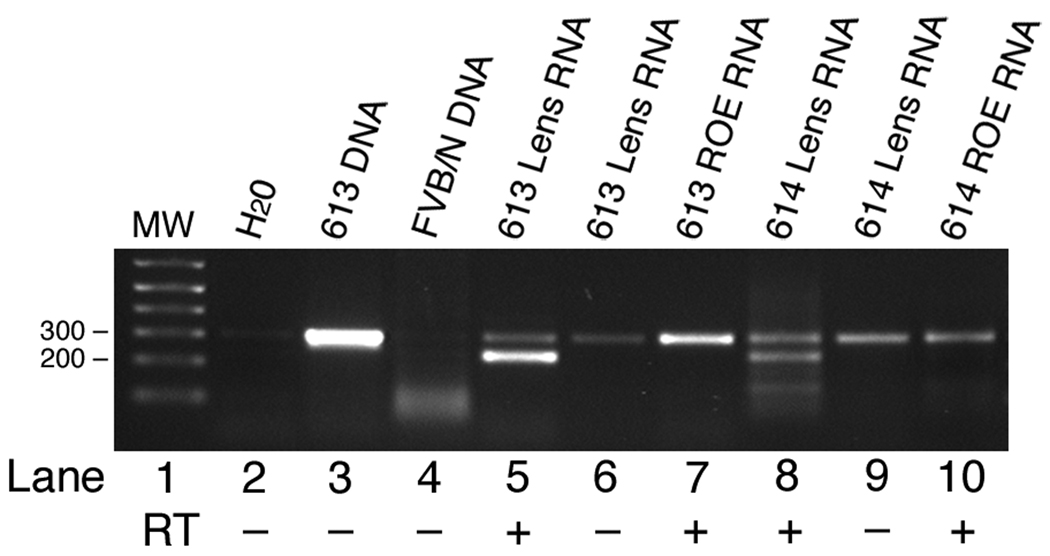
Transgene expression in the eyes of 7-wk-old mice was assayed by RT-PCR. As shown in Figure 3, the transgenic transcript was detected in the lenses, but not in the retina or other parts of the eye in both OVE613 and OVE614 lines. It is likely that homozygous Tg mice from both of these lines die during embryogenesis, since viable homozygotes have not been identified in offspring from Tg to Tg brother × sister mating.
Figure 3.
NT-3 transgene expression in adult eyes. Ocular RNAs were amplified by RT-PCR using primers SV40A and SV40B. These primers amplify a 236 bp fragment from reverse transcribed mRNA in tissues expressing the transgene. The 236 bp fragment is present in reverse transcribed (RT +) lens RNA from the OVE613 and OVE614 transgenic lines (lanes 5 and 8). These primers amplify a band of 300 bp from transgenic genomic DNA (lane 3). No amplification is seen with nontransgenic FVB/N genomic DNA (lane 4). A 300 bp band, most likely representing contaminating genomic DNA, is amplified from lens RNA untreated with reverse transcriptase (RT -) (lanes 6 and 9) and from reverse transcribed total RNA isolated from the rest of the eye (ROE) (lanes 7 and 10).
Elevated levels of NT-3 in the eye
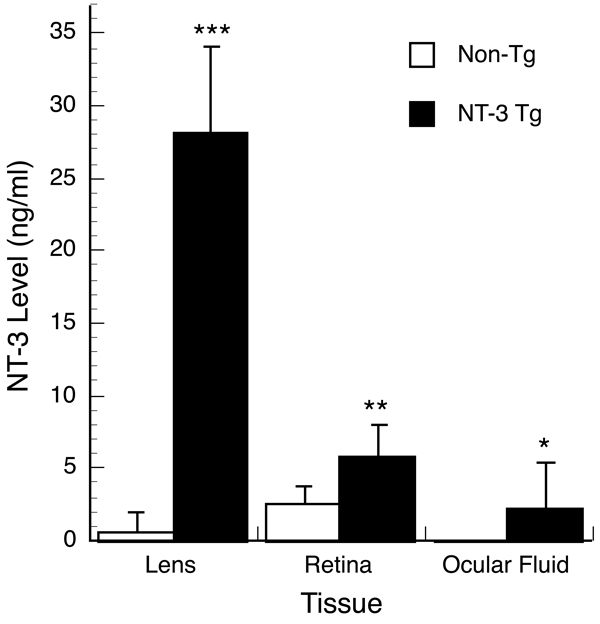
NT-3 levels were measured by ELISA in the lens, ocular fluids and retina of 10 week old mice from OVE613 (Fig. 4) and OVE614 (data not shown). Tg mice from both lines had greater than normal ocular levels. Although the ELISA does not distinguish endogenous from transgene-derived NT-3, the level of NT-3 in the Tg lens was approximately 40-fold higher than that detected in the non-Tg lens. The level of NT-3 detected in the Tg neural retina was 2-fold higher than that detected in the non-Tg retina. The higher than wild-type levels of NT-3 seen in the retina and ocular fluids of the Tg mice suggested that NT-3 was being secreted from the lens through the lens capsule and into the ocular media. This notion is supported by the presence of corneal abnormalities in these mice despite the fact that the cornea does not express the transgene. Despite expressing appreciable amounts of NT-3, lenses from OVE613 and OVE614 mice remain clear, without cataracts, even in animals over one year of age. It is unclear why more NT-3 was found in the retina than in the ocular fluids (Fig. 4). Explanations include possible binding and concentration of the lens-derived NT-3 by the retina, the inclusion of endogenous NT-3 in the measurements, and the technical difficulty of obtaining ocular fluids in the mouse eye, which gives a much higher variability to these measurements than those of the retina (Fig. 4). The levels of NT-3 expression were similar for lines OVE613 and OVE614 (data not shown). Line OVE613 was used for the neuroprotection studies.
Figure 4.
ELISA measurements of NT-3 expression. NT-3 protein levels were assayed in the lenses, ocular fluids and retinas of Tg and non-Tg mice. The lenses of NT-3 Tg mice contain about 40 times as much NT-3 as non-Tg lenses. The ocular fluids (vitreous and aqueous humor) contain detectable NT-3 in the Tg but not non-Tg mice. The NT-3 content of the retinas in Tg mice is twice that of non-Tg mice. The lenses, retinas and ocular fluids (aqueous and vitreous humors combined) were harvested from 13 separate NT-3 Tg mice and 20 non-Tg mice at P70–75. The tissues from both eyes of each mouse were pooled so that the values shown are per two eyes. *P < 0.005; **P < 5 × 10−6; ***P < 5 × 10−19. The small amount of ocular fluid was difficult to collect, and in 7 of the 13 Tg animals the NT-3 level was below the limit of detection. If these 7 mice are omitted from the calculation, the value of the ocular fluids is 4.81 ± 3.07 ng/ml, and the statistical difference between the Tg and non-Tg mice is more significant (P < 5 × 10−7).
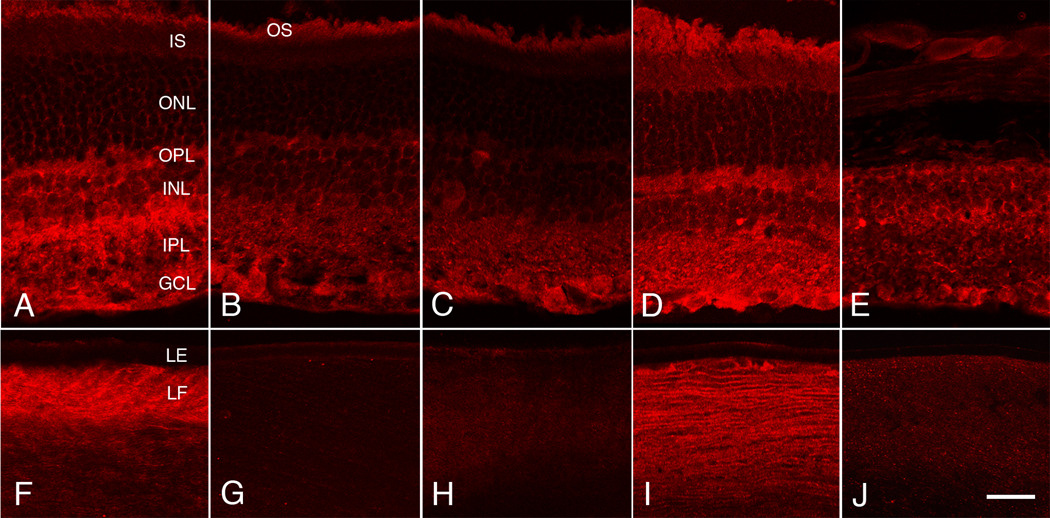
Immunocytochemical localization of NT-3 in the eyes of wild-type mice showed virtually no detectable NT-3 in the lens (Fig. 5G), but a small to moderate level of immunoreactivity was found in the inner retina (Fig. 5B). The immunoreactivity was primarily restricted to the retinal ganglion cell cytoplasm, the cytoplasm of some cells of the inner nuclear layer, the inner and outer plexiform layers and optic nerve fiber layer. Little or no NT-3 was found in the outer nuclear layer or among the photoreceptor inner segments (Fig. 5B). NT-3 has previously been shown to be expressed in some amacrine cells and scattered retinal ganglion cells in the normal mouse eye, similar to our present findings, and an absence of NT-3 expression is found in the lens at all stages of development (Bennett et al., 1999). In the NT-3 Tg mice, strong immunoreactivity was present in the lens fibers, although not in the lens epithelium (Fig. 5F). Much stronger immunoreactivity was found in the retinas of these mice (Fig. 5A) compared to that in the non-Tg mice (Fig. 5B), although the localization was almost the same. In the Tg mouse retinas a small amount of immunoreactivity appeared to be present in the Müller cells or PR processes in the ONL (Fig. 5A). Immunoreactivity in both Tg and non-Tg retinas was present at 5, 8 and 14 months of age.
Figure 5.
Immunocytochemical assays for NT-3. Cryostat sections of retinas (top row) and lenses (bottom row) were assayed for NT-3 protein (red fluorescence) A and F, NT-3 Tg mouse with normal retina at age P409. B and G, non-Tg BALB/c mouse with normal retina at P413. C, D, H and I, mertkkd/+ mice, with normal retinas. C and H are non-Tg for NT-3, P150; D and I are Tg for NT-3, P180. E and J, mertkkd/mertkkd mouse, non-Tg for NT-3, with loss of photoreceptors at P180. Immunoreactivity with antibodies to NT-3 was present in the inner retina of both NT-3 Tg and non-Tg mice (A–E), and in the lenses of only the NT-3 Tg mice (F and I). The immunoreactivity in the lenses of Tg mice was present in the lens fibers (LF), but not in the lens epithelium (LE). In the retinas of NT-3 Tg mice (A and D), the intensity of immunoreactivity was greater than in the non-Tg mice (B, C and E). In both Tg and non-Tg retinas, NT-3 was detected primarily in the outer plexiform layer (OPL) and inner plexiform layer (IPL), with some signal present in neuronal cytoplasm in the inner nuclear layer (INL) and ganglion cell layer (GCL). Little or no signal was found in the outer nuclear layer (ONL) or among the photoreceptor inner segments, although the very small flecks of apparent immunoreactivity in the ONL in the Tg retinas (A and D) may represent Müller cell or PR localization. The apparent immunoreactivity of photoreceptor outer segments (OS) is due either to non-specific binding or autofluorescence, since it was still seen in control experiments in which the primary antibody was omitted (data not shown). Scale bar = 25 µm.
Overexpression of NT-3 has no negative effect on the outer retina
We have examined at least 50 NT-3 Tg mice microscopically ranging in age from P20 to 14 months of age, and in every case the outer retina appeared normal (Fig. 5A and D; Fig. 7A). The number of PR nuclei based on ONL thickness also was indistinguishable from that of wild-type (data not shown).
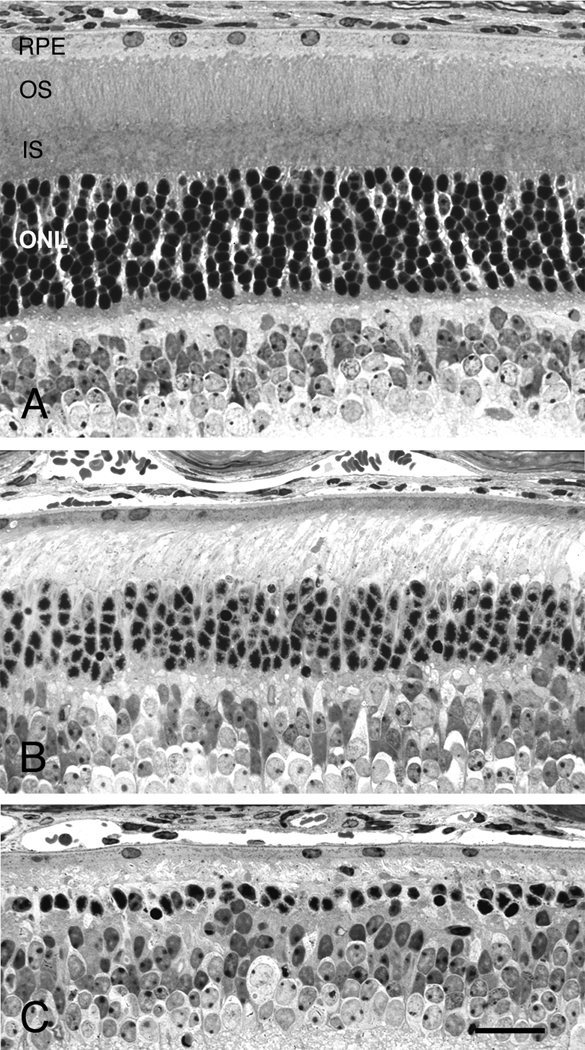
Figure 7.
Overexpression of NT-3 protects photoreceptors from constant light-induced degeneration. Light micrographs of the posterior retinas of NT-3 Tg mice either maintained in cyclic light (A) or exposed to constant light for 3 wks (B), and of a littermate non-Tg mouse exposed to 3 wks of constant light (C). A, The retina of the Tg mouse maintained in cyclic light is indistinguishable from that of wild-type, non-Tg mice. B, After 3 wks of constant light, the retina of the NT-3 Tg mouse shows the loss of most photoreceptor outer segments, but more than 50% of the photoreceptor nuclei in the outer nuclear layer (ONL) survived the light exposure. C, After 3 wks of constant light, the retina of the non-Tg mouse showed significantly greater damage than that of the NT-3 Tg mouse, with only a single row of photoreceptor nuclei surviving. Retinas from all were taken at P79; B and C, mice were placed into constant light at P58. IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium. Scale bar = 25 µm.
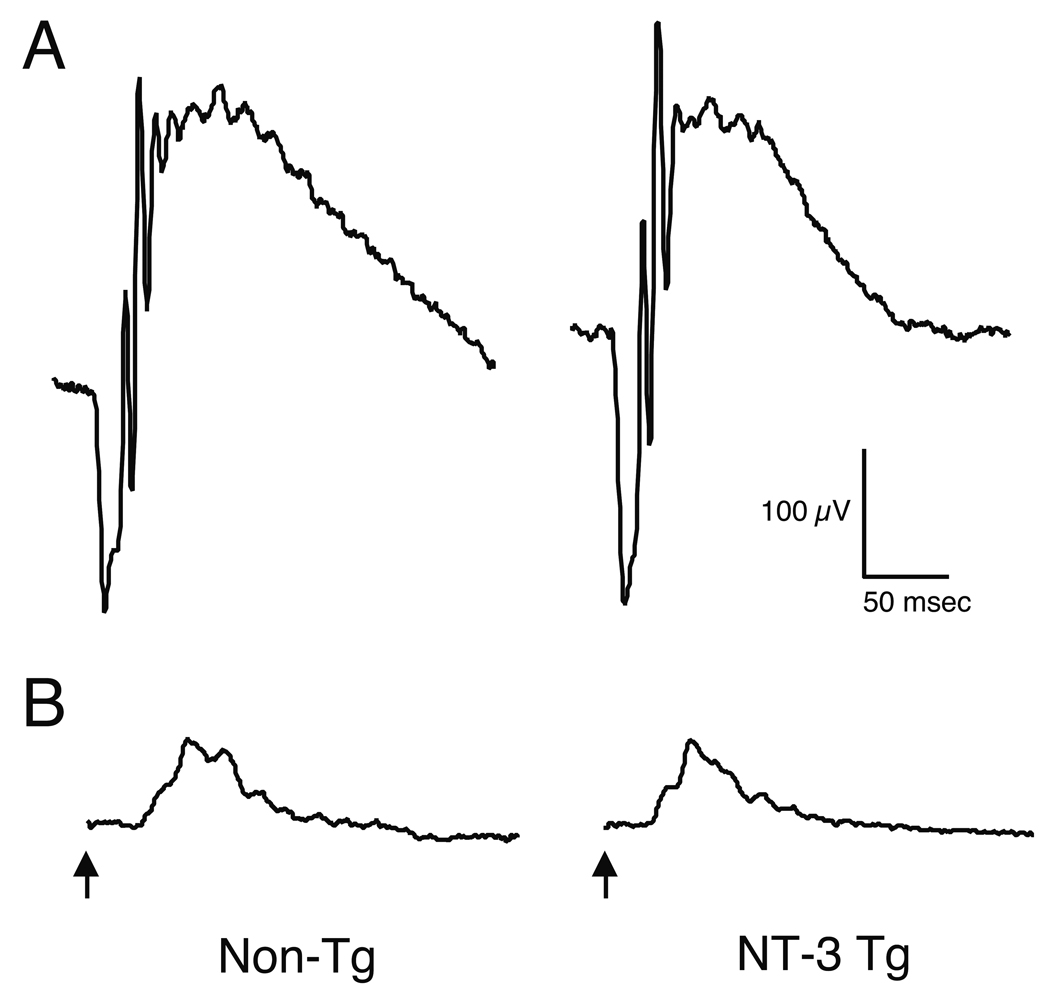
The retinas of NT-3 Tg mice also appear functionally normal when measured by the ERG. At least 3 Tg and 3 non-Tg mice have been examined both at P60 and P180, and the ERG responses from the Tg mice were indistinguishable in threshold, amplitude and timing from the responses of wild-type littermates. Representative scotopic and photopic ERG waveforms of Tg and non-Tg mice at P180 are shown in Fig. 6.
Figure 6.
Representative ERG waveforms from non-Tg and NT-3 Tg mice. Scotopic (A) and photopic (B) waveforms from single mice at the age of P180 are shown. The ERG responses from the Tg mice were indistinguishable in amplitude and timing from the responses of wild-type littermates at this and earlier ages. Arrows indicate the time of stimulus onset.
The apparent absence of structural or functional outer retinal abnormalities in the NT-3 Tg mouse retinas was evident despite the overexpression of NT-3 beginning at embryonic day 12.5 (Overbeek et al., 1985). It should be noted, however, that the NT-3 Tg mouse retinas exhibit accelerated retinal ganglion cell laminar refinement before eye opening, with an increase in dendritic branching and inhibition of dendritic elongation in ON-OFF retinal ganglion cells (X. Liu, A.M. Schreiber, V. Wu, M.L. Robinson, M.M. LaVail, J. Cang and D.R. Copenhagen, personal communication).
Overexpression of NT-3 protects photoreceptors from constant light damage
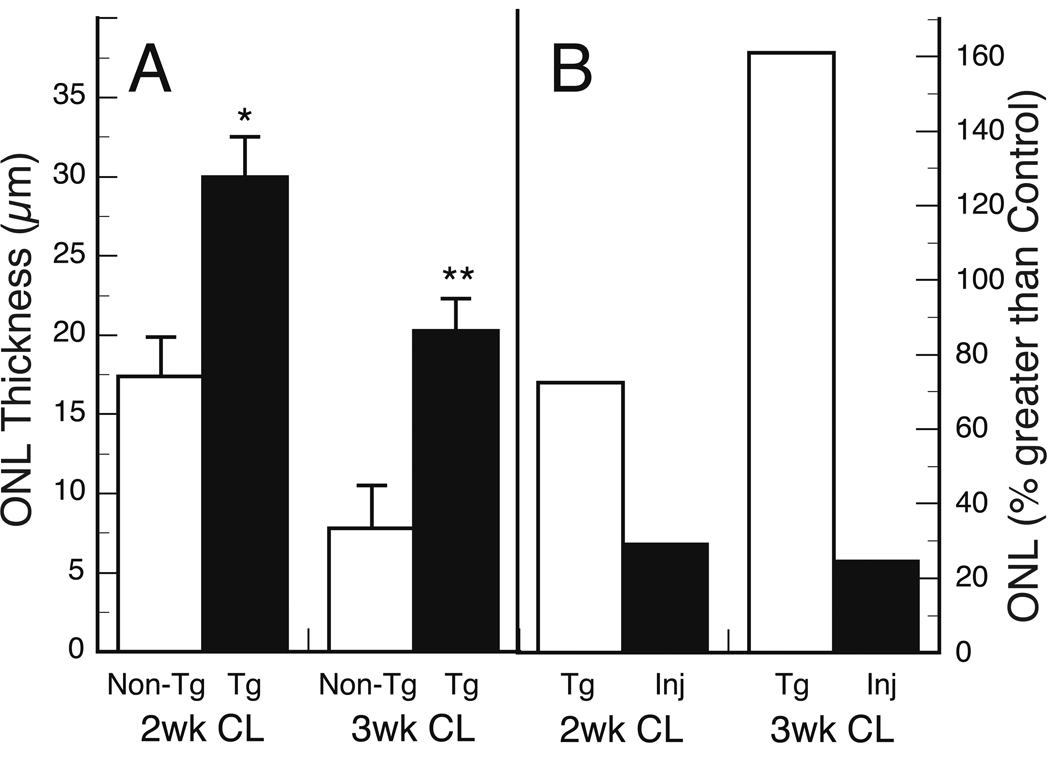
When groups of albino Tg and non-Tg littermates were exposed to constant light for 2- or 3-wk periods, the presence of the Tg slowed the rate of PR loss (Fig. 7 and Fig. 8). Constant light exposure in non-Tg mice caused the loss of PR cells as expected (LaVail et al., 1987a; LaVail et al., 1987b; LaVail et al., 1987c). After 2 wk in constant light, more than 50% of the PRs were lost, and the ONL layer contained about 3–4 rows of nuclei, or about 14–18 µm in thickness (Fig. 8A). After 3 wk of constant light, the ONL was reduced to about 1–2 rows of nuclei (Fig. 7C), or about 6–10 µm in thickness (Fig. 8A). In NT-3 Tg littermates, many more PR nuclei survive. After 2 wk in constant light, about 73% more PRs were present than in non-Tg littermates (Fig. 8A), and after 3 wk of constant light, the number of surviving PR nuclei was 2- to 3-fold the number in non-Tg littermates (Fig. 7A, 7C and Fig. 8A). At both the 2-wk (data not shown) and 3-wk (Fig. 7B and C) exposure period, more PR inner segments survived in the Tg mouse retinas. We have repeated the light damage study on an additional group of OVE613 mice, as well as a group (n=23) of OVE614 mice, with virtually the same results (data not shown).
Figure 8.
Protection from constant light (CL) damage by Tg overexpression of NT-3 and intravitreally injected NT-3. Outer nuclear layer (ONL) thickness is a measure of photoreceptor number. A, NT-3 Tg mouse retinas show survival of significantly more photoreceptor nuclei than non-Tg littermates after 2 or 3 wks of CL. *P < 5 × 10−5; **P < 5 × 10−7 B, Protection by Tg expression compared to protection by intravitreal injection of NT-3. The data from panel A are expressed as “percent greater than control” in B. The NT-3 injected eyes (Inj) show significantly less protection from the damaging effect of CL than that seen in mice with Tg overexpression of NT-3 (P <0.005 at both 2 wk and 3 wk). The difference between the injected and control eyes was significant at both 2 wk and 3 wk (P < 0.005 and P <0.01, respectively). All mice were put into CL at P58, and the data are based on 6–7 mice in each group. The ONL thickness of wild-type mice at this age is 40–45 µm.
To compare Tg overexpression of NT-3 with intravitreal injection of NT-3, BALB/cByJ mice of the same age (P58) were injected with NT-3, then 2 days later placed into constant light for either 2 or 3 wks. As shown in Fig. 8B, the injected NT-3 afforded relatively marginal protection. After 2 wk of constant light, about 29% more PR nuclei survived than in uninjected control mice, and after 3 wk of constant light, about 25% more PRs survived (Fig. 8B). This relatively minimal protection by injected NT-3 is virtually identical to that seen in the light-damaged albino rat (LaVail et al., 1992). By contrast, in the NT-3 Tg mice about 72% and 161% more PRs survived than in control eyes after 2 wk and 3 wk of constant light, respectively (Fig. 8B). Thus, continuous expression of NT-3 from the lens provides a much greater degree of protection from constant light damage to PRs than does bolus injection of NT-3.
Transgenic overexpression of NT-3 fails to slow four inherited retinal degenerations
Breedings were made to transfer the NT-3 transgene into lines of mice with four different inherited RDs to determine if, and to what degree, overexpression of NT-3 would slow the rate of PR degeneration. Three of the RD mutants have PR-specific alterations (rd/rd, Rds/+ and Q344ter) and the fourth is missing expression of a gene in retinal pigment epithelial cells that leads to secondary PR cell death (Mertk knockout). In each case, the eyes of mice were examined histologically using epoxy resin-embedded sections for highest light microscopic resolution. The eyes were examined at ages where any slowing of PR degeneration would be seen (LaVail et al., 1998).
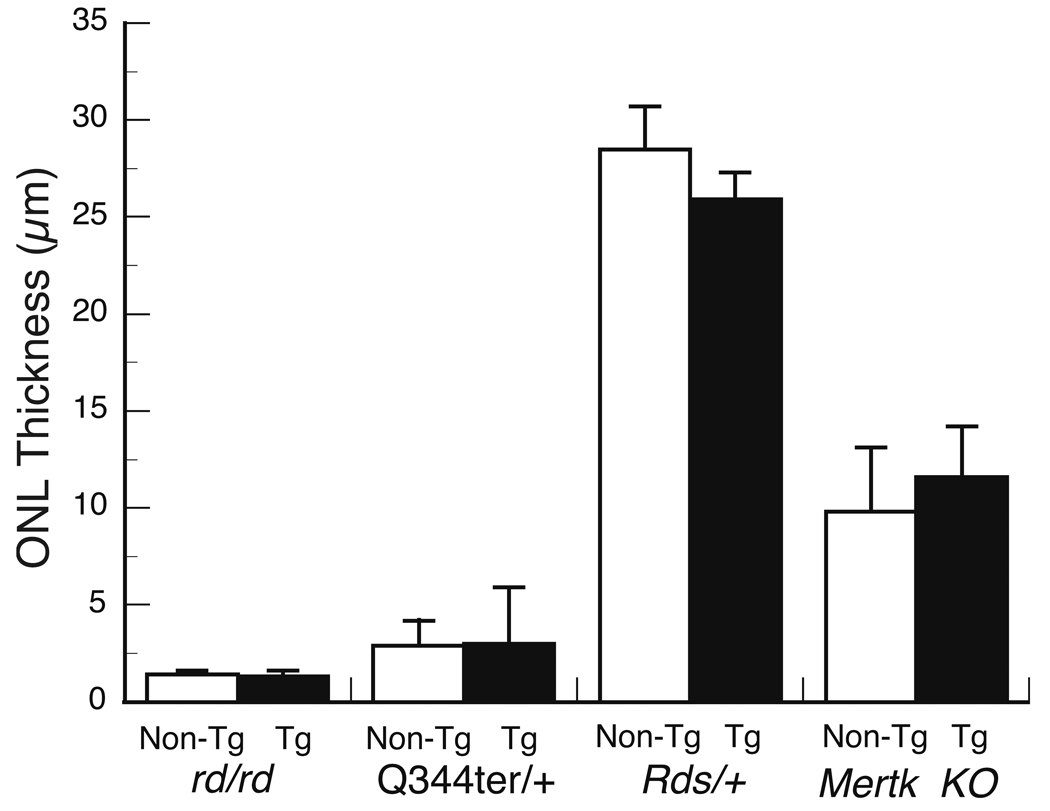
In each of the four crosses, there was no qualitative or quantitative difference between the NT-3 Tg and non-Tg littermates (Fig. 9). In the case of the Rds/+ retinas, we quantified only the the peripheral retina (Hawkins et al., 1985). Hawkins et al. (1985) found that the peripheral retina of Rds/+ mice showed significant reduction in thickness at all ages between P56 and 1 year, and that the difference in ONL thickness between the Rds/+ and wild-type at each age examined was 8–12 µm. Thus, if there had been protection from NT-3, we would have seen it in the Rds/+ mouse.
Figure 9.
Overexpression of NT-3 fails to slow photoreceptor degeneration in 4 inherited models for retinal degeneration. NT-3 Tg mice were bred to rd/rd, Q344ter/+, Rds/+ and mertkkd mice to provide retinal degeneration mutants either expressing the NT-3 transgene (Tg) or not (Non-Tg). The outer nuclear layer (ONL) thickness (mean ± SD) was determined at ages where protection would have been seen. No significant differences were found between the NT-3 Tg and non-Tg mice in any of the mutants. The ages and the number of mice quantified were the following: rd/rd, P20, n = 5 Tg and 5 non-Tg; Q344ter/+, P20, n = 5 Tg and 5 non-Tg; Rds/+, P55-P60, n = 9 Tg and 9 non-Tg; and mertk knockout, P40, n = 5 Tg and 7 non-Tg. As described in the Methods, the mean ONL thickness of each eye was based on 54 measurements around the eye, except for the Rds/+ mice, where 6 measurements were made in the peripheral 500 µm of both the superior and inferior hemispheres (i.e., 12 measurements from each eye). The mean ONL thickness of the peripheral retina of wild-type mice was 37.1 ± 2.9 µm.
In addition to using PCR to identify those mice carrying the NT-3 transgene in these crosses with RD mutant lines, we confirmed by immunocytochemistry that the NT-3 Tg mice contained an overabundance of NT-3 in the lens and retina, and that the non-Tg mice do not, despite the several breeding crosses. For example, in Figs. 5C–E and Figs. 5H-J, heterozygous Mertk knockout mice with normal retinas that were identified as NT-3 Tg by PCR showed increased NT-3 levels in the lens and retina (Fig. 5D and I), as did the wild-type NT-3 Tg mice (Figs. 5A and F). Those mice that did not carry the NT-3 transgene, regardless of their RD genotype, showed no NT-3 immunoreactivity in the lens (Figs. 5H and J) and only endogenous, lower levels of immunoreactivity in the inner retina (Figs. 5C and E), identical to that of non-Tg wild-type mice (Fig. 5B and G).
The crosses to produce experimental mice were made with different strains and lines of animals with different numbers of backcrosses to inbred and mixed strains. As noted above in the case of light damage, the presence of modifying genes that slow PR degeneration can be seen when some of these crosses are made (Danciger et al., 2000; LaVail et al., 1987b). It is highly unlikely that such genes influenced the results in the present experiments with mutant mice, since the state of degeneration for each of the mutants at a given age (Fig. 9) was exactly that expected from previous studies.
DISCUSSION
Overexpression of NT-3 affords greater protection from light damage than injection
In this study, we have generated Tg mice that express NT-3 from the lens fibers under the control of the αA-crystallin promoter. The NT-3 is presumably secreted through the lens capsule, then moves through the vitreous and into the retina. This assumption is based on the presence of extremely high amounts of NT-3 in Tg mouse lenses, the presence of NT-3 in the ocular fluids, where it is absent in non-Tg mice, and the elevated amount of NT-3 in the transgenic neural retina as measured by ELISA. In situ hybridizations revealed expression of the transgene in the lens fibers of embryonic Tg mice, but not in the neural retina or other ocular structures, and RT-PCR of adult eyes similarly shows transgene transcription in the lens, but in no other ocular structures.
Intravitreally injected NT-3 protects PR cells in albino rats from degeneration due to the damaging effects of constant light, but its protective effect is significantly less than other agents, such as BDNF, CNTF and bFGF (LaVail et al., 1992). In the present study with albino mice, a similar low level of protection from light damage was afforded by intravitreal injection of NT-3, even at relatively high concentration. However, the overexpression of NT-3 in albino Tg mice led to significant protection from the damaging effects of constant light on PR cells. This finding adds further evidence that sustained delivery of NTFs is significantly more effective than bolus injection of these agents.
Specificity of NT-3 protection in retinal degenerations
To assess whether NT-3 can also protect PR cells from genetically caused RD, we bred the NT-3 transgene into four lines of mice with inherited RDs. The inherited RDs in these mice represent mutants with PR defects in a structural protein (Rds/+), a transduction enzyme (rd) and the visual pigment, rhodopsin (Q344ter), as well as the knockout of a gene expressed in the retinal pigment epithelium (Mertk). In all of these cases, the overexpression of NT-3 failed to slow the inherited PR degeneration.
There are several implications of these findings. First, our results indicate that different NTFs have different neuroprotective specificities in vivo. CNTF has been found to protect PRs from degeneration in virtually every RD model in which it has been tested, either by intravitreal injection or by continuous delivery (Bok et al., 2002; Cayouette et al., 1998; Cayouette and Gravel, 1997; Liang et al., 2001a; Liang et al., 2001b; Tao et al., 2002; Wang et al., 2002). In contrast, transgenic overexpression of bFGF protects PRs from hyperoxia-induced damage, but does not slow the degeneration in rd/rd or Q344ter Tg mice (Yamada et al., 2001). Likewise, transgenic overexpression of human erythropoietin protects PRs from light-induced damage, but does not protect rd/rd or mutant rhodopsin Tg mice (Grimm et al., 2004). The differing susceptibility of inherited RDs to the protective effect of NTFs has significant ramifications for future therapeutic application of NTFs, suggesting that until some unifying mechanism(s) is discovered, the protective effect of NTFs may have to be determined empirically for specific RDs.
In all RDs that have been examined, PR cell death occurs by apoptosis. Grimm et al. (Grimm et al., 2004) suggest that apoptosis induced by acute light exposure occurs by a different mechanism than apoptosis in genetically based RD. The results of the present study support this possibility. In addition to NT-3 (present study), bFGF (Yamada et al., 2001) and erythropoietin (Grimm et al., 2004), some other agents protect PR cells from oxidative or light stress, but do not slow any or all inherited RDs. These include a free radical trap, phenyl-N-tert-butylnitrone (Ranchon et al., 2003), a nitric oxide synthase inhibitor, L-NAME (Kaldi et al., 2003) and lens epithelium-derived growth factor (Machida et al., 2001). There have also been a number of agents that protect PRs from light-induced damage which have not yet been examined in inherited RD models, such as α2-adrenergic agonists (Wen et al., 1996), a melatonin antagonist (Sugawara et al., 1998), dimethylthiourea and Ginko biloba extract (Ranchon et al., 1999) and various antioxidants (Reviewed by Organisciak and Winkler, 1994; Penn et al., 1987). Clearly, the light-induced apoptotic mechanisms are more susceptible to inhibition than those of most inherited RDs. Therefore, the screening of neuroprotective agents with the light-damage model must be followed by demonstration of effectiveness in inherited RD models to demonstrate relevance to human RDs.
Possible mechanisms of NT-3 specificity
The mechanism by which NT-3 provides selective neuroprotection is unclear. Hao et al. (2002) have demonstrated that light-induced RD has at least two apoptotic pathways. One requires rhodopsin activation but not transducin signaling, and is accompanied by the induction of c-fos and the consequent activation of the AP-1 transcription factor. The other pathway is transducin-dependent. Additional pathways of light damage may also exist given the various additional genes that regulate the degree of light damage (Hao et al., 2002). NT-3 may inhibit an apoptotic pathway induced by light that is not operative in inherited RD. Little is known, thus far, about apoptotic pathways in inherited degenerations, except that c-fos expression has no apparent role in apoptosis in rd/rd (Hafezi et al., 1998) or rhodopsin knockout (Hobson et al., 2000) mice.
It is also important to consider the induced expression of high-affinity neurotrophin receptors and the role of Müller cells in light-damaged and inherited RDs. In normal retinas, the Trk tyrosine kinase receptors to which neurotrophins bind with high affinity are not found in the outer nuclear layer (Cellerino and Kohler, 1997; Perez and Caminos, 1995; Rickman and Rickman, 1996; Rohrer et al., 1999; Suzuki et al., 1998), which has led, in part, to the “Müller cell hypothesis” of NTF protection of PRs (Harada et al., 2002; Harada et al., 2000; Peterson et al., 2000; Wahlin et al., 2001; Wahlin et al., 2000). According to this hypothesis, NTFs bind to high-affinity receptors on Müller cells (Harada et al., 2002; Harada et al., 2000; Taylor et al., 2003), including TrkC to which NT-3 binds (Harada et al., 2000). Neurotrophin binding leads to altered gene expression in Müller cells (Wahlin et al., 2001; Wahlin et al., 2000), and to up-regulation of bFGF (Harada et al., 2000). Müller cells then release bFGF (Harada et al., 2000), which binds to its receptors located on PRs (Fontaine et al., 1998; Kinkl et al., 2002; Kinkl et al., 2001; Valter et al., 2002), thereby protecting them from degeneration. Exogenous bFGF protects PRs from degeneration in some inherited RDs (Akimoto et al., 1999; Faktorovich et al., 1990; Lau et al., 2000) and in light-induced degeneration (Faktorovich et al., 1992; LaVail et al., 1992; Masuda et al., 1995). In light-induced RD, expression of the trkC gene is detected in PR cells (Harada et al., 2000). Thus, in this situation, NT-3 may act both directly on PRs, which may enhance the survival-promoting capability of NT-3 in this form of degeneration. It is not yet known whether trkC expression in PRs occurs in any inherited RD.
The up-regulation of TrkC receptors in Müller cells may also differ in light-induced and inherited RDs. In light-damaged retinas, TrkC expression in Müller cells is up-regulated (Harada et al., 2000), which may augment the protective effect of NT-3 in this form of degeneration. The status of TrkC receptors in Müller cells has not yet been explored in inherited RDs.
The incidence or behavior of activated microglia may also differ in light-induced and inherited RDs. Study of the role of microglia in RDs has grown enormously in the past decade (Reviewed in Langmann, 2007; Schuetz and Thanos, 2004). Strong arguments have been put forth that activated microglia not only phagocytose debris from dying PR cells, but also accentuate or potentiate PR cell death in RDs (Gehrig et al., 2007; Gupta et al., 2003; Roque et al., 1996; Srinivasan et al., 2004; Zeiss and Johnson, 2004; Zeng et al., 2005; Zhang et al., 2004; Zhang et al., 2005). Other studies support a potential protective role of microglia (Harada et al., 2002; Otani et al., 2004; Sasahara et al., 2008), with their secretion of many neurotrophic factors, including NT-3 (Harada et al., 2002; Langmann, 2007). It is clear that different pathways are at play in the activation and regulation of microglia (Langmann, 2007; Schuetz and Thanos, 2004; Yang et al., 2007), and that different etiologies of PR degeneration may result in different types of microglial involvement (Schuetz and Thanos, 2004). Indeed, the complexity of the damaging/protective role of microglia is apparent when it is considered that one of the most potent, presumably toxic, pro-inflammatory cytokines released by activated microglia is tumor necrosis factor alpha (TNFα) (Zeng et al., 2005), and yet TNFα is neuroprotective in light-induced RD (LaVail et al., 1992). A neuroprotective role for TNFα has not been examined in inherited RDs.
In summary, we have tested the hypothesis that sustained delivery of NT-3 in the eye can offer neuroprotection to photoreceptors subjected to either constant light or several different inherited retinal degenerations. Sustained ocular delivery of NT-3 in the eye was achieved using a transgene construct designed to express NT-3 specifically in the lens. Despite production and secretion of NT-3 by the lens, the lens remains clear and morphologically normal. We found that neuroprotection by NT-3 depended on sustained transgene-mediated delivery, as bolus injections of NT-3 were far less effective. Neuroprotection was specific for light-induced photoreceptor damage, as no protection was seen with four different genetic RD models. Therefore these mice provide a model system to distinguish the molecular mechanisms of apoptosis and neuroprotection for light-induced versus inherited retinal degenerations.
ACKNOWLEDGMENTS
The authors thank Cathy Lau-Villacorta, Nancy Lawson, George Nune, John Carter, Hilary Genise, Brad Wagner, Bhavani P. Madakashira and Jose Velarde for technical assistance, and Dr. Ann Acheson of Regeneron Pharmaceuticals for carrying out the ELISA measurements.
Support
This work was supported in part by National Institutes of Health Grants EY01919 (MML), EY02162 (MML), EY00415 (JLD), EY14650 (DV) and EY12995 (MLR); the Bernard A. Newcomb Macular Degeneration Fund (JLD); a Physician Scientist Award from Research to Prevent Blindness (JLD), the Kott Family Foundation (DV), the E. Matilda Ziegler Foundation (DV); the Karl Kirchgessner Foundation (DV); the Foundation Fighting Blindness, Inc. (MML, DV); the Macular Vision Research Foundation (MML); and That Man May See, Inc.
LITERATURE CITED
- Akimoto M, Miyatake S, Kogishi J, Hangai M, Okazaki K, Takahashi JC, Saiki M, Iwaki M, Honda Y. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci. 1999;40:273–279. [PubMed] [Google Scholar]
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- Bok D, Yasumura D, Matthes MT, Ruiz A, Duncan JL, Chappelow AV, Zolutukhin S, Hauswirth W, LaVail MM. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Behn D, Stendtner M, Lachapelle P, Gravel C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Human Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Kohler K. Brain-derived neurotrophic factor/neurotrophin-4 receptor TrkB is localized on ganglion cells and dopaminergic amacrine cells in the vertebrate retina. J Comp Neurol. 1997;386:149–160. [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Danciger M, Matthes MT, Yasumura D, Akhmedov NB, Rickabaugh T, Gentleman S, Redmond TM, LaVail MM, Farber DB. A QTL on distal chromosome 3 that influences the severity of light-induced damage to mouse photoreceptors. Mamm Genome. 2000;11:422–427. doi: 10.1007/s003350010081. [DOI] [PubMed] [Google Scholar]
- Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, Vollrath D. An RCS-like retinal dystrophy phenotype in Mer knockout mice. Invest Ophthalmol Vis Sci. 2003;44:826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V, Kinkl N, Sahel J, Dreyfus H, Hicks D. Survival of purified rat photoreceptors in vitro is stimulated directly by fibroblast growth factor-2. J Neurosci. 1998;18:9662–9672. doi: 10.1523/JNEUROSCI.18-23-09662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig A, Langmann T, Horling F, Janssen A, Bonin M, Walter M, Poths S, Weber BH. Genome-wide expression profiling of the retinoschisin-deficient retina in early postnatal mouse development. Invest Ophthalmol Vis Sci. 2007;48:891–900. doi: 10.1167/iovs.06-0641. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, Gassmann M, Reme C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Hafezi F, Abegg M, Grimm C, Wenzel A, Munz K, Sturmer J, Farber DB, Remé CE. Retinal degeneration in the rd mouse in the absence of c-fos. Invest Ophthalmol Vis Sci. 1998;39:2239–2244. [PubMed] [Google Scholar]
- Hao W, Wenzel A, Obin MS, Chen CK, Brill E, Krasnoperova NV, Eversole-Cire P, Kleyner Y, Taylor A, Simon MI, Grimm C, Reme CE, Lem J. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Hawkins RK, Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985;41:701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Hobson AH, Donovan M, Humphries MM, Tuohy G, McNally N, Carmody R, Cotter T, Farrar GJ, Kenna PF, Humphries P. Apoptotic photoreceptor death in the rhodopsin knockout mouse in the presence and absence of c-fos. Exp Eye Res. 2000;71:247–254. doi: 10.1006/exer.2000.0878. [DOI] [PubMed] [Google Scholar]
- Kaldi I, Dittmar M, Pierce P, Anderson RE. L-NAME protects against acute light damage in albino rats, but not against retinal degeneration in P23H and S334ter transgenic rats. Exp Eye Res. 2003;76:453–461. doi: 10.1016/s0014-4835(02)00334-2. [DOI] [PubMed] [Google Scholar]
- Kinkl N, Hageman GS, Sahel JA, Hicks D. Fibroblast growth factor receptor (FGFR) and candidate signaling molecule distribution within rat and human retina. Mol Vis. 2002;8:149–160. [PubMed] [Google Scholar]
- Kinkl N, Sahel J, Hicks D. Alternate FGF2-ERK1/2 signaling pathways in retinal photoreceptor and glial cells in vitro. J Biol Chem. 2001;276:43871–43878. doi: 10.1074/jbc.M105256200. [DOI] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Lau D, McGee LH, Zhou S, Rendahl KG, Manning WC, Escobedo JA, Flannery JG. Retinal degeneration is slowed in transgenic rats by AAV-mediated delivery of FGF-2. Invest Ophthalmol Vis Sci. 2000;41:3622–3633. [PubMed] [Google Scholar]
- LaVail MM, Battelle BA. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp Eye Res. 1975;21:167–192. doi: 10.1016/0014-4835(75)90080-9. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Repaci MA. Strain differences in sensitivity to light-induced photoreceptor degeneration in albino mice. Curr Eye Res. 1987a;6:826–834. doi: 10.3109/02713688709034850. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Repaci MA, Thomas LA, Ginsberg HM. Genetic regulation of light damage to photoreceptors. Invest Ophthalmol Vis Sci. 1987b;28:1043–1048. [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Repaci MA, Yasumura D. Light-induced retinal degeneration in albino mice and rats: strain and species differences. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Degenerative Retinal Disorders: Clinical and Laboratory Investigations. New York: Alan R. Liss, Inc.; 1987c. pp. 439–454. [PubMed] [Google Scholar]
- LaVail MM, Sidman RL. C57BL/6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974;91:394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung C-H, Steinberg RH. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Dejneka NS, Dudus L, Fisher KJ, Maguire AM, Jacobson SG, Bennett J. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001a;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Dejneka NS, Cohen DR, Krasnoperova NV, Lem J, Maguire AM, Dudus L, Fisher KJ, Bennett J. AAV-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol Ther. 2001b;3:241–248. doi: 10.1006/mthe.2000.0252. [DOI] [PubMed] [Google Scholar]
- Machida S, Chaudhry P, Shinohara T, Singh DP, Reddy VN, Chylack LT, Jr, Sieving PA, Bush RA. Lens epithelium-derived growth factor promotes photoreceptor survival in light-damaged and RCS rats. Invest Ophthalmol Vis Sci. 2001;42:1087–1095. [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Masuda K, Watanabe I, Unoki K, Ohba N, Muramatsu T. Functional rescue of photoreceptors from the damaging effects of constant light by survival-promoting factors in the rat. Invest Ophthalmol Vis Sci. 1995;36:2142–2146. [PubMed] [Google Scholar]
- McGee Sanftner LH, Abel H, Hauswirth WW, Flannery JG. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- Michon JJ, Li ZL, Shioura N, Anderson RJ, Tso MOM. A comparative study of methods of photoreceptor morphometry. Invest Ophthalmol Vis Sci. 1991;32:280–284. [PubMed] [Google Scholar]
- Okoye G, Zimmer J, Sung J, Gehlbach P, Deering T, Nambu H, Hackett S, Melia M, Esumi N, Zack DJ, Campochiaro PA. Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Winkler BS. Retinal light damage: practical and theoretical considerations. In: Osborne N, Chader G, editors. Progress in Retinal and Eye Research. Oxford: Pergamon Press; 1994. pp. 1–29. [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek PA, Chepelinsky AB, Khillan JS, Piatigorsky J, Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985;82:7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Naash MI, Anderson RE. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987;44:779–788. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- Perez MT, Caminos E. Expression of brain-derived neurotrophic factor and of its functional receptor in neonatal and adult rat retina. Neurosci Lett. 1995;183:96–99. doi: 10.1016/0304-3940(94)11123-z. [DOI] [PubMed] [Google Scholar]
- Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchon I, Gorrand JM, Cluzel J, Droy-Lefaix MT, Doly M. Functional protection of photoreceptors from light-induced damage by dimethylthiourea and Ginkgo biloba extract. Invest Ophthalmol Vis Sci. 1999;40:1191–1199. [PubMed] [Google Scholar]
- Ranchon I, LaVail MM, Kotake Y, Anderson RE. Free radical trap phenyl-N-tert-butylnitrone protects against light damage but does not rescue P23H and S334ter rhodopsin transgenic rats from inherited retinal degeneration. J Neurosci. 2003;23:6050–6057. doi: 10.1523/JNEUROSCI.23-14-06050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman DW, Rickman CB. Suppression of trkB expression by antisense oligonucleotides alters a neuronal phenotype in the rod pathway of the developing rat retina. Proc Natl Acad Sci USA. 1996;93:12564–12569. doi: 10.1073/pnas.93.22.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995a;121:3959–3967. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995b;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque RS, Imperial CJ, Caldwell RB. Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 1996;37:196–203. [PubMed] [Google Scholar]
- Sanyal S, De Ruiter A, Hawkins RK. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neur. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Otani A, Oishi A, Kojima H, Yodoi Y, Kameda T, Nakamura H, Yoshimura N. Activation of bone marrow-derived microglia promotes photoreceptor survival in inherited retinal degeneration. Am J Path. 2008;172:1693–1703. doi: 10.2353/ajpath.2008.080024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz E, Thanos S. Microglia-targeted pharmacotherapy in retinal neurodegenerative diseases. Curr Drug Targets. 2004;5:619–627. doi: 10.2174/1389450043345164. [DOI] [PubMed] [Google Scholar]
- Shirke S, Faber SC, Hallem E, Makarenkova HP, Robinson ML, Overbeek PA, Lang RA. Misexpression of IGF-I in the mouse lens expands the transitional zone and perturbs lens polarization. Mech Dev. 2001;101:167–174. doi: 10.1016/s0925-4773(00)00584-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan B, Roque CH, Hempstead BL, Al-Ubaidi MR, Roque RS. Microglia-derived pronerve growth factor promotes photoreceptor cell death via p75 neurotrophin receptor. J Biol Chem. 2004;279:41839–41845. doi: 10.1074/jbc.M402872200. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Sieving PA, Iuvone PM, Bush RA. The melatonin antagonist luzindole protects retinal photoreceptors from light damage in the rat. Invest Ophthalmol Vis Sci. 1998;39:2458–2465. [PubMed] [Google Scholar]
- Sung C-H, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Nomura S, Morii E, Fukuda Y, Kosaka J. Localization of mRNAs for trkB isoforms and p75 in rat retinal ganglion cells. J Neurosci Res. 1998;54:27–37. doi: 10.1002/(SICI)1097-4547(19981001)54:1<27::AID-JNR4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, Stabila PF, Bell WJ, Dean BJ, Kauper KA, Budz VA, Tsiaras WG, Acland GM, Pearce-Kelling S, Laties AM, Aguirre GD. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Müller cells. Brain Res Mol Brain Res. 2003;111:189–197. doi: 10.1016/s0169-328x(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Travis GH, Sutcliffe JG, Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Valter K, van Driel D, Bisti S, Stone J. FGFR1 expression and FGFR1-FGF-2 colocalisation in rat retina: sites of FGF-2 action on rat photoreceptors. Growth Factors. 2002;20:177–188. doi: 10.1080/0897719021000057617. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Adler R, Zack DJ, Campochiaro PA. Neurotrophic signaling in normal and degenerating rodent retinas. Exp Eye Res. 2001;73:693–701. doi: 10.1006/exer.2001.1078. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- Wang F, Xia X, Hu H, Li L, Tian Y, Chen X, Huang Q. [Liposome-mediated gene transfer into retina] Zhonghua Yan Ke Za Zhi. 2002;38:520–522. [PubMed] [Google Scholar]
- Wen R, Cheng T, Li Y, Cao W, Steinberg RH. a2-adrenergic agonists induce basic fibroblast growth factor expression in photoreceptors in vivo and ameliorate light damage. J Neurosci. 1996;16:5986–5992. doi: 10.1523/JNEUROSCI.16-19-05986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Ando A, Esumi N, Bora N, Saikia J, Sung CH, Zack DJ, Campochiaro PA. Fibroblast growth factor-2 decreases hyperoxia-induced photoreceptor cell death in mice. Am J Pathol. 2001;159:1113–1120. doi: 10.1016/S0002-9440(10)61787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO. A possible mechanism of microglia-photoreceptor crosstalk. Mol Vis. 2007;13:2048–2057. [PubMed] [Google Scholar]
- Zeiss CJ, Johnson EA. Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Invest Ophthalmol Vis Sci. 2004;45:971–976. doi: 10.1167/iovs.03-0301. [DOI] [PubMed] [Google Scholar]
- Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci. 2005;46:2992–2999. doi: 10.1167/iovs.05-0118. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2753–2759. doi: 10.1167/iovs.03-1344. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shen JK, Lam TT, Zeng HY, Chiang SK, Yang F, Tso MO. Activation of microglia and chemokines in light-induced retinal degeneration. Mol Vis. 2005;11:887–895. [PubMed] [Google Scholar]