Abstract
Sex differences are pervasive in schizophrenia, ranging from differences in the age of onset and symptoms of the illness to structural brain differences. Yet, there has been very little research on the interaction of these differences with established cognitive sex differences that exist in healthy populations. We tested 25 patients with schizophrenia and 17 healthy controls on a 2-dimensional task of object location memory. It has been previously shown that healthy females outperform healthy males on this task, a result that was upheld in this experiment. However, this female advantage is completely absent in patients with schizophrenia. This finding has important implications for the interpretation of clinical and physiological sex differences present in schizophrenia.
Keywords: Schizophrenia, sex differences, spatial abilities, object location memory
Introduction
It is generally accepted that, on average, males outperform females on most tests of spatial ability including mental rotation, spatial perception and virtual navigation (Voyer et al., 1995; Astur et al., 1998; Burton et al., 2005), while females, on average, have the advantage on verbal tasks such as verbal fluency (Burton et al., 2005; Weiss et al., 2006). Indeed, this spatial dominance by males is expressed not only in humans, but also in other non-human mammals, such as rodents (Beatty, 1979) and baboons (Vauclair et al., 1993). Several hypotheses have been put forth positing biological explanations for these sex differences including differences in gonadal hormones (Beatty, 1979; Williams et al., 1990) and the pacing of brain development (Waber, 1976; Sanders and Soares, 1986).
The developmental pacing hypothesis states that the presence of sex differences on spatial and verbal abilities may be due to an earlier puberty in females, which has been shown to correlate with a verbal specialization at the expense of spatial abilities. To this end, it has been shown that children who reach sexual maturity at a later age become significantly better at spatial tasks than verbal tasks regardless of sex, whereas children who reach sexual maturity at an earlier age show the opposite relation (Waber, 1976; Sanders and Soares, 1986). Moreover, those who mature later demonstrate a stronger lateralization of language abilities than those who mature earlier, suggesting that bilateral dedication of areas to verbal abilities may be impeding the development of spatial abilities (Waber, 1976).
Yet, incongruent with these expected sex differences, women have been shown to outperform men on tests of object location memory (Silverman and Eals, 1992). Specifically, when women and men are given 2-dimensional arrays of objects to study, then asked to examine new arrays in which some of the objects have moved position and requested to identify those objects that have moved, women tend to score significantly higher than men (Silverman and Eals, 1992; Eals and Silverman, 1994; McBurney et al., 1997). One caveat to this female advantage was presented by James and Kimura (1997), in which males and females performed equally on the task if objects in the new array were moved to new positions rather than simply exchanged. However, more recent research contradicts this finding, upholding the female advantage even in the case where objects are shifted to new positions (Levy et al., 2005). A proposed explanation for these results has ascribed women’s advantage to their superior verbal skills and thus ability to better name the objects in question, which could aid in their memory for objects. Alternately, some have argued that these abilities are a byproduct of women’s theorized role as gatherers in early human societies, a role that would be benefited by superior abilities to locate and remember certain objects (i.e. plants), while men derive their spatial abilities from their ancestral roots as hunters, who had to navigate large expanses of territory (Eals and Silverman, 1994).
However, the argument that females rely on their superior verbal skills to complete this task is partially refuted by the evidence that females continue to display significant location memory advantages over men even if the objects in the array are uncommon, thus making it difficult to assign a verbal tag (Eals and Silverman, 1994). The hunter-gatherer theory is equally suspect given that non-human animals, such as rats, display the same male preference for geometric cues in solving mazes, while female rats, like female humans, use landmark cues (Williams et al., 1990). Moreover, homosexual males show the same advantage over heterosexual males as females on the object array task (Rahman et al., 2003).
While the exact etiology of cognitive sex differences is elusive and still under investigation, sex differences also extend from cognitive to psychiatric domains in that there are pronounced sex differences within schizophrenia, in age of onset, clinical presentation and neuroanatomical structure (DeLisi et al., 1989; Cowell et al., 1996; Frederikse et al., 2000; Narr et al., 2001). For example, males with schizophrenia have an earlier age of onset, generally developing the disease 3 to 4 years earlier than females, depending on the diagnostic criteria used (Hafner, 2003). In addition, males with schizophrenia generally have less favorable outcomes and more negative symptoms, while females with schizophrenia have better outcomes and more positive symptoms (Lewine, 1981; DeLisi et al., 1989). In addition to these clinical differences, a range of sex differences in the brain anatomy of patients with schizophrenia have emerged, including larger ventricular volumes in males than females, hippocampal asymmetries in males that are not present in females with schizophrenia, and abnormalities in the expression of sex differences in the inferior parietal lobule (Bryant et al., 1999; Frederikse et al., 1999; Frederikse et al., 2000; Narr et al., 2001).
However, there is some debate surrounding the issue of hippocampal abnormalities in schizophrenia. Some studies report reduced hippocampal volumes in schizophrenia (Nelson et al., 1998; Velakoulis et al., 1999; Narr et al., 2001; Sim et al., 2006), while others report no reductions in hippocampal volume (Kelsoe et al., 1988; Swayze et al., 1992; Tanskanen et al., 2005). Still other studies report functional abnormalities of hippocampal brain activations during fMRI memory tasks (Ongur et al., 2006), specific reductions in the density of nonpyramidal cells in the CA2 region of the hippocampus (Benes et al., 1998), dysfunctional inputs to the hippocampus originating in the amygdala (Benes and Berretta, 2000), and reductions in hippocampal volume that are shifted toward anterior regions of the hippocampal body (Narr et al., 2004). Therefore, while the true nature of the hippocampal abnormalities that accompany schizophrenia may be vastly more complicated than a simple reduction in volume, the majority of evidence indicates the existence of some structural and functional hippocampal differences in schizophrenia as compared to healthy populations. Moreover, the evidence for the differential expression of these differences by sex indicates that hippocampal irregularities could produce cognitive sex differences in patients with schizophrenia (Bryant et al., 1999; Narr et al., 2001).
The effects of schizophrenia on established sex differences in the inferior parietal lobule may also give rise to cognitive sex differences. In healthy populations, males display a leftward asymmetry in the inferior parietal lobule that is not present in females, leading to significantly larger left inferior parietal lobule volumes in males as compared to females (Frederikse et al., 1999). This asymmetry may be relevant to cognitive sex differences as mental rotation tasks have been linked to lateralized activity in the left inferior parietal lobule (Alivisatos and Petrides, 1997). However, male patients with schizophrenia fail to display the leftward asymmetry that is present in healthy males, possibly indicating that sex differences within schizophrenia would differ as compared to those in healthy populations (Frederikse et al., 2000).
Due to the differential expression of the illness across sex and the sex differences in brain anatomy that accompany schizophrenia, particularly in the hippocampus and inferior parietal lobule, there exists the possibility that schizophrenia may have sex specific associations on spatial cognitive tasks. This has been investigated on the delayed-response task, where no sex difference was found between males and females with schizophrenia (Minor and Park, 1999, Myles-Worsley and Park, 2002). However, there were also no sex differences between control subjects on this task (Minor and Park, 1999). Thus it is unclear whether schizophrenia impacts established spatial cognitive sex differences in healthy populations. We addressed this issue by testing a group of males and females with schizophrenia and healthy controls on a 2-dimensional object array task —a spatial task on which healthy females have been shown to hold the advantage over healthy males. By doing so, we hoped to test whether established sex differences are retained or reduced in patients with schizophrenia.
Materials and Methods
Participants
Patients with schizophrenia were recruited by referrals from the Institute of Living, as well as from local advertisements, flyers, and word of mouth. All participants were screened via the Structured Clinical Interview for the DSM-IV-TR (SCID), and symptom assessment was conducted on the people with schizophrenia using the Positive and Negative Syndrome Scale (PANSS). All patients with schizophrenia were currently on a variety of medications to manage their symptoms. The majority of these medications were atypical antipsychotics. All subjects presented with normal or corrected-to-normal vision, no history of traumatic brain injury, and proficiency in English. Control subjects with current substance abuse or dependence or any Axis I disorder were excluded from participating, but past substance abuse or dependence was allowed. The patient group was also free of current substance abuse or dependence. All subjects provided written, informed consent for this study, which was approved by the Hartford Hospital Institutional Review Board. Subjects were compensated for their participation with payments of $20 an hour. 25 patients with schizophrenia or schizoaffective disorder (15 males, average age = 41.5±10.1; 10 females, average age = 45.5±2.8) and 17 healthy control subjects (10 males, average age = 40.2±8.0; 7 females, average age = 44.9±9.0) were recruited for this study. There were no significant differences in handedness between any of the groups (laterality quotient, p > 0.1; right hand ratio, p > 0.1) as confirmed by the Handedness and Right-Left Orientation diagnostic questionnaire. The average PANSS positive symptoms score was 2.5 ± 1.5, and the average negative symptoms score was 2.0 ± 1.4. Patients with schizophrenia had significantly lower IQs as estimated by the Hopkins Adult Reading Test (Full Scale Intelligence Quotient: Sz = 101 ± 12; Controls = 112 ± 7; F(1,37)=12.49, p < 0.001). Moreover, the patients with schizophrenia and controls significantly differed on years of education (Sz = 9.8 + 3.3; Controls = 12.6 + 2.4. However there were no IQ differences between ages or between sexes in either the healthy control or patient group (p > 0.1).
Task
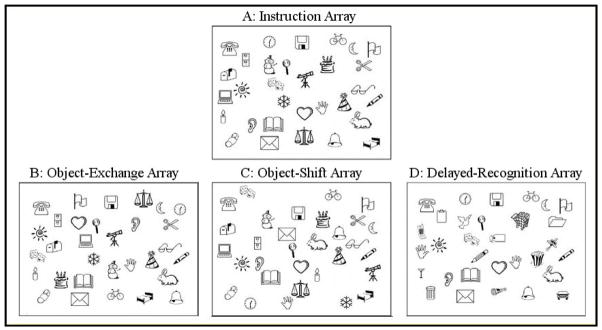
As part of a larger study, participants were presented with a black and white array of recognizable, 2-dimensional objects and asked to study the objects for one minute (see fig. 1A). During this time, participants were explicitly told to remember the location of the objects. After one minute with the original array, participants were presented with a new array in which the locations of some of the objects were exchanged, but no new images were introduced (object-exchange condition, see fig. 1B). Subjects were given one minute to circle the objects that had changed position from the original array. Next, participants were presented with a second test array in which some objects from the original array had moved to new locations that were previously unoccupied by any object (object-shift condition, see fig. 1C). Participants were again given one minute to circle any objects that had changed position from the original array.
Figure 1.
Participants were given one minute to study the instruction array (A), then one minute each to circle exchanged, shifted or new items on the test arrays (B–D).
Participants completed the final task in this experiment following a delay of approximately 20 minutes. After the object-exchange and object-shift tests, subjects were given a series of intervening cognitive tasks, without being told that the object location task would be revisited. However, after the intervening tasks (approx. 20 min.), subjects were told to recall the original array. A third new array was then presented in which new objects appeared in place of some of the objects in the original array (delayed-recognition condition, see fig. 1C). Subjects were given one minute to circle any objects that were new to the array. For each of the tests, score was defined as the number of correct responses minus the number of incorrect responses.
Results
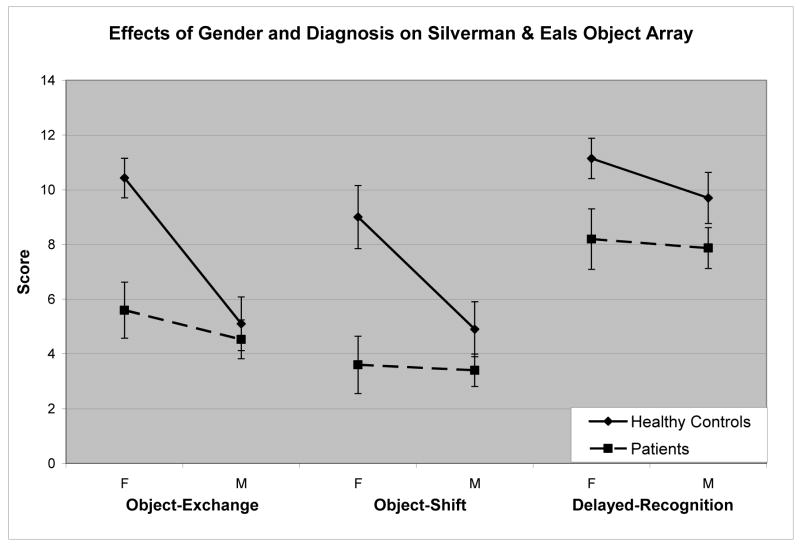
A multivariate analysis of the effects of sex and diagnosis on score (correct minus incorrect) revealed several significant correlations. As expected, women performed significantly better than men on both the object-exchange (F(1,38) = 12.29, P < 0.001) and object-shift (F(1,38) = 5.38, P < 0.05) tasks. However, there was no significant effect of sex on the delayed-recognition task (P > 0.1). The effects of diagnosis were significant on all of the tasks, with schizophrenic patients in each case performing worse than the healthy controls (object-exchange: F(1,38) = 8.75, P < 0.005; object-shift: F(1,38) = 13.85, P < 0.001; delayed-recognition: F(1,38) = 6.45, P < 0.05). However, importantly, there was a significant interaction effect of sex and diagnosis on both the object-exchange (F(1,38) = 5.46, P < 0.05) and object-shift (F(1,38) = 4.43, P < 0.05). Simple effects tests indicate that for both the object-exchange task and the object-shift task, the females healthy control participants perform significantly better than the other three groups (p < 0.001), but the other three groups do not significantly differ from each other (p > 0.05). This indicates that the main effects of group and sex over these two tasks were carried by the female healthy control participants. The delayed-recognition task showed no significant interactions (P > 0.1). Fig. 2 illustrates each of these effects.
Figure 2.
The significant interaction of sex and diagnosis on both the object-exchange and object-shift conditions is due to significantly superior performance by the female healthy control group. On the delayed-recognition condition, patients with schizophrenia performed significantly worse than healthy controls with no significant effect of sex.
The results confirm the previous finding that healthy women outperform healthy men on a 2-dimensional test of object location. Furthermore, they establish that patients with schizophrenia are impaired on this task of object location memory as compared to healthy controls. Most importantly, they indicate that the sex difference present in healthy controls is absent in patients with schizophrenia.
Discussion
This experiment provides evidence for the abolishment of an established cognitive sex difference in patients with schizophrenia. We acknowledge that the sample size in this study is small, limiting the generalizability of our conclusions. However, we believe that this study provides insight into a rarely investigated realm of mental illness in a patient population that is difficult to study. We find an abolishment of the established cognitive sex difference in patients with schizophrenia in spite of the fact that the task chosen was one which favors females, who, in schizophrenia, have later onsets and a generally less severe course of the illness than their male counterparts (Lewine et al., 1981; DeLisi et al., 1989; Hafner, 2003). Indeed, Goldstein et al. (1998) reported that male patients with schizophrenia were significantly impaired on tests of attention, verbal memory, and executive function as compared to female patients. Based on this information and the fact that there was no IQ difference between male and female patients with schizophrenia, there is no reason to believe that the abolishment of the sex difference seen on this task in patients is due to a more disrupted performance in female patients than male patients. Rather, these results point to a specific erosion of the female advantage on this particular spatial task in schizophrenia. This is substantiated by the fact that, on the object-exchange condition, patients with schizophrenia performed as well as healthy males. Thus, these results are not attributable to an overall impairment in schizophrenia, but, in fact, represent the loss of the specific female advantage.
While the cause of this effect is unknown, there are several reasonable explanatory hypotheses. The first is that developmental differences between healthy females and those with schizophrenia underlie the lack of expression of the female advantage on this task in patients with schizophrenia. Specifically, children who later go on to develop schizophrenia display mild, but significant delays in achieving early developmental milestones such as standing, walking and talking (Tarrant et al., 1999) and are deficient in comparison to their peers on tests of attention and verbal short-term memory (Niemi et al., 2003).
Moreover, children who go on to develop schizophrenia exhibit abnormal play behaviors with respect to their peers. Schiffman et al. (2004) who had videotaped high-risk and control children as they interacted, found that children who went on to develop schizophrenia as adults had significantly lower scores on a childhood sociability scale than those who did not. These results are in agreement with other published studies, which identify poor social competence as one trait often present in children who will go on to develop the illness (Tarrant et al., 1999; Niemi et al., 2003). This information, together with the data that links earlier puberty to enhanced abilities on verbal tasks (Waber, 1976; Sanders and Soares, 1986), suggests that female children who are predisposed to schizophrenia may not specialize in verbal skills to the same degree as their peers, possibly due to delayed development and lack of socialization. If superior verbal skills are an important driving factor of female dominance on this task in healthy populations, these developmental differences may be at the root of the lack of expression of such a sex difference in patients with schizophrenia. However, there is some evidence that the sex difference seen on this task may not be entirely due to differences in verbal abilities, casting some doubt on this explanation (Eals and Silverman, 1994). Future experiments will seek to further quantify the contribution of verbal abilities to the female advantage on this task and specifically test the possible verbal deficits suggested by this result in female patients with schizophrenia.
Related to these developmental differences are differences in hormonal expression in patients with schizophrenia, which could be suppressing the female advantage on this 2-dimensional object array task. Females with schizophrenia have lower levels of estrogen than healthy females (Oades and Schepker, 1994; Riecher-Rossler et al., 1994; Goldstein, 2006), and these lower estrogen levels were significantly related to lower scores on tests of verbal memory and verbal fluency (Ko et al., 2006). While the exact relationship between estrogen levels and verbal abilities has not been studied in healthy human populations, there is some evidence that early estrogen exposure may mediate spatial sex differences in rats (Williams et al., 1990). Further studies of this task, combined with hormonal measurements, may be able to address this with greater precision.
Likewise, the reason for the impaired performance of patients with schizophrenia as compared to healthy controls, particularly on the delayed-recognition condition, is also unknown. Possibilities include interference from the psychotic symptoms of the illness, general cognitive impairments which accompany schizophrenia (Nuechterlein et al. 2004; Bowie and Harvey, 2005), and brain abnormalities in patients with schizophrenia, including reduced or abnormal hippocampal volumes (Nelson et al., 1998; Velakoulis et al., 1999). Indeed, based on results of the Hopkins Adult Reading Test, an estimate of IQ, patients with schizophrenia were significantly impaired with respect to healthy controls in this experiment.
In addition to revealing the lack of a sex difference in patients with schizophrenia, this experiment substantiates evidence from Levy et al. (2005) that the female advantage on this task in healthy subjects extends to the object-shift condition, in opposition to evidence from James and Kimura (1997). There are several subtle methodological differences that may account for this discrepancy. First, the array used in this experiment and in Levy et al. (2005), although similar in nature to those in James and Kimura (1997), differed slightly in that we used 31 objects in the array versus 27 objects in James and Kimura (1997) and the objects themselves were different. It is possible, although unlikely, that differences in object salience or the inclusion of four extra objects could have influenced the results. Another difference is that subjects in the James and Kimura experiment only participated in either the object-exchange or object-shift condition, whereas our subjects participated in both, sequentially. It is possible that the first test array in this experiment (object-exchange condition) affected performance on the second array (object-shift condition), either enhancing female performance, possibly through rehearsal of object location, or degrading male performance through interference.
The final methodological difference was in the scoring of results. In the James and Kimura study, participants were required to mark every object that had moved with an “X” and circle every object that had not moved. In this way, the participant was required to register an answer for every object in the array, and score was calculated as the number of objects correctly marked. In the current experiment, participants were instructed only to circle each object that had moved and score was calculated as the number of correctly circled objects minus the number of incorrectly circled objects. While both these methods should acceptably assess behavior, James and Kimura may have induced a more active strategy by forcing a choice on certain objects that the participant may have been unsure of, while in this study participants were allowed the option to leave a particular object blank, thus neither gaining nor losing points. Whether the divergent results between this study and that of James and Kimura are due to one or a combination of the above methodological issues, the methods used in this experiment and that of Levy et al. (2005) provide internally reliable results.
The evidence that patients with schizophrenia do not display the same sex difference as healthy controls on a task of object location memory has important implications for the interpretation of the physiological and phenomenological sex differences that exist within schizophrenia. However, this experiment only encompasses one specific task. In future experiments, it will be interesting to categorize the presence or absence of sex differences in patients with schizophrenia on additional tasks that have established sex differences in healthy populations. In particular, the investigation of tasks on which healthy males have been shown to outperform healthy females would greatly expand our understanding of this phenomenon. Likewise, it would be especially interesting to record the performance of at-risk children and adolescents over time as compared to control groups on tasks that have been shown to invoke a sex difference. Knowing whether these differences pre-exist the symptoms of schizophrenia could help to define the progression of the disease and may lead to behavioral markers that could be used to identify at-risk individuals. Moreover, functional brain imaging during this and other tasks may provide us with more insight into the fundamental brain activity changes that accompany the differences seen in performance both between sexes and between patient and healthy populations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alivisatos B, Petrides M. Functional activation of the human brain during mental rotation. Neuropsychologia. 1997;35(2):111–18. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behavioral Brain Research. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: Organizational and activational influences. Hormones and Behavior. 1979;12:112–163. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Annals of the New York Academy of Sciences. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biological Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. The Psychiatric Clinics of North America. 2005;28(3):613–33. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: A volumetric MRI study. American Journal of Psychiatry. 1999;156(4):603–609. doi: 10.1176/ajp.156.4.603. [DOI] [PubMed] [Google Scholar]
- Burton LA, Henninger D, Hafetz J. Gender differences in relations of mental rotation, verbal fluency, and SAT scores to finger length ratios as hormonal indexes. Developmental Neuropsychology. 2005;28(1):493–505. doi: 10.1207/s15326942dn2801_3. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE. Sex differences in neuroanatomical and clinical correlations in schizophrenia. American Journal of Psychiatry. 1996;153(6):799–805. doi: 10.1176/ajp.153.6.799. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Dauphinais D, Hauser P. Gender differences in the brain: Are they relevant to the pathogenesis of schizophrenia? Comprehensive Psychiatry. 1989;30(3):197–208. doi: 10.1016/0010-440x(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Eals M, Silverman I. The hunter-gatherer theory of spatial sex differences: Proximate factors mediating the female advantage in recall of object arrays. Ethology and Sociobiology. 1994;15:95–105. [Google Scholar]
- Frederikse M, Lu A, Aylward E, Barta P, Pearlson G. Sex differences in the inferior parietal lobule. Cerebral Cortex. 1999;9(8):896–901. doi: 10.1093/cercor/9.8.896. [DOI] [PubMed] [Google Scholar]
- Frederikse M, Lu A, Aylward E, Barta P, Sharma T, Pearlson G. Sex differences in inferior parietal lobule volume in schizophrenia. American Journal of Psychiatry. 2000;157(3):422–27. doi: 10.1176/appi.ajp.157.3.422. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Hormones and Behavior. 2006;50:612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT. Are there sex differences in neuropsychological functions among patients with schizophrenia? American Journal of Psychiatry. 1998;155:1358–1364. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- James TW, Kimura D. Sex differences in remembering the locations of objects in an array: Location-shifts versus location-exchanges. Evolution and Human Behavior. 1997;18:155–163. [Google Scholar]
- Kelsoe JR, Jr, Cadet JL, Pickar D, Weinberger DR. Quantitative neuroanatomy in schizophrenia. A controlled magnetic resonance imaging study. Archives of General Psychiatry. 1988;45(6):533–41. doi: 10.1001/archpsyc.1988.01800300029003. [DOI] [PubMed] [Google Scholar]
- Ko YH, Joe SH, Cho W, Park JH, Lee JJ, Jung IK, Kim L, Kim SH. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology. 2006;53:167–175. doi: 10.1159/000093780. [DOI] [PubMed] [Google Scholar]
- Levy LJ, Astur RS, Frick KM. Men and women differ in object memory but not performance of a virtual radial maze. Behavioral Neuroscience. 2005;119(4):853–862. doi: 10.1037/0735-7044.119.4.853. [DOI] [PubMed] [Google Scholar]
- Lewine RRJ. Sex differences in schizophrenia: Timing or subtypes? Psychological Bulletin. 1981;90(3):432–444. [PubMed] [Google Scholar]
- Lewine RRJ, Strauss JS, Gift TE. Sex differences in age at first hospital admission for schizophrenia: Fact or artifact? American Journal of Psychiatry. 1981;138(4):440–444. doi: 10.1176/ajp.138.4.440. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Gaulin SJC, Devineni T, Adams C. Superior spatial memory of women: Stronger evidence for the gathering hypothesis. Evolution and Human Behavior. 1997;18:165–174. [Google Scholar]
- Minor K, Park S. Spatial working memory: Absence of gender differences in schizophrenia patients and healthy control subjects. Biological Psychiatry. 1999;46:1003–1005. doi: 10.1016/s0006-3223(99)00149-3. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Park S. Spatial working memory deficits in schizophrenia patients and their first degree relatives from Palau, Micronesia. American Journal of Medical Genetics. 2002;114:609–615. doi: 10.1002/ajmg.10644. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Blanton R, Anvar B, Edris A, Krupp R, et al. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: Gender effects. Biological Psychiatry. 2001;50:84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. NeuroImage. 2004;21:1563–75. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Archives of General Psychiatry. 1998;55(5):433–40. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lonnqvist JK. Childhood developmental abnormalities in schizophrenia: Evidence from high-risk studies. Schizophrenia Research. 2003;60:239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of seperable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Oades RD, Schepker R. Serum gonadal steroid hormones in young schizophrenic patients. Psychoneuroendocrinology. 1994;19(4):373–85. doi: 10.1016/0306-4530(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The neural basis of relational memory deficits in schizophrenia. Archives of General Psychiatry. 2006;63:356–65. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD, Abrahams S. Sexual orientation related differences in spatial memory. Journal of the International Neuropsychological Society. 2003;9:376–383. doi: 10.1017/S1355617703930037. [DOI] [PubMed] [Google Scholar]
- Riecher-Rossler A, Hafner H, Stumbaum M, Maurer K, Schmidt R. Can estradiol modulate schizophrenic symptomatology? Schizophrenia Bulletin. 1994;20(1):203–14. doi: 10.1093/schbul/20.1.203. [DOI] [PubMed] [Google Scholar]
- Sanders B, Soares MP. Sexual maturation and spatial ability in college students. Developmental Psychology. 1986;22(2):199–203. [Google Scholar]
- Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: A prospective investigation. American Journal of Psychiatry. 2004;161(11):2021–2027. doi: 10.1176/appi.ajp.161.11.2021. [DOI] [PubMed] [Google Scholar]
- Silverman I, Eals M. Sex differences in spatial abilities: evolutionary theory and data. In: Barkow JH, Cosmides L, Tooby J, editors. The adapted mind. New York: Oxford; 1992. pp. 533–549. [Google Scholar]
- Sim K, DeWitt I, Ditman T, Zalesak M, Greenhouse I, Goff D, Weiss AP, Heckers S. Hippocampal and parahippocampal volumes in schizophrenia: A structural MRI study. Schizophrenia Bulletin. 2006;32(2):332–40. doi: 10.1093/schbul/sbj030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze VW, II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biological Psychiatry. 1992;31(3):221–40. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Tanskanen P, Veijola JM, Piippo UK, Haapea M, Miettenen JA, Pyhtinen J, Bullmore ET, Jones PB, Isohanni MK. Hippocampus and amygdala volumes in schizophrenia and other psychoses in the Northern Finland 1966 birth cohort. Schizophrenia Research. 2005;75:283–94. doi: 10.1016/j.schres.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Tarrant CJ, Jones PB. Precursors to schizophrenia: Do biological markers have specificity? Canadian Journal of Psychiatry. 1999;44(4):335–349. doi: 10.1177/070674379904400403. [DOI] [PubMed] [Google Scholar]
- Vauclair J, Fagot J. Manual and hemispheric specialization in the manipulation of a joystick by baboons (Papio papio) Behavioral Neuroscience. 1993;107(1):201–4. doi: 10.1037//0735-7044.107.1.210. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia. Archives of General Psychiatry. 1999;56:133–40. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Waber DP. Sex differences in cognition: A function of maturation rate? Science. 1976;192:572–574. doi: 10.1126/science.1257795. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Ragland JD, Brensinger CM, Bilker WB, Deisenhammer EA, Delazer M. Sex differences in clustering and switching in verbal fluency tasks. Journal of International Neuropsychological Society. 2006;12:502–509. doi: 10.1017/s1355617706060656. [DOI] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secrections on sexual differentiation in spatial memory. Behavioral Neuroscience. 1990;104(1):84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]