Abstract
Objectives
The IGF axis plays a significant role in normal growth and development and variation in IGFs is associated with health outcomes. Past studies report variation in IGF levels among race/ethnic groups known to differ in disease incidence. This paper reports on race/ethnic variation in serum levels of IGF-I and IGF-BP3 in a nationally representative and ethnically diverse sample of US adults.
Design
Serum IGF-I and IGFBP-3 levels from the fasting subsample (n = 6061) of respondents to the US National Health and Nutrition Examination Survey III (NHANES III) were analyzed using an IGF-I ELISA (Diagnostic Systems Laboratory (DSL) 10–5600) and an IGFBP-3 IRMA (DSL 6600). The NHANES is a combined examination and interview survey of a nationally representative sample of US adults. Regression analyses were used to estimate cross-sectional associations between the IGF axis and demographic variables.
Results
In unadjusted analyses, serum IGF-I levels were higher in males than in females, and IGFBP-3 levels were higher in females than in males. Both analytes were lower in older adults. Univariate analyses indicate that serum levels of IGF-I are lower in female Non-Hispanic Whites (NHW) (256 [4.9]) and Hispanics (249 [6.6]) than in Non-Hispanic Blacks (NHB) (281 [4.9]). However, in males, IGF levels in NHWs (287 [3.6]) and NHBs (284 [4.3]) are similar and levels in Mexican-Americans are only moderately reduced (265 [3.4]). Notably, NHB’s have the highest molar ratio of IGF-I:IGFBP-3 at all ages. After adjustment for age and BMI, gender and race/ethnicity differences persist.
Conclusions
These cross-sectional data support exploration of the IGF axis as an explanation for some race/ethnic differences in cancer incidence.
Keywords: Cancer, Insulin-Like Growth Factor, Race/Ethnicity, Age
Introduction
The insulin-like growth factor (IGF) system plays a significant role in normal growth and development [1,2] and in a variety of health outcomes [3–6]. In addition, it is now widely believed to be involved in carcinogenesis [7,8]. In this report, we focus on associations between the IGF system and a number of demographic and anthropometric variables. These studies could help explain experimental [9–11] and epidemiological evidence suggesting that the IGF axis plays a role in carcinogenesis [10]. For example, epidemiological evidence suggests that higher levels of IGF-I and lower levels of IGF binding protein 3 (IGFBP-3) or changes in their ratio are associated with increased risk of breast [12], colorectal [13] and prostate cancer [13,14]. Note that changes in the ratio of IGF-1:IGFBP-3 are thought to reflect changes in bio-available IGF-I [4,8,15,16]. Moreover, IGF-I signaling stimulates cell proliferation in many in vitro studies of cancer cell cultures [17–19]. Associations between the IGF axis and carcinogenesis could be mediated by genetic, anthropometric, and lifestyle variables.
Age and gender are well known correlates of serum IGF levels, but some recent studies have shown that race/ethnicity also may be associated with serum levels of IGF-I and IGFBP-3 [20–22]. Slattery et al. reported that Hispanic women had significantly lower levels of IGFBP-3 than non-Hispanic white women [22,23], whereas DeLellis et al. reported lower levels of IGF-I and IGFBP-3 in Hispanics compared to other race ethnic groups, including whites [20], and Heald et al. found that Pakistani subjects had lower levels of IGF-I than subjects of European or Afro-Caribbean origin [24]. Additionally, African-American men sometimes [21,25], but not always [26] appear to have lower levels of IGF-I than non-Hispanic white men.
Comparative studies of variation in serum IGF levels [4] have also been conducted in multiple countries including Japan [27], Denmark [28], England [29], and New Zealand [30]. Cross-sectional studies exploring associations between race/ethnic differences in lifestyle, anthropometry and carcinogenesis can help inform longitudinal and experimental studies of the IGF axis.
The present report includes results of serum measurements of IGF-I and IGFBP-3 in a large (n = 6061), nationally representative sample of US adults from the National Health and Nutrition Examination Survey (NHANES III). The sample data set allowed us to compare serum levels of these two factors, in Non Hispanic Whites and Blacks, and Mexican Americans. We present values of IGF levels by age and gender and analysis of the effects of race/ethnicity on serum IGF-I and IGFBP-3, adjusting for the effects of several demographic and anthropometric variables. These results contribute to the debate concerning the role of the IGF axis as a determinant of race/ethnic variation in population patterns of carcinogenesis.
Materials and Methods
We analyzed data from the Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative sample of the US population with a stratified multistage probability design and over-sampling of African- and Mexican-Americans [31]. The survey, carried out from 1988–1994, included questionnaires, serum collection, and physical examination. A subset (n = 6,226) of the total sample of adults (n = 20,024) were selected at random and asked to fast overnight before attending a morning examination at which they supplied a serum sample. Response rates for adults aged 20 years and older were approximately 97% [32].
Serum samples, collected from 1988 to 1994, were divided into two aliquots. Upon collection, one aliquot was shipped to contract labs for analysis of diverse constituents. The remaining aliquot was shipped to Atlanta, defrosted on an ice table, and further aliquoted into four 0.5 ml vials. These samples were stored at −80° C until shipment to Diagnostic Systems Laboratories (DSL) in Webster, Texas. Details of serum handling procedures and quality control protocols have been reported [33]. Each sample underwent two freeze-thaw cycles before analysis. In brief, we used DSL’s IGF-I ELISA (Cat. # 10–5600) and IGFBP-3 IRMA (Catalog # 6600). All assays described were performed by a single technician at the DSL facility. For the IGF-I ELISA, a single batch of reagents sufficient for the entire experiment were frozen at the study onset. The IGFBP-3 IRMA required fresh batches of radioactive tracer during the study. Throughout the study, we reanalyzed samples if the coefficient of variation for replicate samples from a single vial was greater than 15%.
We further extracted data from NHANES records concerning respondent’s age, gender, race/ethnicity, education, income, height, weight, and waist and hip circumferences. In this study age was treated as a continuous variable, the survey design allows categorization in ten year intervals (20–29, 30–39, etc.) (http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm). All the anthropometric variables in this study were obtained by measurement, not from self report. Education level was classified as < High school, High school, and any college. Income was categorized as a percent of the USDA Poverty Ratio (≤100, 100–200, 200–300, 300+). To account for the complex survey design used in NHANES III, data were analyzed using SUDAAN [34], following recommendations discussed in Korn and Graubard [35]. Molar ratio of IGF-I:IGFBP-3 was calculated using the following equivalents for conversion: 1 ng/ml IGF-1 = 0.130 nM IGF-1, and 1 ng/ml IGFBP-3 = 0.036 nM IGFBP-3. Note that the appropriate conversion equivalents depend on the standards used to calibrate the IGF-I and IGFBP-3 assays. Cubic equations are used to characterize associations between the IGF axis and age for different race/ethnic groups. Age, BMI, and other anthropometric variables are normalized to gender specific average values when required. We also report adjusted means and standard errors by race/ethnicity for IGF-I, IGFBP-3, and their molar ratio based on these cubic equations.
Results
Sample characteristics are shown in Table 1. The sample consisted of 3,319 women and 2,742 men. About 75% of the respondents were non-Hispanic whites (NHW), 10% non-Hispanic blacks (NHB), and 5% Mexican American (MA). The remaining respondents were from diverse race/ethnic groups and we do not present further details concerning this heterogeneous group, although they are included in our estimates of mean IGF levels. For women, mean body mass index (BMI, or kg/m2) was 26.3 (median=26.4, 10–90th percentile = 20.4–36.3); for men, mean BMI was 26.8 (median=26.2, 10–90th percentile = 21.4–32.8).
Table 1.
Characteristics of the sample.
| Variablea | Women (n = 3319) | Men (n =2742) |
|---|---|---|
| Age (y) | 44.68 (0.64)b | 43.03 (0.56) |
| Race/Ethnicity | ||
| Non-Hispanic White (%) | 76.62 (1.46) | 77.87 (1.74) |
| Non-Hispanic Black (%) | 11.10 (0.78) | 9.09 (0.67) |
| Mexican American (%) | 4.59 (0.43) | 5.45 (0.60) |
| Other (%) | 7.69 (1.04) | 7.59 (1.34) |
| Height (meters) | 161.91 (0.22) | 175.85 (0.27) |
| Weight (kg) | 69.08 (0.54) | 83.14 (0.62) |
| BMI (kg/m2) | 26.34 (0.20) | 26.81 (0.18) |
| Waist-Hip ratio | 0.86 (0.00) | 0.95 (0.00) |
| Waist circumference (cm) | 88.24 (0.50) | 95.51 (0.47) |
Values weighted to account for the survey design
Mean +/− S.E.
Triceps +Subscapular + Suprailiac + Thigh
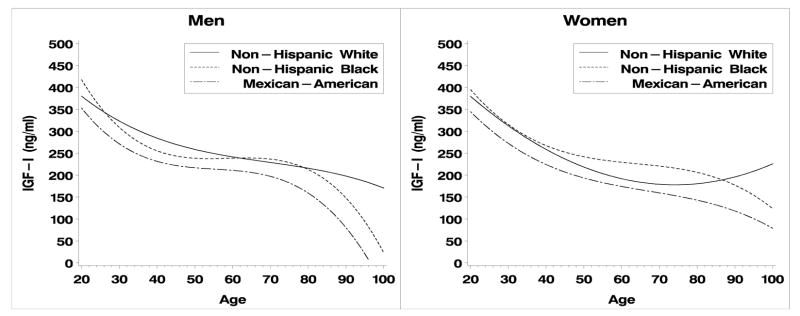
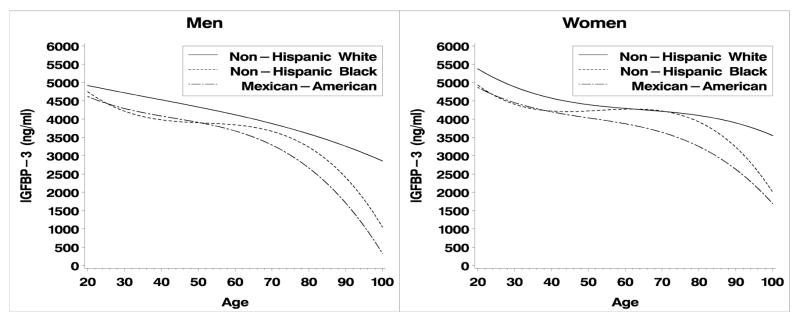
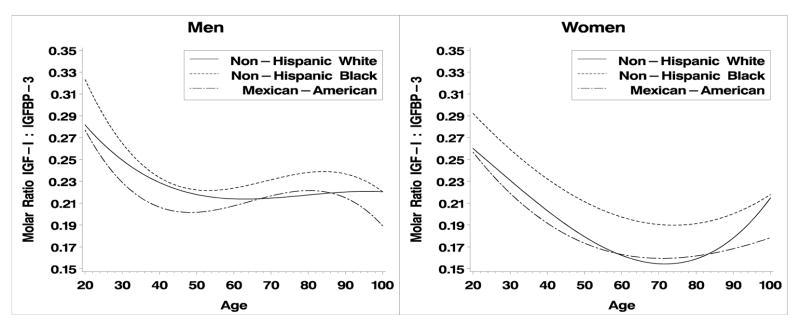
IGF-I, IGBP-3 and their molar ratio exhibit curvilinear relationships with age in both men and women. (Fig. 1, 2; Table 2). Quadratic and cubic terms in these models were statistically significant (p<0.05) or interactions between race/ethnicity and the curvilinear terms were statistically significant (p < 0.05), indicating that non-linear models were required. Interactions between the cubic term and race/ethnicity were statistically significant in men and women for IGF-I and IGFBP-3, therefore we present cubic models for all three outcomes. Overall, IGF-I and IGFBP3 decline with age, except in the case of IGF-I for Non-Hispanic white women (Fig. 1B). In men, the molar ratio of IGF-I:IGFBP-3 declined from ages 20 – ~40 and then plateaus whereas in women the ratio declines steadily until about age 70. Increased values are observed after age 70, particularly in non-Hispanic whites, but these are based on small samples, about 200 male and female respondents are aged 70+).
Figure 1.
Figure 1a, b. IGF-I by age and race/ethnicity for men and women respectively.
Figure 2.
Figure 2a, b. IGFBP-3 by age and race/ethnicity for men and women respectively.
Table 2.
Regression coefficients for cubic equations relating IGF axis and age. For second and higher order terms, Aage is centered near the mean age of male and female respondents (43 and 45 respectively) to reduce colinearity.
| Term* | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| a (S.E.) | b** (S.E.) | c (S.E.) | d (S.E.) | a (S.E.) | b (S.E.) | c (S.E.) | d (S.E.) | |
| A. IGF-I (ng/ml) | ||||||||
| Non-Hispanic White (S.E) | 395.3 (11.4) | −2.78 (0.25) | 0.059 (0.015) | −0.00075 (0.00039) | 418.3 (15.8) | −4.03 (0.28) | 0.068 (0.015) | 0.000028 (0.00046) |
| Non-Hispanic Black (S.E) | 341.5 (29.0) | −2.18 (0.67) | 0.153 (0.020) | −0.00323 (0.00093) | 365.8 (14.5) | −2.52 (0.27) | 0.089 (0.017) | −0.001558 (0.00054) |
| Mexican-American (S.E) | 301.3 (17.7) | −1.77 (0.41) | 0.102 (0.015) | −0.00276 (0.00068) | 343.8 (19.9) | −3.03 (0.36) | 0.072 (0.018) | −0.001081 (0.00056) |
| B. IGFBP-3 (ng/ml) | ||||||||
| Non-Hispanic White (S.E) | 5302.6 (137.9) | −19.34 (3.18) | −0.039 (0.143) | −0.0021 (0.00423) | 5262.7 (173.0) | −17.55 (3.22) | 0.517 (0.153) | −0.009126 (0.00488) |
| Non-Hispanic Black (S.E) | 4350.6 (186.7) | −9.38 (4.33) | 0.587 (0.172) | −0.0230 (0.00605) | 4162.6 (200.0) | 0.99 (4.83) | 0.594 (0.137) | −0.024369 (0.00654) |
| Mexican-American (S.E) | 4763.7 (193.1) | −16.90 (4.59) | 0.019 (0.155) | −0.0152 (0.00906) | 4841.6 (230.2) | −16.20 (5.29) | 0.227 (0.174) | −0.024369 (0.00745) |
| C. Molar Ratio IGF-I/IGFBP-3 | ||||||||
| Non-Hispanic White (S.E) | 0.279 (7.635E-03) | −0.00126 (1.560E-04) | 0.0000436 (1.034E-05) | −4E-7 (2.787E-07) | 0.298 (8.967E-03) | −0.00239 (1.637E-04) | 0.0000269 (9.063E-06) | 4.48E-7 (2.647E-07) |
| Non-Hispanic Black (S.E) | 0.291 (1.650E-02) | −0.00145 (3.789E-04) | 0.0000895 (1.328E-05) | −1.17E-6 (5.751E-07) | 0.315 (1.326E-02) | −0.00208 (2.647E-04) | 0.0000326 (1.349E-05) | 7.76E-8 (5.108E-07) |
| Mexican-American (S.E) | 0.235 (1.819E-02) | −0.00074 (4.217E-04) | 0.0000786 (1.155E-05) | −1.23E-6 (6.423E-07) | 0.264 (1.471E-02) | −0.00182 (2.767E-04) | 0.0000428 (1.259E-05) | −1.94E-7 (4.353E-07) |
a, b, c, and d correspond to coefficients for the equation y = a + bx + c(x−43 or 45)2 + d(x − 43 or 45)3
Serum IGF levels differed significantly by race/ethnicity (Table 3A–C) when adjusted for age and age plus BMI. After adjustment, IGF-I levels were highest in NHW males (289 ng/ml), lower in NHB (279 ng/ml) and lowest in MA males (244 ng/ml) (Table 3A). In females, lower levels were observed in MA’s and higher levels in non-Hispanic blacks between ages 50–80, with overall levels of IGF-I greatest in NHBs (275 ng/ml), lower in NHW (258 ng/ml) and lowest in MAs (226 ng/ml). Levels of IGFBP-3 were greatest in NHW males and were comparable and much lower in NHB and MA males. This pattern was repeated for females. In both genders, differences between MA’s and NHB’s were small or absent until age ~45+. Adjustment for other anthropometric variables (Height and WHR), education, and income levels did not qualitatively alter these patterns. Note these comparisons represent adjusted values at the overall mean BMI.
Table 3.
Crude and adjusted means for IGF-I, IGFBP-3 and their ratio.
| Race/Ethnicity | N | Males* | N | Females* | ||||
|---|---|---|---|---|---|---|---|---|
| Crude Mean (S.E.) | Adj. for Age (S.E.) | Adj. for Age +BMI* (S.E.) | Crude Mean (S.E.) | Adj. for Age (S.E.) | Adj. for Age + BMI (S.E.) | |||
| A. IGF-I (ng/ml) | ||||||||
| NH White | 1156 | 287 (3.6) | 289 (3.4) | 289 (3.3)a | 1401 | 256 (4.9) | 259 (3.7) | 258 (3.7)b |
| NH Black | 702 | 284 (4.3) | 279 (3.5) | 279 (3.5)a | 940 | 281 (4.9) | 272 (4.8) | 275 (4.6)a |
| Mex. Amer. | 777 | 265 (4.2) | 243 (3.5) | 244 (3.4)b | 836 | 249 (6.6) | 224 (4.7) | 226 (4.6)c |
| B. IGFBP-3 (ng/ml) | ||||||||
| NH White | 1156 | 4437 (46) | 4453 (44) | 4453 (44)a | 1401 | 4583 (36) | 4601 (39) | 4605 (38)a |
| NH Black | 702 | 4072 (39) | 4033 (41) | 4034 (41)b | 940 | 4368 (58) | 4331 (55) | 4314 (55)b |
| Mex. Amer. | 777 | 4184 (38) | 3998 (35) | 3996 (35)b | 836 | 4333 (60) | 4158 (52) | 4145 (51)b |
| C. Ratio IGF-I/IGFBP-3 | ||||||||
| NH White | 1156 | 0.234 (0.003) | 0.235 (0.003) | 0.235 (0.003)b | 1401 | 0.201 (0.003) | 0.201 (0.003) | 0.200 (0.003)b |
| NH Black | 702 | 0.252 (0.003) | 0.249 (0.003) | 0.249 (0.003)a | 940 | 0.226 (0.004) | 0.228 (0.004) | 0.231 (0.004)a |
| Mex. Amer. | 777 | 0.240 (0.003) | 0.220 (0.003) | 0.221 (0.003)c | 836 | 0.190 (0.003) | 0.191 (0.003) | 0.194 (0.003)b |
superscripts indicate significantly difference adjusted means
For men as well as women, the ratio of IGF-I:IGFBP-3 was highest for NHB, lower in NHWs and lowest in MAs (Table 3C), with a statistically significant difference between NHW respondents and the other race/ethnic groups in both genders (P < 0.0001). Exclusion of diabetics (n = 335) did not alter these results.
The above results are based on predictive margins adjusting for age and BMI. Inspection of the graphs in Figures 1a–c and Figure 2a–c clearly illustrate the presence of age-race interactions. These patterns were not entirely consistent across all age groups (Figures 1–3), graphical evidence suggests age by race by gender interactions. IGF-I levels were lower in male and female MAs and IGF-BP3 levels are consistently higher in NHW men. Lastly, the ratio of IGF-I:IGFBP-3 is consistently highest in NHB women and lowest in MA below age 60. The data used for these calculations are publicly available (http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm) and reference values for specific combinations of age, gender and race/ethnicity can be extracted for comparison with particular studies.
Figure 3.
Figure 3a, b. IGF-I/IGFBP-3 by age and race/ethnicity for men and women respectively.
Discussion
In this analysis of cross-sectional data from NHANES III, a large nationally representative sample of US adults aged 20 years and older, we document significant associations between race/ethnicity and serum IGF-I and IGFBP-3 levels. The observed differences in the IGF axis are not accounted for by the effects of anthropometric variables, notably BMI. Note that the height and weight avariables in this study were obtained from standardized measurements in the examination center, not from self report. Mexican-Americans had lower levels of IGF-I and NHWs had higher levels of IGFBP-3, resulting in the lowest IGF-I to IGFBP-3 ratio values in male and female Mexican Americans. Among men, non-Hispanic whites had the highest levels of igf-I and IGFBP-3, but Non-Hispanic blacks had the highest molar ration of IGG-I:IGFBP-3 in both men and women. These results are important because the ratio of IGF-I to IGFBP-3 is a measure of bio-available IGF-I and a potential link to variation in cancer incidence at some sites [15]. As in past studies, serum IGF-I and IGFBP-3 levels were lower in older respondents [4]. All three analytes showed curvilinear relationships with age and values for one gender are not uniformly higher than the other.
Because these results are based on a large nationally representative sample of US adults, they should prove invaluable as a guide to calculating reference values for the design and evaluation of future epidemiological studies of the IGF axis in many parts of the world. Details of the distributions for both analytes by age, gender, race/ethnicity, and many other classification variables can be obtained by downloading the public access data (http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm) and calculating values for IGF-I and IGFBP-3 for demographic categories of interest. In the remainder of the paper we focus on race/ethnic differences in serum IGF levels, potential confounders of these differences, and the possibility that population level differences in IGFs may help explain variation in cancer incidence.
Race/ethnic differences in serum IGFs could occur because of genetic differences in IGF regulation among different race/ethnic groups. One study of Finnish twins reports that genetic variation accounted for 38% of the variance in serum IGF-I levels and 60% of the variance in IGFBP-3 [36]. Second, lifestyle, anthropometric, or other variables associated with the IGF axis could account for differences in IGF levels among racial/ethnic groups. For example, diverse anthropometric variables [4,21] and regional fat deposition [37,38] have been associated with IGF-I levels. In our study, after adjustment for age and race/ethnicity, height was positively associated with IGF-I in men (p = 0.021) and waist circumference was negatively associated with IGFBP-3 in women (p = 0.002). Height, BMI, and waist circumference were not significantly associated with IGFBP-3 in men or women. However, adjustment for height, BMI, and waist:hip ratio did not significantly alter the associations between race/ethnicity and the IGF variables measured. Thus there appear to be associations between race/ethnicity and the IGF axis, independent of anthropometric characteristics.
It is possible that dietary intakes explain some of the observed relationships. We found little evidence for associations between food and alcohol consumption estimated from limited dietary assessment instruments available in this study and aspects of the IGF axis measured here (Potischman et al. Personal Communication), despite past studies suggesting that some dietary factors may be associated with the IGF axis [39–42]. Nor did we detect differences between race/ethnic groups and dietary variables. In general, the literature relating dietary factors and IGF-I and IGFBP3 is inconsistent. For example, carbohydrate intake has been shown to be negatively [24,43], positively [39,40] and not associated [41,42,44–46] with IGF-I or IGF-IGFBP3 concentrations. In addition, there are null intervention and cross-sectional studies relating common dietary factors to IGF-I and IGFBP3 [20,47–50] and further work would need to address associations within race/ethnic groups.
Other potential confounders of the association between race/ethnicity and IGF levels include physical activity [42,51], alcohol use [52,53], and smoking [54,55]. However, reports concerning these variables are contradictory and in general suggest that their effects, if present are small [4,21]. It is a major challenge to determine the mechanisms responsible for race/ethnic difference in the IGF axis because of the complexity of IGF regulation [8,56] and the fact that lifestyle and environmental influences on the IGF axis are still poorly understood [8,56].
A growing literature concerning the epidemiology of IGF suggests that the IGF axis and cancer at several sites [13] have small but significant associations. The strongest evidence to date has been found for colon, prostate and pre-menopausal breast cancer. At these sites, serum or plasma levels of IGF-I and these cancers are positively associated. Results for IGFBP-3 are mixed. A recent meta-analysis indicated that IGFBP-3 levels were associated with increased risk of pre-menopausal breast cancer but not with risk for cancer at other sites [13]. Given that IGFBP-3 is the major binding protein of IGF-I, such associations are thought to be consistent with the regulation of IGF-I’s mitogenic effects through binding to IGFBP-3 [8]. Thus, the elucidation of demographic and behavioral factors associated with variation in serum levels of IGF-I and its binding proteins could help explain racial variation in cancer incidence [22,25,57,58].
Past studies have examined race/ethnic differences in serum levels of IGF and one or more of its binding proteins. A small study of middle-aged men found that reported IGF-I levels were higher in Caucasians (224 ng/ml) than in African Americans (205 ng/ml) and Asians (208 ng/ml), and IGFBP-3 levels were lowest in African Americans (3373 ng/ml) and higher in Caucasians (3868 ng/ml) and Asians (3926 ng/ml), resulting in lower ratios of IGF-I:IGFBP-3 in Asians but similar values among the other groups [25]. Platz et al. [25] point out that the lower level of bio-available IGF-I in Asians is consistent with their lower incidence of prostate cancer, but their results do not account for the higher prostate cancer incidence observed in African Americans. Slattery et al. report similar levels of IGF-I, lower levels of IGFBP-3 and comparable ratios of IGF-I:IGFBP-3 in Hispanic compared to non-Hispanic white women [59]. One large study of men and women aged 45–75 years (The Multi-Ethnic Cohort (MEC)) focusing on racial/ethnic variation in cancer risk factors has examined the IGF axis. This study, of men and women aged 45–75 years, set in Hawaii and Los Angeles [20,57,60], has reported significant associations between race/ethnicity and both IGF-I and IGFBP-3 levels [20]. Systematic review of race/ethnic variation in the IGF axis might help identify current challenges to teasing apart environmental and genetic influences on this key molecule [4,20,26,61,62].
Overall, the results of this study match some cancer data according to ethnic group incidence rates. Colorectal, pre-menopausal breast and prostate cancer risk have been linked to the IGF axis and have incidence rates that vary by race and ethnicity [63]. Most notably, the incidence of all three cancers is lowest in Hispanics and Mexican-Americans in population-based cancer registries in the US [63]. In our national data, the concentration of IGF-I and IGF-I:IGFBP-3 ratio is also lowest in this ethnic group in younger men and older women. Prostate cancer incidence is markedly higher in non-Hispanic Blacks (234 per 100,000) than non-Hispanic Whites or Mexican Americans (152 and 134 per 100,000, respectively) [63]. The IGF-I:IGFBP-3 ratio is also higher in non-Hispanic Blacks than the other two groups. Compared to Non-Hispanic Whites and Mexican Americans, colorectal cancer is higher in non-Hispanic Black males and females and the IGF-I:IGFBP-3 ratio is also highest in non-Hispanic Blacks at all ages, except ages ~40–50 in men.
These considerations suggest that the IGF axis could be related to variation in cancer incidence among these race/ethnic groups. Our estimates of serum IGF levels are based on NHANES data whereas these estimates of race/ethnic variation incidence from SEER, nevertheless, both surveys are population-based with highly standardized data collection and processing systems necessary for research purposes. Only a few longitudinal studies of variation in the IGF axis have been conducted. Thus, it is difficult to determine whether cross-sectional studies reflect long-term exposures. One study indicates that IGF-I levels show little intra-individual variation over the short term (about 40 days) in relative values despite significant declines with over the long term (as subjects aged) [54]. The Cardia Male Hormone study has examined longitudinal change in IGF-I and IGFBP-3 [21]. However, they have not yet reported an analysis of repeatability over this time period.
The results presented here were obtained from a cross-sectional study and the serum samples analyzed had been stored for 10–16 years before analysis. Quality control and sample degradation characteristics are discussed in some detail in an earlier publication [33]. Mean levels of IGF-I and IGFBP-3 observed in this study are comparable to those seen in many past studies [4,66], but we cannot rule out differential levels of sample degradation in specific demographic subgroups.
In sum, these results provide strong evidence for race/ethnic variation in serum levels of IGF-I and IGFBP-3 in a large nationally representative sample of US adults. Furthermore, they offer an introduction to an easily accessible public use data resource that allows calculation of reference values for IGF-I and IGFBP-3 stratified by gender, age and race ethnicity. This should be of some utility for evaluating data quality in future epidemiological studies. Our results provide strong support for further efforts to explore variations in the IGF axis related to race and ethnicity and their association with cancer incidence at several sites. NHANES III also contains data concerning many more serum components and diverse measures of health status and health behavior and these data are freely available for analysis ((http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm).
Acknowledgments
We thank Viraj Patel for her tireless work analyzing these samples and Lisa Kahle for programming support. David Berrigan acknowledges the Cancer Prevention Fellowship Program for support during the early portions of this project, Geraldine McQuillan of NCHS for facilitating our use of the NHANES III surplus serum, and Michael Nicar for advice about laboratory analyses. Tanya Agur-Collins, Anne Rodgers, and two anonymous reviewers provided useful comments on the MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeRoith D, McGuinness M, Shemer J, et al. Insulin-like growth factors. Biol Signals. 1992;1:173–181. doi: 10.1159/000109323. [DOI] [PubMed] [Google Scholar]
- 2.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 3.Rosen CJ. Serum insulin-like growth factors and insulin-like growth factor-binding proteins: clinical implications. Clin Chem. 1999;45:1384–1390. [PubMed] [Google Scholar]
- 4.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 7.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 8.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 9.Dunn SE, Kari FW, French J, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 10.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 11.Hursting SD, Lavigne JA, Berrigan D, et al. Diet-gene interactions in p53-deficient mice: insulin-like growth factor-1 as a mechanistic target. J Nutr. 2004;134:2482S–2486S. doi: 10.1093/jn/134.9.2482S. [DOI] [PubMed] [Google Scholar]
- 12.Shi R, Yu H, McLarty J, Glass J. IGF-I and breast cancer: a meta-analysis. Int J Cancer. 2004;111:418–423. doi: 10.1002/ijc.20233. [DOI] [PubMed] [Google Scholar]
- 13.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 14.Shi R, Berkel HJ, Yu H. Insulin-like growth factor-I and prostate cancer: a meta-analysis. Br J Cancer. 2001;85:991–996. doi: 10.1054/bjoc.2001.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CY. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models With Binary and Continuous Outcomes. University of California; Los Angeles: 2002. PhD Dissertation. [Google Scholar]
- 16.Yu H, Mistry J, Nicar MJ, et al. Insulin-like growth factors (IGF-I, free IGF-I and IGF-II) and insulin-like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J Clin Lab Anal. 1999;13:166–172. doi: 10.1002/(SICI)1098-2825(1999)13:4<166::AID-JCLA5>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, Dai B, Yallampalli U, Lu X, Schroy PC. Proliferation and differentiation of a human colon cancer cell line (CaCo2) is associated with significant changes in the expression and secretion of insulin-like growth factor (IGF) IGF-II and IGF binding protein-4: role of IGF-II. Endocrinology. 1996;137:1764–1774. doi: 10.1210/endo.137.5.8612513. [DOI] [PubMed] [Google Scholar]
- 20.DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1444–1451. [PubMed] [Google Scholar]
- 21.Gapstur SM, Kopp P, Chiu BC, Gann PH, Colangelo LA, Liu K. Longitudinal associations of age, anthropometric and lifestyle factors with serum total insulin-like growth factor-I and IGF binding protein-3 levels in Black and White men: the CARDIA Male Hormone Study. Cancer Epidemiol Biomarkers Prev. 2004;13:2208–2216. [PubMed] [Google Scholar]
- 22.Slattery ML, Baumgartner KB, Byers T, et al. Genetic, anthropometric, and lifestyle factors associated with IGF-1 and IGFBP-3 levels in Hispanic and non-Hispanic white women. Cancer Causes Control. 2005;16:1147–1157. doi: 10.1007/s10552-005-0318-2. [DOI] [PubMed] [Google Scholar]
- 23.Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365:36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 24.Heald AH, Cade JE, Cruickshank JK, Anderson S, White A, Gibson JM. The influence of dietary intake on the insulin-like growth factor (IGF) system across three ethnic groups: a population-based study. Public Health Nutr. 2003;6:175–180. doi: 10.1079/PHN2002414. [DOI] [PubMed] [Google Scholar]
- 25.Platz EA, Pollak MN, Rimm EB, et al. Racial variation in insulin-like growth factor-1 and binding protein-3 concentrations in middle-aged men. Cancer Epidemiol Biomarkers Prev. 1999;8:1107–1110. [PubMed] [Google Scholar]
- 26.McGreevy K, Hoel B, Lipsitz S, Bissada N, Hoel D. Racial and anthropometric differences in plasma levels of insulin-like growth factor I and insulin-like growth factor binding protein-3. Urology. 2005;66:587–592. doi: 10.1016/j.urology.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 27.Teramukai S, Rohan T, Eguchi H, Oda T, Shinchi K, Kono S. Anthropometric and behavioral correlates of insulin-like growth factor I and insulin-like growth factor binding protein 3 in middle-aged Japanese men. Am J Epidemiol. 2002;156:344–348. doi: 10.1093/aje/kwf069. [DOI] [PubMed] [Google Scholar]
- 28.Johnsen SP, Hundborg HH, Sorensen HT, et al. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90:5937–5941. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- 29.Bray I, Gunnell D, Holly JM, Middleton N, Davey SG, Martin RM. Associations of childhood and adulthood height and the components of height with insulin-like growth factor levels in adulthood: a 65-year follow-up of the Boyd Orr cohort. J Clin Endocrinol Metab. 2006;91:1382–1389. doi: 10.1210/jc.2005-1722. [DOI] [PubMed] [Google Scholar]
- 30.Bagg W, Aoina J, Cross PA, et al. Serum IGF-I levels are similar in Samoan, Maori and European populations despite differences in body composition. Growth Horm IGF Res. 2006;16:57–60. doi: 10.1016/j.ghir.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics. NCHS plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Vital Health Stat. 1994;1(32) [PubMed] [Google Scholar]
- 32.National Center for Health Statistics. Analytic and Reporting Guidelines: the Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) U.S. Department of Health and Human Services; Hyattsville, MD: 1996. [Google Scholar]
- 33.Berrigan D, Potischman N, Dodd KW, et al. Serum levels of insulin-like growth factor-I and insulin-like growth factor-I binding protein-3: quality control for studies of stored serum. Cancer Epidemiol Biomarkers Prev. 2007;16:1017–1022. doi: 10.1158/1055-9965.EPI-07-0044. [DOI] [PubMed] [Google Scholar]
- 34.Shah BV, Barnwell G, Bieler GS. SUDAAN User’s Manual, Release 8.0. Research Triangle Institute; Research Triangle Park, NC: 1997. [Google Scholar]
- 35.Korn EL, Graubard BI. Analysis of Health Surveys. John Wiley and Sons; New York: 1999. [Google Scholar]
- 36.Harrela M, Koistinen H, Kaprio J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin P, Kvist H, Lindstedt G, Sjostrom L, Bjorntorp P. Low concentrations of insulin-like growth factor-I in abdominal obesity. Int J Obes Relat Metab Disord. 1993;17:83–89. [PubMed] [Google Scholar]
- 38.Schoen RE, Schragin J, Weissfeld JL, et al. Lack of association between adipose tissue distribution and IGF-1 and IGFBP-3 in men and women. Cancer Epidemiol Biomarkers Prev. 2002;11:581–586. [PubMed] [Google Scholar]
- 39.Giovannucci E, Pollak M, Liu Y, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–89. [PubMed] [Google Scholar]
- 40.Gunnell D, Oliver SE, Peters TJ, et al. Are diet-prostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF-I and IGFBP-3 in healthy middle-aged men. Br J Cancer. 2003;88:1682–1686. doi: 10.1038/sj.bjc.6600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson SC, Wolk K, Brismar K, Wolk A. Association of diet with serum insulin-like growth factor I in middle-aged and elderly men. Am J Clin Nutr. 2005;81:1163–1167. doi: 10.1093/ajcn/81.5.1163. [DOI] [PubMed] [Google Scholar]
- 42.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 43.Kaklamani VG, Linos A, Kaklamani E, Markaki I, Koumantaki Y, Mantzoros CS. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. J Clin Oncol. 1999;17:3291–3298. doi: 10.1200/JCO.1999.17.10.3291. [DOI] [PubMed] [Google Scholar]
- 44.Baibas N, Bamia C, Vassilopoulou E, Sdrolias J, Trichopoulou A, Trichopoulos D. Dietary and lifestyle factors in relation to plasma insulin-like growth factor I in a general population sample. Eur J Cancer Prev. 2003;12:229–234. doi: 10.1097/00008469-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Maskarinec G, Takata Y, Kaaks R. The relation between nutritional factors and insulin-like growth factor-I in premenopausal women of different ethnicity. Eur J Nutr. 2005;44:105–113. doi: 10.1007/s00394-004-0500-4. [DOI] [PubMed] [Google Scholar]
- 46.Probst-Hensch NM, Wang H, Goh VH, Seow A, Lee HP, Yu MC. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003;12:739–746. [PubMed] [Google Scholar]
- 47.Kaaks R, Bellati C, Venturelli E, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr. 2003;57:1079–1088. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 48.Gann PH, Kazer R, Chatterton R, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- 49.Maskarinec G, Takata Y, Murphy SP, Franke AA, Kaaks R. Insulin-like growth factor-1 and binding protein-3 in a 2-year soya intervention among premenopausal women. Br J Nutr. 2005;94:362–367. doi: 10.1079/bjn20051525. [DOI] [PubMed] [Google Scholar]
- 50.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Dietary soy and fats in relation to serum insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 levels in premenopausal Japanese women. Nutr Cancer. 2003;45:185–189. doi: 10.1207/S15327914NC4502_07. [DOI] [PubMed] [Google Scholar]
- 51.Holmes MD, Pollak MN, Hankinson SE. Lifestyle correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:862–867. [PubMed] [Google Scholar]
- 52.Lavigne JA, Wimbrow HH, Clevidence BA, et al. Effects of alcohol and menstrual cycle on insulin-like growth factor-I and insulin-like growth factor binding protein-3. Cancer Epidemiol Biomarkers Prev. 2004;13:2264–2267. [PubMed] [Google Scholar]
- 53.Lavigne JA, Baer DJ, Wimbrow HH, et al. Effects of alcohol on insulin-like growth factor I and insulin-like growth factor binding protein 3 in postmenopausal women. Am J Clin Nutr. 2005;81:503–507. doi: 10.1093/ajcn.81.2.503. [DOI] [PubMed] [Google Scholar]
- 54.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]
- 55.Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, et al. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 56.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 57.DeLellis K, Ingles S, Kolonel L, et al. IGF1 genotype, mean plasma level and breast cancer risk in the Hawaii/Los Angeles multiethnic cohort. Br J Cancer. 2003;88:277–282. doi: 10.1038/sj.bjc.6600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slattery ML, Murtaugh M, Caan B, Ma KN, Neuhausen S, Samowitz W. Energy balance, insulin-related genes and risk of colon and rectal cancer. Int J Cancer. 2005;115:148–154. doi: 10.1002/ijc.20843. [DOI] [PubMed] [Google Scholar]
- 59.Slattery ML, Kinney AY, Levin TR. Factors associated with colorectal cancer screening in a population-based study: the impact of gender, health care source, and time. Prev Med. 2004;38:276–283. doi: 10.1016/j.ypmed.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Stram DO, Hankin JH, Wilkens LR, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jernstrom H, Deal C, Wilkin F, et al. Genetic and nongenetic factors associated with variation of plasma levels of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10:377–384. [PubMed] [Google Scholar]
- 62.Jernstrom H, Chu W, Vesprini D, et al. Genetic factors related to racial variation in plasma levels of insulin-like growth factor-1: implications for premenopausal breast cancer risk. Mol Genet Metab. 2001;72:144–154. doi: 10.1006/mgme.2000.3130. [DOI] [PubMed] [Google Scholar]
- 63.Ries LAG, Harkins D, Krapcho M, et al. SEER cancer statistics review, 1975–2003, National Cancer Institute, 2006. Available at: http://seer.cancer.gov/csr/1975_2003/
- 64.Hussey PS, Anderson GF, Osborn R, et al. How does the quality of care compare in five countries? Health Aff (Millwood) 2004;23:89–99. doi: 10.1377/hlthaff.23.3.89. [DOI] [PubMed] [Google Scholar]
- 65.Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166:518–526. doi: 10.1093/aje/kwm124. [DOI] [PubMed] [Google Scholar]
- 66.Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf) 1994;41:85–93. doi: 10.1111/j.1365-2265.1994.tb03788.x. [DOI] [PubMed] [Google Scholar]