Abstract
Calcineurin is a calcium-dependent, serine/threonine phosphatase that is involved in a variety of signaling pathways. Calcineurin is distinct among phosphatases because its activity requires calcium and is not sensitive to inhibition by compounds that block the related phosphatases PP1A and PP2A. Therefore, the most common methods to measure calcineurin activity rely on calcium-dependent dephosphorylation of a substrate derived from the RII subunit of protein kinase A in the presence of PP1A/PP2A inhibitors. However, current techniques quantify activity by measurement of released radioactive phosphate or detection of free phosphate with malachite green. Both methods involve technical challenges and have undesirable features. We report a new calcineurin fluorimetric assay that utilizes a fluorescently labeled phosphopeptide substrate and separation of dephosphorylated peptide product by titanium-oxide. The method is rapid, quantitative, involves no radioactivity and is suitable for high throughput assays. Furthermore, with the use of a standard curve, precise measurements of calcineurin activity can be obtained.
Introduction
Calcineurin is a calcium-dependent, serine/threonine phosphatase. The most common method for detection of calcineurin activity is based on earlier published work of Fruman et al [1] who established optimal reaction conditions for calcineurin activity. In brief, a peptide substrate is generated and then incubated with protein kinase A and 32Pγ[ATP] under appropriate conditions to result in phosphorylation of the peptide with a radioactive residue. The labeled substrate is then purified and used within a short period of time as a substrate for calcineurin. To measure calcineurin activity, equal parts of cell lysate, reaction mixture, and labeled substrate are incubated at 30°C for 10 minutes before the reaction is terminated. To determine how much of the phosphorylated peptide has been dephosphorylated, individual columns are prepared for each reaction containing pre-charged ion-exchange resin. Reactions are loaded on the column and unincorporated phosphate, which does not bind the resin, is eluted. The amount of radioactivity in the eluted fractions is then measured in a scintillation counter and used to quantify calcineurin activity. In general, the method has several drawbacks including the use of radioactive phosphate for labeling of the peptide substrate, background due to unincorporated phosphate, reliance upon ion exchange to separate phosphorylated from non-phosphorylated peptide, and the final measurement of free phosphate to represent calcineurin activity. These factors increase variability of the data and reduce reproducibility of the assay.
We report a new assay to determine calcineurin activity using well-characterized reaction conditions, a fluorescently labeled phosphopeptide substrate, and separation of dephosphorylated substrate by titanium-oxide. The method is fast, involves no radioactivity, and is suitable for high throughput assays. Furthermore, with the use of a standard curve, precise measurements of calcineurin activity can be obtained.
Methods
Materials and Reagents
Recombinant calcineurin was purchased from Calbiochem (San Diego, CA) and all other chemicals were obtained from Sigma (St Louis, MO). Titanium oxide (TiO4) coated plates were obtained from Glygen (Colombus, MA). The RII peptide, Fluoresceinyl-DLDVPIPGRFDRRVSVAAE, and its phosphorylated analog (Fluoresceinyl-DLDVPIPGRFDRRVpSVAAE where pS=L-phosphoserine) were synthesized at the Emory University Microchemical and Proteomics Facility by Fmoc-based solid-phase peptide synthesis using model Liberty microwave-assisted peptide synthesizer (CEM Corporation, Matthews, NC). The peptides were purified to apparent homogeneity by reversed-phase HPLC and their masses were confirmed by mass spectrometry. Peptide was diluted in: Tris 50 mM, 100 mM NaCl, 0.5 mM DTT, and 0.1 mg/ml BSA to a final concentration of 30 ng/ml. Reaction buffer consisted of 0.1 mg/ml BSA, 35 mM Tris pH 7.5, 25 mM NaCl, 2.0 mM MgCl2, 270 μM DTT, 500 μM EDTA, 419 nM okadaic acid (in 0.63% ethanol), 25 mM CaCl2 or 500 uM EGTA. TiO4 plates were pre-incubated with a binding buffer consisting of 0.1% acetic acid in 10% acetonitrile.
Protocol
20 μl diluted peptide substrate, 20 μl of reaction buffer, and 20 μl of sample were loaded into individual wells of a 96-well plate and incubated at 30°C for 10 minutes. During the reaction time, a 96-well plate with TiO4 - coated wells was prepared by adding 50 μl binding buffer (0.1% acetic acid in 10% acetonitrile) per well. After the incubation period, reactions were transferred into prepared TiO4 - coated wells following by gentle shaking for 5 minutes. The contents of each well were then removed to a white 96-well plate preloaded with 20 μl of 3 N ammonium hydroxide. The amount of fluorescent label that did not bind to the TiO4 matrix was quantified by fluorimetry at 485 nm excitation 528 nm emission.
Experimental Models
Primary mouse renal fibroblasts were obtained from minced kidneys and propagated in culture using RPMI supplemented with 5% serum and pen/strep antibiotics. Lysates were prepared using a hypotonic lysis buffer as described [2, 3]. Wildtype, adult mice are maintained in the animal facility at the Atlanta Veterans Administration Hospital in accordance with Institutional Care and Use Committee Guidelines. Where indicated, wildtype mice were treated by sub-cutaneous injection with either vehicle alone (10% ethanol in Ringer’s lactate solution) or with 10 mg/kg body weight cyclosporin A daily for 3 days. Organs were harvested and calcineurin activity determined as previously described [4].
Mass Spectrometry
The system used for the analysis was an Ultimate capillary HPLC system (LC Packings) with a FAMOS autosampler. An 0.5 × 150 mm C18SB-300 Zorbax (Agilent, Technologies, Palo Alto, CA) reversed-phase column was used as the analytical column. The LC eluent was directly sprayed into the 4000QTRAP mass spectrometer using a TurboV electrospray ion source (Applied Biosystems, Foster City, CA). Elution from the column was accomplished with an acetonitrile gradient from 2% to 80% with 0.1% formic acid as a counter ion for HPLC. The flow rate was set at 15 μl/min. The total LC run time was 60 minutes including equilibration. The 4000Qtrap was operated both in the information dependent acquisition (IDA) mode and straight MS mode. In IDA, for each cycle, a single MS spectrum was acquired followed by up to two MS/MS spectra based upon observed ions in the MS spectrum. The MS spectrum was acquired over the m/z range of 350 to 1,350. Each MS/MS spectrum was acquired over the m/z range of 50 to 1,350. Precursors were determined by each cycle’s MS spectrum from the m/z range of 375 to 1,100. Straight MS was performed over the m/z range of 350 to 1,350. For each sample, extracted ion chromatograms (XIC) were generated for the phosphorylated and non-phosphorylated versions of the RII peptide. The width used for the XIC was 1 Dalton. Based on the areas of the peaks from each XIC, the relative quantity of phosphorylated to non-phosphorylated peptide was determined.
Results and Discussion
Calcineurin is an important enzyme but available assays to detect calcineurin activity are hampered by several problems. In the most commonly used assay, a radioactively-labeled peptide substrate is mixed with desired sample and reaction buffer. The most common drawbacks to this method are inconsistent labeling of the peptide with recombinant protein kinase A, increased background due to incorporated 32P-phosphate, and quantification of “released” phosphate as a marker of calcineurin activity. An improved method would be one where the peptide substrate is uniformly labeled, does not require radioactivity, and quantitatively measures the amount of dephosphorylated peptide.
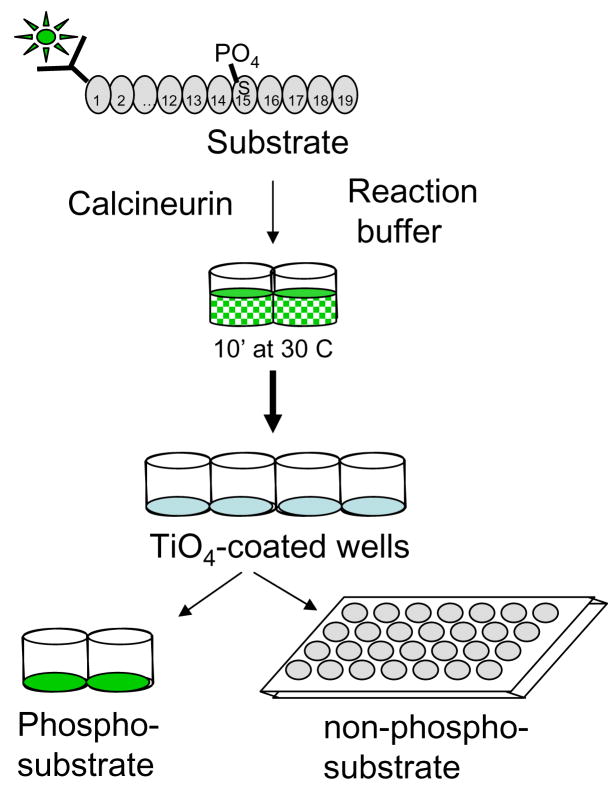
Figure 1 illustrates the steps we propose to solve these problems. First, a peptide is synthesized that is phosphorylated on Ser-15 during synthesis, thus eliminating the need for enzymatic labeling. The peptide was also generated with a fluorescein moiety at its amino-terminus. Next, the tagged, labeled peptide is incubated with the desired lysate and well-characterized reaction conditions [1] for 10 minutes at 30°C.
Figure 1. Diagram of fluorimetric calcineurin assay.
The RII peptide substrate is synthesized with a phospho-Serine 15 residue and an amino-terminus fluorescein tag. In a 96-well plate, the labeled substrate is mixed in equal parts with reaction buffer and sample and allowed to incubate at 30°C for 10 minutes. Each well is then transferred to a 96-well plate coated with titanium-oxide (TiO4) followed by gentle shaking to allow binding of phosphorylated substrate. Finally, the total contents of each well is then moved to a new 96-well plate and the amount of dephosphorylated peptide determined by fluorimetry at 485 nm excitation and 528 nm emission.
Recently, TiO4 has been demonstrated to be highly specific for binding of phosphorylated peptides [5, 6]. Therefore, to separate phosphorylated from non-phosphorylated peptide, plates coated with titanium oxide are utilized. Reactions are transferred to the TiO4 plate followed by gentle shaking at room temperature for 5 minutes to allow binding of the phosphorylated peptide. Dephosphorylated peptide (which does not bind to the TiO4 matrix) is then transferred to a new plate and quantified by fluorimetry of the fluorescein tag.
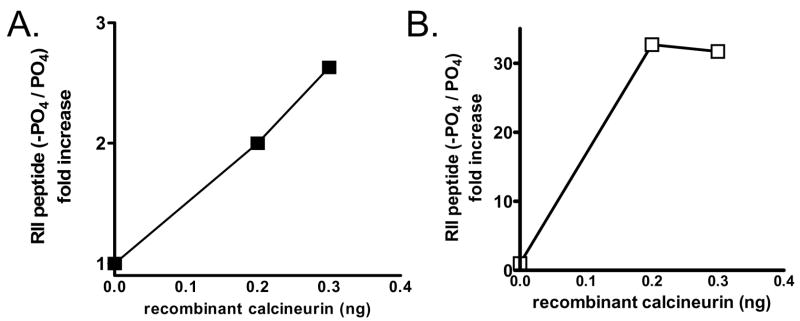
To validate this new method, we first verified by mass spectrometry that the labeled, tagged peptide can be dephosphorylated by calcineurin. Phosphorylated, fluorescein-tagged peptide was used as a substrate for calcineurin under established reaction conditions. After stopping the reaction with 0.1% acetic acid in 10% acetonitrile, the samples were analyzed by mass spectrometry. Figure 2A shows that recombinant calcineurin stimulated a dose-responsive increase in the relative amount of dephosphorylated to phosphorylated peptide. Next, we verified that the TiO4 matrix effectively separated dephosphorylated from phosphorylated peptide. Reactions were performed identically as in panel (A), but with the additional step of incubating the reactions in TiO4 coated wells for 5 minutes. The amount of dephosphorylated peptide in the unbound fraction was analyzed by mass spectrometry. Figure 2B shows that there is a 30-fold increase in the amount of dephosphorylated peptide in the unbound fraction with the addition of recombinant calcineurin.
Figure 2. Validation of fluorescein-labeled RII peptide by mass spectrometry.
A) Reactions were carried out with 0, 0.2, or 0.3 ng of recombinant calcineurin per reaction and then the relative amount of dephosphorylated to phosphorylated peptide was determined by mass spectrometry. Data shown is the ratio of the area under the curve for dephosphorylated RII and phosphorylated RII with each condition. B) Reactions were carried identically as in (A) and then incubated with TiO4 matrix in a 96-well plate. After binding, the samples were removed and the relative amount of dephosphorylated to phosphorylated peptide was determined by mass spectrometry. Data shown is the ratio of the area under the curve for dephosphorylated RII and phosphorylated RII with each condition.
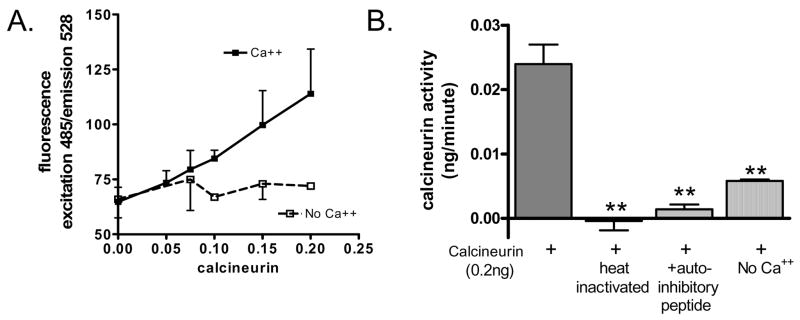
After confirming that the labeled peptide could be dephosphorylated and the TiO4 effectively separated phosphorylated from non-phosphorylated peptide, we proceeded with characterizing the new assay. First, we analyzed reactions containing increasing amounts of recombinant calcineurin in either normal reaction buffer or calcium-free reaction buffer. Calcineurin in normal buffer resulted in dephosphorylation of the peptide in a dose-dependent manner. In the absence of calcium, however, there was no increase in dephosphorylation (Figure 3A). Next, we performed reactions using 0.2 ng of recombinant calcineurin along with standard controls for calcineurin activity including heat inactivation of the recombinant enzyme, addition of an autoinhibitory peptide, absence of calcium, and chelation of calcium with EGTA. Dephosphorylation by 0.2 ng calcineurin was significantly reduced (ANOVA) by each of these conditions (Figure 3B).
Figure 3. Dose-response with recombinant calcineurin.
A) Calcineurin assays were performs with increasing amounts of recombinant calcineurin in the presence or absence of calcium. Recombinant calcineurin resulted in increased amounts of dephosphorylated peptide in a dose-dependent manner. This activity was dependent upon calcium. Data shown are the mean +/− SEM of triplicate reactions. B) Reactions containing 0.2 ng of recombinant calcineurin were carried out along with standard controls to verify calcineurin activity. Heat inactivation of the enzyme, addition of an autoinhibitory peptide, or absence of calcium all significantly reduced activity (ANOVA). Data shown are the mean +/− SEM of triplicate samples compared to a standard curve.
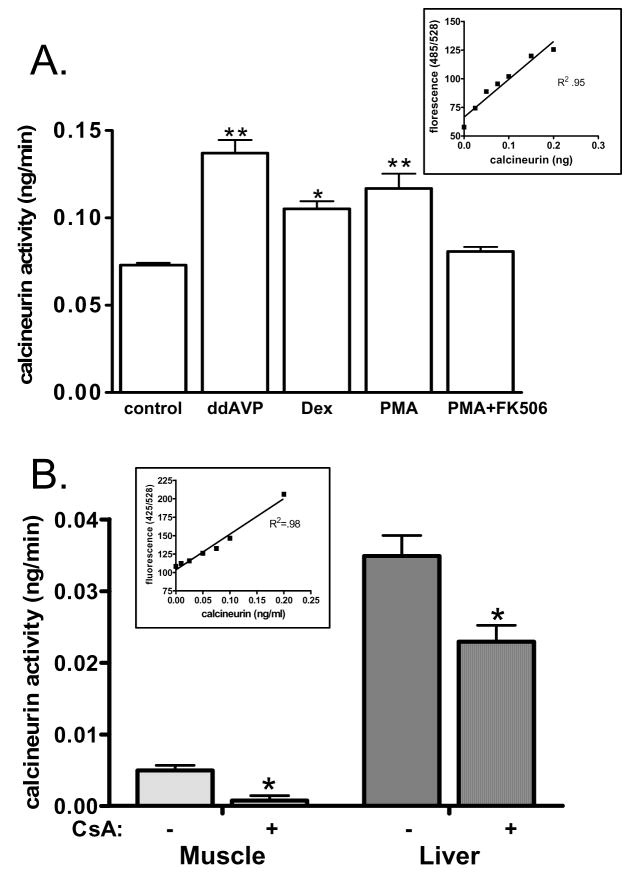
Finally, we explored the use of the new calcineurin assay in two model systems. Figure 4A shows the result of calcineurin stimulation by a variety of agents in cultured primary renal fibroblasts. When the absorbance values obtained were compared to a standard curve of recombinant calcineurin run simultaneously, a determination of calcineurin activity in each condition can be made. Arginine vasopressin (AVP), dexamethasone, and phorbol myristate acetate (PMA) each significantly stimulated calcineurin activity. Pre-treatment with the calcineurin inhibitor FK506 blocked stimulation by PMA. Next, protein lysates were collected from liver and muscle samples of mice treated with vehicle alone or treated daily with 20 mg/kg cyclosporin A. Calcineurin assays were performed on 1 μg of total protein. Results indicated that there is significantly more calcineurin activity per μg protein in the liver compared to muscle. Cyclosporin treatment significantly decreased calcineurin activity in both tissues (Figure 4B).
Figure 4. Detection of calcineurin-mediated dephosphorylation.
A) Cultured renal fibroblasts were treated with a variety of stimuli including arginine vasopressin (AVP), dexamethasone, and phorbol myristate acetate (PMA) to induce calcineurin activity. In addition, some cells were pre-treated with calcineurin inhibitors prior to addition of PMA. Cells were lysed according to previously established methods [2, 3] and then calcineurin activity was determined. Data shown is the mean +/− SEM of triplicate samples compared to a standard curve. B) Mice were treated with cyclosporin A for three days to inhibit calcineurin activity and then liver and muscle samples were harvested. Tissues were lysed according to previously established methods [4, 7] and calcineurin activity determined. Data shown are the mean +/− SEM of triplicate samples compared to a standard curve.
While our method offers several improvements over current methods, there are a several points that are worth brief discussion. First, acidic peptides (other than phosphopeptides) also show affinity for TiO4 as per literature and our own experience. The RII peptide is, in fact, rather acidic not including pSer-15 (5 negative and 3 positive charges); however this does not seem to negatively influence the ruggedness and reproducibility of the assay. Second, 32P-based assays are very specific as they measure inorganic phosphate released upon the action of the phosphatase. This assay, on the other hand, measures fluorescence of the probe attached to unbound peptide released to solution due to the loss of phosphate (loss of binding affinity to TiO4). However, it is possible that the fluorescence probe may be released in solution by the action of a protease, e.g. a tryptic-like enzyme present in the biological material, cleaving the substrate at the C-terminal side of arginines. In such case, peptide fragments such as Fluoresceinyl-DLDVPIPGR will be released simulating phosphatase activity (false positive). Hence, inclusion of protease inhibitors is highly advisable in order to minimize such artifacts. Finally, the fluorimetric method in fact is not limited to the incorporation of a fluorescein tag and may be modified with other fluorescent moieties. Fluorescein can be quenched by common reagents including dithiothreitol and its excitation can be altered with pH and light exposure. Use of fluorescein requires the reaction to be protected from light and neutralized prior to fluorimetry. Other tags such as Tamra may be less pH- and light-sensitive.
To further evaluate the reproducibility of the method, we analyzed 14 separate samples with 6 replicate reactions. The intra-assay variability was 9.35%. In our hands, this is a far better variability rate than we achieved with previous methodologies.
In conclusion, we present a novel method for the determination of calcineurin activity that has several important benefits compared to standard methods. First, the technique is non-radioactive and utilizes a peptide substrate that is phosphorylated during synthesis. Next, the peptide is synthesized with a fluorescein moiety at peptide’s NH2-terminus to facilitate measurement by fluorimetry. Finally, phosphorylated and non-phosphorylated peptides are separated by TiO4 which is embedded in coating at the base of a 96-well plate. Combined, these innovations results in an assay that is rapid, non-radioactive, quantitative, and reproducible.
Acknowledgments
The authors wish to acknowledge Ashalk Shulka for his advice and input and Matt Reed for his technical expertise. Funding for this work was provided by NIH/NIDDK DK066422-02 and DK074854 (JLG) and by NIH-NCRR RR022440 (JP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fruman DA, Pai S-Y, Klee CN, Burakoff SJ, Bierer BE. Measurement of calcineurin phosphatase activity in cell extracts. Methods in Enzymology. 1996;9:146–154. doi: 10.1006/meth.1996.0020. [DOI] [PubMed] [Google Scholar]
- 2.Gooch JL, Tang Y, Ricono JM, Abboud HE. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurin-dependent mechanism. J Biol Chem. 2001;276:42492–500. doi: 10.1074/jbc.M102994200. [DOI] [PubMed] [Google Scholar]
- 3.Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–70. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- 4.Gooch JL, Toro JJ, Guler RL, Barnes JL. Calcineurin A-alpha but not A-beta is required for normal kidney development and function. Am J Pathology. 2004;165:1755–1765. doi: 10.1016/s0002-9440(10)63430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroda I, Shintani Y, Motokawa M, Abe S, Furuno M. Phosphopeptide-selective column-switching RP-HPLC with a titania precolumn. Anal Sci. 2004;20:1313–9. doi: 10.2116/analsci.20.1313. [DOI] [PubMed] [Google Scholar]
- 6.Pinkse MW, Uitto PM, Hilhorst MJ, Ooms B, Heck AJ. Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal Chem. 2004;76:3935–43. doi: 10.1021/ac0498617. [DOI] [PubMed] [Google Scholar]
- 7.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284:F144–54. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]