Abstract
Rapid technical developments, and an expanding list of applications that have supplanted less accurate or more invasive diagnostic tests, have led to a dramatic increase in the use of body CT imaging in medical practice since its introduction in 1975. Our purpose here is to discuss medical justification of the small risk associated with the ionizing radiation used in CT and to provide perspectives on practice-specific decisions that can maximize overall patient benefit. In addition, we review available dose management and optimization technique.
I. Considerations of Risk
In 2006, the estimated number of CT scans performed in the U.S was approximately 62 million, up from 46 million in 2000 and 13 million in 1990 [1]. This increased use of CT is largely due to the tremendous contributions of increasingly powerful CT imaging methods to modern healthcare. However, in spite of measurable health benefits, considerable media attention has been given to the very small potential health risk associated with the ionizing radiation from a CT exam [1-3].
The radiation dose associated with a CT scan (approximately 1-14 mSv) is comparable to the annual dose received from naturally occurring sources of radiation, such as radon and cosmic radiation (1-10 mSv) [4]. More importantly, conservative estimations of risk (i.e. any required assumptions are made toward the direction of overestimating risk, rather than underestimating it) demonstrate that the risk of dying from a CT scan is less than that of drowning or a pedestrian dying from being struck by any form of ground transportation, both of which most Americans consider to be an extremely unlikely event. Table 1 provides a comparison of the statistical odds of dying from various radiological examinations relative to other causes of death [5-11].
Table 1.
Estimated lifetime risk of death from various sources (adapted from Circulation. 2009 Feb 24;119(7):1056-65, with permission)
| Cause of death | Estimated number of deaths per 1000 individuals |
|---|---|
| Cancer (U.S. American Cancer Society Data 2008) | 228 |
| Motor vehicle accidents | 11.9 |
| Radon in Home | |
| US average | 3 |
| High exposure (1-3%) | 21 |
| Arsenic in drinking water | |
| 2.5 ug/L (U.S. estimated average) | 1 |
| 50 ug/L (acceptable limit before 2006) | 13 |
| Radiation-induced fatal cancer | |
| Routine abdomen/pelvis CT scan | 0.5 |
| - single phase, approximately 10 mSv effective dose) | |
| Annual dose limit for a radiation worker | |
| - 10 mSv (recommended yearly average) | 0.5 |
| - 50 mSv (limit in any single year) | 2.5 |
| Pedestrian Accident | 1.6 |
| Drowning | 0.9 |
| Bicycling | 0.2 |
| Lightning strike | 0.013 |
The most troubling aspect of many media reports is the underlying implication that because CT scans are increasing, death rates due to radiation induced cancers are not far behind. Such analyses fail to realize two critical aspects relating to potential risks of CT: non-transferability of risk and mortality reduction through disease identification and treatment. Non-transferability of risk means that medically-related dose given to one individual does not transfer risk of cancer induction to those never undergoing CT. Thus, the focus must be on those individuals undergoing CT scans - not all Americans - as occurred in media reports subsequent to the release of NCRP Report No 160, which documented a six-fold increase in the dose delivered to members of the U.S. public from medical sources [12]. Secondly, the small theoretical risk of cancer induction must be considered in the context of the potential incremental (survival) benefit from undergoing CT. From Table 1, it can be seen that the lifetime risk of a fatal cancer from all causes is 22.8%, while the lifetime risk of a fatal cancer from the radiation associated with a body CT exam is approximately 0.05%. However, in a patient with a known cancer, the risk of cancer death is already much higher than average. In these patients, CT is used to stage cancer—with an aim towards cure and extending life. As an example, cure from colorectal cancer is not possible unless hepatic metastases can be diagnosed and treated. In these patients, the use of CT is a critical means of reducing mortality. Thus, the benefit-to-risk ratio for any patient will be driven by the benefit and appropriateness of the CT exam.
Although informing the public of potential health risks—even small risks—is appropriate, journalistic responsibility should ensure that the data are presented in a manner that puts the risk into perspective. Stating that a CT scan is the equivalent of 600 chest x-rays may be an accurate estimate, however such statements imply that a CT scan delivers “a lot” of radiation simply because 600 of anything seems like a relatively large number. The implication is that CT is a high dose examination and presents a substantial risk to the recipient. Quite the opposite is true. In their position statement on radiation risk [13], the Health Physics Society, a nonprofit scientific professional organization whose mission is excellence in the science and practice of radiation safety, states “In accordance with current knowledge of radiation health risks, the Health Physics Society recommends against quantitative estimation of health risks below an individual dose of 5 rem [50 mSv] in one year or a lifetime dose of 10 rem [100 mSv] above that received from natural sources. ... below 5-10 rem [50 - 100 mSv] ... risks of health effects are either too small to be observed or are nonexistent.”
Thus, our purpose is to counter the alarmist statements being made in both general and professional forums regarding the potential dangers to public health from the increased use of CT imaging. We address this topic in terms of the two fundamental principles of radiation protection as applied to medicine: justification and optimization.
II. Justification
The field of radiation protection, which seeks to minimize the radiation dose levels to exposed persons, embraces three guiding principles when applied to medical exposures:
Justification: The exam or procedure must be medically indicated.
Optimization: The exam or procedure must use doses that are As Low As Reasonably Achievable (ALARA), without compromising the diagnostic task.
Limitation: In medicine, upper limits to dose levels are typical only for occupationally exposed individuals (i.e. the radiologist or technologist). Limits are rarely established for medically-necessary exams or procedures. One example where patient dose limits have been established is in screening mammography. However, when a screening mammogram, physical examination, or patient symptoms indicate the need for a diagnostic mammogram, no dose limits are applied. The philosophy of the U.S. Food and Drug Administration (FDA) is not to establish dose limits because, as with any medicine or medical intervention, the medical practitioner must be able to tailor the exam to the particular patient and medical concern.
A. Maximizing Benefit-to-Risk Ratio
A CT exam should be performed only when the radiation dose is deemed to be justified by the potential clinical benefit to the patient. An alternate way of stating this is that the risk of not performing the exam (e.g. delayed or inaccurate diagnoses or treatment) must exceed the risk associated with the examination. Medical justification includes a consideration of evidence-based recommendations for relevant clinical scenarios and an understanding of the risk of disease for each patient. Where the health risks and likelihood of a disease are high, increased risk from radiation and intravenous contrast media is justified if CT can detect the disease (e.g., hospitalized patients with sepsis). Additionally, increased benefits from higher tube currents (and radiation dose) are justified when they permit diagnostic quality images (e.g., in morbidly obese patients). Low-dose techniques are justified when the patient is asymptomatic or when image quality does not require discrimination between structures with soft tissue attenuation (e.g., CT colonography, repeat CT for renal stone disease). Justification should also take into account potential alternatives, such as ultrasound, MR, or optical imaging, as well as urgency and clinical availability. Once the determination is made that there is an appropriate CT exam that can benefit the patient, CT parameters should be optimized and dose-reduction techniques employed to perform the diagnostic task at the lowest level of radiation dose. These strategies are discussed in Section III.
B. Symptomatic Patients
The American College of Radiology Appropriateness Criteria and others have provided evidence-based guidelines to help physicians recommend an appropriate imaging test [14, 15]. Table 2 delineates some of the indications for which the ACR Gastrointestinal (GI) Expert Panel considered CT the most appropriate imaging option [16] (see Appendix 1 for a full listing). To illustrate their erudite considerations of justification and optimization, we consider two clinical scenarios—small bowel obstruction and suspected hepatic metastases.
Table 2.
List of all clinical scenarios to which the ACR Gastrointestinal expert panel considered CT as the most appropriate imaging modality. The appropriateness rank for CT (1 to 9, 9 being the most appropriate) is also listed
| General clinical scenario | Scenario variants to which CT is the most appropriate exam (appropriateness rank, 1 - 9)* | Scenario variants to which CT is not the most appropriate exam (Most appropriate modality, appropriateness Rank, CT appropriateness rank)* |
|---|---|---|
| Acute abdominal diffuse pain and fever or suspected abdominal abscess in adults | -Postoperative patient with fever (8) -Postoperative patient with persistent fever and no abscess seen on CT scan within the last 7 days (8). -Patient presenting with fever, non-localizing abdominal pain, and no recent operation (8). |
-Pregnancy (US abdomen, 8, 5) |
| Suspected Liver Metastases | -Initial imaging test following detection of primary tumor (8). -Surveillance following treatment of primary tumor (8). -Abnormal surveillance US, CT, or MRI, in PVP: high suspicion of malignancy (8, MR and percutaneous biopsy have the same score). -Abnormal surveillance US, CT, or MRI in PVP: high suspicion of benignancy (8, MR has the same score). |
|
| Liver Lesion Characterization | -Indeterminate on initial imaging, >1 cm, no suspicion or evidence of extrahepatic malignancy or liver disease. (CT or MR depending on availability, 8) -Indeterminate mass on initial imaging, >1 cm, known or suspected liver disease associated with a high risk of hepatocellular carcinoma (chronic hepatitis, cirrhosis, hemochromatosis, etc). (CT or MR depending on availability, 8) |
-Typical benign on initial imaging, no history of malignancy (No imaging at this time, 8, 4). -Typical benign on initial imaging, known history of extrahepatic malignancy (No imaging at this time, 8, 5). -Typical malignant hepatic mass on initial imaging (No imaging at this time, 7, 6). -Indeterminate solitary mass on initial imaging, >1 cm, known history of extrahepatic malignancy (Percutaneous biopsy liver, 8, 7). -Small lesion on initial imaging, <1 cm (No imaging at this time, 8, 5). |
| Small Bowel Obstruction | -Suspected complete or high-grade partial SBO (8). -Suspected intermittent or low-grade SBO (7). |
-Suspected intermittent or low-grade SBO (small bowel follow through and enteroclysis (7). |
| Crohn’s disease | -Adult; initial presentation (abdominal pain, fever, or diarrhea); Crohn’s disease suspected (8). -Initial presentation of a child (less than 14 years of age); Crohn’s disease suspected (8). -Adult with known Crohn’s disease and fever, increasing pain, leukocytosis, etc (8). -Child (less than 14 years of age) with known Crohn’s disease and fever, increasing pain, leukocytosis, etc (8). -Adult with known Crohn’s disease; stable, mild symptoms (7). |
-Child (less than 14 years of age) with known Crohn’s disease; stable, mild symptoms (US abdomen and pelvis, 6, 5). |
Small-bowel obstruction (SBO) accounts for 20% of all acute surgical admissions, potentially resulting in bowel ischemia, strangulation, or death, particularly if diagnosis is delayed. Due to its diagnostic accuracy for high-grade SBO (greater than 90%) and its ability to identify causative etiology, CT is recommended as the first line test in the initial evaluation of high grade SBO [16-19] (Table 2). If, on the other hand, clinical symptoms suggest a low grade small bowel obstruction, the accuracy of routine CT is much lower [18]. In such circumstances CT enteroclysis with larger volumes of oral contrast or fluoroscopy with real-time visualization of gut motility are likely more helpful in identifying points of low-grade obstruction [16]. MR enterography may be preferred for pregnant patients or in practices with greater MR experience [20, 21].
Suspected hepatic metastasis is a frequent indication for abdominal CT. The detection of hepatic metastases determines therapeutic decisions, with early detection and treatment of some metastases now resulting in cure as a result of subsequent chemotherapy, resection or radiofrequency ablation [22-24]. The theoretical increased risk of additional radiation in these scenarios is overwhelmed by the potential benefits of accurate detection and characterization of liver lesions. The GI expert panel ranked CT with contrast as the most appropriate initial imaging test following detection and treatment of a primary tumor. Multiple other tests (MRI with gadolinium, PET, percutaneous biopsy) were considered equally appropriate when a liver lesion was found on surveillance imaging, however they are also associated with some level of risk (e.g. nephrogenic systemic fibrosis, radioisotopes, or infection, respectively) [16].
To maximize patient benefit, CT acquisition parameters and patient preparation should be tailored to the individual patient and indication (i.e., to detect and/or characterize liver lesions). Portal-phase imaging is usually sufficient [25, 26] unless a patient’s primary tumor is hypervascular (e.g., neuroendocrine tumors) [27, 28], while higher spatial resolution imaging may be warranted in looking for some metastases or HCC [29, 30]. Higher tube currents may be needed if thinner sections are chosen (to offset increased image noise) and oral contrast agents may be needed to maximize the detection of other metastases in the bowel [31].
C. Asymptomatic Patients
Asymptomatic patients are a unique group of individuals to consider for CT exams, as their risk for disease is much lower [32]. CT colonography (CTC) is one screening exam that has undergone extensive scrutiny. Justification of CTC as a screening exam include the high mortality from colon cancer, its long preclinical course, and the potential for polypectomy to eliminate the progression to invasive cancer [32]. Given its performance characteristics [33-35], CTC was endorsed as an acceptable colorectal cancers screening test by the American Cancer Society in 2008 [36]. The potential risks of CTC are small, but include ionizing radiation, perforation and unnecessary treatment/workup of extracolonic findings. These must be balanced against the anticipated lifetime risk of colorectal cancer (5-6% [37]) and prevalence of advanced colorectal neoplasia (3-9% [38, 39]). Brenner and Georgsson, using the conservative linear non-threshold model, estimated the potential risk of radiation-induced malignancy from CTC to be 0.14% in a 50-year-old and 0.07% for a 70-year-old, with these risks falling further when optimized protocols are used [37]. They concluded that for CTC, the benefit-to-risk ratio was positive. The risk of perforation at screening CTC is also extremely low, probably slightly less than optical colonoscopy at 0.001-0.02% [40, 41]. Extracolonic findings may be beneficial [42, 43], but may also increase financial burden or morbidity [44, 45], resulting in ongoing efforts to minimize these effects [46].
D. Practice Decisions That Individualize and Maximize Patient Benefit
Each CT exam should be tailored and effectively implemented for each patient, based on clinical history, suspected disease (and pathophysiology), patient size, radiologic conspicuity, and morbidities affecting the use of intravenous and oral contrast agents, while also taking into account the acquisition capabilities of the specific CT system to be used. The complexity and interrelatedness of these multiple decisions argue for the central role for radiologists in guiding and coordinating these decisions. Such leadership maximizes the benefit-to-risk ratio for patients and involves coordinating knowledge transfer and communication between many members of the patient care team--including the referring clinician, radiologist, medical physicist, technologists, and nurses. In the past (before MDCT), this wide range of considerations had minimal consequences in the resultant CT exam. However now, with the advanced level of MDCT technology available at even small facilities, differences in patient preparation, exam acquisition and post-processing can have considerable impact on disease conspicuity, and ultimately, patient care.
The development of new CT technologies has facilitated the rapid growth of CT in medical practice. Faster scanning and improved spatial resolution have led to the incorporation of the CT scanner into the Emergency Department (as a method for triaging trauma), as well as the development of a wide array of organ-specific CT exams (e.g. CT angiography, enterography and urography) that guide management decisions. Emerging CT applications, such as CT cholangiography and dual energy CT, will continue to arise as radiologists and primary care providers incorporate new technological possibilities into patient care. Practices can maximize patient benefit by creating mechanisms that facilitate innovation as well as insure exam appropriateness, including the establishment of common CT acquisition protocols and quality programs while eliminating non-beneficial (inappropriate) exams.
The establishment of common CT acquisition and reconstruction protocols, which are tailored to each individual scanner model to deliver comparable image quality (section width, spatial resolution, temporal resolution, image noise, etc.), and contrast enhancement protocols (oral and/or IV), can improve clinical benefits for patients for several reasons. First, standardization reduces variations in the resultant images due to radiologist-, technologist-, or scanner-dependent factors. The ability to diagnose interval changes in follow-up examinations is thus greatly enhanced. Second, the use of common exam protocols allows for knowledge transfer from sub-specialized radiologists (e.g., a GI radiologist) to general radiologists elsewhere in the practice. Creation of standardized protocols requires input from medical physicists (to maximize image quality and ensure proper use of dose reduction techniques) and referring clinicians (to maximize impact on clinical decision-making). Interdisciplinary collaboration often leads to the conclusion that image quality and lesion detection are more important than lower radiation doses, particularly when the implications for misdiagnosis can be high (e.g., suspected pancreatic cancer or hepatocellular carcinoma). Third, standardized organ- or disease-specific protocols permit scheduling triage to the appropriate level of scanner technology in those practices where such options exist. For many CT exams, the difference in exam quality is minimally affected as long as some minimum level of CT technology is used. However, for some examinations, such as multiphase CT enterography (Figure 1) or obese patient scanning, patients should be preferentially scheduled on higher-detector-row systems (e.g. 64-MDCT or above) having higher x-ray power capabilities to allow multi-phase imaging at the proper time intervals with thin image widths (< 3mm) and low noise levels in large patients.
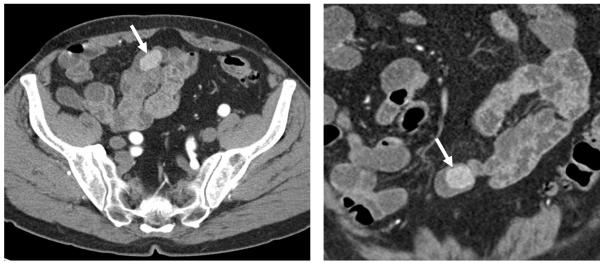
Figure 1.
84-year-old man with occult gastrointestinal blood loss and negative upper and lower endoscopies. A multiphase CT enterography was triaged to a 128-slice CT scanner due to isotropic spatial resolution and greater power compatibility permitting multiphase scanning of the abdomen and pelvis. A) axial and B) coronal images from the enteric phase demonstrate an enhancing ileal mass, which turned out to be a tubulovillous adenoma at surgical resection.
Quality programs that track compliance with practice-based decisions and imaging protocols can be extremely useful in identifying areas for improving patient care. We periodically monitor our general CT practice, as well as all CT colonography exams, for compliance with general guidelines and scanner-specific protocols [47]. Our experience with these programs is that they can identify areas needing improvement, minimize inter-observer variability or lack of communication between members of the healthcare team, and improve patient education and report timeliness and clarity [48]. Examples of changes we have made in our practice as a result of these quality programs include standardizing oral contrast for a variety of CT indications, and use of low-dose renal stone imaging for patients with a prior positive CT exam demonstrating urolithiasis.
Eliminating non-beneficial and inappropriate CT exams likely represents the most important method towards reducing CT risk. Evidence-based recommendations [15] or decision support tools [49] are employed by many practices to facilitate appropriate referral for CT imaging. These electronic tools can significantly reduce the number of low-utility exams [49] and seem to have wide clinical acceptability [50]. Non-beneficial CT exams ordered as a result of defensive medicine practices or self-referral are more problematic. Both defensive medicine and self-referral often result in an increased number of marginally beneficial imaging studies, and increases both cost as well as patient risk [51-53]. Successfully addressing these practices would likely require actions by third-party payers or the government [54]. Because it is unrealistic to expect that all patients referred for CT imaging will have entirely appropriate indications, practices should establish mechanisms for transferring patients to MR or ultrasound when these exams are more appropriate.
III. Optimization
Technical aspects of medically justified exams should be optimized, such that the required level of image quality is obtained while keeping dose as low as possible [55]. The radiology community (radiologists, medical physicists and manufacturers) has worked to implement ALARA principles in CT imaging [56-60], the most basic of which is the required adjustment of technique factors based on patient size (attenuation characteristics) [61, 62]. Appropriate dose management in CT means ensuring that the right dose is delivered for the specific patient attenuation and the specific diagnostic task.
A. Dose per Exam: The Historical View
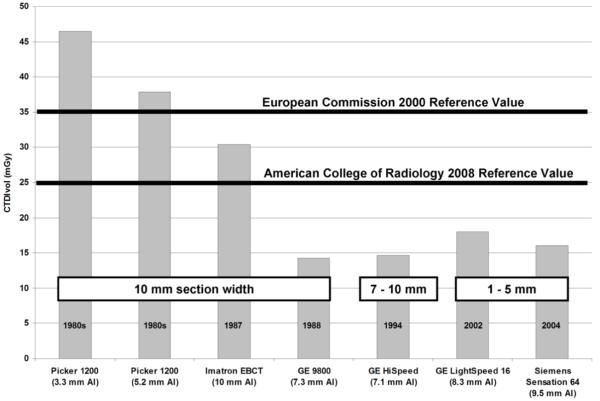
The radiation output from a CT scanner has been measured and reported in a very standardized manner since the early days of CT using a parameter known as the CT Dose Index (CTDI) [63-65]. The Volume CTDI (CTDIvol) is a newer metric of the radiation output of a scanner that takes into account overlap or gaps between consecutive rotations of the x-ray tube [66-68]. Because of this consistency in the CT dose metric, it is relatively straightforward to assess how the dose per exam has changed over the past two decades. Our large CT practice has operated over 100 different CT scanners since the original EMI Mark I head CT scanner was installed in 1973. Using CTDI data measured and stored at our institution (using the same sets of CTDI phantoms and with the same model of CTDI ionization chambers), and with knowledge of the routine scan parameters used for an abdominal CT scan, we calculated the CTDIvol for routine abdominal exams from the mid 1980s to 2004. The data shown in Figure 2 support the general statement that dose tends to decrease as the half-value layer of the beam spectrum increases, although other factors such as geometry and detector type also play an important role [69]. In 2000, the European Commission set their diagnostic reference value, which is an investigation level at the upper 75th percentile of doses used in clinical practice, at 35 mGy [70].
Figure 2.
Historical trends in radiation dose at the Mayo Clinic Rochester as measured using CTDIvol using standard phantoms during routine acceptance testing. Note decreased dose over time even given much smaller slice thicknesses over the last decade (adapted with the author’s permission from Health Phys. 2008 Nov;95(5):508-17).
Recently, the American College of Radiology’s CT Accreditation Program decreased their reference value from 35 mGy to 25 mGy, reflecting the downward trend in body CT doses over the last decade [71]. Newer multi-detector row CT systems have eliminated the dose inefficiency of the early multi-detector row systems (“4-slice” scanners) for thin images, and automatic exposure control (AEC) systems can lower patient dose dramatically (20-40%). Even in large patients, dose reductions can still be achieved using AEC because the dose is distributed more effectively [72]. Finally, in the 1980s, 10 mm images and multiple breath-holds were standard. Now, a 5 mm image width and a single-breath-hold is considered routine, with 2-3 mm image widths used for some applications (e.g. CT enterography) and reconstructions as narrow as 1 mm or less for multi-planar reformations or 3D renderings. Hence, today’s CT systems provide thinner image widths, improved spatial resolution and decreased partial volume averaging at a fraction of the scan time and patient dose than the lower quality, thicker slice width exams of older scanners.
B. CT Scanner Accreditation
A CT scanner’s radiation dose levels and image quality should fall within manufacturer specifications and comply with applicable regulatory requirements. Regional and national health care payers have begun to require that sites demonstrate satisfactory equipment performance and clinical use through programs such as the ACR’s CT Accreditation Program, which addresses both adult and pediatric practice. A thorough equipment quality assurance program should be designed and overseen by a qualified medical physicist, and supported with technologists who coordinate protocol development and perform basic image quality checks on a routine basis.
C. Fixed tube current (technique charts) and patient size
Unlike film-based radiographic imaging, a CT image never looks “over-exposed” in the sense of being too dark or too light. As a consequence, CT users are not technically compelled to decrease the tube-current-time product (mAs) for small patients, as is required in film-based radiography in order to avoid over-exposing the film (i.e. a too darkened film). Nonetheless, it remains the responsibility of the CT operator to take patient size into account when selecting parameters that affect radiation dose and image quality, the most basic of which is the tube-current-time product (mAs) [56, 60].
For most CT applications, it is common to standardize the tube potential (kV) and gantry rotation time (s): the fastest rotation time is typically used to minimize motion blurring or artifact and the lowest kV selected to maximize image contrast, provided that the patient size and tube current limits are adequate to provide sufficient mAs [72-76]. Thus, tube current, which simply scales the dose rate up or down, is the primary parameter that is used to take into consideration patient size.
Tube current should be adjusted as a function of patient size based on the overall attenuation of the anatomy of interest, as opposed to patient weight, which is not a perfect surrogate for attenuation [61, 62, 77]. Importantly, the evaluation of images obtained across a range of patient sizes demonstrated that radiologists do not find the same image noise level acceptable for all patient sizes [62]. Radiologists tend to demand lower image noise in children relative to larger patients because children often lack adipose tissue between organs and tissue planes and have smaller anatomic dimensions, [61, 62, 77, 78]. For body CT imaging, a reduction in mAs of a factor of 4 to 5 from adult techniques is generally acceptable in infants, while for obese patients, an increase of at least a factor of 2 is required [77]. To achieve sufficient exposure levels for obese patients, either the rotation time or the tube potential may need to be increased.
D. Angular and longitudinal (x, y, z) tube current (mAs) modulation
Angular mA modulation deals with the variations in x-ray attenuation within a scan plane (e.g. in the A.P. versus lateral direction). The mA is varied as the x-ray tube rotates according to the patient attenuation at the same level. Attenuation information is determined from the CT radiograph (e.g. scout image) or from the previous 180° projection. Longitudinal (z) mA modulation takes into account variations in attenuation among different regions (e.g. shoulders versus abdomen) by varying the mA along the patient’s long axis. The operator must, however, prexcribe the desired level of image quality. The paradigms used to accomplish this are at present relatively manufacturer-specific. Combining angular and longitudinal (x,y,z) mA modulation is the most comprehensive approach to CT dose reduction.
E. Automatic exposure control (AEC)
Modern CT systems adjust the x-ray tube current in response to variations in patient attenuation [79-82]. Methods of adapting the tube current to patient attenuation, known generically as AEC systems, are analogous to photo-timing in general radiography and have demonstrated reductions in dose of about 20-40% when image quality is appropriately specified. An exception to this trend occurs with obese patients, in which the radiation output of the system is increased to ensure adequate image quality. In obese patients, however, much of the additional x-ray dose is absorbed by excess adipose tissue. Thus, doses to internal organs do not increase linearly with increases in tube current [83]. AEC is a broad term that encompasses not only tube current modulation (to adapt to changes in patient attenuation), but also determining and delivering the “right” dose for any patient (infant to obese) in order to achieve the diagnostic task. Because the specific implementations of AEC differ by manufacturer, users need to take the time to understand how their particular system functions and to set up the image quality reference appropriately for differing diagnostic tasks (e.g. CT colonography vs routine abdomen or CT enterography). In addition, the image noise/quality requirements should be adapted for pediatric and obese patients, as prior studies have demonstrated that the same level of image noise is not clinically accepted across large variations in patient size [62, 72, 78, 84, 85].
F. Adjusting kV based on patient size
Several investigators have studied the use of lower tube potential (kV) CT imaging to improve image quality or reduce radiation dose. The physics principle behind lower kV imaging is that the attenuation coefficient of iodine increases as photon energy decreases toward the k-edge energy of 33 keV. In CT exams involving the use of iodinated contrast media, the superior enhancement of iodine at lower tube potentials improves the conspicuity of hypervascular or hypovascular pathologies. However, images obtained using lower tube potentials tend to be much noisier, mainly due to the higher absorption of low-energy photons by the patient, unless the tube current is adequately adjusted [56, 73, 75, 76, 80-82, 86]. This continues to be an area of active investigation, particularly in pediatric CT (Figure 3).
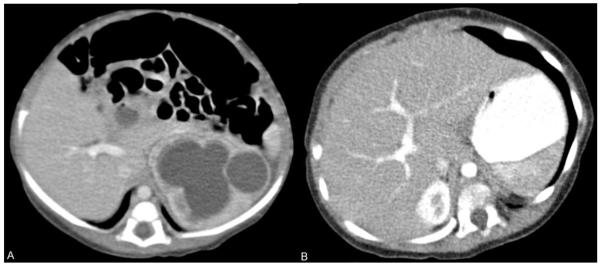
Figure 3.
1-year-old male undergoing treatment for fibrosarcoma underwent two contrast-enhanced CT scans.
A) axial contrast-enhanced 3 mm slice at the level of the right portal vein obtained using 120 kV and CTDIvol of 3.5 mGy. The left hydronephrosis resolved following resection of the pelvic tumor (not shown).
B) follow-up CT scan 9 months later showing axial contrast-enhanced 3 mm slice at the level of the right portal vein shows similar image quality using 80 kV and a CTDIvol of 1.69 mGy (a 50% dose savings). Lowering tube potential in smaller patients is a means of improving image contrast while simultaneously reducing radiation dose.
G. Examples of patient-specific dose reduction strategies
Pregnant patients
Imaging of the pregnant patient includes the need to consider the radiation risk to the embryo/fetus. Common indications for CT in a pregnant patient include suspected pulmonary embolism, appendicitis or urinary tract calculi. For scanning regions outside the abdomen and pelvis, such as for suspected pulmonary embolism, the dose to the fetus is very low (< 0.1 mGy) as only scattered radiation reaches the fetus. For all indications, the scan volume should be restricted to the necessary anatomy, and dual-pass (with and without contrast) studies should be avoided, if possible [87]. Fetal dose can be reduced with careful selection of the scan parameters (e.g. use of wider beam collimation, higher pitch, lower mAs, lower kV or reduced scan range). It is important, however, to realize that for a routine bi-phase CT exam of the abdomen and pelvis, the probability of birthing a healthy baby decreases by only 0.1%, from 96.00 to 95.90%, and the probability that the baby will be born healthy and not develop childhood cancer changes by only 0.5%, from 95.93 to 95.43% [87-89].
Pediatric patients
When a CT examination is deemed to be needed in a pediatric patient, the scan protocol must be specifically tailored for use in children [90], using dose reduction features such as tube current modulation [72, 79], a child-size bowtie filter and scan field of view (FOV), or a weight or size-based technique chart to determine the appropriate kV and/or mAs [91, 92]. For pediatric patients, the noise level does not increase significantly with the decrease of kV. Because of this, the dose reduction or image quality improvement from low kV imaging is much more significant than in adult patients [61, 76, 91, 92]. It is essential, however, that a weight or size-based kV/mAs technique chart is used in order to avoid excessive noise levels and avoid motion artifacts or long scan times. Thus, the lower-kV scan protocol has to be carefully evaluated by radiologists and physicists for every type of pediatric exam.
Selective in-plane shielding
Products are commercially available to selectively shield radiation sensitive tissues and organs during CT scanning. However, investigations that consider both image quality and dose have demonstrated that tube current modulation, or simply reduced tube current, can achieve greater dose reductions relative to the in-plane shielding for the same resultant image noise. In addition, decreasing the tube current does not introduce the beam-hardening and steak artifacts observed with use of in-plane shielding, some of which have been shown to significantly alter CT number accuracy [93, 94].
IV. Conclusion
In recent years, the media has focused on the potential danger of radiation exposure from CT while ignoring the potential for great individual benefit. The attention drawn to CT dose issues resulted in increased awareness within the radiology community of the need to carefully adapt scan parameters to the individual patient and exam. However, an increased level of patient anxiety has also ensued, occasionally resulting in a refusal to undergo a medically indicated exam. Reliable data are not available as to the frequency of this occurrence, however anecdotal reports - such as an 84 year old patient scheduled for aortic dissection repair who refused a pre-surgical stent-planning CT because of “that cancer causing stuff” - are alarming. The benefits of CT imaging - including the avoidance of exploratory or negative surgeries - cannot simply be dismissed in the introductory statements of articles that go on to emphasize the risks associated with ionizing radiation. Neither can exams such as MRI or ultrasound be endorsed simply because they avoid ionizing radiation. Truly mitigating the risk of CT means maximizing individual patient benefit, working with providers to ensure appropriate referral, and individualizing patient preparation and CT acquisition and post-processing techniques to maximize disease conspicuity and impact therapeutic decisions. Dose reduction strategies described in this paper must be well understood and properly used, but require broad-based practice strategies that extend beyond the CT scanner console and default, generic manufacturer settings. In the final analysis, physicians must request the imaging examination that best addresses the specific medical question, without allowing worries about radiation to dissuade them or their patients from obtaining needed CT examinations. Ongoing efforts to ensure that CT examinations are both medically justified and optimally performed must continue, and education must be provided to the medical community and general public that put both the risks - and benefits - of CT exams into proper perspective.
V. Acknowledgments
The authors thank Kris Nunez for her help with manuscript preparation and Dr. Jeremy McBride for his suggestions. We also wish to thank the Calouste Gulbenkian Foundation (Lisbon, Portugal), which provided salary support for Dr. Luís Guimarães.
Appendix
Table.
Appendix
| General clinical scenario | Scenario variants to which CT is the most appropriate exam (appropriateness rank, 1 - 9)* | Scenario variants to which CT is not the most appropriate exam (Most appropriate modality, appropriateness Rank, CT appropriateness rank)* |
|---|---|---|
| Acute abdominal diffuse pain and fever or suspected abdominal abscess in adults | Postoperative patient with fever (8) Postoperative patient with persistent fever and no abscess seen on CT scan within the last 7 days (8). Patient presenting with fever, non-localizing abdominal pain, and no recent operation (8). |
Pregnancy (US abdomen, 8, 5) |
| Acute pancreatitis | Severe abdominal pain, elevated amylase lipase, 48 hours later assuming no improvement or degradation (assume no prior imaging) (8). Severe abdominal pain, elevated amylase lipase, fever and elevated white blood cell count (9). Severe abdominal pain, elevated amylase lipase, hemoconcentration, oliguria, tachycardia (9). |
Etiology unknown, first episode of pancreatitis (US abdomen, 8, 6). Severe abdominal pain, elevated amylase lipase, no fever or evidence of fluid loss at admission; clinical score pending (US abdomen, 8, 7). |
| Blunt Abdominal Trauma | Stable patient (8) Hematuria >35 RBC/HPF (stable) (8). |
Unstable patient (US screen for hemoperitoneum, 7, 4). |
| Crohn’s disease | Adult; initial presentation (abdominal pain, fever, or diarrhea); Crohn’s disease suspected (8). Initial presentation of a child (less than 14 years of age); Crohn’s disease suspected (8). Adult with known Crohn’s disease and fever, increasing pain, leukocytosis, etc (8). Child (less than 14 years of age) with known Crohn’s disease and fever, increasing pain, leukocytosis, etc (8). Adult with known Crohn’s disease; stable, mild symptoms (7). |
Child (less than 14 years of age) with known Crohn’s disease; stable, mild symptoms (US abdomen and pelvis, 6, 5). |
| Dysphagia | Oropharyngeal dysphagia with an attributable cause (X-ray barium swallow modified, 8, CT not mentioned). Unexplained oropharyngeal dysphagia (X-ray pharynx dynamic and static imaging, 8, CT not mentioned). |
|
| Jaundice | Painless; one or more of the following: weight loss, fatigue, anorexia, duration of symptoms greater than 3 months. Patient otherwise healthy (9). Painless; one or more of the following: weight loss, fatigue, anorexia, duration of symptoms greater than 3 months. Patient will not tolerate radical surgical procedure (9). |
Acute abdominal pain; at least one of the following: fever, history of biliary surgery, known cholelithiasis (US abdomen, 9, 7). Clinical condition and laboratory examination make mechanical obstruction unlikely (US abdomen, 8, 5). Confusing clinical picture; patient not described in previous scenarios (US abdomen, 8, 7). |
| Left Lower Quadrant Pain | Older patient with typical clinical presentation for diverticulitis (8). Acute, severe, with or without fever (9). Chronic, intermittent, or low grade (8). Obese patient (8). |
Woman of childbearing age (US abdomen transabdominal with graded compression, 8, 7). |
| Liver Lesion Characterization | Indeterminate on initial imaging, >1 cm, no suspicion or evidence of extrahepatic malignancy or liver disease. (CT or MR depending on availability, 8) Indeterminate mass on initial imaging, >1 cm, known or suspected liver disease associated with a high risk of hepatocellular carcinoma (chronic hepatitis, cirrhosis, hemochromatosis, etc). (CT or MR depending on availability, 8) |
Typical benign on initial imaging, no history of malignancy (No imaging at this time, 8, 4). Typical benign on initial imaging, known history of extrahepatic malignancy (No imaging at this time, 8, 5). Typical malignant hepatic mass on initial imaging (No imaging at this time, 7, 6). Indeterminate solitary mass on initial imaging, >1 cm, known history of extrahepatic malignancy (Percutaneous biopsy liver, 8, 7). Small lesion on initial imaging, <1 cm (No imaging at this time, 8, 5). |
| Palpable abdominal mass | Palpable abdominal mass (8) | |
| Pretreatment Staging of Colorectal Cancer | Rectal cancer, large lesion (8). Colon cancer, other than rectum (8). |
Rectal cancer, small or superficial (US rectum transrectal, 8, 6). |
| Right Lower Quadrant pain | Fever, leukocytosis, and classic presentation clinically for appendicitis in adults (8). Fever, leukocytosis; possible appendicitis, atypical presentation, adults and adolescents (8). |
Fever, leukocytosis, pregnant woman (US abdomen RLQ, 8, 6) Fever, leukocytosis, possible appendicitis, atypical presentation in children, less than 14 years of age (US abdomen RLQ, 8, 7). |
| Right Upper Quadrant Pain | Fever, elevated WBC, positive Murphy sign (US abdomen, 9, 5). Suspected acalculous cholecystitis (NUC cholescintigraphy, 8, 6). No fever, normal WBC (US abdomen, 8, 7). No fever, normal WBC, ultrasound shows only gallstones (NUC cholescintigraphy, 8, 6). Hospitalized patient with fever, elevated WBC, and positive Murphy sign (US abdomen, 9, 7). |
|
| Suspected Liver Metastases | Initial imaging test following detection of primary tumor (8). Surveillance following treatment of primary tumor (8). Abnormal surveillance US, CT, or MRI, in PVP: high suspicion of malignancy (8, MR and percutaneous biopsy have the same score). Abnormal surveillance US, CT, or MRI in PVP: high suspicion of benignancy (8, MR has the same score). |
|
| Suspected Small Bowel Obstruction | Suspected complete or high-grade partial SBO (8). Suspected intermittent or low-grade SBO (7, small bowel follow-through and enteroclysis have the same score). |
|
References
- 1.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Elliston CD, Hall EJ, Berdon WE. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 3.Rogers LF. Radiation exposure in CT: why so high? AJR Am J Roentgenol. 2001;177:277. doi: 10.2214/ajr.177.2.1770277. [DOI] [PubMed] [Google Scholar]
- 4.National Council on Radiation Protection and Measurements . Ionizing radiation exposure of the population of the United States. National Council on Radiation Protection and Measurements; Bethesda, MD: 1987. Report No. 93. [Google Scholar]
- 5.Committee on Passive Smoking (National Research Council) Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. National Academies Press; Washington, DC: 1986. [PubMed] [Google Scholar]
- 6.FDA [Accessed 3/9/09];Whole body scanning using computed tomography (CT) 2002 wwwfdagov/cdrh/ct/riskshtml.
- 7.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 8.National Safety Council [accessed 3/9/09];Odds of Death Due to Injury. 2005 http://www.nsc.org/research/odds.aspx.
- 9.Nero AV, Schwehr MB, Nazaroff WW, Revzan KL. Distribution of airborne radon-222 concentrations in U.S. homes. Science. 1986;234:992–997. doi: 10.1126/science.3775373. [DOI] [PubMed] [Google Scholar]
- 10.Smith AH, Hopenhayn-Rich C, Bates MN, et al. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subcommittee on Arsenic in Drinking Water (National Research Council) Arsenic in Drinking Water. National Academies Press; Washington, DC: 1999. [PubMed] [Google Scholar]
- 12.National Council on Radiation Protection and Measurements . Ionizing radiation exposure of the population of the United States. National Council on Radiation Protection and Measurements; Bethesda, MD: 2009. Report No. 160. [Google Scholar]
- 13.Health Physics Society . Position Statement of the Health Physics Society: PS010-1. McLean, VA: 2004. Radiation risk in perspective. [DOI] [PubMed] [Google Scholar]
- 14.The Royal College of Radiologists . Making the best use of clinical radiology services: referral guidelines. 6th ed. London: 2007. [Google Scholar]
- 15.American College of Radiology ACR Appropriateness Criteria, October 2008 Version. 2008 http://wwwacrorg/SecondaryMainMenuCategories/quality_safety/app_criteriaaspx.
- 16.American College of Radiology ACR Appropriateness Criteria for Gastrointestinal Imaging. http://wwwacrorg/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImagingaspx.
- 17.Frager D, Medwid SW, Baer JW, Mollinelli B, Friedman M. CT of small-bowel obstruction: value in establishing the diagnosis and determining the degree and cause. Ajr. 1994;162:37–41. doi: 10.2214/ajr.162.1.8273686. [DOI] [PubMed] [Google Scholar]
- 18.Maglinte DD, Gage SN, Harmon BH, et al. Obstruction of the small intestine: accuracy and role of CT in diagnosis. Radiology. 1993;188:61–64. doi: 10.1148/radiology.188.1.8511318. [DOI] [PubMed] [Google Scholar]
- 19.Megibow AJ, Balthazar EJ, Cho KC, Medwid SW, Birnbaum BA, Noz ME. Bowel obstruction: evaluation with CT. Radiology. 1991;180:313–318. doi: 10.1148/radiology.180.2.2068291. [DOI] [PubMed] [Google Scholar]
- 20.Beall DP, Fortman BJ, Lawler BC, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57:719–724. doi: 10.1053/crad.2001.0735. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe TA, Miller CM, Merkle EM. Practice patterns in imaging of the pregnant patient with abdominal pain: a survey of academic centers. Ajr. 2007;189:1128–1134. doi: 10.2214/AJR.07.2277. [DOI] [PubMed] [Google Scholar]
- 22.Curley SA. Outcomes after surgical treatment of colorectal cancer liver metastases. Semin Oncol. 2005;32:S109–111. doi: 10.1053/j.seminoncol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. Ajr. 2007;189:883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 24.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis IR, Cohan RH, McNulty NJ, et al. Multidetector CT of the liver and hepatic neoplasms: effect of multiphasic imaging on tumor conspicuity and vascular enhancement. Ajr. 2003;180:1217–1224. doi: 10.2214/ajr.180.5.1801217. [DOI] [PubMed] [Google Scholar]
- 26.Sheafor DH, Frederick MG, Paulson EK, Keogan MT, DeLong DM, Nelson RC. Comparison of unenhanced, hepatic arterial-dominant, and portal venous-dominant phase helical CT for the detection of liver metastases in women with breast carcinoma. Ajr. 1999;172:961–968. doi: 10.2214/ajr.172.4.10587129. [DOI] [PubMed] [Google Scholar]
- 27.Miller FH, Butler RS, Hoff FL, Fitzgerald SW, Nemcek AA, Jr., Gore RM. Using triphasic helical CT to detect focal hepatic lesions in patients with neoplasms. Ajr. 1998;171:643–649. doi: 10.2214/ajr.171.3.9725290. [DOI] [PubMed] [Google Scholar]
- 28.Silverman PM. Liver metastases: imaging considerations for protocol development with multislice CT (MSCT) Cancer Imaging. 2006;6:175–181. doi: 10.1102/1470-7330.2006.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher JG, Wiersema MJ, Farrell MA, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81–90. doi: 10.1148/radiol.2291020582. [DOI] [PubMed] [Google Scholar]
- 30.Kawata S, Murakami T, Kim T, et al. Multidetector CT: diagnostic impact of slice thickness on detection of hypervascular hepatocellular carcinoma. Ajr. 2002;179:61–66. doi: 10.2214/ajr.179.1.1790061. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Ha H, Park S, et al. Gastrointestinal Tract Metastasis from Primary Lung Cancer: CT Findings and Clinicopathologic Features. Ajr. 2009 doi: 10.2214/AJR.08.1907. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Obuchowski NA, Graham RJ, Baker ME, Powell KA. Ten criteria for effective screening: their application to multislice CT screening for pulmonary and colorectal cancers. Ajr. 2001;176:1357–1362. doi: 10.2214/ajr.176.6.1761357. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CD, Chen M-H, Toledano AY. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. al. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 36.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA: a cancer journal for clinicians. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 37.Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129:328–337. doi: 10.1053/j.gastro.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy Screening: Implications for CT Colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DH, Pickhardt PJ, Taylor AJ. Characteristics of advanced adenomas detected at CT colonographic screening: implications for appropriate polyp size thresholds for polypectomy versus surveillance. Ajr. 2007;188:940–944. doi: 10.2214/AJR.06.0764. [DOI] [PubMed] [Google Scholar]
- 40.Limburg PJ, Fletcher JG. Making sense of CT colonography-related complication rates. Gastroenterology. 2006;131:2023–2024. doi: 10.1053/j.gastro.2006.10.060. discusion 2024. [DOI] [PubMed] [Google Scholar]
- 41.Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313–316. doi: 10.1148/radiol.2392052002. [DOI] [PubMed] [Google Scholar]
- 42.Xiong T, Richardson M, Woodroffe R, Halligan S, Morton D, Lilford RJ. Incidental lesions found on CT colonography: their nature and frequency. The British journal of radiology. 2005;78:22–29. doi: 10.1259/bjr/67998962. [DOI] [PubMed] [Google Scholar]
- 43.Hassan C, Pickhardt PJ, Laghi A, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Archives of internal medicine. 2008;168:696–705. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 44.Gluecker TM, Johnson CD, Wilson LA, et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology. 2003;124:911–916. doi: 10.1053/gast.2003.50158. [DOI] [PubMed] [Google Scholar]
- 45.Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249:151–159. doi: 10.1148/radiol.2491072148. [DOI] [PubMed] [Google Scholar]
- 46.Berland LL. Incidental extracolonic findings on CT colonography: the impending deluge and its implications. J Am Coll Radiol. 2009;6:14–20. doi: 10.1016/j.jacr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 47. [accessed 3/9/09];ACR NRDR Database. https://nrdr.acr.org/portal/CTC/Main/page.aspx.
- 48.Blackmore CC. Defining quality in radiology. J Am Coll Radiol. 2007;4:217–223. doi: 10.1016/j.jacr.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Sistrom CL, Dang PA, Weilburg JB, Dreyer KJ, Rosenthal DI, Thrall JH. Effect of Computerized Order Entry with Integrated Decision Support on the Growth of Outpatient Procedure Volumes: Seven-year Time Series Analysis. Radiology. 2009 doi: 10.1148/radiol.2511081174. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal DI, Weilburg JB, Schultz T, et al. Radiology order entry with decision support: initial clinical experience. J Am Coll Radiol. 2006;3:799–806. doi: 10.1016/j.jacr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Hillman BJ, Joseph CA, Mabry MR, Sunshine JH, Kennedy SD, Noether M. Frequency and costs of diagnostic imaging in office practice--a comparison of self-referring and radiologist-referring physicians. The New England journal of medicine. 1990;323:1604–1608. doi: 10.1056/NEJM199012063232306. [DOI] [PubMed] [Google Scholar]
- 52.Hillman BJ, Olson GT, Griffith PE, et al. Physicians’ utilization and charges for outpatient diagnostic imaging in a Medicare population. Jama. 1992;268:2050–2054. [PubMed] [Google Scholar]
- 53.Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. Jama. 2005;293:2609–2617. doi: 10.1001/jama.293.21.2609. [DOI] [PubMed] [Google Scholar]
- 54.Dunnick NR, Applegate KE, Arenson RL. The inappropriate use of imaging studies: a report of the 2004 Intersociety Conference. J Am Coll Radiol. 2005;2:401–406. doi: 10.1016/j.jacr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 55.McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for Reducing Radiation Dose in CT. Radiol Clin North Am. 2009;47:27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.FDA FDA public health notification: reducing radiation risk from computed tomography for pediatric and small adult patients. Pediatr Radiol. 2002;32:314–316. doi: 10.1007/s00247-002-0687-6. [DOI] [PubMed] [Google Scholar]
- 57.Frush DP, Donnelly LF, Rosen NS. Computed tomography and radiation risks: what pediatric health care providers should know. Pediatrics. 2003;112:951–957. doi: 10.1542/peds.112.4.951. [DOI] [PubMed] [Google Scholar]
- 58.International Commission on Radiological Protection Managing patient dose in computed tomography, ICRP Publication 87. Ann ICRP. 2000;30:7–45. doi: 10.1016/s0146-6453(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 59.Kalra MK, Maher MM, Toth TL, et al. Strategies for CT radiation dose optimization. Radiology. 2004;230:619–628. doi: 10.1148/radiol.2303021726. [DOI] [PubMed] [Google Scholar]
- 60.Linton OW, Mettler FA., Jr. National conference on dose reduction in CT, with an emphasis on pediatric patients. Am J Roentgenol. 2003;181:321–329. doi: 10.2214/ajr.181.2.1810321. [DOI] [PubMed] [Google Scholar]
- 61.Boone JM, Geraghty EM, Seibert JA, Wootton-Gorges SL. Dose reduction in pediatric CT: a rational approach. Radiology. 2003;228:352–360. doi: 10.1148/radiol.2282020471. [DOI] [PubMed] [Google Scholar]
- 62.Wilting JE, Zwartkruis A, van Leeuwen MS, Timmer J, Kamphuis AG, Feldberg M. A rational approach to dose reduction in CT: individualized scan protocols. Eur Radiol. 2001;11:2627–2632. doi: 10.1007/s003300101039. [DOI] [PubMed] [Google Scholar]
- 63.American Association of Physicists in Medicine . Standardized methods for measuring diagnostic x-ray exposures. AAPM; New York: 1990. [Google Scholar]
- 64.Jucius RA, Kambic GX. Radiation dosimetry in computed tomography. Application of optical instrumentation in medicine (Part VI) Proceedings of the Society of Photo Optical Instrumentation in Engineering. 1977;127:286–295. [Google Scholar]
- 65.Shope TB, Gagne RM, Johnson GC. A method for describing the doses delivered by transmission x-ray computed tomography. Med Phys. 1981;8:488–495. doi: 10.1118/1.594995. [DOI] [PubMed] [Google Scholar]
- 66.American Association of Physicists in Medicine . AAPM Task Group 23 of the Diagnostic Imaging Council CT Committee. College Park, MD: 2008. The measurement, reporting and management of radiation dose in CT. [Google Scholar]
- 67.International Electrotechnical Commission . Medical Electrical Equipment. Part 2-44: Particular requirements for the safety of x-ray equipment for computed tomography. Ed. 2.1. International Electrotechnical Commission (IEC) Central Office; Geneva, Switzerland: 2002. IEC publication No. 60601-2-44. [Google Scholar]
- 68.McNitt-Gray MF. AAPM/RSNA Physics Tutorial for Residents: Topics in CT. Radiation dose in CT. Radiographics. 2002;22:1541–1553. doi: 10.1148/rg.226025128. [DOI] [PubMed] [Google Scholar]
- 69.McCollough CH, Zink FE, Morin RL. Radiation dosimetry for electron beam CT. Radiology. 1994;192:637–643. doi: 10.1148/radiology.192.3.8058927. [DOI] [PubMed] [Google Scholar]
- 70.European Commission . European guidelines on quality criteria for computed tomography (EUR 16262 EN) European Commission; Luxembourg: 2000. [Google Scholar]
- 71.McCollough C, Branham T, Herlihy V, et al. Radiation Doses from the ACR CT Accreditation Program: Review of Data Since Program Inception and Proposals for New Reference Values and Pass/Fail Limits. RSNA; Chicago, IL: 2006. [Google Scholar]
- 72.McCollough CH, Bruesewitz MR, Kofler JM., Jr. CT dose reduction and dose management tools: overview of available options. Radiographics. 2006;26:503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 73.Funama Y, Awai K, Nakayama Y, et al. Radiation dose reduction without degradation of low-contrast detectability at abdominal multisection CT with a low-tube voltage technique: phantom study. Radiology. 2005;237:905–910. doi: 10.1148/radiol.2373041643. [DOI] [PubMed] [Google Scholar]
- 74.Huda W, Ravenel JG, Scalzetti EM. How do radiographic techniques affect image quality and patient doses in CT? Seminars in ultrasound, CT, and MR. 2002;23:411–422. doi: 10.1016/s0887-2171(02)90012-0. [DOI] [PubMed] [Google Scholar]
- 75.Nakayama Y, Awai K, Funama Y, et al. Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology. 2005;237:945–951. doi: 10.1148/radiol.2373041655. [DOI] [PubMed] [Google Scholar]
- 76.Siegel MJ, Schmidt B, Bradley D, Suess C, Hildebolt C. Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape. Radiology. 2004;233:515–522. doi: 10.1148/radiol.2332032107. [DOI] [PubMed] [Google Scholar]
- 77.McCollough CH, Zink FE, Kofler J, Matsumoto JS, Thomas KB, Hoffman AD. Dose optimization in CT: creation, implementation and clinical acceptance of size-based technique charts. Radiology. 2002;225(P):591. [Google Scholar]
- 78.Kalra MK, Maher MM, Toth TL, et al. Techniques and applications of automatic tube current modulation for CT. Radiology. 2004;233:649–657. doi: 10.1148/radiol.2333031150. [DOI] [PubMed] [Google Scholar]
- 79.Gies M, Kalender WA, Wolf H, Suess C, Madsen M. Dose reduction in CT by anatomically adapted tube current modulation: Simulation studies. Medical Physics. 1999;26:2235–2247. doi: 10.1118/1.598779. [DOI] [PubMed] [Google Scholar]
- 80.Haaga JR, Miraldi F, MacIntyre W, LiPuma JP, Bryan PJ, Wiesen E. The effect of mAs variation upon computed tomography image quality as evaluated by in vivo and in vitro studies. Radiology. 1981;138:449–454. doi: 10.1148/radiology.138.2.7455129. [DOI] [PubMed] [Google Scholar]
- 81.Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation: Phantom measurements. Med Phys. 1999;26:2248–2253. doi: 10.1118/1.598738. [DOI] [PubMed] [Google Scholar]
- 82.McCollough CH. Automatic exposure control in CT: are we done yet? Radiology. 2005;237:755–756. doi: 10.1148/radiol.2373051151. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt B, Kalender WA. A fast voxel-based Monte Carlo method for scanner- and patient-specific dose calculations in computed tomography. In: Guerra AD, editor. Physica Medica. European Journal of Medical Physics; Erlangen, Germany: 2002. pp. 43–53. [Google Scholar]
- 84.Kalra MK, Maher MM, D’Souza RV, et al. Detection of urinary tract stones at low-radiation-dose CT with z-axis automatic tube current modulation: phantom and clinical studies. Radiology. 2005;235:523–529. doi: 10.1148/radiol.2352040331. [DOI] [PubMed] [Google Scholar]
- 85.Nyman U, Ahl TL, Kristiansson M, Nilsson L, Wettemark S. Patient-circumference-adapted dose regulation in body computed tomography. A practical and flexible formula. Acta Radiol. 2005;46:396–406. doi: 10.1080/02841850510021193. [DOI] [PubMed] [Google Scholar]
- 86.Huda W, Scalzetti EM, Levin G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology. 2000;217:430–435. doi: 10.1148/radiology.217.2.r00nv35430. [DOI] [PubMed] [Google Scholar]
- 87.Wagner LK, Huda W. When a pregnant woman with suspected appendicitis is referred for a CT scan, what should a radiologist do to minimize potential radiation risks? Pediatric Radiology. 2004;34:589–590. doi: 10.1007/s00247-004-1206-8. [DOI] [PubMed] [Google Scholar]
- 88.McCollough CH, Schueler BA, Atwell TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27:909–917. doi: 10.1148/rg.274065149. discussion 917-908. [DOI] [PubMed] [Google Scholar]
- 89.Wagner LK, Lester RG, Saldana LR. Exposure of the Pregnant Patient to Diagnostic Radiations: A Guide to Medical Management. Medical Physics Publishing; Madison: 1997. [Google Scholar]
- 90.Frush DP, Donnelly LF. Helical CT in children: technical considerations and body applications. Radiology. 1998;209:37–48. doi: 10.1148/radiology.209.1.9769810. [DOI] [PubMed] [Google Scholar]
- 91.Hollingsworth C, Frush DP, Cross M, Lucaya J. Helical CT of the body: a survey of techniques used for pediatric patients. Ajr. 2003;180:401–406. doi: 10.2214/ajr.180.2.1800401. [DOI] [PubMed] [Google Scholar]
- 92.Paterson A, Frush DP. Dose reduction in paediatric MDCT: general principles. Clin Radiol. 2007;62:507–517. doi: 10.1016/j.crad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Geleijns J, Salvado Artells M, Veldkamp WJ, Lopez Tortosa M, Calzado Cantera A. Quantitative assessment of selective in-plane shielding of tissues in computed tomography through evaluation of absorbed dose and image quality. Eur Radiol. 2006;16:2334–2340. doi: 10.1007/s00330-006-0217-2. [DOI] [PubMed] [Google Scholar]
- 94.Leswick DA, Hunt MM, Webster ST, Fladeland DA. Thyroid shields versus z-axis automatic tube current modulation for dose reduction at neck CT. Radiology. 2008;249:572–580. doi: 10.1148/radiol.2492071430. [DOI] [PubMed] [Google Scholar]